Published online Jan 27, 2024. doi: 10.4254/wjh.v16.i1.33
Peer-review started: October 10, 2023
First decision: October 16, 2023
Revised: November 6, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: January 27, 2024
Processing time: 104 Days and 17.2 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common liver disease worldwide, with an estimated prevalence of 31% in Latin America. The presence of metabolic comorbidities coexisting with liver disease varies substantially among populations. It is acknowledged that obesity is boosting the type 2 diabetes mellitus “epidemic,” and both conditions are significant contributors to the increasing number of patients with MASLD. Non-alcoholic steatohepatitis represents a condition of chronic liver inflammation and is considered the most severe form of MASLD. MASLD diagnosis is based on the presence of steatosis, noninvasive scores and altered liver tests. Noninvasive scores of liver fibrosis, such as serum biomarkers, which should be used in pri
Core Tip: Metabolic dysfunction-associated steatotic liver disease must be prevented in primary care by focusing on risk factors for metabolic syndrome and noninvasive fibrosis scores so that early detection is possible. To avoid a late diagnosis, primary care physicians need to reinforce in their routine examinations the need for lifestyle changes through healthy diet and exercise and implement pharmacological treatment when disease progression with the presence of fibrosis is identified. The treatment must be individualized, and in many cases several pharmacological options may be used to avoid disease progression, resulting in multisystemic involvement.
- Citation: Vargas M, Cardoso Toniasso SC, Riedel PG, Baldin CP, dos Reis FL, Pereira RM, Brum MCB, Joveleviths D, Alvares-da-Silva MR. Metabolic disease and the liver: A review. World J Hepatol 2024; 16(1): 33-40
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/33.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.33
Metabolic syndrome (MetS) represents a multifaceted disorder distinguished by cardiovascular risk factors associated with central fat deposition and insulin resistance. These metabolic alterations have ramifications for various organs, with particular emphasis on the liver, a pivotal organ responsible for metabolizing diverse substances[1,2]. Epidemiological evidence indicates that the global diagnosis of MetS exceeds one billion individuals, primarily attributable to lifestyle factors[3]. Within the Brazilian population, the prevalence of MetS stands at 33%, surpassing the international prevalence range of 20%-25%[4].
Type 2 diabetes stands as one of the most pivotal risk factors contributing to the onset of non-alcoholic steatohepatitis (NASH), advanced fibrosis/cirrhosis, hepatocellular carcinoma, and mortality[5]. Emerging evidence underscores the bidirectional relationship between these ailments, wherein the presence of metabolic dysfunction-associated steatotic liver disease (MASLD) heightens the susceptibility to type 2 diabetes.
The etiology of MASLD remains incompletely elucidated. Among the various identified origins, insulin resistance accompanied by subclinical inflammation emerges as a prominent contributor. Within this proinflammatory milieu, an augmented influx of free fatty acids (FFAs) into the liver precipitates hepatic infiltration of lipids, subsequently instigating liver injury through processes such as lipid peroxidation and mitochondrial dysfunction[6].
In MASLD, the accrual of hepatic fat ensues through several pathways: Deficient uptake of circulating lipids; heightened hepatic de novo lipogenesis; inadequate compensatory augmentation in fatty acid oxidation; and alterations in lipid export, particularly as constituents of very low-density lipoprotein (VLDL). The increased uptake of lipids and accelerated rates of de novo lipogenesis within MASLD contribute to augmented hepatic triglyceride accumulation. This process is accompanied by the excessive production and release of voluminous, triglyceride-enriched VLDL particles, facilitating the mobilization and transportation of fat from the liver to peripheral tissues[7].
The overproduction of VLDL particles in the context of MASLD triggers a cascade of plasma lipoprotein irregularities, manifesting as atherogenic dyslipidemia characterized by elevated serum triglyceride levels and diminished high-density lipoprotein (HDL) cholesterol levels. Similarly, an atherogenic lipoprotein phenotype, featuring a preponderance of low-density lipoprotein particles, accumulation of triglyceride-rich lipoproteins and their remnants, and intermediate-density lipoprotein, is evident. These apolipoprotein-B-containing lipoproteins are fundamentally implicated in the progression of atherosclerosis[7].
In MASLD, the accumulation of hepatic fat arises through distinct pathways. Obesity, recognized as a pervasive and epidemic-level chronic ailment on a global scale, has seen a marked escalation in prevalence worldwide. According to findings from a population-based cohort study, individuals classified as overweight or obese, devoid of additional metabolic abnormalities such as diabetes, hypertension, or dyslipidemia, exhibited a two-fold heightened risk of developing MASLD compared to their eutrophic counterparts[8]. Failure to control obesity during the steatosis stages triggers an intrahepatic inflammatory process.
During this phase, immune cells release cytokines that amplify the inflammatory response contributing to the fibrotic process, which becomes evident with prolonged inflammation. Following liver injury, the customary counter-regulatory mechanism typically facilitates the replacement of deceased or apoptotic hepatocytes. However, in instances where this mechanism falters, as observed in sustained obesity, fibrosis ensues, possibly representing an unsuccessful attempt to counteract liver injury and promote tissue regeneration. The cumulative outcome of these ongoing processes manifests as scarring, encompassing cirrhosis and neoplasia[9].
The prevalence of hypertension in MASLD patients spans a range of 40%-70%, and recent evidence underscores a robust association with an elevated risk of incident prehypertension and hypertension[10].
The precise nature of the relationship between MASLD and hypertension remains incompletely elucidated. There are indications that the systemic inflammation accompanying MASLD may trigger the sympathetic nervous system, potentially contributing to hypertension. Additionally, insulin resistance could play a role in promoting hypertension by fostering increased concentrations of FFAs, leading to perivascular fatty deposits in proximity to vessels and the renal sinus. Elevated levels of homocysteine in MASLD coupled with intestinal dysbiosis may incite heightened oxidative stress, further substantiating a link to hypertension[10]. Another study proposed that MASLD and hypertension may share a multifactorial association involving biochemical, genetic, nutritional, and lifestyle factors[11].
In patients with MASLD, cardiovascular disease stands as the predominant cause of mortality. Risk factors for cardiovascular disease, including hypertension, dyslipidemia, insulin resistance, smoking, and central obesity, are intricately connected to MetS and risk factors for MASLD. Screening for these conditions bears significant clinical implications for disease mitigation and the prevention of cardiovascular events[12].
The escalation in the prevalence of MASLD worldwide is intricately linked to sedentary lifestyle choices and the consumption of processed foods[13]. It is noteworthy that MASLD stands as the primary cause of liver-related morbidity and mortality[14]. Recent investigations have honed in on the metabolic facet of fatty liver disease, pinpointing liver fat storage as the unifying factor. A consensus panel of international experts has proposed the substitution of the term non-alcoholic fatty liver disease (NAFLD) with MASLD, aligning it with metabolic comorbidities. This novel nomenclature, grounded in the classification of causative factors, holds promise for refining phenotypic characterization and facilitating the identification of new biomarkers and therapeutic modalities[15,16].
These criteria possess the potential to surmount challenges associated with defining alcohol consumption, catalyzing advancements in our understanding of pathophysiology and streamlining the execution of clinical trials. The diagnosis of MASLD will encompass individuals exhibiting fatty liver and dysmetabolism, irrespective of reported alcohol consumption[15,16]. This diagnosis considers the presence of hepatic steatosis (confirmed through imaging, biomarkers, or histology) alongside at least one of the following features: Overweight/obesity; type 2 diabetes; and metabolic dysregulation. The latter criterion is satisfied when a minimum of two features are present including increased waist circumference, hypertension, hypertriglyceridemia, low HDL cholesterol, prediabetes, insulin resistance, and subclinical inflammation. The criteria for assessing MASLD in lean individuals with fatty liver hinge on the identification of at least two metabolic risk abnormalities[15,16]. While the terminology for MASLD remains subject to ongoing discussions, alternative terms such as metabolic associated liver disease and alcoholic liver disease have been proposed, as noted during the European Association for the Study of the Liver (EASL) Congress in 2023[17].
In Brazil, a survey conducted among individuals in the middle-aged and elderly demographic revealed a prevalence of 35.2% for MASLD[18]. In a meta-analysis encompassing 35599 patients, the prevalence of NAFLD in those with type 2 diabetes was reported at 59.67%, with results ranging from 29.60% to 87.10%[19]. Recently, an extensive meta-analysis, incorporating data from over 24 million individuals, identified an elevated risk of severe liver disease in this cohort. Conversely, a reduced risk of severe liver disease was observed in individuals with a body mass index (BMI) exceeding 30 kg/m². Nonetheless, the study suggested a less favorable prognosis in the presence of central adiposity, particularly among females[20].
A global prevalence assessment of MASLD, drawing upon data from 205307 subjects across 14 countries, indicated a prevalence of 9.7% in lean patients. Moreover, MASLD was found to be more prevalent among middle-aged individuals (45-59 years) and those of Asian descent[21].
Studies categorically delineate MASLD into two classifications: Simple steatosis, which infrequently progresses to cirrhosis; and steatohepatitis, or NASH, a process with the potential to culminate in the development of cirrhosis and hepatocellular carcinoma[22]. Approximately 30% of MASLD patients exhibit steatohepatitis[23], a progressive condition resulting in severe liver dysfunction, including cirrhosis in 20%-25% of cases[17,24]. This progression is marked by the presence of macrovesicular steatosis, lobular inflammation, hepatocyte degeneration, and fibrosis.
Clinical Practice Guidelines for MASLD Management, collaboratively proposed by the EASL, European Association for the Study of Obesity, and European Association for the Study of Diabetes, advocate for a 7% to 10% reduction in body weight for overweight/obese patients with NAFLD[17,25]. A congruent weight reduction target is endorsed by the American Association for the Study of Liver Diseases[26].
Hepatic steatosis is characterized by the presence of more than 5% lipid content in hepatocytes, a diagnosis established through imaging or histological examinations[27]. The three principal sources of FFAs in the liver include non-esterified fatty acids from adipose tissue (60%), de novo lipogenesis in the liver (25%), and FFAs from the diet in the form of chylomicrons (15%). The liver metabolizes fat primarily through the beta-oxidation of FFAs, a process predominantly occurring in mitochondria, peroxisomes, and cytochrome P-450, in situations of energy surplus or through the export of FFAs as VLDLs[27].
Plasma non-esterified fatty acid levels escalate when adipocytes are overloaded, leading to an augmented process of lipolysis. Adipose tissue responds to hormones such as glucagon, epinephrine, and adrenocorticotropic acid by releasing non-esterified fatty acids. Postprandial lipolysis in adipose tissue is inhibited by insulin after meals. In instances of insulin resistance within adipocytes, inadequate postprandial lipolysis transpires. Steatosis induced by impaired beta-oxidation of fatty acids can also result from mitochondrial dysfunction, as observed in conditions like alcoholic steatosis, NASH, and acute fatty liver of pregnancy and through the use of medications such as valproic acid[27].
Steatosis renders the liver parenchyma vulnerable to aggression, manifested through the release of FFAs and oxidative stress, both conducive to cellular injury and the development of steatohepatitis. Genetic polymorphisms, environmental factors, and dietary influences can induce inflammation, fibrosis, and progression to cirrhosis[27].
In summary, the accumulation of fat in the liver can be elucidated by insulin resistance, leading to heightened peripheral lipolysis, increased hepatic lipid uptake, and elevated triglyceride biosynthesis.
NASH denotes a chronic state of liver inflammation, representing an inflammatory subtype within the spectrum of NAFLD. In NASH, steatosis serves as evidence of hepatocyte damage characterized by ballooning and inflammation, with or without concurrent fibrosis[28]. The prevailing hypothesis posits that NASH evolves from NAFLD, precipitated by the so-called “second hit.” The precise manifestation of this second hit remains inconclusive, although prevalent theories implicate oxidative stress, specific cytokines, and lipopolysaccharides. FFAs and hyperinsulinemia synergistically potentiate lipid peroxidation and the release of free radicals, directly inflicting injury upon hepatocytes by recruiting neuroinflammatory mediators. Prolonged liver injury eventually activates stellate cells, laying the groundwork for the development of liver fibrosis.
Given the histological dynamism of NASH, it is imperative to establish a consensus on parameters signifying disease progression, irrespective of its advancement or regression. From a regulatory standpoint, a one-point expansion in fibrosis stage is indicative of deterioration. The extent of scarring does not increase linearly, with a notable surge observed in the progression of fibrosis from stage 2 to stage 3. This bears noteworthy implications for the validation of biomarkers targeting fibrosis measurement rather than its distribution[28]. Although often clinically asymptomatic, steatohepatitis has the potential to evolve into cirrhosis or end-stage liver disease or necessitate liver transplantation[28].
NASH constitutes a multifaceted condition with metabolic complications, rendering its treatment intricate. The ideal therapeutic approach would effectively reverse liver damage and fibrosis, ameliorate additional metabolic parameters, address cardiovascular comorbidities, or at the very least exhibit no deleterious effects. Despite the wealth of information accumulated on the pathogenesis of NASH over the past decade, no approved therapy has yet emerged[28].
Various diagnostic and monitoring modalities have been employed in the assessment of MASLD, including ultrasonography (US), computed tomography, magnetic resonance imaging (MRI), and more recently the controlled attenuation parameter utilized in conjunction with transient elastography and MRI elastography. While US is widely accessible, its interobserver reproducibility is not notably high, and it lacks sensitivity for detecting mild steatosis. Similarly, computed tomography exhibits limited sensitivity in identifying mild steatosis and entails patient exposure to radiation. Although MRI accessibility is constrained, it boasts high reproducibility when employing multi-echo fat quantification techniques and proton spectroscopy. Approximately 25% of individuals with isolated steatosis progress to NASH, with a positively correlated escalation in the degree of steatosis heightening the risk of disease progression. Among those diagnosed with NASH, around 25% advance to chronic hepatopathy, characterized by fibrosis, cirrhosis, and an elevated risk of complications, including portal hypertension and hepatocellular carcinoma[29] (depicted in Figure 1).
An extensive comprehension of the outcomes of clinical trials and the significance of reported treatment effects necessitates a reflection on diverse approaches to diagnose MASLD. Although liver biopsy stands as the gold standard technique for a thorough MASLD diagnosis, enabling the identification of inflammation and the classification of fibrosis stages (F0-4), its invasive nature and associated risks constrain widespread utilization. Consequently, most preceding clinical studies have resorted to US, liver enzymes, or various indices for MASLD diagnosis. It is imperative to develop dependable noninvasive methods for evaluating liver fibrosis and is crucial for estimating disease progression and guiding therapy[30].
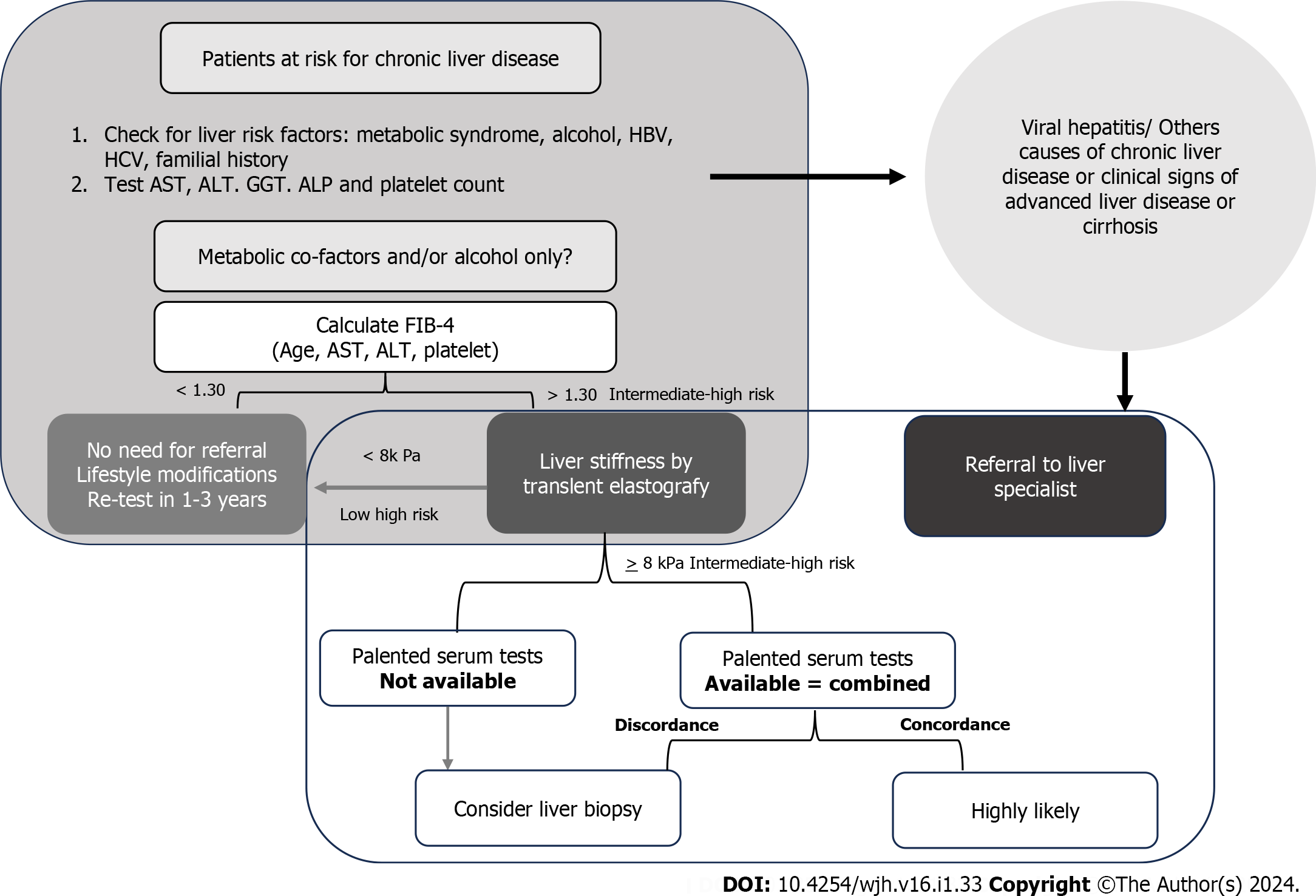
The suspicion of MASLD is grounded in the identification of steatosis via US or abnormal liver test results in patients harboring risk factors (obesity, type 2 diabetes, and/or MetS). The selection of noninvasive tools should follow a sequential approach, guided by local availability and the context of primary healthcare utilization. Simple, economical, and widely accessible serum biomarkers, such as fibrosis-4 (FIB-4) or NAFLD fibrosis score (NFS), exhibiting a high negative predictive value (88%-95%) for excluding advanced fibrosis, should constitute the first-line assessment. Individuals at low risk (FIB-4 < 1.3 or NFS fibrosis score < -1.455) require no further evaluation and are advised to adopt lifestyle modifications and engage in regular exercise. Those at intermediate risk (FIB-4 between 1.30 and 2.67 or NFS -1.455 to 0.672; accounting for 30% of cases) and high risk (FIB-4 > 2.67 or NFS > 0.672; comprising 12%-15% of cases, with a positive predictive value of 75%-90%) of advanced fibrosis should be referred to a specialized center for expert evaluation.
Another noninvasive approach involves estimating liver fibrosis through elasticity assessment. A recent development in this realm is a shear elasticity probe based on one-dimensional transient elastography utilizing ultrasound (5 MHz) and low-frequency (50 Hz) elastic waves, initially termed FibroScan. While initially employed for patients with chronic hepatitis C, ongoing studies are investigating hepatic elastography in MASLD, exploring diverse cutoff values for patients with NASH[31].
Unfortunately, existing noninvasive or minimally invasive biomarkers remain limited[32]. Endeavors have been made to formulate clinical parameters capable of reliably identifying fibrosis in MASLD patient cohorts. Various scores have been devised to ascertain the presence of fibrosis using clinical data and laboratory outcomes, aiming to integrate routine parameters of liver injury (e.g., transaminase activity) and risk characteristics (e.g., diabetes)[33]. The most commonly utilized scores are FIB-4 and the NFS. FIB-4 comprises four straightforward parameters: age; platelets; and the serum transaminases aspartate aminotransferase and alanine aminotransferase. NFS encompasses seven parameters: age; BMI; glycated hemoglobin; serum transaminases; platelets; and albumin. In primary care settings, where the prevalence of advanced fibrosis is low (5%), FIB-4 emerges as the preferred option due to its simplicity, and the fact that serum transaminases and platelet count are routinely requested by physicians in standard examinations. In large populations, a FIB-4 threshold of up to 1.30 effectively excludes the risk of advanced liver fibrosis with a substantial degree of accuracy (60%-80%). Individuals with a FIB-4 between 1.30 and 2.67 are deemed at intermediate risk for advanced fibrosis, warranting further investigations such as elastography, which can be performed before or after referral to a medical specialist. Those with a FIB-4 > 2.67 are classified as high risk and should be directed to specialized services for additional investigations, potentially including liver biopsy. It is noteworthy that guidelines from international hepatology societies advocate for the use of noninvasive strategies, which can simplify case finding and management of high-risk MASLD patients in clinical practice[34].
As MASLD or NAFLD manifests as a multifactorial ailment, various integrated treatment strategies are employed with primary objectives of retarding the progression to severe forms, such as fibrosis (NASH), and managing associated risk factors including obesity, diabetes, dyslipidemia, and hypertension[34].
Initiating therapeutic measures entails lifestyle interventions involving dietary modifications and exercise. Medication may be introduced as a secondary measure, particularly as fibrosis advances, and bariatric surgery becomes a viable option in the tertiary phase. It is noteworthy to underscore the significance of assembling an interdisciplinary team comprising a nutritionist, endocrinologist, physical educator, psychologist/psychiatrist, cardiologist, and hepatologist[36]. The percentage of weight loss is directly correlated with NASH progression, irrespective of the chosen method[17,25].
Consideration for pharmacological intervention arises if diet and exercise prove ineffective in disease control. Following the EASL recommendations (2023)[17], the presence of steatohepatitis, diagnosed through noninvasive methods or liver biopsy, accompanied by macro and microvesicular steatosis, mixed inflammatory infiltrate, hepatocellular ballooning in centrilobular vein areas (Zone III), Mallory’s corpuscles, and fibrosis, warrants pharmacological intervention. This holds true for less severe cases but with a high risk of progression. Given the limited scope of drugs and surgical treatments for NASH, lifestyle changes, including dietary adjustments, increased physical activity, and exercise, remain the cornerstone of its management.
Regular physical activity serves as a pivotal adjunct to metabolic regulation. A favorable correlation exists between sedentary behavior and susceptibility to MASLD. Individuals adhering to a health-conscious lifestyle exhibit diminished likelihood of developing pivotal factors contributing to the onset of the disease, including insulin resistance, diabetes, and glucose intolerance. Additionally, engaging in physical activity facilitates a reduction in visceral and hepatic adipose tissue, along with a decrease in circulating FFAs in the plasma[28].
Dietary considerations, with a particular focus on calorie intake, especially derived from carbohydrates, the primary energy source for the human body, play a crucial role in regulating the body’s glycemic levels. Manipulating dietary carbohydrate intake, either through restriction or substitution with complex carbohydrates, exerts an influence on enhancing serum glucose and triglyceride levels. It contributes to the elevation of HDL levels and exerts an impact on pancreatic β cells involved in insulin elimination[27]. A calorie-restricted diet, meticulously assessed and calculated by a professional considering basal metabolism and accounting for individual physical and behavioral variations, is recommended as a contributing factor to the regression of NAFLD.
Pharmacological intervention is warranted when the disease manifests a moderate degree of fibrosis, denoted as F2 > 2, as determined through transient hepatic elastography or liver biopsy. Based on this data, the initial and economically feasible treatment may involve the use of vitamin E at a dosage of 800 IU and pioglitazone at 30 mg. If applicable, anti-GLP1 medications such as liraglutide or semaglutide are recommended for overweight and obese patients to achieve a reduction of 7%-10% in body weight. Emerging pharmacological options discussed at EASL 2023, such as retatrutide, are being considered. Bariatric surgery stands as a viable treatment option with favorable outcomes, particularly for patients with a BMI > 35[17,25,26].
Pharmacotherapy aims to mitigate the progression from the early stages of NASH to advanced fibrosis. The correlation between fibrosis and overall mortality, cardiovascular risk, and the transition from cirrhosis to hepatocellular carcinoma is significant, particularly in individuals aged over 50 with concurrent diabetes, elevated alanine aminotransferase, MetS, and NASH, where greater inflammatory activity is observed.
MAFLD necessitates preventive measures within the domain of primary healthcare, centering attention on the identification of risk factors associated with MetS and the utilization of noninvasive fibrosis scoring systems to facilitate early detection. Moreover, in instances characterized by moderate to severe fibrosis, it is imperative to advocate for referral to a specialized hepatology department for comprehensive evaluation and subsequent monitoring. To preclude delayed diagnoses, primary care practitioners should consistently underscore, in their routine counselling sessions, the imperative for lifestyle modifications encompassing a wholesome diet and regular exercise. Furthermore, the introduction of pharmacological interventions becomes imperative upon the identification of disease progression, concomitant with the presence of fibrosis. Such therapeutic interventions must be tailored to individual patient profiles, often necessitating the utilization of diverse pharmacological options to forestall disease progression and foster a comprehensive multisystemic approach.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang K, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhao S
| 1. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 2. | Krauss RM. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:2265-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Kao TW, Huang CC. Recent Progress in Metabolic Syndrome Research and Therapeutics. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3852] [Cited by in RCA: 4252] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 5. | Vieira Barbosa J, Lai M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatol Commun. 2021;5:158-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Tang B, Kang P, Guo J, Zhu L, Xu Q, Gao Q, Zhang H, Wang H. [Effects of mitochondrial aldehyde dehydrogenase 2 on autophagy-associated proteins in neonatal rat myocardial fibroblasts cultured in high glucose]. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 385] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 8. | Vusirikala A, Thomas T, Bhala N, Tahrani AA, Thomas GN, Nirantharakumar K. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): A United Kingdom population-based cohort study using the health improvement network (THIN). BMC Endocr Disord. 2020;20:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 809] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 10. | Azzam H, Malnick S. Non-alcoholic fatty liver disease - the heart of the matter. World J Hepatol. 2015;7:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Vasunta RL, Kesäniemi YA, Ylitalo AS, Ukkola OH. High ambulatory blood pressure values associated with non-alcoholic fatty liver in middle-aged adults. J Hypertens. 2012;30:2015-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Li AA, Ahmed A, Kim D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:168-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2598] [Article Influence: 236.2] [Reference Citation Analysis (0)] |
| 14. | Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, Richardson B, Munoz C, Sigurðardóttir S, Coulibaly A, Milan M, Bautista F, Leung NWY, Mooney V, Obekpa S, Bech E, Polavarapu N, Hamed AE, Radiani T, Purwanto E, Bright B, Ali M, Dovia CK, McColaugh L, Koulla Y, Dufour JF, Soliman R, Eslam M. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 15. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (1)] |
| 16. | Bianco C, Romeo S, Petta S, Long MT, Valenti L. MAFLD vs NAFLD: Let the contest begin! Liver Int. 2020;40:2079-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 506] [Article Influence: 56.2] [Reference Citation Analysis (2)] |
| 18. | Karnikowski M, Córdova C, Oliveira RJ, Karnikowski MG, Nóbrega Ode T. Non-alcoholic fatty liver disease and metabolic syndrome in Brazilian middle-aged and older adults. Sao Paulo Med J. 2007;125:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, Lai Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017;96:e8179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 20. | Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, Hanratty B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 21. | Choudhary NS, Duseja A. Screening of Cardiovascular Disease in Nonalcoholic Fatty Liver Disease: Whom and How? J Clin Exp Hepatol. 2019;9:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 23. | Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, Brandman D, Tonascia J, Chalasani N, Neuschwander-Tetri B, Sanyal AJ; NASH Clinical Research Network. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin Gastroenterol Hepatol. 2019;17:1877-1885.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 25. | Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 370] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 27. | Sharma B, John S. Nonalcoholic Steatohepatitis (NASH). 2023 Apr 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] |
| 28. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 999] [Article Influence: 199.8] [Reference Citation Analysis (0)] |
| 29. | Parente DB. Imaging methods in the assessment of nonalcoholic fatty liver disease. Radiol Bras. 2020;53:IX-IX. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 31. | Baranova A, Younossi ZM. The future is around the corner: Noninvasive diagnosis of progressive nonalcoholic steatohepatitis. Hepatology. 2008;47:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 33. | Schulz M, Tacke F. Identifying High-Risk NASH Patients: What We Know so Far. Hepat Med. 2020;12:125-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Castera L, Boursier J. Noninvasive Algorithms for the Case Finding of "At-Risk" Patients with NAFLD. Semin Liver Dis. 2022;42:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:948-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 36. | Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res. 2016;167:257-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |