Published online Jan 27, 2024. doi: 10.4254/wjh.v16.i1.54
Peer-review started: October 2, 2023
First decision: October 9, 2023
Revised: October 22, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 27, 2024
Processing time: 112 Days and 15.1 Hours
Metabolic-associated fatty liver disease (MAFLD) is a liver condition marked by excessive fat buildup in the absence of heavy alcohol use. It is primarily linked with metabolic issues like insulin resistance, obesity, and abnormal lipid levels, and is often observed with other conditions such as type 2 diabetes and cardiovas
To compare the severity of liver fibrosis among different MAFLD subtypes.
A total of 322 adult patients of either gender with fatty liver on ultrasound were enrolled between January to December 2021. MAFLD was defined as per the Asian Pacific Association for the Study of the Liver guidelines. Fibrosis-4 index (Fib-4) and nonalcoholic fatty liver disease fibrosis score (NFS) were employed to evaluate liver fibrosis.
The mean age was 44.84 ± 11 years. Seventy-two percent of the patients were female. Two hundred and seventy-three patients were classified as having MAFLD, of which 110 (40.3%) carried a single, 129 (47.3%) had two, and 34 (12.5%) had all three metabolic conditions. The cumulative number of metabolic conditions was related to elevated body mass index, triglyceride (TG) levels, and glycated hemoglobin, lower high-density lipoprotein (HDL) levels, higher liver inflammation (by aspartate aminotransferase and γ-glutamyl transferase), and higher likelihood of fibrosis (by NFS and Fib-4 scores) (P < 0.05 for all). The proportion of advanced fibrosis also increased with an increase in the number of metabolic conditions (4.1%, 25.5%, 35.6%, and 44.1% by NFS and 6.1%, 10.9%, 17%, and 26.5% by Fib-4 for no MAFLD and MAFLD with 1, 2, and 3 conditions, respectively). Among MAFLD patients, those with diabetes alone were the eldest and had the highest mean value of NFS score and Fib-4 score (P < 0.05), while MAFLD patients diagnosed with lean metabolic dysfunction exhibited the highest levels of TG and alanine aminotransferase but the lowest HDL levels
The study suggests that the severity of liver fibrosis in MAFLD patients is influenced by the number and type of metabolic conditions present. Early identification and management of MAFLD, particularly in patients with multiple metabolic conditions, are crucial to prevent liver-related complications.
Core Tip: This is the first study on the South-Asian population on assessment of fibrosis among metabolic-associated fatty liver disease (MAFLD) patients. The study highlights that as the number of risk factors increases in a patient with MAFLD, it is more likely to have progression of liver fibrosis.
- Citation: Shaikh SS, Qazi-Arisar FA, Nafay S, Zaheer S, Shaikh H, Azam Z. Metabolic puzzle: Exploring liver fibrosis differences in Asian metabolic-associated fatty liver disease subtypes. World J Hepatol 2024; 16(1): 54-64
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/54.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.54
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of diseases ranging from benign accumulation of excessive fat in the liver (steatosis) to the inflammation of liver cells [nonalcoholic steatohepatitis (NASH)]. It can lead to advanced fibrosis, cirrhosis, and subsequent hepatocellular carcinoma (HCC). NAFLD is now one of the common indications for liver transplantation from Western data. It is primarily a diagnosis of exclusion that needs to exclude other causes of liver fat accumulation, for instance, alcohol intake above a certain quantity, medications, viral hepatitis, and autoimmune liver disease[1]. The disease progression from benign fatty liver to inflammation and, ultimately, liver fibrosis is linked with the co-existence of diabetes mellitus (DM), obesity, and metabolic syndrome (MS)[2]. This has resulted in the proposal of this terminology change from NAFLD to metabolic-associated fatty liver disease (MAFLD) (metabolic malfunction associated fatty liver disease)[3]. The Asian Pacific Association for the Study of the Liver (APASL) also endorsed this amendment in nomenclature and the development of "diagnostic criteria" for MAFLD, unlike NAFLD, a diagnosis of exclusion[4].
When evaluating fatty liver and fibrosis, liver biopsy remains the gold standard. Due to its invasive nature, various noninvasive diagnostic tools (based on imaging or biomarkers) are now being used. Among them are the NAFLD fibrosis score (NFS) and fibrosis-4 index (Fib-4), endorsed by various guidelines as preference screening panels for predicting advanced fibrosis[4,5]. A strong body of evidence suggests that MAFLD is more effective than NAFLD in identifying significant liver fibrosis[6,7]. However, whether the subtypes of MAFLD differentially influence liver fibrosis is not very well understood, especially in the Asian population. Therefore, given the recent notion of MAFLD, our objective was to compare the severity of liver fibrosis among different MAFLD subtypes.
This cross-sectional study was conducted at the National Institute of Liver and GI Diseases, located at Dow University Hospital in Karachi, Pakistan. Patients (ranging in age between 18 and 65 years, including both males and females) diagnosed with fatty liver disease between January and December 2021 were included. Those patients with decom
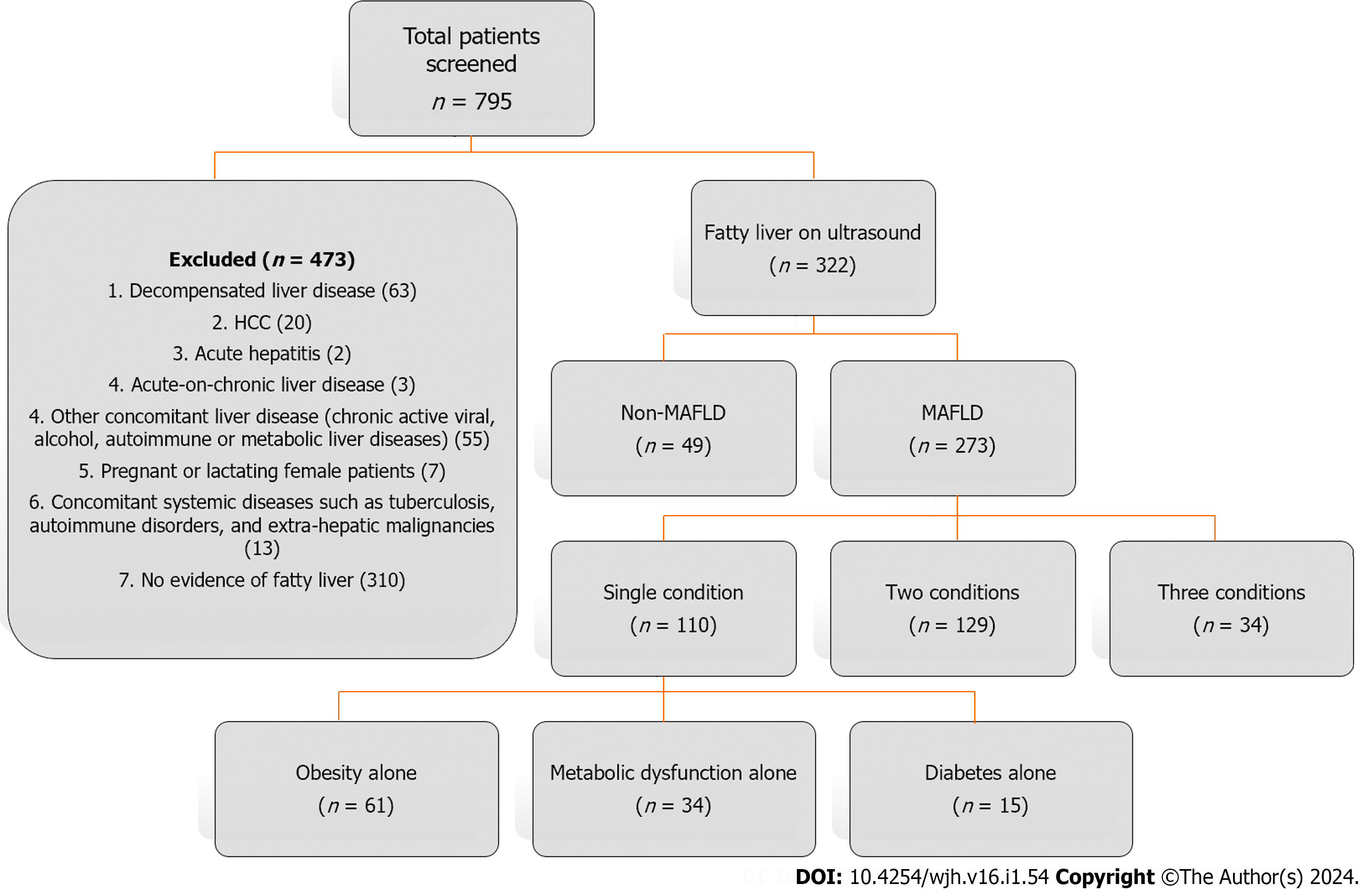
The demographic, clinical, and laboratory data of the patients were collected and analyzed. The main indications to perform an ultrasound examination were symptoms of dyspepsia and right upper quadrant abdominal pain and an evaluation showing deranged liver function tests. The fatty liver finding was confirmed on ultrasound examination based on the diffuse increased hepatic parenchymal echogenicity or "bright texture of liver parenchyma"[8]. According to the APASL guidelines, MAFLD was defined as the presence of fatty liver in conjunction with at least one of the following three conditions: Overweight/obesity, type 2 DM, or evidence of metabolic dysfunction (MD) such as increased waist circumference or an abnormal lipid or glycemic profile[4]. Fib-4 and NFS were noninvasive tools used to assess liver fibrosis in this population with fatty liver disease. Asian cutoffs for body mass index (BMI) were used to classify the subjects as overweight/obese vs lean/normal weight among different MAFLD groups. Figure 1 describes the study flow chart.
The study was approved by the Institutional Review Board of Dow University of Health Sciences (IRB-1842). Informed consent was obtained from all eligible participants. The methods employed in this study were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
The statistical analyses were executed using SPSS software version 26.0. Quantitative variables are expressed as the mean ± SD, while categorical variables are represented as frequencies and percentages. The chi-square test was used to assess categorical variables. The Mann-Whitney U-test was applied to compare the difference between two groups, while the Kruskal-Wallis test was performed to evaluate the difference among three groups. A P value of 0.05 or less was considered significant.
A total of 322 patients with fatty liver were included, with a mean age of 44.84 ± 11 years. The majority were female (72%). The mean BMI was 29.83 ± 5.53 kg/m2, 29.8% had DM, and 9.6% had hypertension.
Out of 322 patients with fatty liver, 273 were classified as having MAFLD. The MAFLD patients were further classified into three categories corresponding to their components of metabolic conditions (i.e., one, two, and three). Out of 273 participants with MAFLD, 110 (40.3%) had a single metabolic condition, 129 (47.3%) had two metabolic conditions, and 34 (12.5%) had all three metabolic conditions (Figure 1).
With the increasing number of metabolic conditions, more patients were diabetic and obese, with the worsening of liver enzymes and lipid profile, as well as increasing hepatic fibrosis scores. With an increase in the cumulative number of metabolic conditions, the patients exhibited a significant elevation in their metabolic parameters such as BMI (28.99 ± 5.19 vs 31.63 ± 5.19 vs 33.59 ± 4.75; P < 0.001) and glycated hemoglobin (Hb1Ac) (5.97 ± 1.13 vs 6.82 ± 1.86 vs 8.22 ± 1.58, P < 0.001). Significant worsening of lipid profile was also noted with the increasing number of metabolic conditions as triglyceride (TG) levels rose (182.45 ± 109.5 vs 198.13 ± 98.8 vs 221.85 ± 102.38, P = 0.002), while high-density lipoprotein (HDL) levels showed a negative trend among MAFLD patients (41.65 ± 15.08 vs 36.05 ± 8.93 vs 32.38 ± 6.62, P < 0.001).
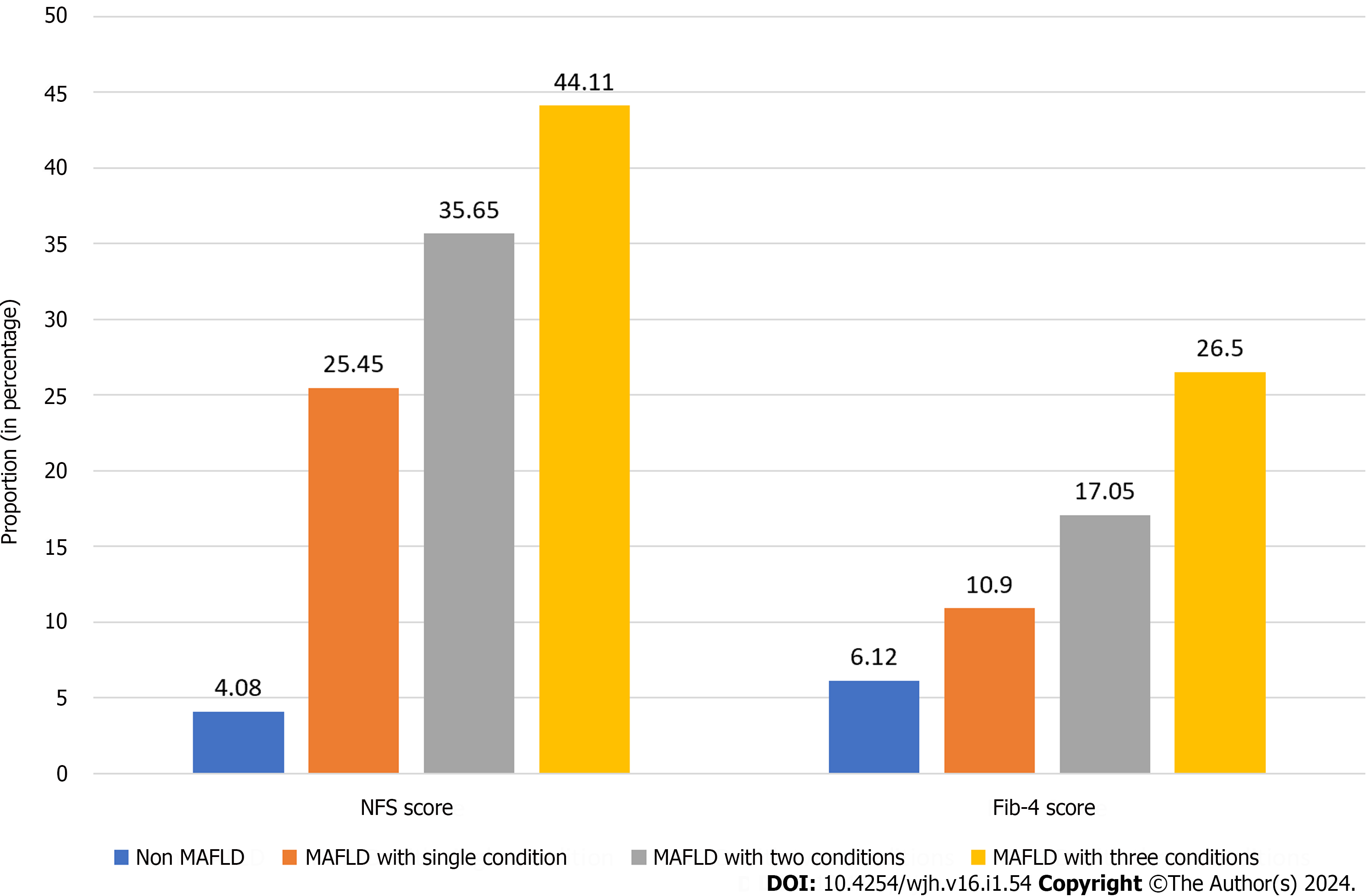
As a consequence of these findings, increasing liver inflammation (as reflected by aspartate aminotransferase (AST) 28.62 ± 20.74 vs 32.29 ± 23.36 vs 40.06 ± 26.74, P = 0.021 and γ -glutamyl transferase 34.93 ± 21.08 vs 51.50 ± 36.44 vs 65.41 ± 38.02, P < 0.001) and liver fibrosis (reflected by the NFS score -2.59 ± 1.59 vs -2.00 ± 1.69 vs -1.39 ± 1.60, P = 0.002 and Fib-4 score 0.79 ± 0.45 vs 0.94 ± 0.86 vs 1.11 ± 0.66, P = 0.041) were seen as the trends of different metabolic categories (Table 1). The proportion of significant fibrosis was also established with the collective number of metabolic conditions. For the NFS score, advanced fibrosis was present in 4.1% of subjects with no fulfilled criteria for MAFLD and in 25.5%, 35.6%, and 44.1% with 1, 2, and 3 MAFLD conditions, respectively, while for the Fib-4 score, advanced fibrosis was present in 6.1% of subjects without MAFLD, and in 10.9%, 17%, and 26.5% with 1, 2, and 3 MAFLD conditions, respectively (Figure 2).
| Characteristic | Total (n = 322) | No MAFLD(n = 49) | MAFLD (n = 273) | P value | |||||
| Single condition (n = 110; 40.3%) | Two conditions (n = 129; 47.3%) | Three conditions (n = 34; 12.5%) | Overall | Single vs two | Single vs three | Two vs three | |||
| Age (yr) | 44.84 ± 11 | 42.69 ± 12 | 44.13 ± 10.47 | 45.53 ± 10.80 | 47.65 ± 10.32 | 0.332 | 0.474 | 0.138 | 0.321 |
| BMI (kg/m2) | 29.83 ± 5.53 | 34.38 ± 2.20 | 28.99 ± 5.19 | 31.63 ± 5.19 | 33.59 ± 4.75 | < 0.001 | < 0.001 | < 0.001 | 0.064 |
| Female gender (%) | 232 (72) | 32 (65.3) | 85 (77.3) | 94 (72.9) | 21 (61.8) | 0.201 | 0.434 | 0.073 | 0.206 |
| Hypertension (%) | 31 (9.6) | 5 (10.2) | 14 (12.7) | 12 (9.3) | 0 (0.0) | 0.086 | 0.397 | 0.029 | 0.065 |
| Diabetes (%) | 96 (29.8) | 0 (0) | 15 (13.6) | 47 (36.4) | 34 (100) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| NFS score | -2.41 ± 1.71 | -3.76 ± 1.19 | -2.59 ± 1.59 | -2.00 ± 1.69 | -1.39 ± 1.60 | 0.002 | 0.033 | < 0.001 | 0.043 |
| Fib-4 score | 0.88 ± 0.67 | 0.75 ± 0.47 | 0.79 ± 0.45 | 0.94 ± 0.86 | 1.11 ± 0.66 | 0.041 | 0.771 | 0.009 | 0.031 |
| DBP | 85.22 ± 12.07 | 82.26 ± 11 | 85.32 ± 13.22 | 86.34 ± 11.59 | 84.91 ± 11.80 | 0.631 | 0.333 | 0.643 | 0.958 |
| SBP | 133.86 ± 19.18 | 129.34 ± 16 | 133.00 ± 17.73 | 134.83 ± 20.8 | 139.56 ± 20.55 | 0.133 | 0.148 | 0.071 | 0.376 |
| Platelet count | 295.77 ± 90.85 | 305.91 ± 73 | 293.20 ± 91.20 | 294.06 ± 92.0 | 295.97 ± 110.13 | 0.888 | 0.644 | 0.856 | 0.811 |
| Total cholesterol | 183.29 ± 45.18 | 184.3 ± 47.11 | 181.03 ± 42.35 | 182.94 ± 48.3 | 190.41 ± 39.58 | 0.375 | 0.705 | 0.266 | 0.161 |
| LDL | 124.81 ± 39.85 | 120.58 ± 38.16 | 123.85 ± 39.87 | 126.52 ± 41.3 | 127.44 ± 37.20 | 0.859 | 0.949 | 0.648 | 0.573 |
| HDL | 38.55 ± 12.08 | 43.01 ± 11.93 | 41.65 ± 15.08 | 36.05 ± 8.93 | 32.38 ± 6.62 | < 0.001 | < 0.001 | < 0.001 | 0.038 |
| TG | 184.79 ± 101.2 | 125.57 ± 51.8 | 182.45 ± 109.5 | 198.13 ± 98.8 | 221.85 ± 102.38 | 0.002 | 0.022 | 0.001 | 0.050 |
| Total bilirubin | 0.54 ± 0.37 | 0.55 ± 0.26 | 0.53 ± 0.35 | 0.57 ± 0.43 | 0.49 ± 0.31 | 0.284 | 0.358 | 0.287 | 0.157 |
| Direct bilirubin | 0.21 ± 0.24 | 0.21 ± 0.23 | 0.19 ± 0.09 | 0.24 ± 0.35 | 0.18 ± 0.08 | 0.601 | 0.417 | 0.732 | 0.417 |
| Serum albumin | 4.41 ± 0.38 | 4.51 ± 0.34 | 4.41 ± 0.37 | 4.37 ± 0.38 | 4.40 ± 0.43 | 0.552 | 0.305 | 0.481 | 0.906 |
| ALT | 40.67 ± 31.69 | 42.2 ± 29 | 37.03 ± 28.70 | 42.30 ± 36.49 | 44.09 ± 24.15 | 0.058 | 0.229 | 0.022 | 0.097 |
| AST | 31.84 ± 22.6 | 32.22 ± 20 | 28.62 ± 20.74 | 32.29 ± 23.36 | 40.06 ± 26.74 | 0.021 | 0.361 | 0.004 | 0.040 |
| GGT | 46.02 ± 34.77 | 42.45 ± 43.65 | 34.93 ± 21.08 | 51.50 ± 36.44 | 65.41 ± 38.02 | < 0.001 | < 0.001 | < 0.001 | 0.016 |
| ALP | 110.82 ± 51.76 | 105.3 ± 40 | 111.90 ± 59.42 | 108.22 ± 46.1 | 125.24 ± 58.81 | 0.169 | 0.802 | 0.089 | 0.070 |
| HbA1c | 6.44 ± 1.65 | 5.32 ± 0.4 | 5.97 ± 1.13 | 6.82 ± 1.86 | 8.22 ± 1.58 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The age of the patients increases somewhat as the number of metabolic diseases increases, with more men afflicted, but these results were not statistically significant across the categories. There was also no significant difference in ALT, platelets, total cholesterol, low-density lipoprotein (LDL) cholesterol, total bilirubin, or alkaline phosphatase levels.
Furthermore, MAFLD patients with a single metabolic condition (n = 110, 40.3%) were sub-classified into three categories: Obesity alone (n = 61, 55.5%), lean MD (n = 34, 30.9%), and DM alone (n = 15, 13.6%). Among MAFLD patients with a single metabolic condition, those established with DM alone were the oldest and those with obesity alone were the youngest (mean age 50.73 ± 9.04 for DM vs 45.53 ± 10.60 for lean MD and 41.72 ± 10.03 for obesity alone, P = 0.005). Similarly significant differences were noted in platelet count, which was within the normal range but the lowest in the DM group (245.40 ± 50.70 vs 275.44 ± 81.92 in lean MD vs 314.85 ± 97.95 in obesity alone, P = 0.004), TG levels, which were the highest in the lean MD group (269.02 ± 120.03 vs 176.13 ± 132.33 in DM vs 135.75 ± 57.72 in obesity, P < 0.001), HDL levels, which were the lowest in the lean MD group (39.96 ± 21.71 vs 42.30 ± 8.73 in obesity vs 42.50 ± 11.14 in DM, P = 0.026), and ALT levels, which were the highest in lean MD (43.94 ± 28.41 vs 34.13 ± 19.04 in DM vs 33.89 ± 30.47 in obesity, P = 0.043). Similarly, diabetic MAFLD had the highest Hb1Ac levels (8.03 ± 1.71 vs 5.77 ± 0.48 vs 5.56 ± 0.46, P < 0.001) than others (Table 2).
| Characteristic | Obesity alone (n = 61) | Lean MD (n = 34) | DM alone (n = 15) | P value | |||
| Overall | Obesity vs lean MD | Obesity vs DM | Lean MD vs DM | ||||
| Age (yr) | 41.72 ± 10.03 | 45.53 ± 10.60 | 50.73 ± 9.04 | 0.005 | 0.082 | 0.002 | 0.116 |
| BMI (kg/m2) | 32.33 ± 4.66 | 24.86 ± 1.63 | 24.81 ± 1.58 | < 0.001 | < 0.001 | < 0.001 | 0.965 |
| Female Gender (%) | 51 (83.6) | 23 (67.6) | 11 (73.3) | 0.191 | 0.072 | 0.358 | 0.691 |
| Hypertension (%) | 8 (13.1) | 6 (17.6) | 0 (0.0) | 0.230 | 0.551 | 0.138 | 0.082 |
| NFS score | -2.86 ± 1.74 | -2.50 ± 1.40 | -1.61 ± 0.81 | 0.017 | 0.309 | 0.005 | 0.054 |
| Fib-4 score | 0.68 ± 0.35 | 0.92 ± 0.56 | 0.95 ± 0.48 | 0.027 | 0.050 | 0.017 | 0.761 |
| DBP | 84.26 ± 15.41 | 87.03 ± 10.43 | 85.73 ± 8.61 | 0.499 | 0.262 | 0.548 | 0.728 |
| SBP | 131.03 ± 17.46 | 135.82 ± 19.94 | 134.60 ± 12.89 | 0.306 | 0.255 | 0.181 | 0.828 |
| Platelet count | 314.85 ± 97.95 | 275.44 ± 81.92 | 245.40 ± 50.70 | 0.004 | 0.032 | 0.002 | 0.298 |
| Total cholesterol | 176.91 ± 37.71 | 190.97 ± 49.33 | 175.00 ± 41.93 | 0.336 | 0.160 | 0.879 | 0.313 |
| LDL | 120.53 ± 35.87 | 126.76 ± 44.83 | 130.73 ± 44.80 | 0.626 | 0.473 | 0.433 | 0.688 |
| HDL | 42.50 ± 11.14 | 39.96 ± 21.71 | 42.30 ± 8.73 | 0.026 | 0.010 | 0.678 | 0.079 |
| TG | 135.75 ± 57.72 | 269.02 ± 120.03 | 176.13 ± 132.33 | < 0.001 | < 0.001 | 0.213 | 0.001 |
| Total bilirubin | 0.52 ± 0.43 | 0.55 ± 0.24 | 0.50 ± 0.22 | 0.171 | 0.055 | 0.698 | 0.467 |
| Direct bilirubin | 0.19 ± 0.10 | 0.18 ± 0.08 | 0.20 ± 0.09 | 0.613 | 0.480 | 0.549 | 0.424 |
| Serum albumin | 4.41 ± 0.34 | 4.40 ± 0.33 | 4.44 ± 0.59 | 0.731 | 0.779 | 0.526 | 0.428 |
| ALT | 33.89 ± 30.47 | 43.94 ± 28.41 | 34.13 ± 19.04 | 0.043 | 0.016 | 0.264 | 0.288 |
| AST | 25.75 ± 15.38 | 34.76 ± 29.26 | 26.33 ± 13.60 | 0.086 | 0.025 | 0.724 | 0.302 |
| GGT | 32.32 ± 21.22 | 37.85 ± 20.37 | 39.15 ± 22.28 | 0.139 | 0.083 | 0.169 | 0.849 |
| ALP | 113.61 ± 68.86 | 107.03 ± 39.59 | 116.00 ± 58.23 | 0.948 | 0.880 | 0.734 | 0.888 |
| HbA1c | 5.56 ± 0.46 | 5.77 ± 0.48 | 8.03 ± 1.71 | < 0.001 | 0.006 | < 0.001 | < 0.001 |
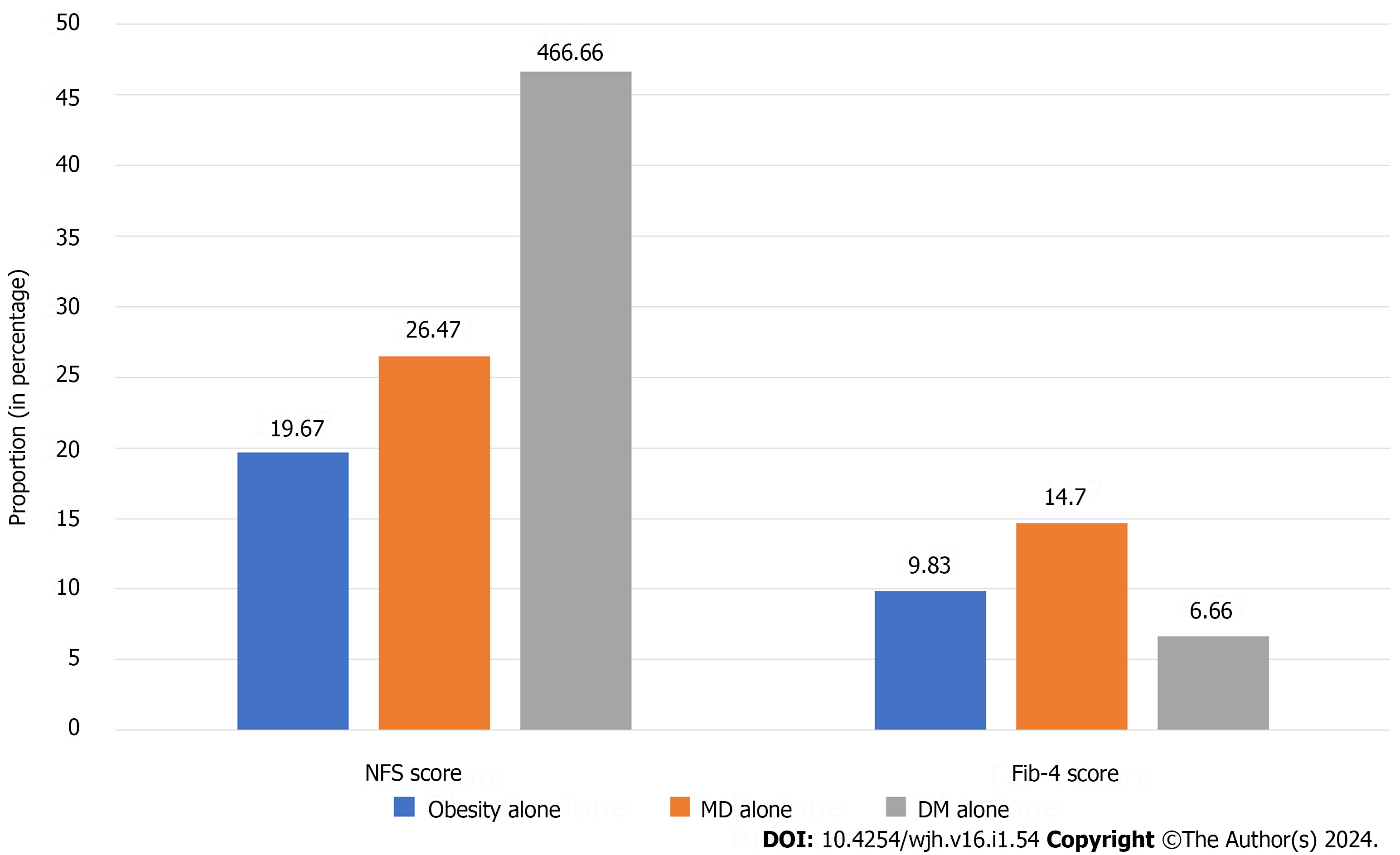
When compared among the three subtypes of MAFLD, the proportion of advanced liver fibrosis was significantly higher among diabetic MAFLD patients according to the NFS score (46.6% vs 26.5% for MD alone and 19.7% for obesity alone), whereas patients with lean MD had the highest proportion of advanced fibrosis according to the Fib-4 score (14.7% vs 9.8% for obesity alone vs 6.7% for DM alone) (Figure 3).
No significant differences were observed in gender distribution, education awareness, history of hypertension, blood pressure, and blood levels of cholesterol, LDL, bilirubin, albumin, AST, and alkaline phosphate between these respective three groups.
The present study provides valuable insights into the progression of MAFLD and its subtypes in the Pakistani population. Our findings demonstrated that as the cumulative number of metabolic conditions increased, there was a corresponding escalation in the NFS and Fib-4 scores. This trend aligns with the work of Yamamura et al[6], which also reported that patients with multiple metabolic conditions exhibited a higher risk of advanced fibrosis.
In our study, around 60% of patients had more than one metabolic condition, which is comparable to a recent study of the NHANES III database in which more than 70% of all patients with MAFLD had more than one metabolic condition. Additionally, having more than one metabolic condition was associated with abnormal liver function tests and kidney diseases[9]. The same study found that there were an increasing number of metabolic conditions in the higher age group. In our study, there was only a non-significant association between older age and comorbidities. This may be attributed to the different demographic spectra in our population (North American vs Southeast Asian). Recent meta-analytical evidence lends further credence, delineating the clinical characteristics of NAFLD in Asian populations. It demonstrates that the pooled mean age of NAFLD patients was 52.07 years (95%CI: 51.28-52.85), which contrasts with a notably younger mean age of 42.66 years (95%CI: 32.23-53.11) observed in patients from Southeast Asia, indicating regional age-related disparities among NAFLD patients[10].
The degree of liver fibrosis varies across MAFLD subtypes[11], with an increased risk of liver-related death as fibrosis progresses[12]. Hence, we further classified the MAFLD into subtypes according to the type of metabolic conditions. Interestingly, the sub-classification of MAFLD based on individual metabolic conditions revealed distinct profiles. Diabetic MAFLD patients tended to be older, have higher TG levels, and exhibit more pronounced fibrosis compared to those with other MAFLD subtypes, echoing the finding of Chhabra et al[13] that diabetes is a strong predictor of advanced fibrosis in MAFLD. Studies have consistently shown a higher proportion of advanced liver fibrosis in diabetic MAFLD patients compared to other MAFLD subtypes[9,14]. The relationship between DM, MAFLD, and advanced fibrosis is likely a multifactorial chronic process, with insulin resistance and older age playing significant roles[15,16]. This relationship is reflected in the Fib-4 and NFS scores, which incorporate age as a variable, leading to higher scores in older individuals. These findings underscore the importance of considering diabetes as a risk factor for advanced fibrosis in MAFLD patients, particularly among older individuals. Early identification and management of diabetes and MAFLD are crucial to prevent liver-related complications and improve patient outcomes.
Elevated TG, ALT, and AST levels in lean MAFLD indicate that lean MAFLD has clinical implications and is associated with liver inflammation or injury. Lean MAFLD patients have a more detrimental metabolic profile compared to lean non-MAFLD patients[17]. Lean MAFLD is independently associated with an increased risk of overall mortality [hazard ratio (HR): 1.296; 95%CI: 1.064-1.578][18], as well as liver-specific mortality (HR: 2.84; 95%CI: 2.72-2.97) as compared to other MAFLD subtypes[19]. Furthermore, this impact was also observed in post-liver transplant, as lean NASH patients have worse post-liver transplant overall survival compared to non-lean NASH (HR: 0.17; 95%CI: 0.03-0.86, P = 0.0142)[20]. These findings highlight the importance of recognizing lean MAFLD as a distinct clinical entity with significant adverse health outcomes. Early identification and management of lean MAFLD are crucial to prevent liver-related complications and improve patient outcomes.
On the other hand, individuals with obesity as the sole metabolic condition presented with a younger age and less severe fibrosis, suggesting a potential protective effect of youth or a longer disease trajectory before significant fibrosis develops, which has been suggested by Yang et al[21].
Our study did not find statistically significant differences in ALT, total cholesterol, LDL, bilirubin, or alkaline phosphatase levels across the MAFLD subtypes, which diverges from the findings of Wong et al[22], who reported dyslipidemia and elevated liver enzymes as common features in MAFLD patients. This discrepancy could be attributed to the genetic or dietary factors unique to our study population, underlining the complexity of MAFLD phenotypes as noted by Eslam et al[23].
The diagnostic performance of Fib-4 and NFS for advanced fibrosis can be influenced by various factors, including age, DM, and BMI. In particular, the inclusion of overweight or obesity as a criterion for MAFLD has impacted the BMI component in NFS, leading to differences in the sensitivity and specificity of the two scores in identifying advanced fibrosis. A recent study found that although the overall performance of Fib-4 and NFS in diagnosing liver fibrosis was similar between lean and non-lean individuals, the sensitivity and specificity of NFS varied according to BMI quartile ranges. Specifically, NFS was found to be less sensitive in lean individuals compared to Fib-4[24]. Another study found that the diagnostic ability of NFS was lower among individuals with diabetes compared to Fib-4 [area under the receiver operating characteristic curve (AUROC) 0.717 vs 0.809; P = 0.002]. This suggests that NFS may not be as effective in identifying advanced fibrosis in patients with diabetes[25]. A recent study also found that Fib-4 was superior to NFS in accurately classifying non-obese NAFLD patients with F2–4 fibrosis (AUROC 81.5% vs 73.7%, P < 0.001). This suggests that Fib-4 may be a better choice for diagnosing advanced fibrosis in this patient population[26]. Overall, the evidence suggests that Fib-4 may be a more reliable tool for diagnosing advanced fibrosis than NFS, particularly in lean individuals and patients with diabetes. Further research is needed to confirm these findings and to determine the optimal use of both scores in clinical practice.
The strengths of this study are multifaceted, encompassing stringent participant selection, methodological robustness, ethical integrity, and analytical rigor. First, the study employed rigorous inclusion and exclusion criteria, ensuring a well-defined study population that accurately represented the target demographic for MAFLD. This strategic participant selection minimized confounding variables, thereby enhancing the validity of the findings. Second, the adoption of a cross-sectional study design facilitated the examination of the prevalence and association patterns of liver fibrosis with metabolic conditions at a specific point in time. Moreover, this is the first study on the South Asian population to high
The present study, while contributing valuable insights into the long-term implications of MAFLD subtypes on hepatic fibrosis, is not without its limitations that merit acknowledgment. First, the study's design was observational, precluding any assertions of causality between MAFLD subtypes and the progression of hepatic fibrosis. Second, the reliance on existing clinical datasets limits the scope to fully capture the nuances of patients’ longitudinal metabolic changes and their direct impact on liver pathology. Another constraint is the study's dependence on non-invasive markers of hepatic fibrosis, which, while clinically relevant, cannot substitute for the histopathological assessment through liver biopsy or transient elastography, the gold standard for fibrosis evaluation. The use of surrogate endpoints, therefore, necessitates cautious interpretation of the findings. However, Fib-4 and NFS are widely used and endorsed by various guidelines for screening MAFLD patients for advanced fibrosis, and they are superior to other scores like aspartate aminotransferase to platelet ratio index (APRI) and BMI, AST/ALT ratio, and diabetes mellitus (BARD)[29,30]. Even though, they may not be as effective due to limitations by risk factors like age and BMI scores[1]. Lastly, the study's geographic and demographic concentration may restrict the generalizability of the findings across different populations and ethnicities.
These limitations highlight areas for future research, emphasizing the need for prospective studies, using a longitudinal study design, larger sample size with a more diverse demographic distribution, integration of transient elastography with existing non-invasive markers like NFS and Fib-4, and comparative analyses juxtaposing patients with MAFLD against control groups without MD, to delineate the specific contributory pathways leading to fibrosis within the context of MS.
This research has rigorously demonstrated that the severity of liver fibrosis in MAFLD patients is influenced by the number and type of metabolic conditions present. Early identification and management of MAFLD, particularly in patients with multiple metabolic conditions, are crucial to prevent liver-related complications.
Metabolic-associated fatty liver disease (MAFLD) is a medical condition characterized by the presence of fatty liver along with overweight/obesity and/or diabetes and/or metabolic dysfunction. However, whether the subtypes of MAFLD based on the metabolic disorder differentially impact on liver fibrosis is not well explicated, especially in the Asian population.
Different subgroups of MAFLD present distinct clinical spectra and risks of advanced liver fibrosis, which can influence their treatment strategies. Metabolic syndrome is related to higher deaths in nonalcoholic fatty liver disease (NAFLD) patients. Moreover, the high fibrotic burden in fatty liver disease is associated with a higher risk of development of hepatocellular carcinoma, liver-related mortality, and cardiovascular disease. Hence, it is worth classifying the MAFLD patients depending on the number of metabolic conditions at the beginning. This helps to stratify patients with MAFLD according to the long-term risk of significant liver fibrosis.
To compare the severity of liver fibrosis among different MAFLD subtypes.
This was a cross-sectional investigation carried out at the National Institute of Liver and GI Diseases, located at Dow University Hospital in Karachi, Pakistan. All patients aged between 18 and 65 years, irrespective of gender, who were diagnosed with fatty liver between January and December 2021 were included. Patients with decompensated liver disease, hepatocellular carcinoma, acute hepatitis, acute-on-chronic liver disease, and other concomitant liver disease (chronic active viral, alcohol, autoimmune, or metabolic liver diseases) were excluded. Pregnant or lactating female patients and patients with concomitant systemic diseases such as tuberculosis, autoimmune disorders, and extra-hepatic malignancies were also excluded from the study. MAFLD was defined according to the Asia Pacific Association for the Study of the Liver guidelines, and fibrosis-4 index (Fib-4) and NAFLD fibrosis score (NFS) were used to assess liver fibrosis. Asian cutoffs were used for body mass index to classify the subjects into overweight/obese vs lean/normal weight MAFLD groups.
Out of 322 patients with fatty liver, 273 were classified as having MAFLD. The MAFLD patients were segregated into three categories according to their number of metabolic conditions (i.e., one, two, and three). Out of 273 participants with MAFLD, 110 (40.3%) carried a single metabolic condition, 129 (47.3%) had two metabolic conditions, and 34 (12.5%) had all the three metabolic conditions. The proportion of significant fibrosis increased with the cumulative number of metabolic conditions. For the NFS score, advanced fibrosis was 4.1%, 25.5%, 35.6%, and 44.1% for no MAFLD and MAFLD with 1, 2, and 3 conditions, respectively, while for Fib-4 score, the proportion of advanced fibrosis was 6.1%, 10.9%, 17%, and 26.5% for no MAFLD and MAFLD with 1, 2, and 3 conditions, respectively. Furthermore, MAFLD patients with a single metabolic condition (n = 110, 40.3%) were sub-classified into three categories: Obesity alone (n = 61, 55.5%), lean metabolic dysfunction (MD) (n = 34, 30.9%), and diabetes mellitus (DM) alone (n = 15, 13.6%). When compared among the three subtypes of MAFLD, the proportion of advanced liver fibrosis was significantly higher among diabetic MAFLD patients according to the NFS score (46.6% vs 26.5% for MD alone and 19.7% for obesity alone), whereas patients with lean MD had the highest proportion of advanced fibrosis according to the Fib-4 score (14.7% vs 9.8% for obesity alone vs 6.7% for DM alone).
The increased number of metabolic conditions increases the likelihood of fibrosis in patients with MAFLD. The severity of liver fibrosis varies among different subtypes of MAFLD. Patients with diabetes and MAFLD have the highest risk of developing fibrosis.
The direction of future research in this area involves several key questions that need to be addressed. Investigating the specific diagnostic markers for different subgroups within MAFLD, such as those with obesity, lean individuals, and those with type 2 diabetes. Further exploration is needed regarding the pathogenesis of MAFLD/metabolic dysfunction-associated steatohepatitis (MASH). By conducting thorough investigations into these areas, researchers can gain a better understanding of the complexities surrounding non-alcoholic fatty liver disease and its associated MD. Future research should focus on identifying effective pharmacotherapeutic interventions for MAFLD/MASH, as there is currently no approved treatment for this condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Ji G, China S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Kaya E, Yilmaz Y. Epidemiology, natural history, and diagnosis of metabolic dysfunction-associated fatty liver disease: a comparative review with nonalcoholic fatty liver disease. Ther Adv Endocrinol Metab. 2022;13:20420188221139650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 3. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2819] [Article Influence: 563.8] [Reference Citation Analysis (1)] |
| 4. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 553] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 5. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4939] [Article Influence: 705.6] [Reference Citation Analysis (9)] |
| 6. | Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 7. | Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 8. | Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23:290-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Huang J, Ou W, Wang M, Singh M, Liu Y, Liu S, Wu Y, Zhu Y, Kumar R, Lin S. MAFLD Criteria Guide the Subtyping of Patients with Fatty Liver Disease. Risk Manag Healthc Policy. 2021;14:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Kam LY, Huang DQ, Teng MLP, Takahashi H, Tanaka K, Yasuda S, Fung J, Lee TY, Hyogo H, Ono M, Saruwatari J, Oniki K, Yeo YH, Barnett S, Henry L, Li J, Zou B, Cheung RC, Kumada T, Yuen MF, Eguchi Y, Toyoda H, Nguyen MH. Clinical Profiles of Asians with NAFLD: A Systematic Review and Meta-Analysis. Dig Dis. 2022;40:734-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Lim TS, Chun HS, Kim SS, Kim JK, Lee M, Cho HJ, Kim SU, Cheong JY. Fibrotic Burden in the Liver Differs Across Metabolic Dysfunction-Associated Fatty Liver Disease Subtypes. Gut Liver. 2023;17:610-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1427] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 13. | Chhabra S, Singh SP, Singh A, Mehta V, Kaur A, Bansal N, Sood A. Diabetes Mellitus Increases the Risk of Significant Hepatic Fibrosis in Patients With Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2022;12:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kim M, Yoon EL, Cho S, Lee CM, Kang BK, Park H, Jun DW, Nah EH. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 2022;42:1536-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 711] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 16. | Li X, Jiao Y, Xing Y, Gao P. Diabetes Mellitus and Risk of Hepatic Fibrosis/Cirrhosis. Biomed Res Int. 2019;2019:5308308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, Liang XY, Li X, Liu ZL, Xiao J, Wei JY, Ma CY, Wu KN, Ran L, Kong LQ. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Chen X, Chen S, Pang J, Tang Y, Ling W. Are the different MAFLD subtypes based on the inclusion criteria correlated with all-cause mortality? J Hepatol. 2021;75:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Chung GE, Yu SJ, Yoo JJ, Cho Y, Lee KN, Shin DW, Kim D, Kim YJ, Yoon JH, Han K, Cho EJ. Lean or diabetic subtypes predict increased all-cause and disease-specific mortality in metabolic-associated fatty liver disease. BMC Med. 2023;21:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Qazi-Arisar FA, Uchila R, Chen C, Yang C, Chen SY, Karnam RS, Azhie A, Xu W, Galvin Z, Selzner N, Lilly L, Bhat M. Divergent trajectories of lean vs obese non-alcoholic steatohepatitis patients from listing to post-transplant: A retrospective cohort study. World J Gastroenterol. 2022;28:3218-3231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Yang A, Jung N, Kim S, Lee JE. Association Between Non-invasive Diagnostic Methods of Liver Fibrosis and Type 2 Diabetes in Pediatric Patients With Non-alcoholic Fatty Liver Disease. Front Pediatr. 2022;10:825141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 23. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 24. | Park H, Yoon EL, Ito T, Jo AJ, Kim M, Lee J, Kim HL, Arai T, Atsukawa M, Kawanaka M, Toyoda H, Ishigami M, Yu ML, Jun DW, Nguyen MH. Diagnostic Performance of the Fibrosis-4 Index and Nonalcoholic Fatty Liver Disease Fibrosis Score in Lean Adults With Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2023;6:e2329568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Park H, Yoon EL, Kim M, Lee J, Kim JH, Cho S, Jun DW, Nah EH. Comparison of diagnostic performance between FIB-4 and NFS in metabolic-associated fatty liver disease era. Hepatol Res. 2022;52:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Tan EX, Lee JW, Jumat NH, Chan WK, Treeprasertsuk S, Goh GB, Fan JG, Song MJ, Charatcharoenwitthaya P, Duseja A, Imajo K, Nakajima A, Seki Y, Kasama K, Kakizaki S, Lesmana LA, Zheng KI, Zheng MH, Koh CJ, Ho KY, Goh KL, Wong VW, Dan YY. Non-obese non-alcoholic fatty liver disease (NAFLD) in Asia: an international registry study. Metabolism. 2022;126:154911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Majumdar A, Tsochatzis EA. Changing trends of liver transplantation and mortality from non-alcoholic fatty liver disease. Metabolism. 2020;111S:154291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Cotter TG, Dong L, Holmen J, Gilroy R, Krong J, Charlton M. Nonalcoholic fatty liver disease: impact on healthcare resource utilization, liver transplantation and mortality in a large, integrated healthcare system. J Gastroenterol. 2020;55:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Wu YL, Kumar R, Wang MF, Singh M, Huang JF, Zhu YY, Lin S. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol. 2021;27:5753-5763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 30. | Chen X, Goh GB, Huang J, Wu Y, Wang M, Kumar R, Lin S, Zhu Y. Validation of Non-invasive Fibrosis Scores for Predicting Advanced Fibrosis in Metabolic-associated Fatty Liver Disease. J Clin Transl Hepatol. 2022;10:589-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |