Published online Aug 27, 2023. doi: 10.4254/wjh.v15.i8.964
Peer-review started: March 20, 2023
First decision: May 18, 2023
Revised: May 26, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 27, 2023
Processing time: 154 Days and 16.1 Hours
Tenofovir alafenamide (TAF) has a serum lipid-raising effect in patients with HIV; however, its effect on serum lipids and nonalcoholic fatty liver disease (NAFLD) risk in patients with chronic hepatitis B (CHB) is unclear.
To compare the effects of TAF and entecavir (ETV) on serum lipid levels in patients with CHB.
In this retrospective cohort study, the data including the clinical features, serum lipids, and metabolic factors of patients with CHB at baseline and approximately 1 year after TAF or ETV treatment were collected and analyzed. We used prope
A total of 336 patients (75.60% male) were included; 63.69% received TAF and 36.31% received ETV. Compared with the ETV group, the TAF group had significantly higher TCHO levels after treatment (4.67 ± 0.90 vs 4.36 ± 1.05, P = 0.006). In a propensity score-matched model for body mass index, age, sex, smoking, drinking, presence of comorbidities such as NAFLD, cirrhosis, diabetes mellitus, and hypertension, TAF-treated patients had significantly increased TCHO levels compared to that at baseline (P = 0.019). There was no difference for the ETV group. Body mass index, sex, hypertension, baseline TCHO, and creatine kinase-MB isoenzyme levels were significantly associated with elevated TCHO levels in logistic regression analysis. However, 1-year TAF treatment did not increase the incidence of NAFLD.
A greater increase in TCHO was observed in patients with CHB receiving TAF compared to those receiving ETV. However, TAF-induced dyslipidemia did not increase the incidence of NAFLD.
Core Tip: This study compared the effects of tenofovir alafenamide (TAF) and entecavir on serum lipid levels in chronic hepatitis B patients. The results suggested that TAF-treated patients had significantly increased triglycerides, and total cholesterol levels compared to that at baseline, while there was no difference in the entecavir group. However, dyslipidemia caused by TAF therapy did not increase the incidence of nonalcoholic fatty liver disease. Our findings indicated that the potential impact of anti-viral therapy on the lipid profile may be an important consideration in the treatment choices for chronic hepatitis B patients with abnormal metabolic factors.
- Citation: Lai RM, Lin S, Wang MM, Li N, Zhou JH, Lin XY, Chen TB, Zhu YY, Zheng Q. Tenofovir alafenamide significantly increased serum lipid levels compared with entecavir therapy in chronic hepatitis B virus patients. World J Hepatol 2023; 15(8): 964-972
- URL: https://www.wjgnet.com/1948-5182/full/v15/i8/964.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i8.964
Hepatitis B virus (HBV) infects approximately 240 million people worldwide, including approximately 86 million people in China[1]. Chronic hepatitis B (CHB) may lead to decompensation of cirrhosis and hepatocellular carcinoma (HCC), which are the leading causes of mortality in patients with CHB[2]. Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome (MetS), with a cumulative prevalence of 24% worldwide[3]. In recent decades, the prevalence of NAFLD has significantly increased in China, leading to the coexistence of NAFLD and CHB. Dyslipidemia, which is characterized by high triglyceride (TG), high total cholesterol (TCHO), low high-density lipoprotein (HDL), and high low-density lipoprotein levels, is strongly associated with NAFLD and MetS[4].
Interferon and nucleoside analog therapies cannot completely eradicate HBV infection[5]. Many patients require long-term anti-HBV therapy with potent oral drugs tenofovir alafenamide (TAF) and entecavir (ETV), which are recommended as first-line treatment in HBV clinical practice guidelines[6]. TAF has also recently been incorporated into antiretroviral regimens for people with HIV, and we observed its impact on serum lipid levels in these individuals. A prospective cohort study showed that patients with HIV infection treated with a TAF-containing regimen had significantly worse blood lipid levels, especially those with higher LDL and TCHO[7]. In a recent real-world study, switching from tenofovir disoproxil fumarate (TDF) to a TAF-containing regimen in HIV-infected patients resulted in a significant increase in serum lipid profiles[8]. However, data on the effects of TAF on serum lipid levels in patients with HIV may be limited by the potentially confounding effects of concomitant antiretroviral HIV drugs.
The effect of ETV on serum lipid profiles has not yet been reported in postmarketing studies. A retrospective cohort study showed greater reductions in TCHO, LDL, and HDL levels in patients with CHB treated with TDF than in those treated with ETV[9]. TAF is considered the successor of TDF; however, no studies have compared the effects of TAF and ETV on lipid profiles in HBV-treated patients. Meanwhile, there are limited data on the effects of TAF on metabolism-related complications in real-world settings. Therefore, this retrospective cohort study aimed to characterize the effect of TAF on serum lipid levels and NAFLD risk in patients with CHB, and we compared the pretreatment and post-treatment serum lipid profile changes after initiation of either TAF or ETV anti-viral therapy.
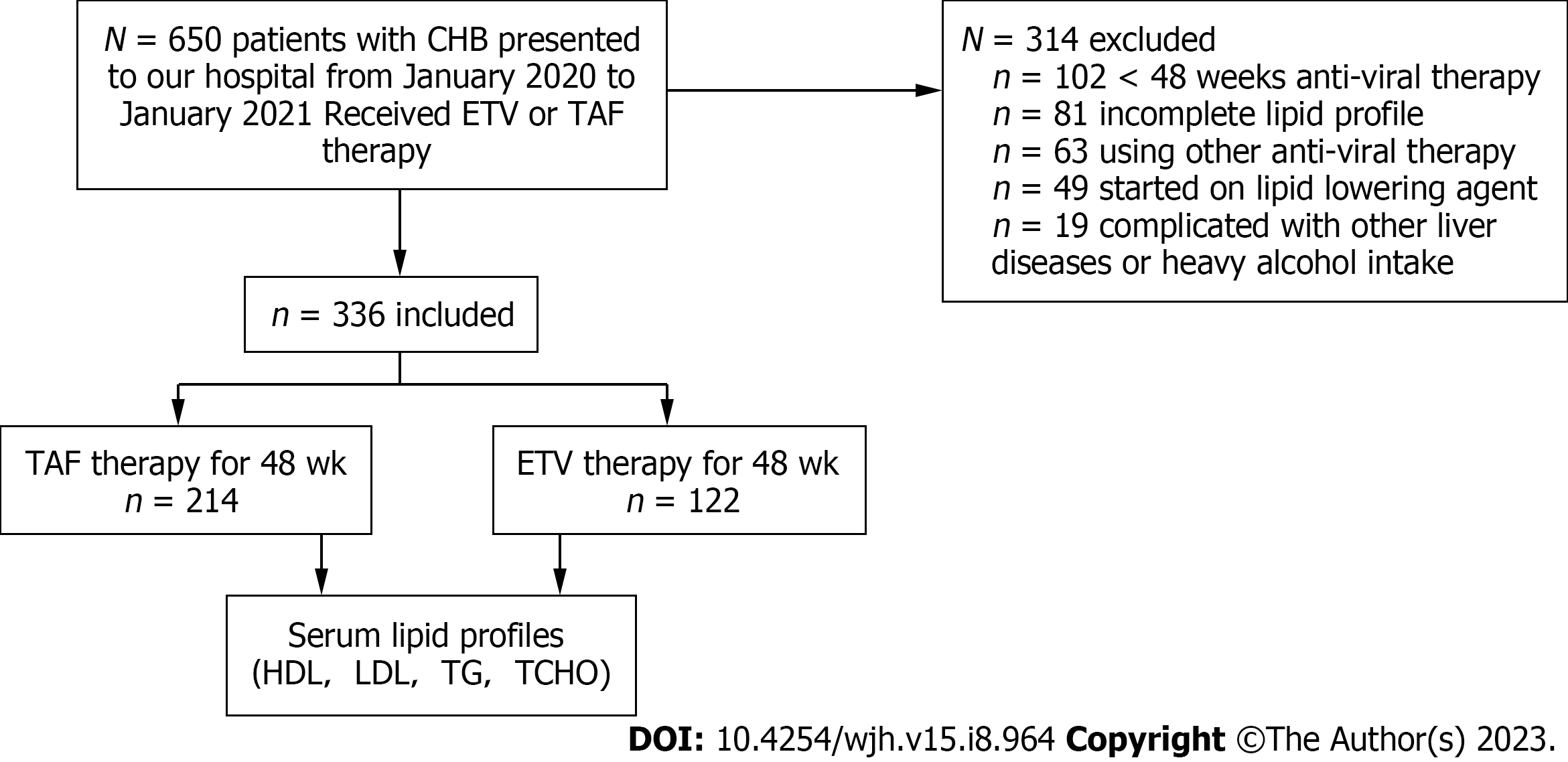
This study included all patients with CHB older than 18 years who visited the outpatient department of the Hepatology Research Institute of the First Affiliated Hospital of Fujian Medical University between January 2020 and January 2021. Information such as patient demographics, treatment history, laboratory data, and comorbidities was extracted from the electronic medical record system. CHB was defined according to the Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2019) of China[10]. Diagnosis of decompensated cirrhosis was confirmed by ultrasonography or imaging for inclusion in the study. Finally, the study included 336 participants (Figure 1) who were taking TAF or ETV and had a pretreatment serum lipid profile and repeated serum lipid assessment after initiating anti-viral therapy for 1 year. Exclusion criteria were as follows: (1) Less than 1 year of anti-viral therapy; (2) Use of other oral anti-viral drugs during the study period; (3) Use of lipid-lowering drugs; (4) Complicated with other liver disease or pregnancy; (5) Heavy alcohol intake (amounting to ethanol consumption of ≥ 40 g/day for males and ≥ 20 g/day for females).
The collected clinical and demographic data included age, sex, body mass index (BMI), drinking and smoking habits, date of anti-viral treatment initiation, cirrhosis, and comorbidities [diabetes mellitus (DM), hypertension, and NAFLD]. A fatty liver was identified using ultrasonography. Clinical laboratory information included HBV-DNA, hepatitis B surface antigen, aspartate aminotransferase, alanine aminotransferase, glomerular filtration rate, uric acid, creatinine, creatine kinase (CK), CK-MB isoenzyme, fasting serum lipid profiles (TCHO, TG, HDL, and LDL), and baseline fasting blood glucose levels. The parameters were measured by the clinical laboratory of the First Affiliated Hospital of Fujian Medical University, and data were collected before and 1 year after the initiation of anti-viral therapy. This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, China.
Statistical analyses were performed using SPSS 23.0. Normally distributed continuous variables were presented as mean ± standard deviation, which were further evaluated by Student’s t-test for the different treatment groups. Categorical variables were described using frequencies and proportions, and Pearson’s χ2 test was used to compare categorical variables. The paired t-test and McNemar’s test were used to assess the differences between the levels before and after treatments in the same treatment group. We calculated the pretreatment and post-treatment differences in each lipid profile component in order to evaluate the impact of anti-viral therapy on lipid profile.
Propensity score-matched models were used to assess the effect of treatment type (TAF vs ETV) on lipid profile component changes. All propensity score-matched models were adjusted for BMI, age, sex, fatty liver disease, cirrhosis, DM, hypertension, smoking, and drinking. We presented the average changes in the model coefficients. Finally, logistic regression analysis was used to estimate the odds ratio of the association between baseline factors and elevated TCHO levels. Statistical P values less than 0.05 were considered significant.
Overall, 336 CHB patients receiving anti-viral therapy (TAF, n = 214 vs ETV, n = 122) were included in the study. The mean age was 46.67 years, 75.60% were male, and 30.95% were cirrhotic at baseline. Patients were older in the ETV group (P = 0.001), but the two groups had a similar rate of NAFLD (TAF: 35.05% vs ETV: 26.23%, P = 0.122) and BMI (TAF: 22.97 vs ETV: 23.78, P = 0.152). However, hypertension and cirrhosis were more prevalent in the ETV group (17.21% vs 7.94%, P = 0.016 and 40.98% vs 25.23%, P = 0.004, respectively). TAF and ETV had similar levels of hepatitis B surface antigen (3.07 vs 3.04, P = 0.787) and HBV-DNA (1.94 vs 1.86, P = 0.560) (Table 1) as well as no statistically significant differences in serum alanine aminotransferase and aspartate aminotransferase levels.
| Characteristic | TAF, n = 214 | ETV, n = 122 | P value |
| Age in yr | 43.38 ± 10.42 | 49.96 ± 11.82 | 0.001 |
| Male | 158 (73.83) | 96 (78.69) | 0.387 |
| BMI in kg/m2 | 22.97 ± 2.93 | 23.78 ± 3.30 | 0.152 |
| Smoking | 16 (7.48) | 15 (12.30) | 0.203 |
| Drinking | 13 (6.07) | 14 (11.48) | 0.123 |
| ALT in U/L | 36.60 ± 36.87 | 30.44 ± 20.00 | 0.089 |
| AST in U/L | 28.01 ± 26.41 | 24.50 ± 13.58 | 0.172 |
| logHBsAg in ng/mL | 3.07 ± 0.92 | 3.04 ± 0.84 | 0.787 |
| logDNA in IU/mL | 1.94 ± 1.23 | 1.86 ± 1.05 | 0.56 |
| CREA in μmol/L | 73.68 ± 15.87 | 74.82 ± 16.78 | 0.535 |
| UA in μmol/L | 353.52 ± 89.10 | 357.23 ± 84.29 | 0.708 |
| GFR in mL/min | 103.48 ± 15.01 | 97.86 ± 14.17 | 0.001 |
| CK in U/L | 157.23 ± 444.75 | 122.91 ± 71.31 | 0.401 |
| FBG in mmol/L | 5.18 ± 0.80 | 5.52 ± 1.61 | 0.011 |
| Concurrent diseases | |||
| Hypertension | 17 (7.94) | 21 (17.21) | 0.016 |
| DM | 20 (9.35) | 13 (10.66) | 0.844 |
| NAFLD | 75 (35.05) | 32 (26.23) | 0.122 |
| Cirrhosis | 54 (25.23) | 50 (40.98) | 0.004 |
TCHO, TG, LDL, and HDL values in the TAF-treated and ETV-treated individuals were comparable at baseline (prior to anti-viral medication). In the TAF-treated group, post-treatment serum lipoprotein levels were considerably higher than the pretreatment levels for TCHO (4.51 ± 0.93 vs 4.67 ± 0.90, P = 0.001) and TG (1.25 ± 0.67 vs 1.37 ± 0.81, P = 0.014), whereas HDL and LDL levels did not change in the TAF group over the duration of our study. Further, the TAF group showed significantly higher post-treatment TCHO levels compared with that in the ETV group (4.67 ± 0.90 vs 4.36 ± 1.05, P = 0.006). However, TAF treatment did not increase the incidence of NAFLD after 1 year of follow-up (Table 2). In the ETV cohort, there was no significant difference between the pretreatment and post-treatment serum lipoprotein levels.
| Characteristic | TAF, n = 214, statistic | P value1 | ETV, n = 122, statistic | P value1 | P value2 |
| Pre-Tx TCHO in mmol/L | 4.51 ± 0.93 | 0.001 | 4.41 ± 1.03 | 0.275 | 0.376 |
| Post-Tx TCHO in mmol/L | 4.67 ± 0.90 | 4.36 ± 1.05 | 0.006 | ||
| Pre-Tx TG in mmol/L | 1.25 ± 0.67 | 0.014 | 1.33 ± 0.75 | 0.052 | 0.35 |
| Post-Tx TG in mmol/L | 1.37 ± 0.81 | 1.24 ± 0.61 | 0.126 | ||
| Pre-Tx HDL in mmol/L | 1.32 ± 0.40 | 0.794 | 1.26 ± 0.40 | 0.879 | 0.246 |
| Post-Tx HDL in mmol/L | 1.32 ± 0.36 | 1.27 ± 0.41 | 0.285 | ||
| Pre-Tx LDL in mmol/L | 3.12 ± 0.90 | 0.785 | 3.06 ± 1.01 | 0.078 | 0.543 |
| Post-Tx LDL in mmol/L | 3.14 ± 0.92 | 3.15 ± 1.00 | 0.906 | ||
| Pre-Tx NAFLD | 75 (35.05) | 0.125 | 32 (26.23) | 1 | 0.122 |
| Post-Tx NAFLD | 70 (32.71) | 31 (25.41) | 0.16 |
Using propensity score-matched models for BMI, age, sex, smoking, drinking, and presence of comorbidities such as NAFLD, cirrhosis, DM, and hypertension, we assessed the impact of TAF compared to ETV on achieving an increase in the levels of the serum lipid profile (Table 3). Patients treated with TAF had a statistically significant increase in TCHO levels (P = 0.019), which was 5% higher than that in the baseline lipid profile.
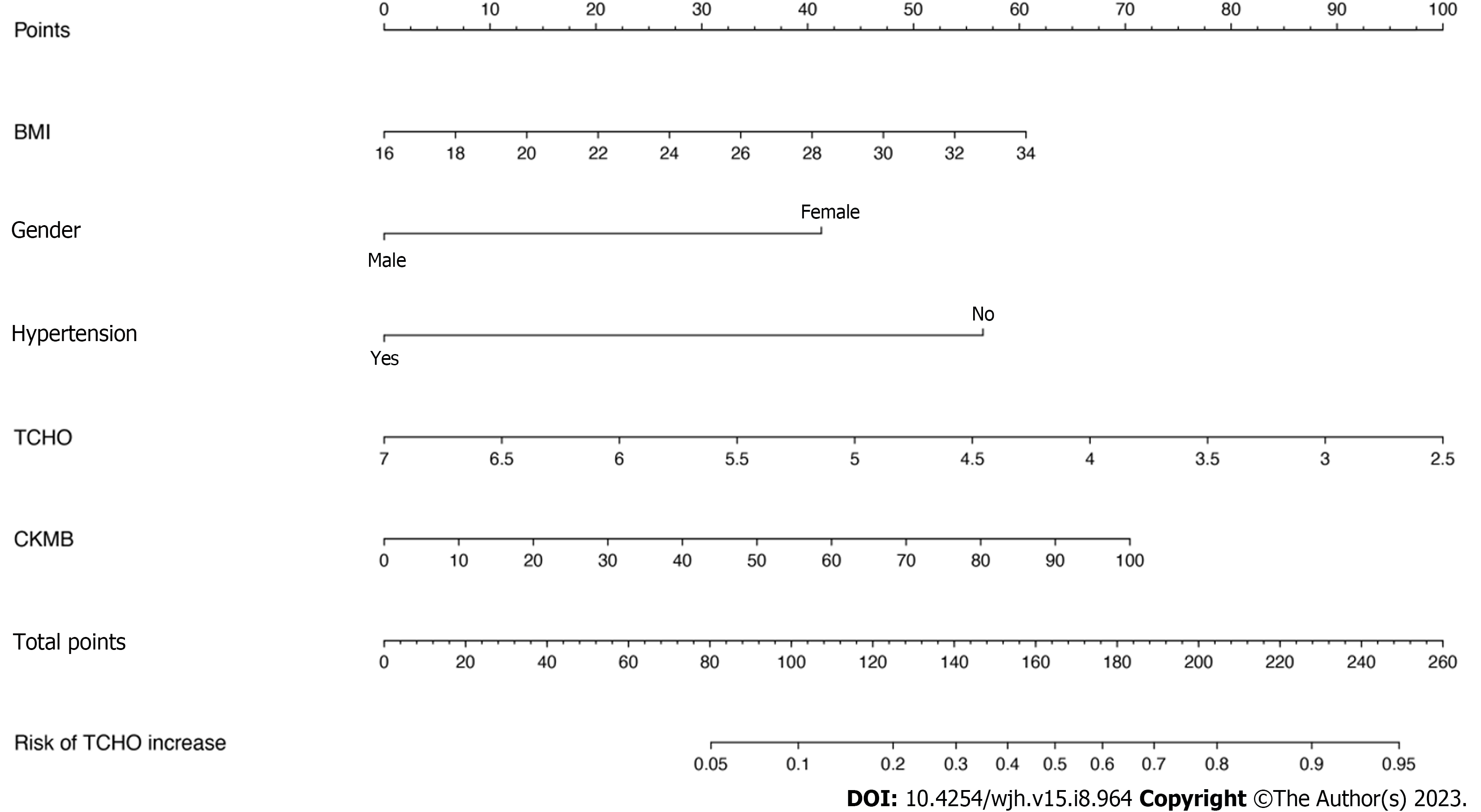
Logistic regression analysis was used to evaluate the risk factors associated with the worsening of TCHO levels (Figure 2). BMI, sex, hypertension, the baseline TCHO, and CK-MB levels were significantly associated with elevated TCHO levels. Furthermore, a nomogram incorporating statistically significant parameters in the logistic regression analysis was constructed, and the total points predicted the probability of elevated TCHO levels in individual patients.
In this real-life retrospective cohort study of 336 patients with CHB who received anti-viral therapy for 1 year, we compared fasting serum lipid profiles before and after initiation of either TAF or ETV treatment. In the TAF-treated cohort, we found a significant increase in the serum TCHO and TG levels compared with no difference in patients who received ETV. However, there were no concomitant significant changes in serum HDL or LDL levels in TAF-treated patients. Recent data have demonstrated that MetS increased the risk of progression of liver fibrosis independent of viral factors in patients with CHB[11,12]. Elevated TCHO and TG levels as MetS risk factors were related to a high risk of cirrhotic events in patients with CHB; thus, the results of this investigation may affect the decision regarding anti-viral therapy in CHB patients with cirrhotic risk factors.
TAF has been used as first-line therapy for CHB and HIV infections. Dyslipidemia and metabolic disorders have been linked to the prolonged use of TAF to treat HIV infection. Previous studies found that the serum lipid profile increased in HIV patients switching from TDF to TAF therapy, and the effect of increased serum lipids on the risk of cardiovascular events cannot be ignored[13-15]. Therefore, whether dyslipidemia due to TAF treatment in patients with CHB would lead to an increased risk of NAFLD is also a concern. Studies have reported that patients with CHB and NAFLD have better liver-related outcomes and overall mortality than those with CHB alone[16]. It is increasingly recognized that metabolic factors, which are precursors of NAFLD, can also be used to evaluate the risk of HCC in patients with CHB[17,18].
In our study, patients who received TAF therapy for 1 year did not have a significantly increased risk of developing NAFLD. However, owing to the short follow-up period of our study, the effect of TAF on increasing the incidence of NAFLD still needs to be evaluated over a longer follow-up period, and more data are required to better elucidate this.
It was unclear how TAF increased lipid concentrations, but the mechanism was independent of the patient’s HIV status because a similar effect of TAF has been reported in HIV-negative patients receiving CHB therapy. Notably, a higher proportion of TAF-treated CHB patients experienced higher levels of LDL than the TDF group at the 48-wk follow-up[19,20]; however, the use of lipid-lowering agents was not described. In our study, the increased TCHO levels with TAF treatment represented a small change in the propensity score-matched model (5% higher than baseline, P = 0.019), but we did not find a significant change in the LDL profile compared to the ETV treatment group. Regular serum lipid measurements might not accurately capture the intricate alterations in lipid metabolism brought on by anti-viral medication. A recent study showed that TDF modulates lipid metabolism by upregulating hepatic CD36 via PPAR-a activation in patients with HBV infection[21]. However, the mechanism by which TAF increases serum lipid profiles remains unclear, and further studies are required.
In addition, in multivariate logistic regression analysis, metabolic factors, including BMI, hypertension, baseline TCHO, and CK-MB levels, were significantly associated with elevated TCHO levels, demonstrating an important impact of metabolic factors in terms of the elevated lipid profile caused by TAF. Previous studies have reported the effects of MetS on the adverse outcomes of fibrosis in patients with CHB[22,23]. Furthermore, some studies have confirmed that metabolic factors positively affected the risk of HCC in patients with HBV infection[17,24]. Based on the results mentioned above, the treatment options for patients with CHB who have the aforementioned aberrant metabolic variables may need to consider the potential effects of TAF medication on blood lipid levels.
Our study had some limitations. First, not all relevant clinical data were obtained from the treatment databases, and important data elements, such as lifestyle risk factors (i.e. exercise and diet) and family history, were not fully documented. Second, this was not a multicenter study, and we adjusted for baseline factors through a propensity score-matched model. Therefore, the observed increase in the TCHO profile may be due to the effect of anti-viral treatment. Third, the effect of TAF on the incidence of NAFLD cannot be elucidated well because of the short follow-up time. However, this study provided real-world data from China regarding the relationship between TAF-treated patients with CHB and changes in serum lipid levels. Large-scale multicenter prospective studies should be conducted in the future to further evaluate the effects of CHB and anti-HBV therapies on the risk of dyslipidemia, NAFLD, cirrhosis, and HCC.
In this real-life retrospective cohort study in China, we found that TAF was significantly associated with higher TCHO levels, whereas ETV had no effect in patients with CHB treated for 1 year. Risk factors (BMI, sex, baseline TCHO and CK-MB levels) were significantly associated with elevated TCHO levels. In the future, we will focus on increased NAFLD risk and not just changes in serum lipid profiles. Further studies will help elucidate the effect of TAF on long-term metabolic-related complications in patients with CHB.
In recent decades, the prevalence of nonalcoholic fatty liver disease (NAFLD) has significantly increased in China, leading to the coexistence of NAFLD and chronic hepatitis B (CHB). Many patients require long-term anti-hepatitis B virus (HBV) therapy with potent oral drugs tenofovir alafenamide (TAF) and entecavir (ETV), which are recommended as first-line treatment in HBV clinical practice guidelines. However, no studies have compared the effects of TAF and ETV on lipid profiles in HBV-treated patients. Meanwhile, there are limited data on the effects of TAF on metabolism-related complications in real-world settings.
Many patients require long-term anti-HBV therapy with the potent oral drugs TAF and ETV, which are recommended as first-line treatment in HBV clinical practice guidelines. TAF has a serum lipid-raising effect in patients with HIV; however, its effect on serum lipids and NAFLD risk in patients with CHB is unclear.
This retrospective cohort study aimed to characterize the effect of TAF on serum lipid levels and NAFLD risk in patients with CHB, and we compared the pretreatment and post-treatment serum lipid profile changes after initiation of either TAF or ETV anti-viral therapy.
The data including the clinical features, serum lipids, and metabolic factors of patients with CHB at baseline and approximately 1 year after TAF or ETV treatment were collected and analyzed. We used propensity score-matched models to assess the effects on high-density lipoprotein, low-density lipoprotein, triglycerides, and total cholesterol (TCHO).
Compared with the ETV group, the TAF group had significantly higher TCHO levels after treatment (4.67 ± 0.90 vs 4.36 ± 1.05, P=0.006). In a propensity score-matched model, TAF-treated patients had significantly increased TCHO levels compared to that at baseline (P = 0.019), while there was no difference for the ETV group. Body mass index, sex, hypertension, baseline TCHO, and creatine kinase-MB isoenzyme levels were significantly associated with elevated TCHO levels in logistic regression analysis. However, 1-year TAF treatment did not increase the incidence of NAFLD.
Our study found that a greater increase in TCHO was observed in patients with CHB receiving TAF than in those receiving ETV; however, TAF-induced dyslipidemia did not increase the incidence of NAFLD.
This was not a multicenter study, and most of patients with CHB in this study were Asians. Large-scale multicenter prospective studies should be conducted in the future to further evaluate the effects of CHB and anti-HBV therapies on the risk of dyslipidemia, NAFLD, cirrhosis, and hepatocellular carcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Akbar SMF, Japan; El-Arabey AA, Egypt; Mogulkoc R, Turkey S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (2)] |
| 2. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 3. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3800] [Article Influence: 542.9] [Reference Citation Analysis (2)] |
| 4. | Lozano-Bartolomé J, Llauradó G, Rodriguez MM, Fernandez-Real JM, Garcia-Fontgivell JF, Puig J, Maymó-Masip E, Vendrell J, Chacón MR. Reduced circulating levels of sTWEAK are associated with NAFLD and may affect hepatocyte triglyceride accumulation. Int J Obes (Lond). 2016;40:1337-1345. [PubMed] [DOI] [Full Text] |
| 5. | Isorce N, Lucifora J, Zoulim F, Durantel D. Immune-modulators to combat hepatitis B virus infection: From IFN-α to novel investigational immunotherapeutic strategies. Antiviral Res. 2015;122:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 7. | Lacey A, Savinelli S, Barco EA, Macken A, Cotter AG, Sheehan G, Lambert JS, Muldoon E, Feeney E, Mallon PW, Tinago W; UCD ID Cohort Study. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS. 2020;34:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Kauppinen KJ, Kivelä P, Sutinen J. Switching from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide Significantly Worsens the Lipid Profile in a Real-World Setting. AIDS Patient Care STDS. 2019;33:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Shaheen AA, AlMattooq M, Yazdanfar S, Burak KW, Swain MG, Congly SE, Borman MA, Lee SS, Myers RP, Coffin CS. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther. 2017;46:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 11. | Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, Chan HY, Tse CH, Wong VW. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Lemieux I, Després JP. Metabolic Syndrome: Past, Present and Future. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 13. | Squillace N, Ricci E, Menzaghi B, De Socio GV, Passerini S, Martinelli C, Mameli MS, Maggi P, Falasca K, Cordier L, Celesia BM, Salomoni E, Di Biagio A, Pellicanò GF, Bonfanti P; CISAI Study Group. The Effect of Switching from Tenofovir Disoproxil Fumarate (TDF) to Tenofovir Alafenamide (TAF) on Liver Enzymes, Glucose, and Lipid Profile. Drug Des Devel Ther. 2020;14:5515-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, Elion R, Cavassini M, Madruga JV, Brunetta J, Shamblaw D, DeJesus E, Orkin C, Wohl DA, Brar I, Stephens JL, Girard PM, Huhn G, Plummer A, Liu YP, Cheng AK, McCallister S; GS-US-292-0109 team. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Milinkovic A, Berger F, Arenas-Pinto A, Mauss S. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS. 2019;33:2387-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 17. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, To KF, Wong VW. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 19. | Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, Mehta R, Gane E, Flaherty JF, Massetto B, Gaggar A, Kitrinos KM, Lin L, Subramanian GM, McHutchison JG, Lim YS, Acharya SK, Agarwal K; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 20. | Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 21. | Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, Miyoshi H, Kimura M, Maehara O, Yamada R, Kitagataya T, Yamamoto K, Shigesawa T, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N; NORTE Study Group. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol. 2021;56:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 22. | Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome: how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr Opin Clin Nutr Metab Care. 2009;12:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Yoon H, Lee JG, Yoo JH, Son MS, Kim DY, Hwang SG, Rim KS. Effects of metabolic syndrome on fibrosis in chronic viral hepatitis. Gut Liver. 2013;7:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006-1017.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |