Published online Aug 27, 2023. doi: 10.4254/wjh.v15.i8.973
Peer-review started: May 27, 2023
First decision: June 14, 2023
Revised: June 19, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: August 27, 2023
Processing time: 86 Days and 12.7 Hours
Hepatitis C virus (HCV) is defined as a public health problem by the World Health Organization (WHO) and since then has defined targets through the HCV elimination. The HCV cascade of care highlights the progress towards these goals and essential interventions that need to be delivered along this continuum care.
To document the treatment cascade for patients with HCV infection at the Hospital Nossa Senhora da Conceição (HNSC), defining the percentage of antibody-positive patients who collected molecular biology tests (polymerase chain reaction), attended outpatient clinic assistance, underwent treatment, and achieved a virologic cure termed sustained virologic response (SVR).
With the retrospective cohort design, patients diagnosed with HCV infection in the period between January 1, 2015 and December 31, 2020 were included. Data from HCV notification forms, electronic medical records, Computerized Laboratory Environment Manager System, and Medicine Administration System (evaluation of special medications) were collected in 2022 and all information up to that period was considered. The data were analyzed with IBM SPSS version 25, and Poisson regression with robust simple variance was performed for analysis of variables in relation to each step of the cascade. Variables with P < 0.20 were included in the multivariate analysis with P < 0.05 considered significant. Pearson’s chi-square test was applied to compare the groups of patients who persisted in follow-up at the HNSC and who underwent follow-up at other locations.
Results were lower than expected by the WHO with only 49% of candidates receiving HCV treatment and only 29% achieving SVR, despite the 98% response rate to direct acting antivirals documented by follow-up examination. The city of origin and the place of follow-up were the variables associated with SVR and all other endpoints. When comparing the cascade of patients who remained assisted by the HNSC vs external patients, we observed superior data for HNSC patients in the SVR. Patients from the countryside and metropolitan region were mostly assisted at the HNSC and the specialized and continuous care provided at the HNSC was associated with superior results, although the outcomes remain far from the goals set by the WHO.
With the elaboration of the HCV cascade of care using local data, it was possible to stratify and evaluate risk factors associated with losses between each step of the cascade, to inform new strategies to guide elimination efforts in the future.
Core Tip: Hepatitis C virus (HCV) is defined as a public health problem by the World Health Organization and since then has defined targets through the HCV elimination. The present study aimed to document the treatment cascade for patients with HCV infection at a hospital in southern Brazil. With the retrospective cohort design, patients diagnosed with HCV infection between 2015 and 2020 were included to create the HCV cascade of care described as five exposure columns according to the stages of HCV care. Variables were related to each step of the cascade to identify obstacles for patients to reach the last step. With the elaboration of the HCV cascade of care using local data, it was possible to stratify and evaluate risk factors associated with losses between each step of the cascade, to inform new strategies to guide elimination efforts in the future.
- Citation: Vaucher MB, Silva CU, Varella IRS, Kim AYS, Kliemann DA. Stages of care for patients with chronic hepatitis C at a hospital in southern Brazil. World J Hepatol 2023; 15(8): 973-984
- URL: https://www.wjgnet.com/1948-5182/full/v15/i8/973.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i8.973
The World Health Organization (WHO) set the goal of diagnosing 90% of cases of viral hepatitis and treating 80% of diagnosed cases with the aim of reducing the incidence by 90% and mortality attributable to hepatitis by 65% by 2030[1]. The hepatitis C virus (HCV) care cascade represents the care that patients receive in the respective health services and consequently illustrates the basic indicators of the WHO targets[2]. In the first stage are people with HCV infection, in the second are the patients aware of the diagnosis of HCV, in the third, those who underwent treatment, and in the fourth stage, those who achieved cure with viral suppression from 12 wk to 24 wk after the end of treatment[2-4]. Other stages can be added, such as retention in care and after cure monitoring, but it is difficult to standardize the criteria, hindering the possibility of later comparison[3].
The elaboration of the treatment cascade facilitates the identification of barriers and groups of risks which we must work with[3,5]. The correlation between sociodemographic variables and results between stages is an important tool for the analysis of the HCV cascade of care[6]. Mental health problems, change in follow-up location, and restriction of information about the disease were detected as causes of failures in the stages of chronic HCV treatment in a study by health professionals[7]. Therefore, the construction of local cascades is necessary for the understanding of gaps in current practices and the elaboration of changes[8].
Brazil joined the Hepatitis C Elimination Plan in 2017[9]. Since then, the country has been developing and imple
The South region of Brazil is responsible for the highest detection rate of confirmed HCV infection in the country and also for the highest mortality rate, with higher rates than national data[11]. Within this region, the city of Porto Alegre, in 2020 was the second capital with the highest HCV detection rate, and in 2021 the first, even higher than the national rate[11]. The Hospital Nossa Senhora da Conceição (HNSC) in Porto Alegre is a tertiary hospital that has an infectology service which is a reference in treatment services for patients with human immunodeficiency virus (HIV) infection and viral hepatitis[12]. The objective of this study was to define the continuity of care or treatment cascade for patients with chronic HCV infection at the HNSC and to define sociodemographic variables that influence follow-up between each step of the cascade.
This referred retrospective cohort study included patients diagnosed with chronic HCV infection between 2015 and 2020 at the HNSC. All hospitalized patients and outpatients above 16 years of age notified by the HNSC epidemiological center for viral hepatitis with positive anti-HCV or HCV-polymerase chain reaction (PCR) results detectable during this period were analyzed. Patients under 16 years of age were excluded, as they would be followed up at a pediatric hospital attached to the HNSC. Patients who died, who were not connected with the HNSC, or who were not located in the electronic medical records of the hospitals were excluded.
The Information System for Notifiable Diseases (SINAM) for Viral Hepatitis, electronic medical records from the HNSC, computerized Laboratory Environment Management System (GAL), and Medicine Administration System (AME) provided data such as age, gender, race, education level, history of pregnancy and drug use, profession, city of origin, institutionalization situation, and co-infection with HIV and hepatitis B virus (HBV). Specific data of the disease under study were also obtained, such as date of diagnosis of HCV infection (anti-HCV test), history of consultation with a specific outpatient clinic for the treatment of hepatitis, initial quantitative HCV-PCR and final quantitative HCV-PCR (after the 12th wk since the end of treatment), genotyping, history of cirrhosis or hepatocellular carcinoma, specific regimen, and date of treatment prescribed for HCV. Data were collected in 2022 and all information up to that period was considered.
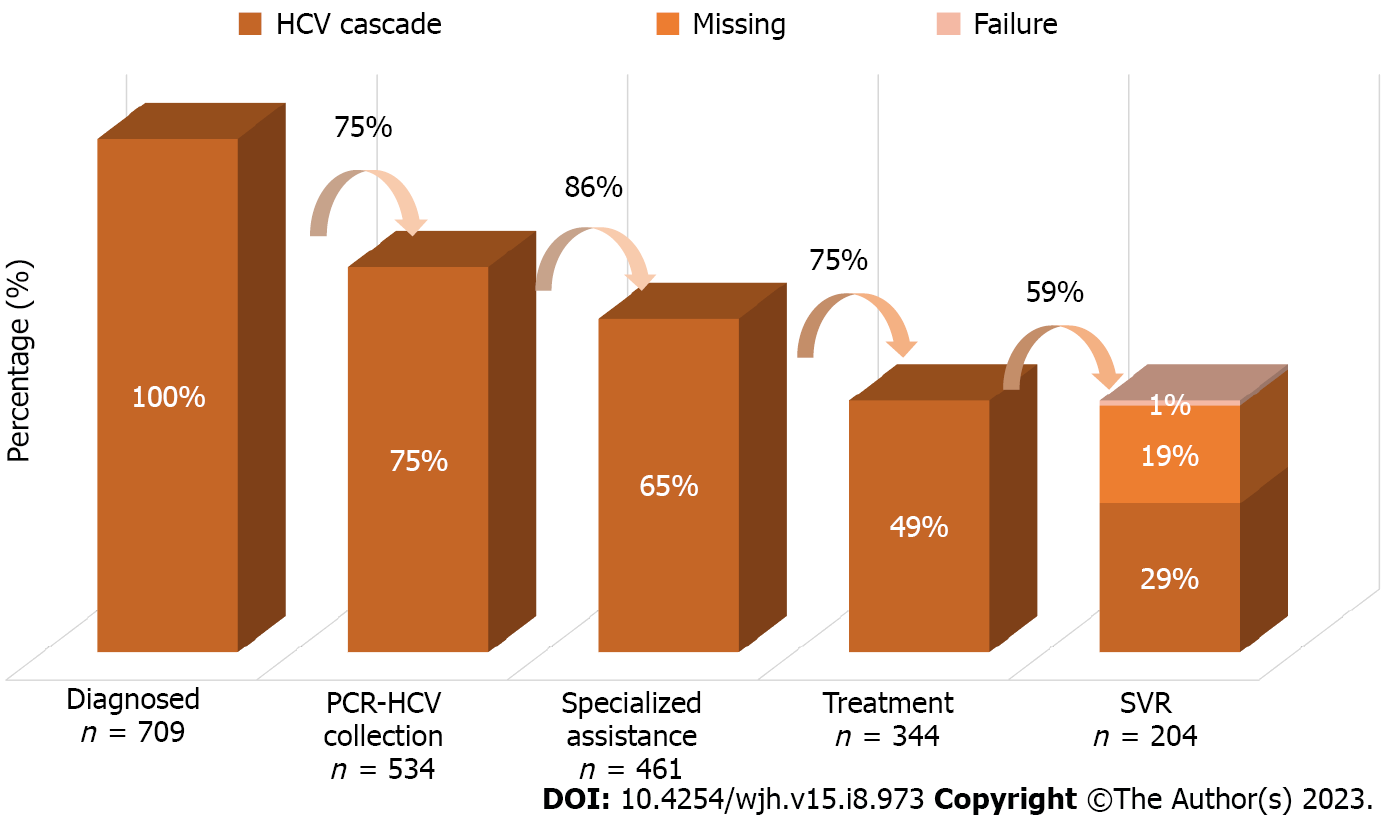
Regarding the HCV cascade of care, five exposure columns were built according to the stages of HCV care. The first stage covers all people diagnosed with chronic HCV infection, that is, patients with positive anti-HCV or detectable quantitative HCV-PCR in the analyzed period. The second includes patients who underwent some quantitative PCR collection, and the third includes patients who underwent consultation at a specialized outpatient clinic for monitoring hepatitis C. The fourth step integrates all who underwent treatment with specific antivirals for chronic HCV infection according to the established protocol. The fifth step ends with all patients who achieved a sustained virological response, that is, those who obtained an undetectable quantitative HCV-PCR test after the 12th wk since the end of treatment. Patients who did not collect this exam after treatment were not included in the fifth step, being presented in the cascade as “missing” and subsequently analyzed within the group of those who were not cured. The percentages were then calculated using the “n” of the first step and the “n” of the previous step as denominator, thus obtaining two percentages for analysis, being represented using a series of unidirectional columns.
Using the IBM SPSS version 25 program, Poisson regression with robust simple variance was performed to estimate the incidence ratio (IR) at a 95% confidence interval (95%CI) for the variables of gender, age group, race, education, city of residence, place of follow-up, presence of cirrhosis, institutionalization, year of diagnosis, and co-infection with HIV/HBV related to each step of the cascade: PCR-HCV collection, bond, treatment, and sustained virologic response (SVR). All variables that had a value of P < 0.20 in the simple analyzes were included in the multivariable model, and in this model only variables with P < 0.05 were considered significant.
Two more cascades were also built, discriminating between patients who underwent treatment at the HNSC and those who underwent treatment at other locations after the diagnosis. The comparison of the sociodemographic characteristics between the groups, HNSC and external, was performed using Pearson’s chi-square test and results with P < 0.05 were considered significant. The study was approved by the research ethics committee of the Hospitalar Conceição Group, under number 51462421.8.0000.5530, and informed consent was waived, subject to the patient’s commitment to confidentiality.
By searching the HNSC viral hepatitis notification database between 2015 and 2020 at the HNSC, 2498 patients were identified. A total of 487 patients who died, with decompensated cirrhosis, hepatocellular carcinoma, renal failure, and sepsis as the main etiologies reported, were excluded. Another 1232 patients were also excluded because they had a diagnosis of other viral hepatitis, an HCV diagnosis prior to 2015, a false anti-HCV test result, no attachment to the HNSC, or being younger than 16 years old. A total of 779 patients diagnosed with HCV infection during the analyzed period were included, but of these 70 had spontaneous cure and, as they did not require treatment, were disregarded for further analyses.
For the HCV cascade of care, 709 patients were analyzed, showing a sociodemographic profile of being predominantly male (54.3%), white (76.6%), and from Porto Alegre (44.7%), and just having had completed elementary education (67.4%). The mean age of the patients was 53 years. Only 22 patients had a history of pregnancy, 13% co-infection with the HIV, 10.3% co-infection with HBV, 17.8% had a history of cirrhosis, and only 2% a diagnosis of hepatocellular carcinoma. It was identified that 24.9% had a history of drug use and 33 patients of institutionalization. Regarding the genotype, 44.5% of the patients did not have an identified genotype and, among the available genotypes, genotype 1 was the most prevalent (60%), followed by genotype 3 (34.6%) and finally genotype 2 (5.3%).
Regarding the total of 709 patients, 534 (75.3%) collected quantitative PCR, 461 (65%) consulted at a specialized clinic, 344 (48.5%) underwent treatment for HCV infection, and 204 (28.7%) reached SVR. When considering the previous column as the denominator, the percentages would be, respectively, 75% with RT-PCR collection, 86% consulted, 75% treated, and 59% with SVR confirmed by post-treatment examination. Both percentages are represented in Figure 1. The results of the simple and multivariate analyzes of the variables in relation to each step of the cascade are described in Tables 1 and 2, respectively.
| Total (%) | PCR-HCV collection | P value | Specialized assistance | P value | Treatment | P value | SVR | P value | |
| Total | 709 | 534 (75.3%) | 461 (65.0%) | 344 (48.5%) | 204 (28.7%) | ||||
| Gender | |||||||||
| Female | 324 (45.7%) | 238 (73.5%); 0.95 (0.87-1.04) | 0.295 | 221 (68.2%); 1.09 (0.98-1.21) | 0.101 | 175 (54.0%); 1.23 (1.05-1.43) | 0.007 | 109 (62.3%); 1.10 (0.92-1.32) | 0.253 |
| Male | 385 (54.3%) | 296 (76.9%); 1.0 | 240 (62.3%); 1.0 | 169 (43.9%); 1.0 | 95 (56.2%); 1.0 | ||||
| Age (yr) | |||||||||
| 17-39 | 118 (16.6%) | 90 (76.3%); 1.0 | 79 (66.9%); 1.0 | 0.420 | 52 (44.1%); 1.0 | 27 (51.9%); 1.0 | |||
| 40-59 | 348 (49.1%) | 267 (76.7%); 1.00 (0.89-1.13) | 0.920 | 225 (64.7%); 0.96 (0.83-1.12) | 0.646 | 170 (48.9%); 1.10 (0.88-1.39) | 0.380 | 103 (60.6%); 1.16 (0.87-1.55) | 0.294 |
| > 60 | 243 (34.3%) | 177 (72.8%); 0.95 (0.84-1.08) | 0.479 | 157 (64.6%); 0.96 (0.82-1.12) | 0.657 | 122 (50.2%); 1.13 (0.89-1.44) | 0.284 | 74 (60.7%); 1.16 (0.86-1.57) | 0.307 |
| Race | |||||||||
| White | 543 (76.6%) | 405 (74.6%); 1.0 | 353 (65.0%); 1.0 | 269 (49.5%); 1.0 | 164 (61.0%); 1.0 | ||||
| Non-white | 154 (21.7%) | 118 (76.6 %); 1.02 (0.92-1.13) | 0.598 | 97 (63.0%); 0.96 (0.84-1.11) | 0.649 | 65 (42.2%); 0.85 (0.69-1.04) | 0.123 | 32 (49.2%); 0.80 (0.62-1.05) | 0.113 |
| Education | |||||||||
| Illiterate | 39 (5.5%) | 28 (71.8%); 0.88 (0.71-1.08) | 0.238 | 26 (66.7%); 0.95 (0.74-1.22) | 0.741 | 20 (51.3%); 0.96 (0.68-1.35) | 0.834 | 13 (65.0%); 0.95 (0.66-1.36) | 0.804 |
| Elementary school | 478 (67.4%) | 344 (72%); 0.88 (0.80-0.97) | 0.011 | 301 (63.0%); 0.90 (0.79-1.03) | 0.134 | 219 (45.8%); 0.86 (0.71-1.03) | 0.110 | 120 (54.8%); 0.80 (0.66-0.98) | 0.031 |
| High school and university | 141 (19.9%) | 115 (81.6%); 1.0 | 98 (69.5%); 1.0 | 75 (53.2%); 1.0 | 51 (68.0%); 1.0 | ||||
| City of origin | |||||||||
| Porto Alegre | 317 (44.7%) | 215 (67.8%); 1.0 | 174 (54.9%); 1.0 | 121 (38.2%); 1.0 | 49 (40.5%); 1.0 | ||||
| Metropolitan region | 247 (34.8%) | 190 (76.9%); 1.13 (1.02-1.25) | 0.016 | 166 (67.2%); 1.22 (1.07-1.39) | 0.003 | 126 (51.0%); 1.33 (1.11-1.60) | 0.002 | 81 (64.3%); 1.58 (1.23-2.04) | 0 |
| Countryside | 134 (18.9%) | 118 (88.1%); 1.29 (1.17-1.43) | 0 | 112 (83.6%); 1.52 (1.34-1.72) | 0 | 91 (67.9%); 1.77 (1.48-2.13) | 0 | 68 (74.7%); 1.84 (1.44-2.36) | 0 |
| Follow-up site | |||||||||
| HNSC | 341 (48.1%) | 320 (93.8%); 1.61 (1.47-1.76) | 0 | 340 (99.7%); 3.03 (2.62-3.50) | 0 | 237 (69.5%); 2.39 (2.00-2.84) | 0 | 166 (70.0%); 1.97 (1.50-2.58) | 0 |
| External | 121 (17.1%) | 214 (58.2%); 1.0 | 121 (32.9%); 1.0 | 107 (29.1%); 1.0 | 38 (35.5%); 1.0 | ||||
| Institutionalized | |||||||||
| Yes | 33 (4.7%) | 25 (75.8%); 1.0 | 18 (54.5%); 1.0 | 12 (36.4%); 0.74 (0.46-1.17) | 0.198 | 8 (66.7%); 1.12 (0.74-1.70) | |||
| No | 676 (95.3%) | 509 (75.3%); 1.00 (0.82-1.22) | 0.952 | 443 (65.5%); 0.83 (0.60-1.14) | 0.255 | 332 (49.1%); 1.0 | 196 (59.0%); 1.0 | 0.561 | |
| Co-infection with HBV | |||||||||
| Yes | 73 (10.3%) | 61 (83.6%); 1.12 (1.00-1.25) | 0.041 | 43 (58.9%); 1.0 | 30 (41.1%); 0.83 (0.62-1.10) | 0.208 | 18 (60.0%); 1.01 (0.74-1.37) | 0.935 | |
| No | 636 (89.7%) | 473 (74.4%); 1.0 | 418 (65.7%); 0.89 (0.73-1.09) | 0.282 | 314 (49.4%); 1.0 | 186 (59.2%); 1.0 | 0.935 | ||
| Co-infection with HIV | |||||||||
| Yes | 93 (13.1%) | 75 (80.6%); 1.08 (0.97-1.20) | 0.158 | 63 (67.7%); 1.04 (0.90-1.22) | 0.541 | 35 (37.6%); 0.75 (0.57-0.98) | 0.039 | 17 (48.6%); 0.80 (0.56-1.14) | 0.222 |
| No | 616 (86.9%) | 459 (74.5%); 1.08 | 398 (64.6%); 1.0 | 309 (50.2%); 1.0 | 187 (60.5%); 1.0 | ||||
| Cirrhosis | |||||||||
| Yes | 126 (17.8%) | 112 (88.9%); 1.22 (1.13-1.33) | 0 | 106 (84.1%); 1.38 (1.25-1.52) | 0 | 82 (65.1%); 1.44 (1.23-1.69) | 0 | 60 (73.2%); 1.33 (1.12-1.57) | 0.001 |
| No | 583 (82.2%) | 422 (72.4%); 1.0 | 355 (60.9%); 1.0 | 262 (44.9%); 1.0 | 144 (55.0%); 1.0 | ||||
| Date of diagnosis | |||||||||
| 2015 | 149 (21.0%) | 111 (74.5%); 1.0 | 102 (68.5%); 1.0 | 76 (51%); 1.0 | 42 (55.3%); 1.0 | ||||
| 2016 | 136 (19.2%) | 113 (83.1%); 1.11 (0.98-1.25) | 0.076 | 101 (74.3%); 1.08 (0.93-1.25) | 0.278 | 72 (52.9%); 1.03 (0.83-1.29) | 0.744 | 51 (70.8%); 1.28 (0.99-1.64) | 0.052 |
| 2017 | 121 (17.1%) | 92 (76.0%); 1.02 (0.89-1.17) | 0.771 | 84 (69.4%); 1.01 (0.86-1.19) | 0.865 | 68 (56.2%); 1.10 (0.88-1.37) | 0.393 | 45 (66.2%); 1.19 (0.91-1.56) | 0.181 |
| 2018 | 121 (17.1%) | 80 (66.1%); 0.88 (0.75-1.04) | 0.140 | 72 (59.5%); 0.86 (0.72-1.04) | 0.133 | 53 (43.8%); 0.85 (0.66-1.10) | 0.244 | 30 (56.6%); 1.04 (0.75-1.39) | 0.880 |
| 2019 | 128 (18.1%) | 90 (70.3%); 0.94 (0.81-1.09) | 0.440 | 67 (52.3%); 0.76 (0.62-0.93) | 0.008 | 49 (38.3%); 0.75 (0.57-0.98) | 0.038 | 22 (44.9%); 0.81 (0.56-1.17) | 0.272 |
| 2020 | 54 (7.6%) | 48 (88.9%); 1.19 (1.04-1.36) | 0.009 | 35 (64.8%); 0.94 (0.75-1.18) | 0.634 | 26 (48.1%); 0.94 (0.68-1.29) | 0.723 | 14 (53.8%); 0.97 (0.64-1.46) | 0.901 |
| PCR-HCV collection | P value | Specialized assistance | P value | Treatment | P value | SVR | P value | |
| Gender | ||||||||
| Female | 0.06 (0.97-1.16) | 0.148 | 1.20 (1.03-1.40) | 0.014 | ||||
| Male | 1.00 | 1.00 | ||||||
| Race | ||||||||
| White | 1.0 | 1.0 | ||||||
| Non-white | 0.99 (0.81-1.21) | 0.931 | 0.95 (0.72-1.25) | 0.734 | ||||
| Education | ||||||||
| Illiterate | 0.83 (0.67-1.03) | 0.103 | 0.87 (0.73-1.04) | 0.152 | 0.76 (0.53-1.09) | 0.145 | 0.63 (0.35-1.12) | 0.120 |
| Elementary school | 0.89 (0.81-0.97) | 0.014 | 0.94 (0.85-1.04) | 0.244 | 0.85 (0.72-1.02) | 0.082 | 0.79 (0.65-0.95) | 0.014 |
| High school and university | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| City of origin | ||||||||
| Porto Alegre | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Metropolitan region | 1.11 (1.00-1.23) | 0.033 | 1.12 (1.00-1.24) | 0.034 | 1.19 (0.98-1.45) | 0.074 | 1.54 (1.19-1.98) | 0.001 |
| Countryside | 1.19 (1.08-1.33) | 0.001 | 1.14 (1.03-1.25) | 0.009 | 1.38 (1.14-1.68) | 0.001 | 1.62 (0.24-2.12) | 0.000 |
| Follow-up site | ||||||||
| HNSC | 1.58 (1.43-1.75) | 0.000 | 3.00 (2.58-3.50) | 0.000 | 2.19 (1.80-2.65) | 0.000 | 1.59 (1.20-2.09) | 0.001 |
| External | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Institutionalized | ||||||||
| Yes | 0.70 (0.42-1.17) | 0.178 | ||||||
| No | 1.0 | |||||||
| Co-infection with HBV | ||||||||
| Yes | 1.11 (0.97-1.27) | 0.121 | ||||||
| No | 1.0 | |||||||
| Co-infection with HIV | ||||||||
| Yes | 1.11 (0.97-1.27) | 0.121 | 0.80 (0.59-1.08) | 0.156 | ||||
| No | 1.0 | 1.0 | ||||||
| Cirrhosis | ||||||||
| Yes | 1.02 (0.94-1.11) | 0.504 | 0.96 (0.90-1.03) | 0.327 | 1.07 (0.91-1.62) | 0.363 | 1.18 (0.91-1.36) | 0.275 |
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Date of diagnosis | ||||||||
| 2015 | 1.0 | 1.0 | 1.0 | 1.0 | 1.000 | |||
| 2016 | 1.02 (0.90-1.16) | 0.662 | 0.90 (0.80-1.01) | 0.083 | 0.82 (0.65-1.03) | 0.102 | 1.05 (0.80-1.37) | 0.701 |
| 2017 | 0.97 (0.84-1.12) | 0.711 | 0.93 (0.81-1.06) | 0.311 | 0.96 (0.76-1.20) | 0.730 | 1.06 (0.80-1.41) | 0.652 |
| 2018 | 0.90 (0.77-1.05) | 0.213 | 0.92 (0.79-1.07) | 0.321 | 0.83 (0.64-1.07) | 0.153 | 0.93 (0.67-1.30) | 0.685 |
| 2019 | 1.02 (0.88-1.19) | 0.717 | 0.88 (0.76-1.03) | 0.118 | 0.81 (0.62-1.05) | 0.125 | 0.81 (0.57-1.16) | 0.267 |
| 2020 | 1.27 (1.09-1.48) | 0.002 | 1.03 (0.86-1.25) | 0.698 | 1.02 (0.75-1.39) | 0.879 | 0.96 (0.66-1.40) | 0.862 |
Patients with incomplete primary education had lower rates of HCV-PCR collections after the diagnosis of HCV infection in the simple analysis (P < 0.20), as well as patients who underwent diagnosis in 2018; however, these variables were not significant in multivariate analysis. Patients living in the metropolitan area and countryside regions, who consulted at an outpatient clinic at the HNSC, co-infected with HIV and HBV, with a history of cirrhosis, and who were diagnosed in 2016 and in 2020, had higher collections of PCR-HCV in the first analysis (P < 0.20). In the second analysis, patients from the metropolitan area and countryside regions, patients from the HNSC, and those diagnosed in 2020 were more likely to obtain HCV-PCR (P < 0.05).
Despite linkage being the subsequent step in the cascade, there were 22 patients who consulted and did not collect any HCV-PCR test. The variables that showed a difference in favor of creating a link to a specialized outpatient clinic were female gender, living outside the city of Porto Alegre, having cirrhosis, and consulting at the HNSC. The diagnoses in 2018 and in 2019, as well as having only elementary school, were factors contrary to consulting with specialists. In the multivariate analysis, only being from the countryside or metropolitan area and consulting the HNSC were significant (P < 0.05). Through the records in the GAL and AME, we identified that 121 patients were followed up in other places after the diagnosis in the hospital.
Being female, having a history of cirrhosis, living outside the city of Porto Alegre, and consulting at the HNSC were protective factors in the simple analysis for undertaking the treatment. As risk factors for not undergoing HCV treatment, co-infection with HIV, being institutionalized, being non-white, having had only completed elementary school, and having been diagnosed in 2019 were identified. Consulting at the HNSC and being from the countryside remained significant protective factors (P < 0.05). The mean time between diagnosis and initiation of treatment was approximately 2 years. The DAAs most used in treatment were sofosbuvir and ledispasvir (26.0%), sofosbuvir and velpatasvir (22.0%), sofosbuvir and daclatasvir (17.0%), and sofosbuvir, daclatasvir, and ribavirin (15.0%). Only eight patients (1.1%) underwent more than one treatment. Among the 22 pregnant women in the study, only nine underwent treatment, but timing of treatment before or after pregnancy was unknown. No history of vertical transmission was found in these cases, since the GAL system evaluated the newborns of the respective pregnant women.
Out of all 344 patients who underwent treatment, only 204 reached SVR; however, 136 patients had no record in the GAL of collection of HCV-PCR control after the 12th wk since the end of treatment. Only four had SVR failure, resulting in a documented DAA response rate of 98%. The risk factors in the simple analysis for not achieving documented SVR were being of non-white race and having only elementary education. As variables favorable to SVR, living in the metropolitan area, in the countryside, and consultation at the HNSC were found, in addition to having been diagnosed with HCV infection in 2015 and in 2016. In the multivariate analysis, only the places of residence (countryside and metropolitan area) and consultation at the HNSC were significant (P < 0.05).
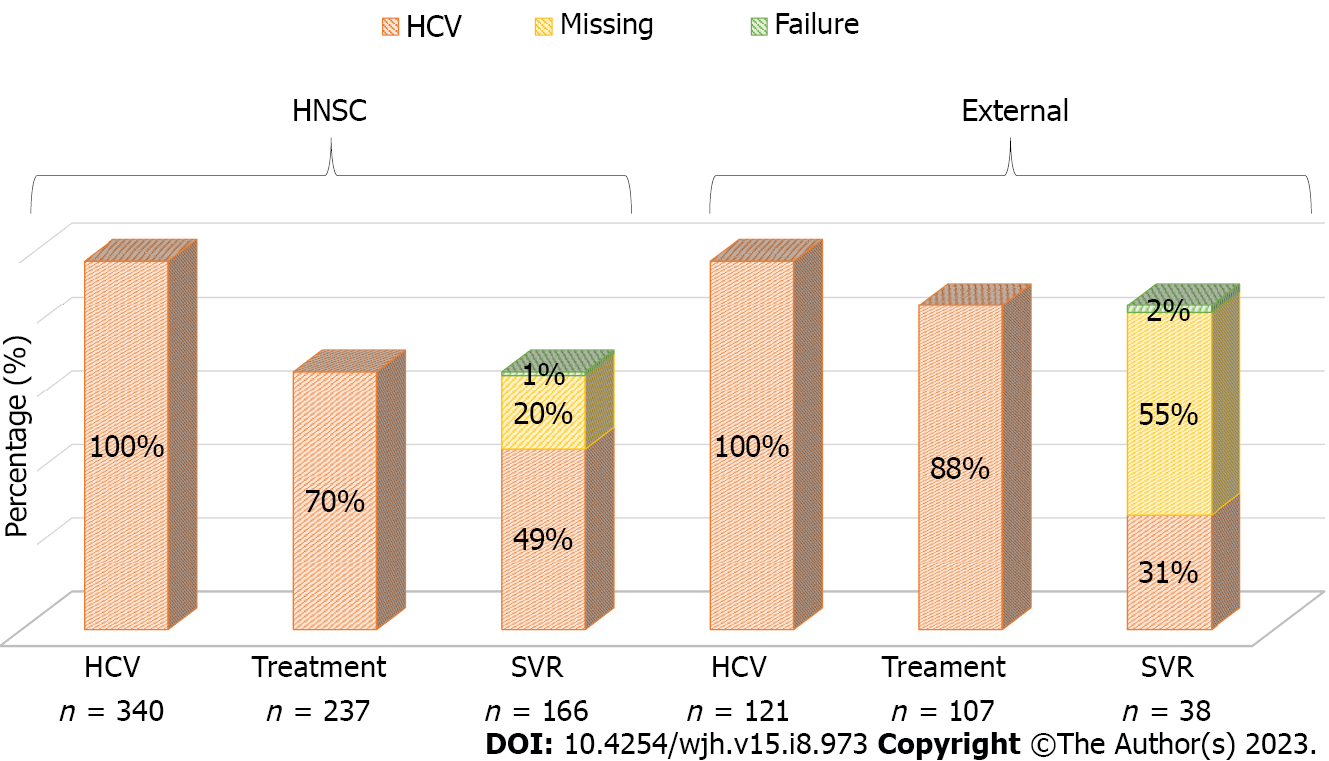
Because significance was found in all stages of HCV care regarding the place of follow-up, we constructed two separate cascades of care: One for patients who remained under follow-up at the HNSC and another for patients who chose to be assisted in other places. As previously mentioned, 340 patients remained at the HNSC, of whom 70% underwent treatment and 49% achieved SVR, 1% showed failure, and 20% did not collect a PCR test after treatment. Among 121 outpatients, 88% underwent treatment and 31% had SVR, but 55% did not perform a control PCR collection and 2% had SVR failure (Figure 2). As variables favorable to SVR, living in the metropolitan area, in the countryside, and consultation at the HNSC were found, in addition to having been diagnosed with HCV infection in 2015 and in 2016. In the multivariate analysis, only the places of residence (countryside and metropolitan area) and consultation at the HNSC were significant (P < 0.05) (Table 3).
| HNSC | External | P value | |
| Total | 340 (73.8%) | 121 (26.2%) | |
| Gender | 0.654 | ||
| Female | 161 (47.2%) | 60 (49.6%) | |
| Age (yr) | 0.854 | ||
| < 40 | 60 (17.6%) | 19 (15.7%) | |
| 40-59 | 167 (49.0%) | 59 (48.8%) | |
| > 60 | 114 (33.4%) | 43 (35.5%) | |
| Race | 0.450 | ||
| White | 262 (79.2%) | 91 (75.8%) | |
| Education | 0.411 | ||
| Illiterate | 22 (7.1%) | 4 (3.5%) | |
| Elementary school | 218 (69.9%) | 83 (73.5%) | |
| High school and university | 72 (23.1%) | 26 (23.0%) | |
| City of origin | 0.006 | ||
| Porto Alegre | 121 (36.4%) | 53 (43.8%) | |
| Metropolitan region | 116 (34.9%) | 51 (42.1%) | |
| Countryside | 95 (28.6%) | 17 (14.0%) | |
| Cirrhosis | 0.000 | ||
| Yes | 100 (29.3%) | 7 (5.8%) |
We conducted this study in our local hospital to identify the HCV care cascade and relevant characteristics for progressing from diagnosis to successful cure, to provide representative data from Rio Grande do Sul and mainly from the city of Porto Alegre[11,13]. The highest prevalence of HCV infection was found in patients who are male, white, and aged over 40 years, consistent with both data from the state of Rio Grande do Sul and national statistics[11,14]. Also, higher prevalence of HCV was observed in patients with only elementary education[11]. Regarding co-infection with HIV, we present data that are very close to those of the South region in 2021 (10.1%)[11]. Groups most vulnerable to infection by the HCV, such as people living with HIV, institutionalized people, and drug users, were considerably represented, corroborating the importance of focusing on testing and preventive actions for these particular subpopulations[9,14]. Genotypes 1 and 3 were the most prevalent, as expected according to national and local data[14].
According to the WHO, the second step of the HCV cascade of care would be represented by patients aware of their diagnosis, with a target of 90%[1]. However, in this study, we did not carry out this estimate and included patients from the diagnosis of chronic HCV infection which we performed at the HNSC. Overall, the WHO target of patients on treatment (80%) was not reached. But when analyzing the cascade broken down by place of follow-up, patients being followed up outside the HNSC reached the goal. This can be explained by a possible data collection bias, where all patients with prescriptions for treatment in the AME system from other locations were linked to other services. In addition, HNSC patients may have even started treatment due to the lack of clinical conditions, such as neoplasms or serious comorbidities. Not having genotyping or HCV-PCR test within a year are also bureaucratic reasons that interfere with the delay in starting HCV therapy, which is unfortunate given the possibility of simplified protocols with pangenotypic regimens[15,16].
About 90% of patients with HCV infection are cured with the new DAAs, resulting in a markedly decreased risk of liver-related morbidity and mortality and also a drastic reduction of onward transmission[5]. Despite the high response rate to DAAs, the SVR percentages are surprisingly low in this study, which represents the biggest “gap” among all the cascade steps. This is due to the large number of patients with missing data after treatment, making it impossible to confirm SVR. In addition to the collection of HCV-PCR control outside the Brazilian Unified Health System (SUS), another reason for the ignored data would be the loss to follow-up of the patients after the completion of the treatment, identifying the importance of implementing a strategy to enhance the rate of return of the patients after the exams[17]. The coronavirus disease 2019 pandemic had an important impact on the follow-up of these patients after treatment[18]. HNSC patients had a higher SVR and a lower number of ignored HCV-PCR tests, possibly due to less loss to follow-up.
National data and data from the state of Rio Grande do Sul have shown a progressive decrease in reported cases of viral hepatitis with anti-HCV reagents and concomitant HCV-PCR reagents, which may demonstrate an increase in notifications of cases of serological cure, or even less access to confirmatory HCV-PCR tests[11,13]. Patients who were connected to the hospital collected significantly more PCR-HCV, which may have been facilitated by the logistics of collecting the test inside the hospital under study, after the diagnosis was made. This can also be explained by the greater severity of the patients, as patients with comorbidities, such as HBV and HIV infections and cirrhosis, were shown to have greater access to the test. Living in the countryside and in the metropolitan area were also significant protective factors in this analysis for HCV-PCR collection, as most of these patients have consultations at the HNSC, possibly due to less access to confirmatory HCV-PCR tests outside the capital Porto Alegre[13]. Patients ended up collecting more exams in 2020, demonstrating more accessibility to the exam that year or even greater concern for investigating the disease of patients throughout the pandemic.
Gaps occur at all stages of HCV care, with dropouts in care occurring before and after linking to specialized care[19]. Consultation at a specialized outpatient clinic is recommended in the treatment lines established in the country; however, more recent guidelines describe the intention of training non-specialist physicians[10,14]. HNSC patients may have found it easier to create a bond with an infectology or gastroenterology outpatient clinic because they were diagnosed in the same hospital. On the other hand, patients from the countryside and metropolitan area, who mostly have consultations at the HNSC, sought this service possibly due to a shortage or lack of specialized professionals in their cities of origin.
Having hepatocellular carcinoma, decompensated cirrhosis, or other neoplasms interfere with the assessment of the patient’s profile and with the recommendation of treatment[14]. Cirrhosis was shown to be a positive factor in the simple analysis, which is justified by the recent finding that having advanced cirrhosis was a necessary criterion for undertaking treatment[14]. On the other hand, co-infection with HIV and institutionalized individuals proved to be risk factors for not undergoing treatment, and it should be noted that they are vulnerable groups[14]. The non-white race in the simple analysis also proved to be a vulnerable group for not undergoing treatment, which reminds us of the need to consider race in the implementation of public policies[20]. In the final analysis, the female sex obtained higher data in the performance of treatment; however, this data contradicts the results seen previously where women, mainly young people, tend to face barriers to engaging in any form of health care[21]. Furthermore, HCV infection rates in women of childbearing potential have increased, making prenatal diagnosis a priority[22]. Being connected to the HNSC was associated with a significantly higher rate of undergoing treatment, which may be related to the maintenance of follow-up at a specialized outpatient clinic for gastroenterology and infectology services.
Having a low level of education, only elementary school, proved to be an obstacle to collecting HCV-RNA and achieving SVR, indicating the importance of education for the perception of their health status. Counterintuitively, it is possible that having more knowledge about the natural history of the disease is associated with greater stigma of HCV infection, another barrier to be addressed in the continuous care of patients. The patients at the HNSC were mostly from the countryside and metropolitan area and had cirrhosis inferring the need and demand for specialized care. Despite these results, the training of primary care professionals is able to increase the rate of treatment of patients, with HCV cure results in the decentralization of care similar to specialty outpatient clinics[22].
Among the limitations of the study is our inability to determine the true rate of SVR due to the high number of missing follow-up HCV-PCR data. Furthermore, other missing data from the HNSC medical records and external sites, important for a thorough analysis of sociodemographic variables, are also a considerable limitation of the referred study. This study identified that the sociodemographic characteristics of patients diagnosed with HCV infection at the HNSC are similar to regional and national data. It was also possible to stratify relevant risk factors for patients failing to proceed along the treatment cascade, thereby elucidating potential targeted strategies to improve care. According to the results of the hepatitis treatment at the HNSC, the importance of specialized care was highlighted. To make hepatitis treatment more accessible to patients in the countryside and metropolitan area, teams need to be properly trained. Given that we are far from the goals defined by the WHO necessary for elimination of HCV as a public health problem, it is critical to strengthen the treatment lines and facilitate care for patients not only at the HNSC, but also throughout the South region.
Hepatitis C virus (HCV) is defined as a public health problem by the World Health Origination (WHO) and since then has defined targets through the HCV elimination.
The South region of Brazil is responsible for the highest detection rate of confirmed HCV infection in the country and also for the highest mortality rate, with higher rates than national data. Within this region, the city of Porto Alegre, in 2020 was the second capital with the highest HCV detection rate, and in 2021 the first, even higher than the national rate.
To define the continuity of care or treatment cascade for patients with chronic HCV infection at the Hospital Nossa Senhora da Conceição (HNSC) and to define sociodemographic variables that influence follow-up between each step of the cascade.
With the retrospective cohort design, patients diagnosed with HCV infection in the period between January 1, 2015 and December 31, 2020 were included. Data from HCV notification forms, electronic medical records, Computerized Laboratory Environment Manager System and Medicine Administration System (evaluation of special medications) were collected in 2022 and all information up to that period was considered. The data were analyzed with IBM SPSS version 25, and Poisson regression with robust simple variance was performed for analysis of variables in relation to each step of the cascade. Variables with P < 0.20 were included in the multivariate analysis with P < 0.05 considered significant. Pearson’s chi-square test was applied to compare the groups of patients who persisted in follow-up at the HNSC and who underwent follow-up at other locations.
Results were lower than expected by the WHO with only 49% of candidates receiving HCV treatment and only 29% achieving sustained virologic response (SVR), despite the 98% response rate to direct acting antivirals documented by follow-up examination. The city of origin and the place of follow-up were the variables associated with SVR and all other endpoints. When comparing the cascade of patients who remained assisted by the HNSC vs external patients, we observed superior data for HNSC patients in the SVR. Patients from the countryside and metropolitan region were mostly assisted at the HNSC and the specialized and continuous care provided at the HNSC was associated with superior results, although the outcomes remain far from the goals set by the WHO.
This study identified that the sociodemographic characteristics of patients diagnosed with HCV infection at the HNSC are similar to regional and national data. It was also possible to stratify relevant risk factors for patients failing to proceed along the treatment cascade, thereby elucidating potential targeted strategies to improve care. According to the results of the hepatitis treatment at the HNSC, the importance of specialized care was highlighted. To make hepatitis treatment more accessible to patients in the countryside and metropolitan area, teams need to be properly trained.
We have the perspective that other places carry out their HCV cascade of care for stratification of local risk factors, thus helping to eliminate hepatitis C.
We would like to thank the Hospital Nossa Senhora do Conceição for being a supporter of the work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Health care sciences and services
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Qi XS, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | World Health Organization. Global Health Sector Strategy on Viral Hepatitis, 2016–2021: towards ending viral hepatitis. April 26, 2018. [cited 1 June 2023]. Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf. |
| 2. | World Health Organization. Global Hepatitis Report 2017. 2017. [cited 1 June 2023]. Available from: https://www.who.int/publications-detail-redirect/9789241565455. |
| 3. | Safreed-Harmon K, Blach S, Aleman S, Bollerup S, Cooke G, Dalgard O, Dillon JF, Dore GJ, Duberg AS, Grebely J, Boe Kielland K, Midgard H, Porter K, Razavi H, Tyndall M, Weis N, Lazarus JV. The Consensus Hepatitis C Cascade of Care: Standardized Reporting to Monitor Progress Toward Elimination. Clin Infect Dis. 2019;69:2218-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Global reporting system for hepatitis (GRSH)–project description. 2018. [cited 1 June 2023]. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hepatitis/strategic-information/global-reporting-system. |
| 5. | World Health Organization. Progress report on access to hepatitis C treatment: focus on overcoming barriers in low- and middle-income countries. March 2018. [cited 1 June 2023]. Available from: https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf?sequence=1. |
| 6. | Balakrishnan M, Kanwal F. The HCV Treatment Cascade: Race Is a Factor to Consider. J Gen Intern Med. 2019;34:1949-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hawks L, Norton BL, Cunningham CO, Fox AD. The Hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 9. | Brazil Ministry of Health. Secretary of Health Surveillance. Prevention and Control of Sexually Transmitted Infections, HIV/AIDS and Viral Hepatitis. Plan for the Elimination of Hepatitis C in Brazil. Brasília: Ministry of Health, 2018. [cited 1 June 2023]. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_clinico_hiv_sifilis_hepatites.pdf. |
| 10. | Coalition for Global Hepatitis Elimination. Perfil Nacional De Eliminação. Das Hepatites Virais. Julho, 2021. [cited 1 June 2023]. Available from: https://www.globalhep.org/sites/default/files/content/news/files/2021-07/National%20Hepatitis%20Elimination%20Profile_Brazil_July%2028.pdf. |
| 11. | BRAZIL. Ministry of Health. Secretary of Health Surveillance. Department of Surveillance, Prevention and Control of STIs, HIV/AIDS and Viral Hepatitis. Epidemiological Bulletin of Viral Hepatitis. Brasilia: Ministry of Health, 2022. [cited 1 June 2023]. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2022/boletim-epidemiologico-de-hepatites-virais-2022-numero-especial. |
| 12. | Grupo Hospitalar Conceição. Letter of Services to the Citizen. 2017. [cited 1 June 2023]. Available from: https://www.ghc.com.br/files/cartacidadao.pdf.. |
| 13. | Government of the State of Rio Grande do Sul. State Health Surveillance Center. Epidemiological Bulletin Viral Hepatitis, Rio Grande do Sul. July, 2022. [cited 1 June 2023]. Available from: https://saude.rs.gov.br/upload/arquivos/202207/25120953-boletim-epidemiologico-hepatites-virais-2022.pdf. |
| 14. | Capraru C, Feld JJ. Remaining challenges in HCV elimination. J Hepatol. 2021;74:964-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Solomon SS, Wagner-Cardoso S, Smeaton L, Sowah LA, Wimbish C, Robbins G, Brates I, Scello C, Son A, Avihingsanon A, Linas B, Anthony D, Nunes EP, Kliemann DA, Supparatpinyo K, Kityo C, Tebas P, Bennet JA, Santana-Bagur J, Benson CA, Van Schalkwyk M, Cheinquer N, Naggie S, Wyles D, Sulkowski M. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2022;7:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Kamali I, Shumbusho F, Barnhart DA, Nyirahabihirwe F, Gakuru JP, Dusingizimana W, Nizeyumuremyi E, Habinshuti P, Walker S, Makuza JD, Serumondo J, Nshogoza Rwibasira G, Ndahimana JD. Time to complete hepatitis C cascade of care among patients identified during mass screening campaigns in rural Rwanda: a retrospective cohort study. BMC Infect Dis. 2022;22:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Romero-Hernández B, Martínez-García L, Rodríguez-Dominguez M, Martínez-Sanz J, Vélez-Díaz-Pallarés M, Pérez Mies B, Muriel A, Gea F, Pérez-Elías MJ, Galán JC. The Negative Impact of COVID-19 in HCV, HIV, and HPV Surveillance Programs During the Different Pandemic Waves. Front Public Health. 2022;10:880435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Blanding DP, Moran WP, Bian J, Zhang J, Marsden J, Mauldin PD, Rockey DC, Schreiner AD. Linkage to specialty care in the hepatitis C care cascade. J Investig Med. 2021;69:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Pawlotsky JM, Ramers CB, Dillon JF, Feld JJ, Lazarus JV. Simplification of Care for Chronic Hepatitis C Virus Infection. Semin Liver Dis. 2020;40:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Pearce ME, Bartlett SR, Yu A, Lamb J, Reitz C, Wong S, Alvarez M, Binka M, Velásquez Garcia H, Jeong D, Clementi E, Adu P, Samji H, Wong J, Buxton J, Yoshida E, Elwood C, Sauve L, Pick N, Krajden M, Janjua NZ. Women in the 2019 hepatitis C cascade of care: findings from the British Columbia Hepatitis Testers cohort study. BMC Womens Health. 2021;21:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Luetkemeyer AF, Wyles DL. CROI 2019: highlights of viral hepatitis. Top Antivir Med. 2019;27:41-49. [PubMed] |
| 22. | Saine ME, Szymczak JE, Moore TM, Bamford LP, Barg FK, Forde KA, Schnittker J, Holmes JH, Mitra N, Lo Re V 3rd. The impact of disease-related knowledge on perceptions of stigma among patients with Hepatitis C Virus (HCV) infection. PLoS One. 2021;16:e0258143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |