Published online Sep 27, 2023. doi: 10.4254/wjh.v15.i9.1013
Peer-review started: April 7, 2023
First decision: June 25, 2023
Revised: July 17, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 27, 2023
Processing time: 167 Days and 16.9 Hours
Surrogate endpoints are needed to estimate clinical outcomes in primary sclerosing cholangitis (PSC). Serum alkaline phosphatase was among the first markers studied, but there is substantial variability in alkaline phosphatase levels during the natural history of PSC without intervention. The Mayo risk score incorporates noninvasive variables and has served as a surrogate endpoint for survival for more than two decades. Newer models have better test performance than the Mayo risk score, including the primary sclerosing risk estimate tool (PREsTo) model and UK-PSC score that estimate hepatic decompensation and transplant free survival, respectively. The c-statistics for transplant-free survival for the Mayo risk model and the long-term UK-PSC model are 0.68 and 0.85, respectively. The c-statistics for hepatic decompensation for the Mayo risk model and PREsTo model are 0.85 and 0.90, respectively. The Amsterdam-Oxford model included patients with large duct and small duct PSC and patients with PSC-autoimmune hepatitis overlap and had a c-statistic of 0.68 for transplant-free survival. Other noninvasive tests that warrant further validation include magnetic resonance imaging, elastography and the enhanced liver fibrosis score. Prognostic models, noninvasive tests or a combination of these surrogate endpoints may not only serve to be useful in clinical trials of investigational agents, but also serve to inform our patients about their prognosis.
Core Tip: Several noninvasive prognostic models have been validated that improve upon serum alkaline phosphatase and the Mayo risk score or include subgroups of patients not validated by these tests. The UK-PSC score has superior test performance compared to the Mayo risk score for short and long term transplant free survival. The Primary sclerosing risk estimate tool (PREsTo) has excellent test performance for risk of hepatic decompensation. The Amsterdam-Oxford model includes patients with small duct primary sclerosing cholangitis (PSC) and PSC-autoimmune hepatitis overlap. Elastography and magnetic resonance imaging show promise as prognostic tools.
- Citation: Russo MW. Noninvasive prognostic models, imaging, and elastography to predict clinical events in primary sclerosing cholangitis: A review. World J Hepatol 2023; 15(9): 1013-1020
- URL: https://www.wjgnet.com/1948-5182/full/v15/i9/1013.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i9.1013
Primary sclerosing cholangitis (PSC) is a cholestatic liver disease associated with diffuse inflammation of the biliary treat that may lead to cirrhosis, complications from portal hypertension and cholangiocarcinoma. The diagnosis is typically established on cholangiogram obtained during endoscopic retrograde cholangiography or magnetic resonance cholangiography and less commonly from findings on liver biopsy. The estimated prevalence of PSC varies by geographic location and ranges from 0.1-13.6 per 100000 with higher rates seen in Scandinavian countries and the United States[1]. The median transplant free survival is 21 years and there is no effective medical therapy that improves upon this outcome[2].
The etiology of PSC is not known but proposed mechanisms include dysregulation of the immune system, alterations in bile duct transporters that result in accumulation of toxic bile salts, gut microbiome interactions and immune mediated injury to the biliary epithelium, or environment triggers in genetically susceptible individuals[3].
There have been a number of drugs that have been evaluated for the treatment of PSC[4-6]. Because it would take years or even decades to evaluate the effect of a medication on liver related events on survival surrogate endpoints are needed. A consensus group suggested noninvasive surrogate endpoints are needed for clinical trials, which are preferred to more invasive surrogate endpoints such as liver biopsy or ERCP[7]. Serum alkaline phosphatase (ALP) and the Mayo model, which initially included liver histology, were among the first surrogate endpoints for PSC[8,9]. Since a prior review on this topic noninvasive prognostic models have been developed and validated with excellent test performance[10]. The most common clinical outcomes that surrogate endpoints have been associated with include liver transplant free survival, hepatic decompensation and cholangiocarcinoma. A review of the topic of noninvasive prognostic tests and models is timely because there are a number of molecules under development and noninvasive surrogate tests are recommended as endpoints in clinical trials[7].
The focus of this invited review is to discuss noninvasive surrogate endpoints for patients with PSC and how they compare to ALP and Mayo Risk Score. Key words or search terms used to identify relevant articles published in English from January 1, 2000 to January 1, 2023 that were entered into PUBMED, OVID and EMBASE included “primary sclerosing cholangitis” and “biomarkers”, “primary sclerosing cholangitis” and “prognostic score”, “primary sclerosing cholangitis” and “model” and “prognosis”, “primary sclerosing cholangitis” and “elastography”. The references of articles were reviewed for additional relevant articles.
A reduction or normalization of ALP has been associated with improved outcomes in patients with PSC. In a study of 86 patients with PSC, 38 (44%) achieved ALP normalization within 12 months of diagnosis[11]. Normalization of ALP was not associated with ursodeoxycholic acid (UDCA) therapy or therapeutic endoscopic retrograde cholangiography. Persistent ALP normalization was associated with a 79% lower risk of death, hepatobiliary neoplasia, or liver transplantation. In a separate study, patients with PSC who achieved an ALP less than 1.5 times the upper limit of normal had lower rate of a composite outcome (liver decompensation, liver transplantation, liver related deaths, cholangiocarcinoma) compared to those who did not have a reduction in ALP, 6% vs 38%, P = 0.0002[12]. Among 692 patients with PSC, an ALP > 1.3 × upper limit of normal (ULN) at 1 year of follow-up was associated with a 2-fold greater risk of liver transplant or PSC related death (death from end stage liver failure, cholangiocarcinoma or liver surgery)[13]. A reduction in ALP is associated with improved transplant free survival in patients with PSC with or without dominant strictures[14].
Among UDCA treated PSC patients who did and did not have ALP levels decrease by 40% or more after 1 year, 12 year survival was 90% and 47%, respectively, P = 0.001[15]. Patients in the placebo group had better survival if they had a 40% reduction or more in ALP after 1 year compared to those who did not have a decline in ALP. In a study that included patients who were and were not treated with UDCA no patient with persistently normal ALP reached a clinical endpoint (cholangiocarcinoma, liver transplantation or death) compared to 33% with persistent ALP abnormalities[16].
Patients with PSC have substantial variability in ALP levels over 5 years with 65% and 34% of patients achieving an ALP < 1.5 × ULN or normalizing ALP, respectively[17]. Despite variability in ALP levels, an ALP that declined to < 1.5 × ULN was independently associated with death, liver transplantation, hepatic decompensation or cholangiocarcinoma. However, others have shown that ALP reductions of 40% or more from baseline are seen in 15%-18% of patients with PSC at 2 years that are not associated with disease progression[18].
Total bilirubin is associated with lower survival in patients with PSC, but studies demonstrating this association have included patients with advanced disease, thus limiting its usefulness in patients with early stage PSC[19,20].
Enhanced liver fibrosis (ELF) score (R&D systems, Orion diagnostics, Espoo Finland) and ELF test (Siemens Medical Solutions Diagnostics Inc., Tarrytown, NY, United States) are derived from algorithms that include tissue inhibitor of metalloproteinase I, hyaluronic acid, and propeptide of type III procollagen. The association between transplant free survival and ELF score was derived and validated in 167 and 138 PSC patients, respectively. ELF score was independent of Mayo Risk Score and had a c-statistic of 0.82 for transplant free survival. A score of 10.6 or higher was associated with lower transplant free survival independent of Mayo Risk Score[21]. The ELF test was better than the ELF score at identifying the group at low risk for clinical endpoints. In a clinical trial that randomized patients with PSC to simtizumab or placebo, an ELF test ≥ 9.8 was associated with PSC-related progression events (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, hepatic encephalopathy, ascending cholangitis, cholangiocarcinoma, hepatocellular carcinoma, liver transplantation, and death)[22]. Among those with an ELF test ≥ 9.8, 34% experienced a clinical event compared to 11% of those with scores below this threshold.
Among patients with PSC and cholangiocarcinoma, the ELF test was higher compared to those with PSC alone, 11.4 and 9.9, respectively P < 0.001[23]. In multivariable analysis an ELF test ≥ 9.8 was associated with a diagnosis of cholangiocarcinoma in patients with PSC (OR = 4.91, 95%CI: 1.19-20.21, P = 0.021).
The Mayo Risk Score was developed and validated in 405 patients and 124 patients, respectively with PSC from five centers[24]. The earlier Mayo Model for PSC required liver biopsy because histologic stage of PSC is a variable in the model[8,9]. The Mayo Risk Score includes age, total bilirubin, aspartate aminotransferase, variceal bleeding (yes/no), and albumin (Table 1). In the Mayo risk score study median follow up was 36 mo and the outcome was overall survival up to 4 years. Newer models that have been compared to the Mayo Risk Score will be discussed in further detail below.
| Serum markers | Models | Elastography | Imaging |
| Alkaline phosphatase; Total bilirubin; Enhanced liver fibrosis score | Mayo Risk Score; UK-PSC score; Amsterdam-Oxford Model; PREsTo score | Vibration controlled transient elastography; Magnetic resonance elastography | Magnetic resonance imaging-Anali score |
The Amsterdam-Oxford model was developed and validated among 956 patients with PSC from 44 Dutch hospitals or referral centers[25]. Large duct PSC was diagnosed in 91% of patients, 4% had PSC-autoimmune hepatitis overlap, 71% had inflammatory bowel disease, and 80% of the derivation cohort was taking UDCA. Median follow-up was 110 mo and the primary outcome was a composite outcome of liver transplant or PSC-related death (death from end-stage liver failure, death from liver surgery, death from cholangiocarcinoma or death from colorectal carcinoma).
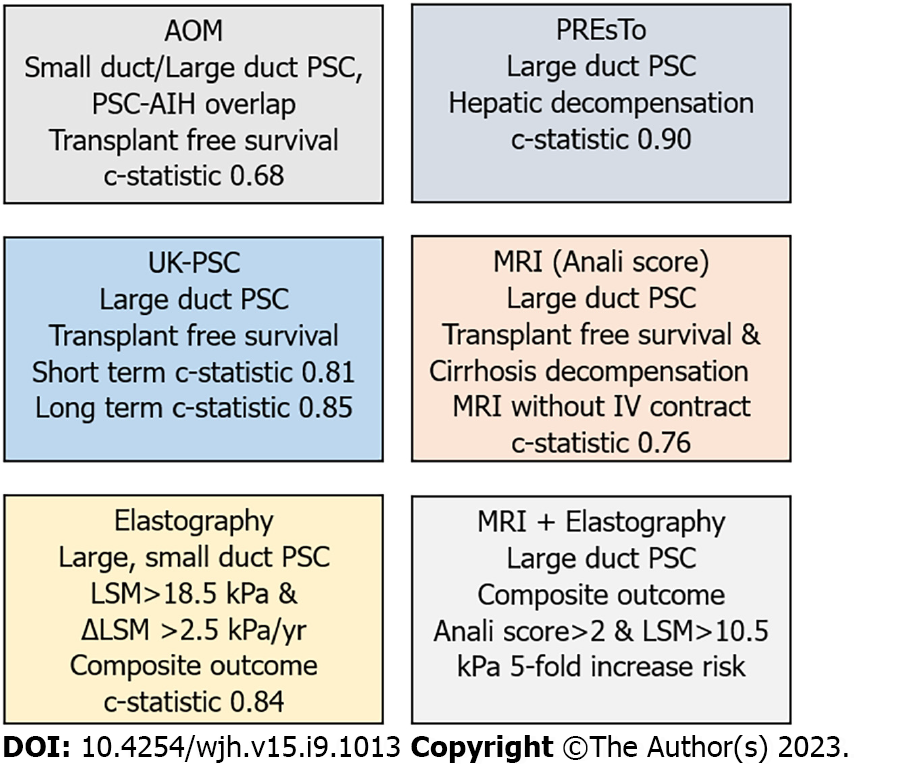
The Amsterdam-Oxford model includes PSC subtype, age at diagnosis, albumin, platelets, aspartate aminotransferase, ALP, and total bilirubin (Table 2). The c-statistics for the primary outcome at 3 years of follow-up in the validation cohort were 0.66 with similar c-statistics at 1 and 2 years of follow-up (Figure 1).
| MRS | AOM | PREsTo | UK-PSCST | UK-PSCLT | |
| Age | √ | √ | |||
| PSC subtype | √ | √ | |||
| Albumin | √ | √ | √ | √ | |
| ALP | √ | √ | √ | ||
| AST | √ | √ | |||
| Hemoglobin | √ | √ | |||
| Platelets | √ | √ | √ | √ | |
| Sodium | √ | ||||
| Total bilirubin | √ | √ | √ | √ | √ |
| Variceal bleed | √ | √ |
A study from three centers in Italy, Belgium and The Netherlands evaluated the test performance of the Amsterdam-Oxford model and compared it to the Mayo Risk Score[26]. The cohort included 534 patients of which 3% had small duct PSC, 10% had PSC-autoimmune hepatitis overlap, 60% had inflammatory bowel disease and 92% were on UDCA therapy. The primary outcome was transplant free survival. The c-statistics for Amsterdam-Oxford model and Mayo Risk Score at 5 years of follow-up in the validation cohort were 0.76 and 0.79, respectively.
Primary sclerosing cholangitis risk estimate tool (PREsTo) was developed and validated in 787 patients with PSC from centers in North America and Norway[27]. Patients with small duct PSC or PSC-autoimmune hepatitis overlap were excluded and approximately 70% of patients had inflammatory bowel disease. The number of patients on UDCA was not provided. Median follow-up was 6 and 4 years for the derivation and validation cohorts, respectively. The primary outcome was hepatic decompensation defined as variceal hemorrhage, hepatic encephalopathy, or ascites.
The authors employed artificial intelligence and used gradient boosting machines, a machine learning technique, to identify variables associated with hepatic decompensation. Variables included in the PREsTo model include total bilirubin, ALP, albumin, alanine aminotransferase, platelets, sodium, and hemoglobin (Table 1). Total bilirubin, albumin and ALP had the highest relative importance in the PREsTo model. In the validation cohort the c-statistic was 0.90 for PREsTo for 5-year risk of decompensation compared to c-statistics of 0.72, 0.85, and 0.65 for model for end stage liver disease score, Mayo Risk Score, and ALP < 1.5 × ULN, respectively.
The UK-PSC score was developed and validated in 1452 patients with PSC from 155 sites throughout the United Kingdom[28]. All patients had large duct PSC, 73% had inflammatory bowel disease, and 57% were on UDCA. Median follow-up ranged from 6-14.8 years in the validation and derivation cohorts. The primary outcome was transplant-free survival.
A short-term model for 2-year outcome and long-term model for 10-year outcomes were developed. The variables in the short-term UK-PSC score include total bilirubin, albumin, hemoglobin and platelet count (Table 2). The long-term model includes baseline and year 2 total bilirubin, platelet count, ALP, disease type (presence or absence of extrahepatic biliary disease) and history of variceal bleed (yes/no). In the validation cohort the c-statistics for short term UK-PSC model, Mayo Risk Score, model for end stage liver disease score were 0.81, 0.73, and 0.78, respectively. The c-statistics for long term UK-PSC model, Mayo Risk Score and aspartate aminotransferase platelet ratio index were 0.85, 0.69, and 0.70, respectively (Figure 1).
Features on magnetic resonance imaging (MRI) with cholangiography have been associated with outcomes in patients with PSC called the Anali score developed by Ana Ruiz and Lionel Arrive[29]. Based 289 MRI images from 64 patients with a median follow-up of 4 years a model for findings on MRI without and with contrast were developed to predict radiologic progression. The Anali score without gadolinium includes dilatation of intrahepatic bile ducts, dysmorphy, and portal hypertension while the score with gadolinium includes dysmorphy and parenchymal enhancement heterogeneity. Dysmorphy was defined as significant atrophy of either the right or left hepatic lobe and/or marked lobulations of the liver surface and/or increase in the caudate/right lobe ratio. The c-statistics for the Anali scores with and without gadolinium were 0.83 and 0.80, respectively (Figure 1)[29].
The MRI derived Anali score was validated in a study that included 338 patients with large duct PSC from France, Canada, Italy and the United Kingdom equally divided between a derivation and validation cohort[30]. The primary endpoint was transplant free survival or cirrhosis decompensation. The c-statistics for the primary outcome for the Anali score with and without gadolinium in the validation cohort were 0.73 and 0.76, respectively[30].
Liver stiffness measurements (LSM) obtained by vibration controlled transient elastography at baseline and during follow-up are associated with outcomes. A prospective study that included patients with large duct, small duct PSC (9%) or PSC-autoimmune hepatitis (3%) overlap reported adverse outcomes associated with baseline LSM and change in LSM[31]. Adverse outcomes were defined as a composite of death, liver transplantation, ascites, hepatic encephalopathy, gastrointestinal bleeding related to portal hypertension, cholangiocarcinoma or hepatocellular carcinoma. All patients were on UDCA and 68% had inflammatory bowel disease. The LSMs with highest accuracy for adverse outcomes were LSM > 18.5 kPa and change in LSM > 4 kPa/yr.
The group that developed PREsTo demonstrated an increase in magnetic resonance elastography (MRE) score is associated with hepatic decompensation (ascites, variceal hemorrhage or hepatic encephalopathy)[32]. In this study of 204 patients with PSC of which 82% had inflammatory bowel disease and 34% were on UDCA reported an increase of LSM > 0.34 kPa/yr had a c-statistic of 0.79 for hepatic decompensation. Combining a LSM > 4.32 kPa at baseline and an increase > 0.34 kPa/yr had a c-statistic of 0.93 for hepatic decompensation.
In a retrospective study that included 162 patients with PSC from 3 centers, Anali score without gadolinium and vibration controlled transient elastography (VCTE) were combined to risk stratify patients at risk for liver transplantation or cirrhosis decompensation[33]. Patients were categorized into three groups: Anali score ≤ 2 and LSM < 10.5 kPa, Anali score > 2 or LSM > 10.5 kPa, or Anali score > 3 and LSM score > 10.5 kPa. An Anali score > 2 and LSM > 10.5 kPa was associated with a 5-year risk of liver transplantation, death or cirrhosis decompensation of 38% compared to 8% for those with an Anali score ≤ 2 and LS ≤ 10.5 kPa, P < 0.001.
Despite the development and validation of several prognostic models and the evolution of imaging and elastography, ALP has persevered as a surrogate marker for disease progression in PSC. ALP or changes in ALP remain as an outcome in clinical trials of investigational agents for PSC[34-39]. The simplicity and availability of ALP make it an attractive biomarker in clinical practice. However, there is variability in ALP that occurs over time without any intervention, and it has inferior test performance compared to more recently validated prognostic models. Despite these limitations, ALP, it remains a variable in Amsterdam-Oxford model, PREsTo and UK-PSCLT models (Table 2). The ELF score has been associated with survival or cholangiocarcinoma but requires further validation. Furthermore, ELF is associated with added cost compared to noninvasive prognostic models where variables and lab data are usually already available.
The Mayo Risk Score has stood the test of time, but a criticism has been that the study cohort included a large number of patients with advanced PSC and the time span for the model is limited to 4 years. The PREsTo and UK-PSC scores provide estimates for outcomes at 5 and 10 years of follow-up. The test performance of UK-PSC and PREsTo models are better compared to the Mayo Risk Score.
Each of the models has its role in informing our patients with PSC about their prognosis (Figure 1). The UK-PSC model provides short-term and long-term estimates of transplant free survival. The PREsTo score provides risk of hepatic decompensation over 5 years. The Amsterdam-Oxford model provide transplant free survival and included patients with small duct PSC and PSC-autoimmune hepatitis overlap, although there were very small numbers in each group.
MRI and VCTE are attractive as prognostic tools because they are frequently obtained during clinical care. The Anali score can be readily obtained from MRI of the abdomen with or without gadolinium because imaging is commonly obtained as part of clinical practice. Results from VCTE or MRE, including baseline measurements as well as annual changes can provide prognostic information, although data on VCTE and MRE are limited to those derived from retrospective studies.
A number of novel biomarkers involved in inflammation, fibrosis or the gut barrier have been studied that are not commercially available but may warrant further study, including third generation anti-neutrophil cytoplasmic antibodies to serine protease-3[40-44]. Future studies could combine results for the prognostic models that include clinical and laboratory data with scores from MRI and elastography (e.g. UK-PSC or PREsTo+Anali score+elastography score). As the test performance of these noninvasive prognostic models improve they may not only serve as surrogate endpoints in clinical trials, but they can also be used to inform our patients about their prognosis. Other cutting-edge techniques, including artificial intelligence may be employed to identify findings on imaging associated with disease progression, survival or patients at risk for cholangiocarcinoma[44].
In conclusion, a number of noninvasive prognostic models for PSC are available that can be used in clinical trials as surrogate endpoints or as tools to inform patients about their disease progression. The models can be tailored to a specific trial endpoint, such as PREsTo score for hepatic decompensation or UK-PSC score for transplant-free survival. In the future, combining these models with results from elastography may improve test performance. Other areas warranting further investigation include novel molecular diagnostics, composition of the gut microbiome and its association with clinical outcomes as well as exploring the role of artificial intelligence in identifying imaging findings associated with disease progression or cholangiocarcinoma.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stojanovic B, Serbia; Xie Q, China S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Charatcharoenwitthaya P, Lindor KD. Primary sclerosing cholangitis: patients with a rising alkaline phosphatase at annual follow-up. Clin Gastroenterol Hepatol. 2007;5:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY; EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 3. | Park JW, Kim JH, Kim SE, Jung JH, Jang MK, Park SH, Lee MS, Kim HS, Suk KT, Kim DJ. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 380] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Rahimpour S, Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A Triple Blinded, Randomized, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis: a Pilot Study. J Gastrointestin Liver Dis. 2016;25:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Ghonem NS, Auclair AM, Hemme CL, Gallucci GM, de la Rosa Rodriguez R, Boyer JL, Assis DN. Fenofibrate Improves Liver Function and Reduces the Toxicity of the Bile Acid Pool in Patients With Primary Biliary Cholangitis and Primary Sclerosing Cholangitis Who Are Partial Responders to Ursodiol. Clin Pharmacol Ther. 2020;108:1213-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Ponsioen CY, Chapman RW, Chazouillères O, Hirschfield GM, Karlsen TH, Lohse AW, Pinzani M, Schrumpf E, Trauner M, Gores GJ. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 306] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | de Vries EM, Beuers U, Ponsioen CY. Biomarkers for disease progression of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2015;31:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Hilscher M, Enders FB, Carey EJ, Lindor KD, Tabibian JH. Alkaline phosphatase normalization is a biomarker of improved survival in primary sclerosing cholangitis. Ann Hepatol. 2016;15:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | de Vries EM, Wang J, Leeflang MM, Boonstra K, Weersma RK, Beuers UH, Geskus RB, Ponsioen CY. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 2016;36:1867-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rupp C, Rössler A, Halibasic E, Sauer P, Weiss KH, Friedrich K, Wannhoff A, Stiehl A, Stremmel W, Trauner M, Gotthardt DN. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Lindström L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Bakhshi Z, Hilscher MB, Gores GJ, Harmsen WS, Viehman JK, LaRusso NF, Gossard AA, Lazaridis KN, Lindor KD, Eaton JE. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J Gastroenterol. 2020;55:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Trivedi PJ, Muir AJ, Levy C, Bowlus CL, Manns MP, Lu X, Crans G, Chung C, Subramanian GM, Myers RP, Goodman Z, Chalasani N, Vierling JM, Guha IN, Hirschfield GM. Inter- and Intra-individual Variation, and Limited Prognostic Utility, of Serum Alkaline Phosphatase in a Trial of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2021;19:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 21. | Vesterhus M, Hov JR, Holm A, Schrumpf E, Nygård S, Godang K, Andersen IM, Naess S, Thorburn D, Saffioti F, Vatn M, Gilja OH, Lund-Johansen F, Syversveen T, Brabrand K, Parés A, Ponsioen CY, Pinzani M, Färkkilä M, Moum B, Ueland T, Røsjø H, Rosenberg W, Boberg KM, Karlsen TH. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology. 2015;62:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Muir AJ, Levy C, Janssen HLA, Montano-Loza AJ, Shiffman ML, Caldwell S, Luketic V, Ding D, Jia C, McColgan BJ, McHutchison JG, Mani Subramanian G, Myers RP, Manns M, Chapman R, Afdhal NH, Goodman Z, Eksteen B, Bowlus CL; GS-US-321-0102 Investigators. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology. 2019;69:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 23. | Saffioti F, Roccarina D, Vesterhus M, Hov JR, Rosenberg W, Pinzani M, Pereira SP, Boberg KM, Thorburn D. Cholangiocarcinoma is associated with a raised enhanced liver fibrosis score independent of primary sclerosing cholangitis. Eur J Clin Invest. 2019;49:e13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, LaRusso NF, Lindor KD, Dickson ER. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 244] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | de Vries EM, Wang J, Williamson KD, Leeflang MM, Boonstra K, Weersma RK, Beuers U, Chapman RW, Geskus RB, Ponsioen CY. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut. 2018;67:1864-1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Goet JC, Floreani A, Verhelst X, Cazzagon N, Perini L, Lammers WJ, de Vries AC, van der Meer AJ, van Buuren HR, Hansen BE. Validation, clinical utility and limitations of the Amsterdam-Oxford model for primary sclerosing cholangitis. J Hepatol. 2019;71:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Eaton JE, Vesterhus M, McCauley BM, Atkinson EJ, Schlicht EM, Juran BD, Gossard AA, LaRusso NF, Gores GJ, Karlsen TH, Lazaridis KN. Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) Predicts Outcomes of the Disease: A Derivation and Validation Study Using Machine Learning. Hepatology. 2020;71:214-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Goode EC, Clark AB, Mells GF, Srivastava B, Spiess K, Gelson WTH, Trivedi PJ, Lynch KD, Castren E, Vesterhus MN, Karlsen TH, Ji SG, Anderson CA, Thorburn D, Hudson M, Heneghan MA, Aldersley MA, Bathgate A, Sandford RN, Alexander GJ, Chapman RW, Walmsley M; UK-PSC Consortium, Hirschfield GM, Rushbrook SM. Factors Associated With Outcomes of Patients With Primary Sclerosing Cholangitis and Development and Validation of a Risk Scoring System. Hepatology. 2019;69:2120-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Ruiz A, Lemoinne S, Carrat F, Corpechot C, Chazouillères O, Arrivé L. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology. 2014;59:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Lemoinne S, Cazzagon N, El Mouhadi S, Trivedi PJ, Dohan A, Kemgang A, Ben Belkacem K, Housset C, Chretien Y, Corpechot C, Hirschfield G, Floreani A, Motta R, Gallix B, Barkun A, Barkun J, Chazouillères O, Arrivé L. Simple Magnetic Resonance Scores Associate With Outcomes of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2019;17:2785-2792.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Corpechot C, Gaouar F, El Naggar A, Kemgang A, Wendum D, Poupon R, Carrat F, Chazouillères O. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146:970-9; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Eaton JE, Sen A, Hoodeshenas S, Schleck CD, Harmsen WS, Gores GJ, LaRusso NF, Gossard AA, Lazaridis KN, Venkatesh SK. Changes in Liver Stiffness, Measured by Magnetic Resonance Elastography, Associated With Hepatic Decompensation in Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2020;18:1576-1583.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Cazzagon N, Lemoinne S, El Mouhadi S, Trivedi PJ, Gaouar F, Kemgang A, Ben Belkacem K, Floreani A, Hirschfield G, Chretien Y, Housset C, Motta R, Russo FP, Chazouillères O, Arrivé L, Corpechot C. The Complementary Value of Magnetic Resonance Imaging and Vibration-Controlled Transient Elastography for Risk Stratification in Primary Sclerosing Cholangitis. Am J Gastroenterol. 2019;114:1878-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Eksteen B, Bowlus CL, Montano-Loza AJ, Lefebvre E, Fischer L, Vig P, Martins EB, Ahmad J, Yimam KK, Pockros PJ, Feld JJ, Minuk G, Levy C. Efficacy and Safety of Cenicriviroc in Patients With Primary Sclerosing Cholangitis: PERSEUS Study. Hepatol Commun. 2021;5:478-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, Shrestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Schiano T, Jin Y, Pencek R, MacConell L, Shapiro D, Bowlus CL; AESOP Study Investigators. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 36. | Trauner M, Bowlus CL, Gulamhusein A, Hameed B, Caldwell SH, Shiffman ML, Landis C, Muir AJ, Billin A, Xu J, Liu X, Lu X, Chung C, Myers RP, Kowdley KV. Safety and Sustained Efficacy of the Farnesoid X Receptor (FXR) Agonist Cilofexor Over a 96-Week Open-label Extension in Patients With PSC. Clin Gastroenterol Hepatol. 2023;21:1552-1560.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Hirschfield GM, Chazouillères O, Drenth JP, Thorburn D, Harrison SA, Landis CS, Mayo MJ, Muir AJ, Trotter JF, Leeming DJ, Karsdal MA, Jaros MJ, Ling L, Kim KH, Rossi SJ, Somaratne RM, DePaoli AM, Beuers U. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: A multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 38. | Arndtz K, Corrigan M, Rowe A, Kirkham A, Barton D, Fox RP, Llewellyn L, Athwal A, Wilkhu M, Chen YY, Weston C, Desai A, Adams DH, Hirschfield GM; BUTEO trial team. Investigating the safety and activity of the use of BTT1023 (Timolumab), in the treatment of patients with primary sclerosing cholangitis (BUTEO): A single-arm, two-stage, open-label, multi-centre, phase II clinical trial protocol. BMJ Open. 2017;7:e015081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Dhillon AK, Kummen M, Trøseid M, Åkra S, Liaskou E, Moum B, Vesterhus M, Karlsen TH, Seljeflot I, Hov JR. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019;39:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A, Par G, Par A, Orosz P, Szalay F, Lakatos PL, Papp M. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol. 2017;23:5412-5421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 41. | Friedrich K, Baumann C, Wannhoff A, Rupp C, Mehrabi A, Weiss KH, Gotthardt DN. Serum miRNA-122 is an independent biomarker of survival in patients with primary sclerosing cholangitis. J Gastrointestin Liver Dis. 2018;27:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Dhillon AK, Rupp C, Bergquist A, Voitl R, Folseraas T, Trøseid M, Midttun Ø, Ueland PM, Karlsen TH, Vesterhus M, Kummen M, Hov JR. Associations of neopterin and kynurenine-tryptophan ratio with survival in primary sclerosing cholangitis. Scand J Gastroenterol. 2021;56:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Lopens S, Wunsch E, Milkiewicz M, Röber N, Zarske G, Nasser A, Conrad K, Laass M, Rödiger S, Krawczyk M, Roggenbuck D, Milkiewicz P. PR3-ANCAs Detected by Third-Generation ELISA Predicts Severe Disease and Poor Survival in Primary Sclerosing Cholangitis. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Hu C, Iyer RK, Juran BD, McCauley BM, Atkinson EJ, Eaton JE, Ali AH, Lazaridis KN. Predicting cholangiocarcinoma in primary sclerosing cholangitis: using artificial intelligence, clinical and laboratory data. BMC Gastroenterol. 2023;23:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |