Published online Aug 27, 2023. doi: 10.4254/wjh.v15.i8.1001
Peer-review started: April 30, 2023
First decision: June 7, 2023
Revised: June 18, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: August 27, 2023
Processing time: 113 Days and 16.6 Hours
Non-alcoholic fatty liver disease (NAFLD) has become a prevalent cause of chronic liver disease and ranks third among the causes of transplantation. In the United States alone, annual medical costs are approximately 100 billion dollars. Unfortunately, there is no Federal Drug Administration (FDA)-approved medi
To assess the efficacy of cyclophilin inhibitors, fibroblast growth factor 21 analogs (FGF21), and dual and pan peroxisome proliferator-activated receptor (PPAR) agonists for treating NAFLD.
A comprehensive literature search using keywords including cyclophilin inhibitor, FGF agonist, pan-PPAR agonists, dual-PPAR agonist, NAFLD, non-alcoholic steatohepatitis, and fatty liver was conducted on October 29, 2022, in PubMed, EMBASE, Cochrane Library, Scopus and Web of Science. Animal and human research, case reports, and published articles in English from all countries with patients aged 18 and above were included. Only articles with a National Institutes of Health (NIH) Quality Assessment score of five or higher out of eight points were included. Articles that were narrative or systematic reviews, abstracts, not in English, focused on patients under 18 years old, did not measure outcomes of interest, were inaccessible, or had a low NIH Quality Assessment score were excluded. Each article was screened by two independent researchers evaluating relevance and quality. Resources were scored based on the NIH Quality Assessment Score; then, pertinent data was extracted in a spreadsheet and descriptively analyzed.
Of the 681 records screened, 29 met the necessary criteria and were included in this review. These records included 12 human studies and 17 animal studies. Specifically, there were four studies on cyclophilin inhibitors, four on FGF agonists/analogs, eleven on pan-PPAR agonists, and ten on dual-PPAR agonists. Different investigational products were assessed: The most common cyclophilin inhibitor was NV556; FGF agonists and analogs was Efruxifermin; pan-PPAR agonists was Lanifibranor; and dual-PPAR agonists was Saroglitazar. All classes were found to be statistically efficacious for the treatment of NAFLD, with animal studies demonstrating improvement in steatosis and/or fibrosis on biopsy and human studies evidencing improvement in different metabolic parameters and/or steatosis and fibrosis on FibroScan (P < 0.05).
The data analyzed in this review showed clinically significant improvement in individual histological features of NAFLD in both animal and human trials for all four classes, as well as good safety profiles (P < 0.05). We believe this compilation of information will have positive clinical implications in obtaining an FDA-approved therapy for NAFLD.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) has become a significant global health issue. There is no medication approved by the Federal Drug Administration for the treatment of NAFLD. However, there are several therapeutic classes currently being studied in clinical trials. In this systematic review, we analyze the scientific data of cyclophilin inhibitors, fibroblast growth factor 21 analogs, and dual and pan peroxisome proliferator-activated receptor agonists for the treatment of NAFLD.
- Citation: Tidwell J, Balassiano N, Shaikh A, Nassar M. Emerging therapeutic options for non-alcoholic fatty liver disease: A systematic review. World J Hepatol 2023; 15(8): 1001-1012
- URL: https://www.wjgnet.com/1948-5182/full/v15/i8/1001.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i8.1001
Non-alcoholic fatty liver disease (NAFLD) is a prevalent chronic liver disease affecting approximately 30% of the world's population[1]. It is characterized by the buildup of more than 5% of fat in hepatocyte histology[1]. NAFLD encompasses a range of conditions, such as non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), and cirrhosis[2]. NAFL is defined as hepatic steatosis without inflammation, based on liver biopsy histology[2].
Approximately 20% of patients with NAFL will develop NASH, which is the presence of hepatic steatosis, lobular inflammation, and hepatocyte ballooning[1,2]. This persistent liver cell injury leads to progressive fibrosis and cirrhosis in approximately 10%–20% of patients, converting NAFLD into the quickest-growing cause of hepatocellular carcinoma (HCC)[1,3]. NAFLD is currently liver transplantation's third most common cause[1]. Unlike other causes of HCC, which start with fibrosis, up to one-third of patients with NASH and HCC are non-cirrhotic and are more advanced, making treatment difficult[3].
NAFLD is a liver condition that is closely linked with metabolic syndrome[1]. It is often seen in people who have type 2 diabetes, are insulin resistant, have high levels of triglycerides and cholesterol, and are obese[1]. The main risk factors for developing NAFLD are a diet high in fats and sugars and a sedentary lifestyle[1]. Some experts have started using metabolic-associate fatty liver disease to describe this condition because of its strong link with metabolic dysfunction. Still, for clarity purposes, we will stick with the NAFLD nomenclature throughout this review[4].
Approximately 70% of diabetics, overweight patients, and 90% of patients with dyslipidemia and morbid obesity will develop NAFLD[1,4]. NAFLD is also associated with systemic pathologies such as chronic kidney disease, cardiovascular disease, and reduced mineral density[1]. Cardiovascular disease is the most common cause of death in NAFLD patients; however, they also have an increased overall mortality rate compared to the general population[1]. It is of utmost concern that there is an increase in the prevalence of adolescents with NAFLD, leading to earlier end-stage liver disease[5].
In the United States alone, $100 billion of annual medical costs are attributed to NAFLD. Searching for an approved medical therapy for NAFLD is a pressured race[4]. The lack of an authorized agent could be secondary to the limited understanding of a multifactorial disease process and the absence of dependable non-invasive biomarkers[4]. Due to the acknowledgment of an increasing epidemic and the severity of NAFLD, several trials are ongoing to identify possible pharmacologic agents[3]. Most of the agents target the known metabolic associations with NAFLD, such as adipose tissue dysfunction, insulin resistance, de novo lipogenesis, lipid exportation in the liver, and imbalance between energy intake and expenditure[5].
There is growing interest in future combined medications targeting multiple critical pathways involved in developing NAFLD[5]. Precise identification of the drivers of this disease is crucial for developing new agents, and it is hoped that registered therapy for NAFLD will become available in the next few years[2]. Clinicians must be aware of the emerging agents for the treatment of NAFLD and the need for further human research to characterize better the efficacy, dosage, length of treatment, etc. This systematic review will delve into the scientific data behind four innovative therapeutic classes currently being studied for treating NAFLD: Cyclophilin inhibitors, fibroblast growth factor 21 analogs (FGF21), and dual and pan peroxisome proliferator-activated receptor (PPAR) agonists.
This review analyzed animal and human research, case reports, and published articles in English from all countries with patients aged 18 and above. Only articles with a five or higher National Institutes of Health (NIH) Quality Assessment score were included. Articles that were narrative or systematic reviews, abstracts, not in English, focused on patients under 18 years old, did not measure outcomes of interest, were inaccessible, or had a low NIH Quality Assessment score were excluded.
A comprehensive literature search using broad search criteria was conducted in five databases: PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science (October 29, 2022). Our search terms were as follows: (rencofilstat OR "cyclophilin inhibitor" OR "cyclophilin inhibition" OR lanifibranor OR "PPAR agonist" OR "peroxisome proliferator activated receptor agonist" OR "pan-ppar agonist" OR "pan-peroxisome proliferator activated receptor agonist" OR efruxifermin OR "FGF-21 inhibitor" OR "fibroblast growth factor-21 inhibitor" OR "fibroblast growth factor 21 inhibitor" OR "FGF21 inhibitor" OR "FGF21 inhibition" OR "FGF-21 inhibition") AND (NASH or "fatty liver" or "hepatic steatosis" or steatohepatitis OR NAFLD OR "non-alcoholic fatty liver disease" OR "Non-alcoholic Fatty Liver Disease" OR "Fatty Liver, Non-alcoholic" OR "Fatty Livers, Non-alcoholic" OR "Liver, Non-alcoholic Fatty" OR "Non-alcoholic Fatty Liver" OR "Non-alcoholic Fatty Livers" OR "Non-alcoholic Steatohepatitis" OR "Non-alcoholic Steatohepatitides" OR "Steatohepatitides, Non-alcoholic" OR "Steatohepatitis, Non-alcoholic" OR "liver, fatty" OR "steatosis of liver" OR "visceral steatosis" OR "steatosis, visceral" OR "steatoses, visceral" OR "visceral steatosis" OR "liver steatosis" OR "liver steatoses" OR "steatosis, liver" OR "steatoses liver").
For the study selection process, Covidence was used, a platform that facilitates the importation of citations and screening of titles, abstracts, and full text. Each article was initially screened by title and abstract by two independent researchers (J.T. and N.B.) to exclude studies irrelevant to our aim. Next, each article was screened by full text by the same two independent researchers (J.T. and N.B.) to exclude studies that were finally irrelevant to our aim. Once both researchers completed all screening stages, any conflicts were registered in Covidence, and the discrepancies were reviewed and resolved.
The following data were collected separately by J.T. and N.B. from all eligible studies and recorded in Excel: First author, digital object identifier, study design, number of participants, name of therapy, mechanism of action, side effects, and statistical data about liver enzymes, cholesterol panels, weight reduction, NAFLD activity score (NAS), Fibroscan controlled attenuation parameter (CAP) and kPa, fibrosis stage, fibrotic markers, and quality assessment scores. J.T. and N.B. resolved all discrepancies in the collected data. The quality of included studies was assessed by the NIH Quality Assessment tool. We included articles with a score greater than or equal to five out of eight points.
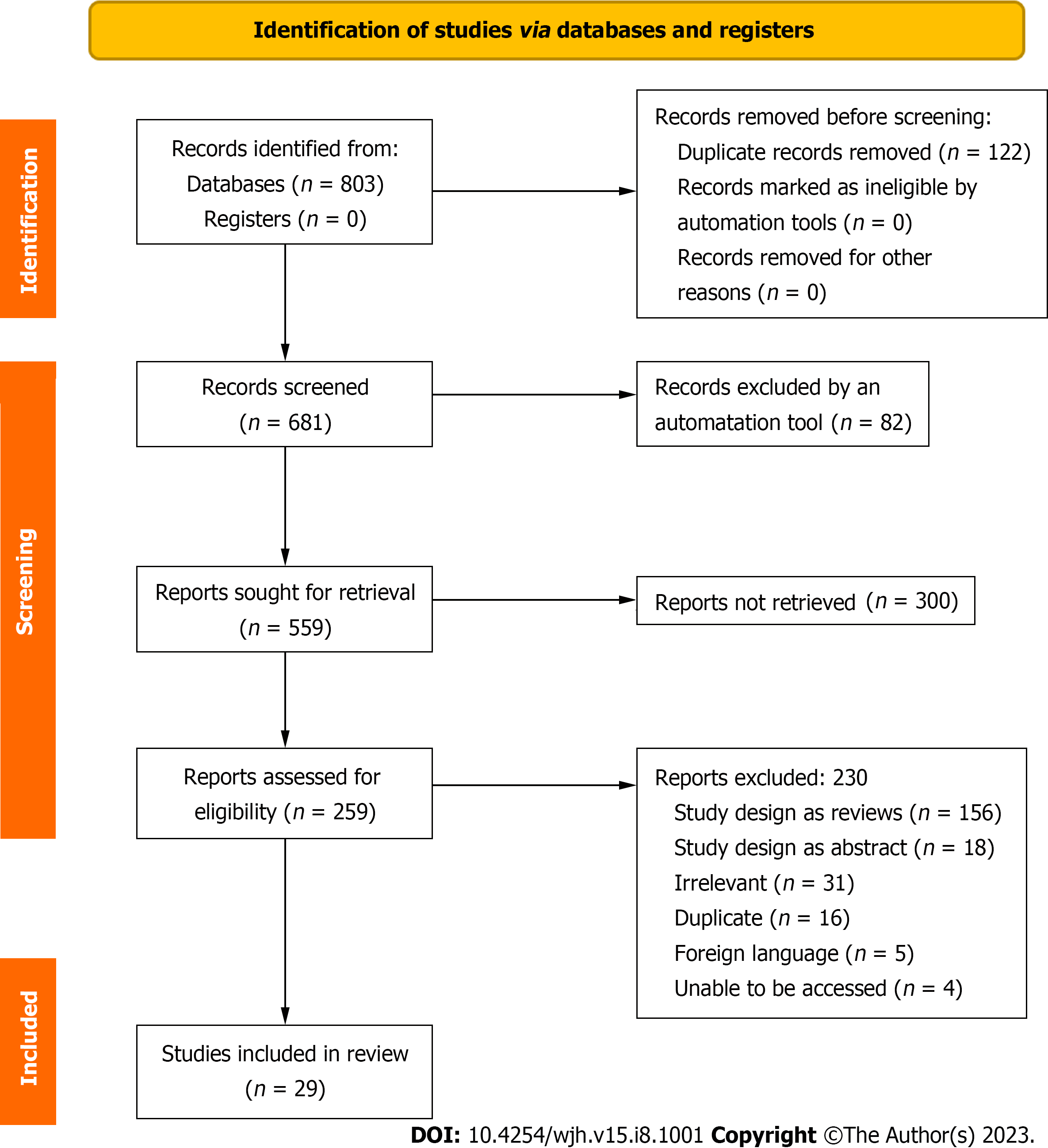
Records were identified from 5 databases: PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science. One hundred twenty-two duplicate records were removed before the screening. Six hundred eighty-one records were screened, out of which eighty-two were excluded by an automation tool. Five hundred fifty-nine reports were sought for retrieval, out of which three hundred were not retrieved. Two hundred fifty-nine reports were assessed for eligibility, out of which two hundred and thirty were excluded. The most common reason for exclusion was review articles (156) followed by irrelevant articles (31), abstracts (18), duplicates (16), foreign language (5), and unable to be accessed (4). Twenty-nine of the two hundred and fifty-nine records assessed for eligibility were included (Figure 1). Most studies, including human and animal participants, were small (n < 100). Some studies enrolled patients with NAFLD and others with NASH. Articles were included when the NIH Quality Assessment Score was greater than or equal to five points. The majority of articles included were scored as six or seven points. Reasons for lower scores included unknown publication bias and no rating by two independent reviewers. Most studies did not report harmful outcomes.
Four studies evaluated cyclophilin inhibitors (Table 1), four evaluated FGF analogs (Table 2), eleven evaluated pan-PPAR agonists (Table 3), and ten evaluated dual-PPAR agonists (Table 4). Different investigational products were assessed; the most common for cyclophilin inhibitors was NV556, for FGF agonists/analogs was Efruxifermin (EFX), for pan-PPAR agonists was Lanifibranor, and for dual-PPAR agonists was Saroglitazar.
| Ref. | Human or animal | Study design | Number of participants | Key inclusion criteria | Investigational product/dose | Study end points | Key findings |
| Harrison et al[9], 2022 | Human | Randomized, single-blind, placebo-controlled, phase 2a study; Duration: 4 wk | n = 49 | Patients with presumed F2/F3 NASH | Rencofilstat Placebo vs Rencofilstat (75 or 225 mg daily) | Evaluate the effect of Rencofilstat on ALT, Pro-C3, liver steatosis, and fibrosis measured by FibroScan | ALT in the placebo vs 75 vs 225 mg group was 70.67 vs 42.5 vs 30.56 IU/L. Pro-C3 was reduced in stratified patients with Pro-C3 > 15 (P < 0.01). Fibrosis was 22 vs 14 vs 12 kPa. Steatosis was 351 vs 337 vs 329 dB/m |
| Kuo et al[6], 2019 | Animal | Duration: 30 wk | n = 10 | High-fat diet-induced NASH mouse model (n = 10) | CRV431: Control vs 50 mg/kg daily | Evaluate the effect of CRV431 on liver fibrosis measured by Sirius red staining in liver biopsy sections | Fibrosis levels were 37%–46% lower in the treatment vs control group (P < 0.05) |
| Kuo et al[8], 2019 | Animal | Duration: 6 wk | n = 9 | CCL4-induced liver fibrosis mouse model (n = 9) | CRV431: Control vs 50 mg/kg daily | Evaluate the effect of CRV431 on liver fibrosis measured by Sirius red staining in liver biopsy sections | Liver fibrosis was lowered by 43% in the treatment vs control group (P < 0.01) |
| Kuo et al[8], 2019 | Animal | Duration: 6 wk | n = 8 | High-fat diet-induced NASH mouse model | NV556: Control vs 50 mg/kg daily | Evaluate the effect of NV556 on liver collagen and fibrosis measured by Sirius red staining in liver biopsy sections | Fibrosis was reduced by 60% in the treatment vs control group (P = 0.0281) |
| Simón Serrano et al[7], 2019 | Animal | Duration: 7 wk | n = 20 | Choline-deficient high-fat diet-induced model of NASH in mice (n = 10 per group) | NV556: Control vs 100 mg/kg daily | Effect of NV556 on liver fibrosis and collagen production measured by Sirus red staining | Reduction of liver fibrosis by 25% (2% in control vs 1.5% in treatment group P < 0.01) |
| Ref. | Human or animal | Study design | Number of participants | Key inclusion criteria | Investigational product/dose | Study endpoints | Key findings |
| Harrison et al[10], 2021 | Human | Randomized, double-blind, placebo-controlled, phase 2a BALANCED study. Duration: 16 wk | n = 80 | Patients with biopsy-confirmed NASH (F1-F3) | Efruxifermin: Placebo vs EFX (28, 50, 70 mg) | Absolute change from baseline in HFF measured as MRI-proton density fat fraction at 12 wk of EFX | The mean relative change in HFF at week 12 was -63.2% -70.9%, and -72.3%, respectively, in the treatment groups of 28, 50, and 70 mg (P < 0.0001) |
| Bao et al[12], 2018 | Animal | Duration: 15 d | n = 10 | Choline-deficient high-fat diet-induced model of NASH in mice (n = 5 per group) | PsTag600 Control vs 3.7 mg/kg-1 daily | Effect of PSTag600 on attenuation of the development of NASH measured by NAS and oil red O staining | Decrease in NAS in control vs treatment group of 5 vs 1 and area of oil red O of 26% vs 3%, respectively (P < 0.05) |
| Le et al[11], 2018 | Animal | Duration: 4 wk | n = 8 | MCD diet-induced NASH mouse model | LY2405319 Control vs 1.5 mg/kg daily | Evaluate the attenuation of fibrosis with the administration of LY2405319 by measuring levels of a-SMA and GPR91 (cells and receptors involved in hepatic fibrogenesis) on liver biopsy after 8 wk | The expression of α-SMA and GPR91 in the liver of mice fed with MCD diet was increased. The treatment group had an attenuated increase of collagen type 1, α-SMA, and GPR91 protein levels (P < 0.05). LY2405319 intraperitoneal administration for 4 wk daily ameliorated hepatic steatosis and fibrosis that was induced by MCD diet |
| Puengel et al[13], 2022 | Animal | Duration: 6 wk | n = 12 | Choline-deficient high-fat diet-induced model of NASH in mice (n = 6 per group) | BMS-986171: Control vs 0.6 mg/kg twice weekly | Effect of BMS-986171 on liver steatosis and fibrosis measured NAS on biopsy | NAS of the control vs. treatment group was 5 vs 4 (P < 0.05), hepatic steatosis 2.5 vs 1.5 (P < 0.01), inflammation 3.5 vs 2.5 and ballooning 1.2 vs 0.75 (P < 0.001) respectively |
| Ref. | Human or animal | Study design | Number of participants | Key inclusion criteria | Investigational product/dose | Study endpoints | Key findings |
| Abitbol et al[21], 2016 | Human | Double-blind, randomized, placebo-controlled, parallel-group study. Duration: 4 wk | n = 45 | Patients with biopsy-confirmed NASH and type 2 diabetes on stable doses of metformin | IVA337 (Lanifibranor). Placebo vs IVA337 (400, 800, or 1200 mg daily) | Metabolic effects of IVA337 in diabetic patients | Reduction in triglycerides by 32% and ALT by 10% (P < 0.05) |
| Cooreman et al[14], 2022 | Human | Post-hoc analysis of the phase 2b NATIVE study. Duration: 24 wk | n = 247 | Patients with non-cirrhotic biopsy-confirmed NASH | Lanifibranor Placebo vs Lanifibranor (800 or 1200 mg daily) | Effect of Lanifibranor on glycemic control and NASH markers. Efficacy in NASH was measured with SAF score and fibrosis staging | NASH resolution and fibrosis improvement in the treatment group vs placebo was 26% vs 7%, respectively, and a 41% reduction of HbA1c from baseline (P < 0.001) |
| Francque et al[18], 2021 | Human | Randomized, double-blind, placebo-controlled, phase 2b trial. Duration: 24 wk | n = 247 | Patients with noncirrhotic, highly active NASH (SAF ≥ 1 or higher for steatosis, hepatocellular ballooning, and lobular inflammation on liver biopsy) | Lanifibranor Placebo vs Lanifibranor (800 or 1200 mg daily) | Decrease of at least 2 points in the SAF score without worsening of fibrosis | 48% of patients in the 800 mg group and 55% in the 1200 mg group had a decrease of at least 2 points in the SAF score vs 33% in the placebo group (P = 0.007) |
| An et al[22], 2017 | Animal | Duration: 3 wk | n = 5 | Genetically obese mice | MHY2013: Control vs 5 mg/kg daily | Reduction of hepatic steatosis measured via liver triglycerides on biopsy | Liver triglycerides were 10 mg/100 mg of protein in the control vs 7 mg/100 mg of protein in the treatment group (P < 0.05) |
| An et al[25], 2018 | Animal | Duration: 3 wk | n = 6 | Aged model mice | MHY2013: Control vs MHY2013 (1 or 3-5 mg/kg daily) | Evaluate the attenuation of hepatic lipid accumulation measured by liver biopsy | The ratio of liver weight/body weight was 0.035, 0.03, and 0.025 in control, 1 and 3-5 mg/kg groups, respectively (P < 0.01) |
| Barbosa-da-Silva et al[16], 2015 | Animal | Duration: 4 wk | n = 20 | High-fat diet mice (n = 10 per group) | Bezafibrate: Control vs 100 mg/kg daily | Effect of Bezafibrate on hepatic lipid metabolism measured by liver TG and steatosis on biopsy | Reduction in TG levels and liver steatosis of 30% and 50%, respectively, in the treatment group (P < 0.0001) |
| Boubia et al[19], 2018 | Animal | Duration: 3 wk | n = 16 | CCI4-induced liver fibrosis in mice (n = 8 per group) | Lanifibranor: Control vs 30 mg/kg daily | Efficacy of Lanifibranor in reducing fibrosis in NASH measured by hepatic collagen on biopsy | Reduction in hepatic collagen deposition from 0.6% of the area to 0.3% in the control vs treatment group (P < 0.01) |
| Lefere et al[15], 2020 | Animal | Duration: 6 wk | n = 16 | Choline-deficient high-fat diet-induced NASH mouse model (n = 8). Isolated hepatic macrophages (n = 8) | Lanifibranor Control vs 30 mg/kg daily | Effect on NAFLD measured by the NAFLD activity score, fibrosis by the Sirus red staining, and hepatic macrophages assessed by IHC | Reduction of NAFLD activity score from 6 to 2 in the treatment vs control group (P < 0.0001), collagen by 5% to 3% (P < 0.01), and liver macrophages from 22% to 8% (P < 0.0001) |
| Møllerhøj et al[20], 2022 | Animal | Duration: 12 wk | n = 13 | Gubra-Amylin NASH diet-induced obese mouse with biopsy-confirmed NASH | Lanifibranor: Control vs 30 mg/kg daily | Change in NAS and fibrosis stage measured on biopsy | At least a 2-point improvement in the steatosis score, and only 20% of hepatocytes had lipid droplets vs 80% in the control group (P < 0.001). 50% of mice had a 1-point improvement in fibrosis (P < 0.05) |
| Nagasawa et al[17], 2006 | Animal | Duration: 5 wk | n = 7 | Choline-deficient high-fat diet-induced NASH mouse model | Benzafibrate: Control vs Benzafibrate (50, 100 mg/kg daily) | Effect on hepatic lipid content and histopathological changes measured on biopsy by the number of activated hepatic stellate cells | Liver TG was 25, 20, and 55 mg/g in the 50, 100 mg/kg vs placebo groups, respectively (P < 0.01). The activated hepatic stellate cells were 11 number/15 fields vs 1 number/15 fields, respectively |
| Wettstein et al[24], 2017 | Animal | Duration: 3 wk | n = 20 | Choline-deficient high-fat diet-induced model of NASH in mice (n = 10 per group) | IVA337 (Lanifibranor) Control vs 30 mg/kg daily | Evaluate the effects of IVA337 on hepatic features associated with NASH measured by hepatic lipid droplet count and lobular inflammation foci count | Prevention of steatosis in 98% of mice and inflammation in 75% of mice (P < 0.001) |
| Ref. | Human or animal | Study design | Number of participants | Key inclusion criteria | Investigational product/dose | Study endpoints | Key findings |
| Boeckmans et al[34], 2019 | Human | In vitro study Duration: N/A | N/A | Hepatic cells generated from human skin-derived precursors with induced NASH | Elafibranor | Effect on hepatic steatosis and inflammatory chemokines | Reduction in hepatic lipid load, as well as the expression and secretion of inflammatory chemokines, which are responsible for the recruitment of immune cells |
| Boeckmans et al[33], 2021 | Human | In vitro study. Duration: N/A | N/A | Hepatic cells generated from human skin-derived precursors with induced NASH | Elafibranor | Effect on hepatic steatosis, inflammatory chemokines, and pro-fibrotic gene expression | Attenuated lipid accumulation, inflammatory chemokine secretion, and pro-fibrotic gene expression |
| Cariou et al[27], 2013 | Human | Multicenter, randomized, single-blind, placebo-controlled, crossover study. Duration: 8 wk | n = 22 | Abdominally obese insulin-resistant males | GFT505: Placebo vs 80 mg daily | Effect on peripheral and hepatic insulin sensitivity with improvement in GIR | Improved peripheral insulin sensitivity with a 21% increase of the GIR (P = 0.048) and enhanced hepatic insulin sensitivity with a 44% increase in insulin suppression of endogenous glucose production (P = 0.006) |
| Chaudhuri et al[32], 2023 | Human | Single-center, prospective, observational, open-label, single-arm study. Duration: 52 wk | n = 76 | Patients with NAFLD and elevated ALT levels along with liver stiffness value ≥ 6 kPa and/or liver steatosis CAP > 290 dB/m | Saroglitazar 4 mg daily | Effect on liver stiffness and steatosis measured by LSM and CAP on FibroScan at baseline, 24 and 54 wk | There was significant improvement of LSM from baseline (11.03 ± 7.19 kPa) to 24-wk (9.29 ± 6.39 kPa) and 52-wk (8.59 ± 6.35 kPa) values, respectively (P < 0.001). There was a significant improvement in median CAP at 24 wk 281 dB/m, (P < 0.001) and 52 wk 287 dB/m, (P < 0.001) as compared with the baseline 328 dB/m |
| Hassan et al[29], 2019 | Animal | Duration: 5 wk | n = 12 | Mice with induced NASH by a high-fat emulsion diet (n = 6 per group) | Saroglitazar: Control vs 4 mg/kg daily | Histopathological effects of Saroglitazar by using light microscopy | In the control vs treatment group, steatosis score was 3 vs 0.5, hepatic ballooning was 2 vs 0.5, lobar hepatitis was 3 vs 1, and portal hepatitis was 3 vs 0.25, respectively (P < 0.05) |
| Padole et al[31], 2022 | Human | Single-center prospective study Duration: 3 mo | n = 91 | Patients with BMI > 23 kg/m2 diagnosed with NAFLD (CAP > 248 dB/m) | Saroglitazar 4 mg daily | Change from baseline of liver biomarker, hepatic steatosis, and fibrosis in patients who lost > 5% of the weight | Patients with > 5% of weight loss had a median AST of 36 vs 40 at baseline (P = 0.038), ALT 44 vs 53 (P < 0.01), kPa 5.9 vs 6.8 (P = 0.336) and CAP 265 vs 311 (P = 0.128) |
| Rajesh et al[28], 2022 | Human | A single-arm, open-label prospective study Duration: 12 wk | n = 85 | Patients with NAFLD (US, CT, or MRI) and type 2 diabetes mellitus, and dyslipidemia | Saroglitazar 4 mg daily | Evaluate the effect of Saroglitazar on liver function test, liver fibrosis score by FibroScan, lipid profiles, and HbA1c | From baseline, there was a reduction in ALT from 49 u/L to 48 (P < 0.05), fibrosis score 10 kPa to 6 (P < 0.0001), TG 359.89 to 103.04 (P = 0.0001), HbA1c 10.29% to 9.85% (P = 0.002) |
| Jain et al[30], 2018 | Animal | Duration: 12 wk | n = 18 | CDHFD-induced model of NASH in mice (n = 9 per group) | Saroglitazar: Control vs 3 mg/kg daily | Reversal of CDHFD-induced NASH after 8 wk | In control vs. treatment, respectively, steatosis score was 2.6 vs 0, ballooning 1.4 vs 0, inflammation 3 vs 1.1 (P < 0.1) |
| Jain et al[30], 2018 | Animal | Duration: 12 wk | n = 16 | CCL4-induced fibrosis model in mice (n = 8 per group) | Saroglitazar: Control vs 4 mg/kg daily | Reversal of CCl4-induced liver fibrosis after 4 wk | Saroglitazar protected mice from CCl4-induced liver fibrosis measured via Hematoxylin and Eosin stains |
| Staels et al[26], 2013 | Animal | Duration: 7 wk | n = 16 | Choline-deficient high-fat diet-induced model of NASH in mice (n = 8 per group) | GFT505: Control vs 10 mg/kg daily | Evaluate the prevention of the development of NASH in CDHFD mice | The percentage of animals with macrosteatosis in control vs treatment was 100% to 0%, inflammation was 100% to 0%, and the percentage of fibrosis was 1.3% to 0.8% (P < 0.01) |
| Staels et al[26], 2013 | Animal | Duration: 7 wk | n = 12 | CCl4-induced liver fibrosis in mice (n = 6 per group) | GFT505: Control vs 30 mg/kg daily | Evaluate the prevention of the development of NASH in CCL4 mice | The fibrotic surface of control vs treatment was 8% vs 4% in CCL4 mice (P < 0.001) |
| Ye et al[23], 2003 | Animal | Duration: 2 wk | n = 6 | High fat-fed rats | Ragaglitazar: 3 mg/kg-1 daily | Evaluate the benefits of Ragaglitazar on insulin sensitivity and lipid metabolism. | Enhanced insulin suppressibility of hepatic glucose output by 79% (P < 0.001), decrease in liver TG from baseline of 23 μmol/g to 7 μmol/g (P < 0.01) |
In terms of cyclophilin inhibitors, four animal studies demonstrated significant improvement in fibrosis on liver biopsy weeks after product use (P < 0.05). The randomized controlled trial (RCT) performed in humans (n = 49) noted similar results, with a reduction in ALT and Pro-C3 levels (P < 0.01), as well as steatosis and fibrosis as measured on FibroScan (P < 0.01).
The three animal studies using FGF analogs demonstrated significant improvement in both steatosis and fibrosis measured on liver biopsy (P < 0.05). The RCT performed in humans (n = 80) measured the change in hepatic fat fraction (HFF) on magnetic resonance imaging at 12 wk of treatment. It noted a greater than 50% reduction in HFF in all treatment dosage groups (P < 0.0001).
Eight animal studies using pan-PPAR agonists evidenced a significant reduction in steatosis on biopsy as measured by the decrease in triglyceride or lipid accumulation in hepatocytes (P < 0.05). There was also a reduction in fibrosis and collagen deposition on liver biopsy (P < 0.05). The human studies included three RCTs that examined the metabolic effects and/or steatosis markers (steatosis activity score) and concluded improved metabolic function, resolution of steatosis, and fibrosis improvement (P < 0.05).
In terms of dual-PPAR agonists, six animal studies reported improvement in steatosis, reduction in fibrosis or progression to fibrosis, and improvement in lipid metabolism and insulin sensitivity (P < 0.05). Two human in-vitro studies on hepatic cells were performed, which demonstrated a reduction in hepatic lipid accumulation, secretion of inflammatory chemokines, and profibrotic gene expression. Four additional human studies, including prospective design and RCTs, showed improved metabolic parameters such as insulin sensitivity, hemoglobin A1c, and lipid profiles (P < 0.05). Additionally, FibroScan results showed improved liver stiffness and steatosis (P < 0.05).
Cyclophilins are thought to contribute to the development of liver fibrosis and cancer. Among these, Cyclophilin B is known to play a role in collagen production, leading to fibrosis. To treat NASH, several investigational products have been developed to target Cyclophilins[6]. The main cyclophilin inhibitors reviewed here are CRV431[6], NV556[7,8], and Rencofilstat[9].
Studies conducted on animals, mainly mice that were administered a cyclophilin inhibitor, have shown positive results in improving liver fibrosis during biopsy[6-8]. In particular, Kuo et al's research indicated a reduction of over 37% in liver fibrosis with CRV431 treatment compared to control on various mouse models[6,8]. Likewise, NV556 also demonstrated a significant decrease in collagen production and liver fibrosis[7,8].
Due to these promising results, researchers conducted a phase 2a RCT with 49 patients who received Rencofilstat (75, 225 mg) or a placebo[9]. The results showed that patients with high baseline Pro-C3 levels (> 15) experienced a decrease in collagen biomarkers, which are predictors for collagen deposition (P < 0.01)[9]. This aligns with previous animal studies, suggesting that cyclophilin inhibitor treatment may reduce liver fibrosis. The patients generally tolerated Rencofilstat well, with only mild side effects reported, such as constipation, diarrhea, back pain, dizziness, and headache[9]. Animal and human studies have shown that various investigational products that inhibit cyclophilin effectively treat patients with NASH. These agents are also well-tolerated and have anti-fibrotic properties that are beneficial.
FGF21 is an active component of organ metabolism. Different variants have been studied for treating fatty liver disease, diabetes, and obesity due to their effect on glucose and lipid metabolism[10]. The main FGF21 analogs and agonists reviewed here are LY2405319[11], PsTag600[12], BMS-986171[13], and EFX[10]. Multiple animal studies involving FGF21 analogs and agonists have demonstrated improved glucose metabolism and reductions in markers of liver injury and fibrosis[11,12]. Le et al[11] performed an animal study using LY2405319 (FGF21 analog), which attenuated increased collagen type 1, alpha-smooth muscle actin, and GPR91 protein levels[11]. These results align with research on PsTag600 (long-acting FGF21) and BMS-986171 (FGF21 agonist)[12,13].
A phase 2a study included 80 patients treated with a placebo or EFX 28 mg, 50 mg, or 70 mg (FGF21 analog) for 12 wk[10]. The results indicated a significant decrease in HFF, with 78% of patients showing a positive response to NAS with an increase of at least 2 points and 48% of patients showing a resolution of NASH[10]. There was also a statistically significant reduction in alanine transaminase (ALT), aspartate aminotransferase (AST), and total cholesterol levels[10]. Compared to Resmetirom, a selective thyroid hormone receptor-β agonist in phase 3 trials, FGF21 analogs/agonists showed similar reductions in HFF and fibrosis[10]. The side effects reported for EFX were mild and included diarrhea, nausea, vomiting, abdominal pain, frequent bowel movements, and fatigue[10]. In conclusion, FGF21 analogs and agonists have numerous benefits for NAFLD, including improved glucose and lipid metabolism, reduced markers of liver injury, and liver fibrosis. They effectively reduce hepatic steatosis and fibrosis, making them a promising treatment for NAFL and NASH.
There are three different isoforms of PPAR, α, γ, δ[14]. PPARα mainly regulates genes that participate in lipid transport, beta-oxidation, gluconeogenesis, and ketogenesis[15]. PPARγ regulates adiponectin, glucose metabolism, adipocyte differentiation, and lipogenesis[15]. PPARδ limits inflammation and regulates hepatic fatty acid oxidation[15]. Single PPAR agonists have had unwanted adverse effects and less effective results, for which investigational products that act on several isoforms have been attractive[15].
The main pan-PPAR agonists reviewed here are Benzafibrate[16,17], Lanifibranor[14,15,18-21], and MHY2013[22,23]. Multiple animal studies involving pan-PPAR agonists have demonstrated increased plasma adiponectin, improvement in hepatic steatosis, and markers of liver injury[15,17,19,20,22-24]. In alignment with the mechanism of pan-PPAR agonists, MHY2013[22,25] and Lanifibranor[19,20,24] also led to a decrease in hepatic steatosis, hepatic inflammation, serum triglycerides, profibrotic and fibrotic genes. In addition to the previously mentioned effects of Lanifibranor, Møllerhøj et al[20] revealed that Lanifibranor resulted in progressive weight loss, a 23% decrease at eight weeks and a 30% decrease at 12 wk[20].
In line with results from animal studies, a study of 45 patients using Lanifibranor (400 mg, 800 mg, or 1200 mg) or placebo for four weeks revealed an increase in adiponectin, a decrease in triglycerides, and ALT[25]. Shortly after, a more significant phase 2b trial was performed on 247 patients with NASH that were randomly assigned to Lanifibranor (800 or 1200 mg) or a placebo daily for 24 wk[18]. Participants had at least a 2-point decrease in the Steatosis, Activity, and Fibrosis score[18]. A comparison of pan-PPAR agonists vs single agents revealed that pan-PPAR agonists were more potent in counteracting fibrosis by combining specific mechanisms of single PPAR agonists[15]. Lanifibranor was generally well tolerated with mild reported side effects, including diarrhea, nausea, peripheral edema, anemia, and weight gain[18]. Based on initial data, pan-PPAR agonists are more effective in improving the histological features of fatty liver disease with fewer adverse side effects than single PPAR agonists. This makes them a desirable option for the treatment of fatty liver disease.
Like pan-PPAR agonists, these agents act on two isoforms of PPAR, allowing for a more targeted effect. Saroglitazar has already been Federal Drug Administration (FDA)-approved for diabetic dyslipidemia and hypertriglyceridemia and has been shown to improve NAFLD, which piqued interest.
The main dual-PPAR agonists reviewed here are Ragaglitazar (α/γ)[23], GFT505 (α/δ)[26,27], Saroglitazar (α/γ)[28-32] and Elafibranor (α/δ)[33,34]. Multiple animal studies involving dual-PPAR agonists have demonstrated promising results, including reduced triglycerides and liver injury markers[23,29]. Ragaglitazar revealed an 88% reduction in triglycerides, increased adiponectin, counteracted an increase in visceral fat mass, and enhanced insulin suppressibility of hepatic glucose output[23]. These outcomes correlate with results seen with GFT505[26] and Saroglitazar[20,29]. Furthermore, Saroglitazar completely normalized AST and ALT, reduced serum TNF-α level by 47.6% and leptin by 58.6%[29].
Human research showed promising results in line with the aforementioned animal studies. GFT505 80 mg/day revealed a statistically significant reduction of fasting plasma triglycerides, LDL, and liver enzyme levels[27]. However, the most studied investigational product is Saroglitazar. A more extensive study in 85 patients revealed reduced ALT and triglycerides[28]. Furthermore, a study of Saroglitazar in 91 patients showed that 57 patients (63%) could reduce ≥ 5% of their weight[31]. There has been discussion regarding pan-PPAR agonists vs dual agents; Boeckmans et al[33] compared Elafibranor vs Lanifibranor (pan-PPAR agonist), which identified Elafibranor as having higher anti-NASH properties[33]. In general, dual-PPAR agonists are safe and effective in treating NAFLD and obesity. Research suggests that Elafibranor may be more effective than pan-PPAR agonists in treating these conditions.
NAFLD has become one of the most common causes of chronic liver disease globally. It's troubling that no FDA-approved treatments are currently available for this condition. Patients are limited to lifestyle changes and managing any concurrent diseases associated with fatty liver. However, there are promising developments in the form of investigational products that are being studied through clinical trials. These products include cyclophilin inhibitors, FGF21 agonists, and pan and dual PPAR agonists. The data analyzed in this review show clinically significant improvement in individual histological features of NAFLD in both animal and human trials for all four classes. These agents were generally well tolerated, with minimal side effects. We believe this compilation of information will have positive clinical implications in obtaining an FDA-approved therapy for NAFLD. However, more extensive trials are needed to further determine their efficacy, proper dosage, duration of therapy, and potential side effects for patients with NAFLD, including those with hepatic steatosis and fibrosis.
Non-alcoholic fatty liver disease (NAFLD) has become a global health issue with significant medical costs. The lack of a Federal Drug Administration (FDA)-approved medication for the treatment of NAFLD has prompted the investigation of several potential therapeutic classes. It is valuable to have a compilation of the data available on their efficacy.
Due to the absence of an approved medication by the FDA for the treatment of NAFLD, several therapeutic classes have been investigated in clinical trials. It is important to understand the mechanisms and statistical significance of the agents being investigated, as NAFLD is extremely prevalent.
To assess the efficacy of cyclophilin inhibitors, fibroblast growth factor 21 analogs (FGF21), and dual and pan peroxisome proliferator-activated receptor (PPAR) agonists as possible therapeutic classes for treating NAFLD.
We searched PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science using keywords including cyclophilin inhibitor, FGF agonist, pan-PPAR agonists, dual-PPAR agonist, NAFLD, non-alcoholic steatohepatitis, and fatty liver. Articles with a National Institutes of Health Quality Assessment score of five or higher were included. Each article was screened by two independent researchers evaluating relevance and quality. Pertinent data were extracted in a sprea
We identified 29 studies that met the necessary criteria and were included in this review. These records included 12 human studies and 17 animal studies. Specifically, there were four studies on cyclophilin inhibitors, four on FGF analogs, 11 on pan-PPAR agonists, and ten on dual-PPAR agonists. All classes were found to be efficacious for the treatment of NAFLD with statistical significance (P < 0.05).
We found that cyclophilin inhibitors, fibroblast growth factor 21 analogs, and dual and pan PPAR agonists are not only statistically efficacious for the treatment of NAFLD but also generally well tolerated. We recommend more extensive human clinical research to further delineate therapy's efficacy, dosage, and duration.
It is to be expected that additional human clinical trials of the therapeutic classes assessed in this review, as well as additional novel agents, will be conducted in the near future. An FDA-approved agent for the treatment of NAFLD is of utmost importance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li H, China; Pham TTT, Viet Nam; Zamani M, Iran S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Filipovic B, Lukic S, Mijac D, Marjanovic-Haljilji M, Vojnovic M, Bogdanovic J, Glisic T, Filipovic N, Al Kiswani J, Djokovic A, Kapor S, Bukumiric Z, Starcevic A. The New Therapeutic Approaches in the Treatment of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Rowe IA, Wong VW, Loomba R. Treatment Candidacy for Pharmacologic Therapies for NASH. Clin Gastroenterol Hepatol. 2022;20:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Lee YA, Friedman SL. Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies. J Intern Med. 2022;291:11-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Nassir F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 5. | Ratziu V, Francque S, Sanyal A. Breakthroughs in therapies for NASH and remaining challenges. J Hepatol. 2022;76:1263-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 6. | Kuo J, Bobardt M, Chatterji U, Mayo PR, Trepanier DJ, Foster RT, Gallay P, Ure DR. A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models. J Pharmacol Exp Ther. 2019;371:231-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Simón Serrano S, Grönberg A, Longato L, Rombouts K, Kuo J, Gregory M, Moss S, Elmér E, Mazza G, Gallay P, Pinzani M, Hansson MJ, Massoumi R. Evaluation of NV556, a Novel Cyclophilin Inhibitor, as a Potential Antifibrotic Compound for Liver Fibrosis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Kuo J, Serrano SS, Grönberg A, Massoumi R, Hansson MJ, Gallay P. Cyclophilin Inhibitor NV556 Reduces Fibrosis and Hepatocellular Carcinoma Development in Mice With Non-Alcoholic Steatohepatitis. Front Pharmacol. 2019;10:1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Harrison SA, Mayo PR, Hobbs TM, Canizares C, Foster EP, Zhao C, Ure DR, Trepanier DJ, Greytok JA, Foster RT. Rencofilstat, a cyclophilin inhibitor: A phase 2a, multicenter, single-blind, placebo-controlled study in F2/F3 NASH. Hepatol Commun. 2022;6:3379-3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA, Hu C, Fong E, de Temple B, Tillman EJ, Rolph TP, Cheng A, Yale K. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 11. | Le CT, Nguyen G, Park SY, Choi DH, Cho EH. LY2405319, an analog of fibroblast growth factor 21 ameliorates α-smooth muscle actin production through inhibition of the succinate-G-protein couple receptor 91 (GPR91) pathway in mice. PLoS One. 2018;13:e0192146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175:3379-3393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Puengel T, Lefere S, Hundertmark J, Kohlhepp M, Penners C, Van de Velde F, Lapauw B, Hoorens A, Devisscher L, Geerts A, Boehm S, Zhao Q, Krupinski J, Charles ED, Zinker B, Tacke F. Combined Therapy with a CCR2/CCR5 Antagonist and FGF21 Analogue Synergizes in Ameliorating Steatohepatitis and Fibrosis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Cooreman MP, Francque S, Dzen L, Huot-Marchand P, Junien JL, Broqua P, Abdelmalek MF. 830-P: The Pan-PPAR Agonist Lanifibranor Improves Nonalcoholic Steatohepatitis (NASH) and Glycemic Control. Diabetes. 2022;71 (Supp 1):830-P. [DOI] [Full Text] |
| 15. | Lefere S, Puengel T, Hundertmark J, Penners C, Frank AK, Guillot A, de Muynck K, Heymann F, Adarbes V, Defrêne E, Estivalet C, Geerts A, Devisscher L, Wettstein G, Tacke F. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages(☆). J Hepatol. 2020;73:757-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 16. | Barbosa-da-Silva S, Souza-Mello V, Magliano DC, Marinho Tde S, Aguila MB, Mandarim-de-Lacerda CA. Singular effects of PPAR agonists on nonalcoholic fatty liver disease of diet-induced obese mice. Life Sci. 2015;127:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 18. | Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, Lanthier N, Alkhouri N, Moreno C, Schattenberg JM, Stefanova-Petrova D, Vonghia L, Rouzier R, Guillaume M, Hodge A, Romero-Gómez M, Huot-Marchand P, Baudin M, Richard MP, Abitbol JL, Broqua P, Junien JL, Abdelmalek MF; NATIVE Study Group. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 19. | Boubia B, Poupardin O, Barth M, Binet J, Peralba P, Mounier L, Jacquier E, Gauthier E, Lepais V, Chatar M, Ferry S, Thourigny A, Guillier F, Llacer J, Amaudrut J, Dodey P, Lacombe O, Masson P, Montalbetti C, Wettstein G, Luccarini JM, Legendre C, Junien JL, Broqua P. Design, Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator Activated Receptor (PPAR) α/γ/δ Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J Med Chem. 2018;61:2246-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Møllerhøj MB, Veidal SS, Thrane KT, Oró D, Overgaard A, Salinas CG, Madsen MR, Pfisterer L, Vyberg M, Simon E, Broermann A, Vrang N, Jelsing J, Feigh M, Hansen HH. Hepatoprotective effects of semaglutide, lanifibranor and dietary intervention in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH. Clin Transl Sci. 2022;15:1167-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Abitbol JL, Broqua P, Junien JL. Metabolic effects and good tolerance of IVA337 a Pan-PPAR agonist in diabetic patients warrant further investigation in NASH. J Hepatol. 2016;64:S189. |
| 22. | An HJ, Lee B, Kim DH, Lee EK, Chung KW, Park MH, Jeong HO, Kim SM, Moon KM, Kim YR, Kim SJ, Yun HY, Chun P, Yu BP, Moon HR, Chung HY. Correction: Physiological characterization of a novel PPAR pan agonist, 2-(4-(5,6-methylenedioxybenzo[d]thiazol-2-yl)-2-methylphenoxy)-2-methylpropanoic acid (MHY2013). Oncotarget. 2017;8:45030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Ye JM, Iglesias MA, Watson DG, Ellis B, Wood L, Jensen PB, Sørensen RV, Larsen PJ, Cooney GJ, Wassermann K, Kraegen EW. PPARalpha /gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab. 2003;284:E531-E540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Wettstein G, Luccarini JM, Poekes L, Faye P, Kupkowski F, Adarbes V, Defrêne E, Estivalet C, Gawronski X, Jantzen I, Philippot A, Tessier J, Tuyaa-Boustugue P, Oakley F, Mann DA, Leclercq I, Francque S, Konstantinova I, Broqua P, Junien JL. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol Commun. 2017;1:524-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | An HJ, Lee B, Kim SM, Kim DH, Chung KW, Ha SG, Park KC, Park YJ, Kim SJ, Yun HY, Chun P, Yu BP, Moon HR, Chung HY. A PPAR Pan Agonist, MHY2013 Alleviates Age-Related Hepatic Lipid Accumulation by Promoting Fatty Acid Oxidation and Suppressing Inflammation. Biol Pharm Bull. 2018;41:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 27. | Cariou B, Hanf R, Lambert-Porcheron S, Zaïr Y, Sauvinet V, Noël B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923-2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Rajesh NA, Drishya L, Ambati MMR, Narayanan AL, Alex M, R KK, Abraham JJ, Vijayakumar TM. Safety and Efficacy of Saroglitazar in Nonalcoholic Fatty Liver Patients With Diabetic Dyslipidemia-A Prospective, Interventional, Pilot Study. J Clin Exp Hepatol. 2022;12:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Hassan NF, Nada SA, Hassan A, El-Ansary MR, Al-Shorbagy MY, Abdelsalam RM. Saroglitazar Deactivates the Hepatic LPS/TLR4 Signaling Pathway and Ameliorates Adipocyte Dysfunction in Rats with High-Fat Emulsion/LPS Model-Induced Non-alcoholic Steatohepatitis. Inflammation. 2019;42:1056-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, Roy SS, Das N, Karmakar E, Wahli W, Patel PR. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Padole P, Arora A, Sharma P, Chand P, Verma N, Kumar A. Saroglitazar for Nonalcoholic Fatty Liver Disease: A Single Centre Experience in 91 Patients. J Clin Exp Hepatol. 2022;12:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Chaudhuri S, Dutta A, Chakraborty SBD. Efficacy and safety of saroglitazar in real-world patients of non-alcoholic fatty liver disease with or without diabetes including compensated cirrhosis: A tertiary care center experience. JGH Open. 2023;7:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 33. | Boeckmans J, Natale A, Rombaut M, Buyl K, Cami B, De Boe V, Heymans A, Rogiers V, De Kock J, Vanhaecke T, Rodrigues RM. Human hepatic in vitro models reveal distinct anti-NASH potencies of PPAR agonists. Cell Biol Toxicol. 2021;37:293-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Boeckmans J, Buyl K, Natale A, Vandenbempt V, Branson S, De Boe V, Rogiers V, De Kock J, Rodrigues RM, Vanhaecke T. Elafibranor restricts lipogenic and inflammatory responses in a human skin stem cell-derived model of NASH. Pharmacol Res. 2019;144:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |