Published online Jun 27, 2023. doi: 10.4254/wjh.v15.i6.826
Peer-review started: December 22, 2022
First decision: February 14, 2023
Revised: March 23, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: June 27, 2023
Processing time: 185 Days and 2.3 Hours
We previously reported national 30-d readmission rates of 27% in patients with decompensated cirrhosis (DC).
To study prospective interventions to reduce early readmissions in DC at our tertiary center.
Adults with DC admitted July 2019 to December 2020 were enrolled and randomized into the intervention (INT) or standard of care (SOC) arms. Weekly phone calls for a month were completed. In the INT arm, case managers ensured outpatient follow-up, paracentesis, and medication compliance. Thirty-day readmission rates and reasons were compared.
Calculated sample size was not achieved due to coronavirus disease 2019; 240 patients were randomized into INT and SOC arms. 30-d readmission rate was 33.75%, 35.83% in the INT vs 31.67% in the SOC arm (P = 0.59). The top reason for 30-d readmission was hepatic encephalopathy (HE, 32.10%). There was a lower rate of 30-d readmissions for HE in the INT (21%) vs SOC arm (45%, P = 0.03). There were fewer 30-d readmissions in patients who attended early outpatient follow-up (n = 17, 23.61% vs n = 55, 76.39%, P = 0.04).
Our 30-d readmission rate was higher than the national rate but reduced by interventions in patients with DC with HE and early outpatient follow-up. Development of interventions to reduce early readmission in patients with DC is needed.
Core Tip: Our 30-d readmission rate was higher than the national rate but reduced by interventions in patients with decompensated cirrhosis (DC) with hepatic encephalopathy and early outpatient follow-up. Development of interventions to reduce early readmission in patients with DC is needed.
- Citation: Pusateri A, Litzenberg K, Griffiths C, Hayes C, Gnyawali B, Manious M, Kelly SG, Conteh LF, Jalil S, Nagaraja HN, Mumtaz K. Randomized intervention and outpatient follow-up lowers 30-d readmissions for patients with hepatic encephalopathy, decompensated cirrhosis. World J Hepatol 2023; 15(6): 826-840
- URL: https://www.wjgnet.com/1948-5182/full/v15/i6/826.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i6.826
Cirrhosis affects approximately 5 million annually[1] and has been reported to be the 8th leading cause of death with more than 40000 deaths annually in the United States[2]. A study on the burden of gastrointestinal (GI), liver, and pancreatic diseases in the United States revealed that liver diseases had the highest mortality at 3.1%[3]. In addition to high mortality, cirrhosis is also associated with high morbidity. The sequelae of decompensated cirrhosis (DC) are often managed during hospital admis
Several studies have demonstrated hospital readmissions in DC place a large financial burden on the United State healthcare system. The 30-d readmission rate has been reported to be 20%-37%[5-14]. We have recently published on early readmission rates up to 27% in patients with DC and developed the Mumtaz readmission risk score based on United States data[15]. We also reported that nearly one-third of patients with HE were readmitted within 30 d, and early readmission adversely impacted healthcare utilization and calendar-year mortality[16].
Interventions to reduce readmissions have been shown to be safe and effective. For instance, Morales et al[17] developed a program including a hepatologist follow-up exam within 7 d after discharge. This program resulted in a reduction in 30-d readmissions, 60-d mortality, emergency department visits and associated costs[17]. Similarly, another group demonstrated that follow-up with a “care management check-up” as opposed to “standard outpatient care” reduced 30-d readmission, 12-mo mortality and saved 1500 euros per patient month of life[18].
There is a paucity of prospective studies on interventions to reduce early readmission rates in patients with DC. Therefore, we prospectively studied 30-d readmission rates in patients with DC and compared various interventions (INT) with standard of care (SOC) to reduce early readmission rates. We hypothesized that DC patients in the INT arm would have decreased 30-d readmission vs the SOC arm.
This study was conducted at the Ohio State University Wexner Medical Center (OSUWMC), Columbus, Ohio from July 2019 to December 2020. Our study was approved by OSUWMC Institutional Review Board. All aspects of the studying involving human participants including informed consent for enrollment were in accordance with the ethical standards of our Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
All patients admitted with DC to the hepatology (inpatient or consult) service were screened for enrollment. Patients meeting inclusion criteria were approached for study consent. Of note, due to the global coronavirus disease 2019 (COVID-19) pandemic, beginning March 2020, only COVID negative patients were approached for informed consent. Elective readmissions for inpatient procedures including endoscopy, trans-arterial chemoembolization, transjugular intrahepatic portosystemic shunt (TIPS), paracentesis or readmissions unrelated to DC such as motor vehicle accidents were excluded.
Study data were collected and managed using research electronic data capture (REDCap) hosted at The Ohio State University Wexner Medical Center[19,20]. Informed consent was obtained from all individual participants included in the study. Consented patients were randomly assigned to either the INT arm or the SOC arm in a 1:1 ratio using the REDCap randomization tool. The following data were collected on all patients via REDCap software including demographics (age, sex, insurance type, income based on the zip code), hospitalization data [date of index admission defined as initial admission during which patient consented for study, reason for admission, length of stay (LOS) defined as difference in days between index admission date and index admission discharge date, discharge disposition, associated cost of care of admission as obtained through medical record billing tab], etiology of cirrhosis (alcoholic and non-alcoholic including viral, non-alcoholic fatty liver disease, autoimmune, primary biliary cirrhosis, primary sclerosing cholangitis or cryptogenic), complications of cirrhosis (HE, AKI, ascites, variceal bleeding, SBP, hepatorenal syndrome, coagulopathy, portal hypertension, hepato-pulmonary syndrome, hepatocellular carcinoma), and procedures performed during admission [esophago-gastro-duodenoscopy, colonoscopy or flexible sigmoidoscopy, paracentesis, TIPS and hemodialysis (HD) on admission and discharge]. We also collected data including Elixhauser comorbidity index, discharge medications, and laboratory data (complete blood counts, serum creatinine, liver function tests including total bilirubin, INR, and sodium). Child Turcotte Pugh (CTP) and Sodium-model for end stage liver disease (MELD-Na) score were calculated from the data. The nurse case manager (CM) also recorded labs & medications at readmission and discharge and associated cost of readmission. Status of early readmission, liver transplantation, and mortality at one year were also collected.
The CM phoned each patient enrolled in either arm weekly for 30 d after index discharge to find out if the patient has been readmitted to OSUWMC or another hospital. In the INT arm, during the call CM also ensured i) early (defined as within 30 d from index admission discharge) outpatient hepatology follow-up ii) compliance of medication, iii) arrangement of outpatient paracentesis if needed, and reviewed outpatient hepatology clinic follow-up records. SOC arm as per our center’s protocol had to be taken care of by the primary inpatient team. This included arranging early outpatient clinic follow-up, providing list of medications, and advice for outpatient paracentesis if needed at the time of discharge. Due to the nature of intervention, the study could not be blinded.
Early readmission was defined as admission within 30 d of index admission discharge. Reasons for readmission were gathered by CM by reviewing the electronic medical record (EMR) of all enrolled patients readmitted at OSUWMC or outside hospital within 30 d of index admission. Predictors of early readmission were also compared in the two arms.
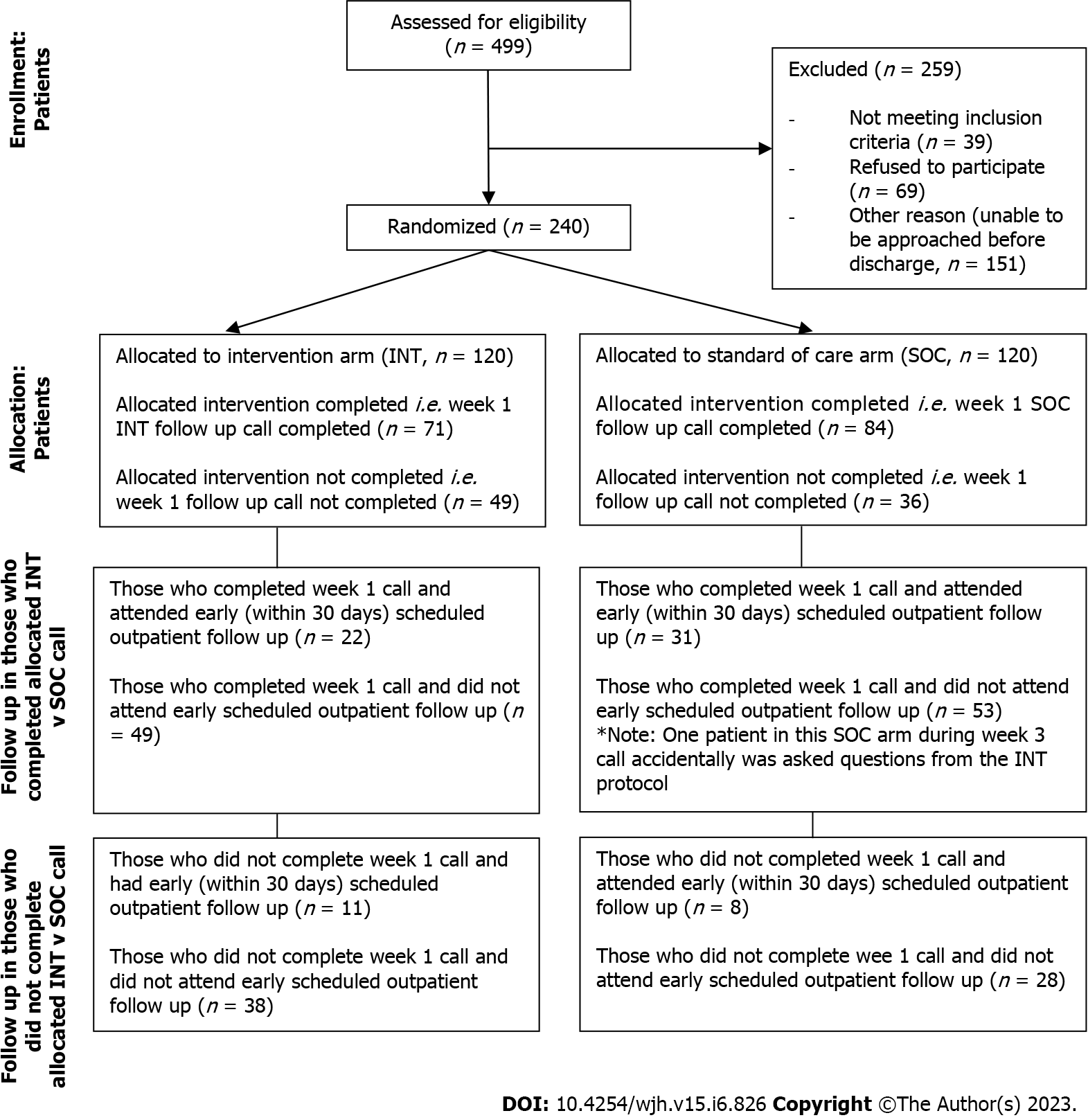
Based on the sample size calculation, target of recruitment for the study was 848 patients, admitted to the hospital with DC under the hepatology (inpatient and consult) services. Patients were randomly assigned in a 1:1 ratio into INT or SOC arms. Based on our previous study using the National Readmissions Administrative Database, we expected a 30-d readmission rate of 27% among patients meeting inclusion criteria, which yield 114/424 patients with 30-d readmission events, thus meeting the target sample size. Based on this calculation, a total sample size of 848 (424 per group) provided 80% power to detect a 30% decrease in 30-d readmission rate (from 27% to 19%) with a type I error rate of 0.05. However, planned sample size could not be achieved due to the COVID-19 pandemic related restriction started in our center in March 2020. Therefore, we end up with available sample size of a total of 240 patients. The modified consort flow diagram for enrollment in our study trial is illustrated in Figure 1.
Means of continuous response variables between two groups were compared using robust t-test (Welch test). Proportions were compared using χ-test or Fisher’s exact test as applicable. Logarithmic transformation was used for comparing the LOS and admission cost across groups. Level of significance was kept at 0.05 for each comparison. JMP Version 15 (SAS Institute, NC) was used for all the analyses.
From July 1, 2019, to December 1, 2020, 1392 patients were screened. Due to the COVID-19 pandemic, recruitment was held from March 2020 to July 2020 and subsequently resumed until December 2020. Out of the patients screened, only 499 (35.85%) were eligible for inclusion; however, 240 patients consented and randomized: 120 each into the INT and SOC arm (Figure 1).
The mean age of patients was 56.34 ± 11.19 years, majority were males (135, 56.25%), belonged to White race (n = 202, 84.17%) and non-Hispanic or Latino ethnicity (n = 227, 94.58%). Almost two-thirds of the patients had public insurance (n = 76, 31.67% on Medicare and n = 70, 29.17% on Medicaid); 73 (30.42%) had private insurance. At admission, the mean MELD-Na score and mean CTP Score were 21.89 ± 8.03 and 9.36 ± 1.96, respectively. Major etiology of cirrhosis was alcohol (n = 121, 50.42%) followed by non-alcoholic fatty liver disease (n = 79, 32.92%) and viral hepatitis (n = 43, 17.92%). Furthermore, 116 (48.33%) patients were actively under evaluation for liver transplantation.
The index admission mean LOS was 8.13 ± 5.83 d (median 6, range 1-43 d). The mean cost of index admission was $60595 ± $47174 (n = 225, median $42932, range $1630-251991). The top five reasons for index admission included volume overload (n = 111, 46.25%), AKI (n = 65, 27.08%), hepatic encephalopathy (n = 45, 18.75%), variceal bleed (n = 42, 17.50%), lower GI bleed (n = 19, 7.92%) and hyponatremia (n = 16, 6.67%). The top five interventions performed were esophago-gastro-duodenoscopy (n = 136, 56.67%), paracentesis (n = 115, 47.92%), colonoscopy/flexible sigmoidoscopy (n = 24, 10 %), HD (n = 15, 6.25%) and TIPS (n = 10, 4.17%). Most patients were discharged from index admission to home (n = 159, 66.25%) followed by home with health care (n = 42, 17.50%) and skilled nursing facility (n = 32, 13.33 %, Table 1).
| Total | Not readmitted (n = 159) | Readmitted (n = 81) | P value | |
| Index admission characteristics | ||||
| Reasons for admission1 | ||||
| Acute kidney injury | 65, 27.08 | 41, 25.79 | 24, 29.63 | 0.54 |
| Hyponatremia | 16, 6.67 | 11, 6.92 | 5, 6.17 | 1.00 |
| Hepatic encephalopathy | 45, 18.75 | 26, 16.35 | 19, 23.46 | 0.22 |
| Volume overload | 111, 46.25 | 81, 50.94 | 30, 37.04 | 0.06 |
| Variceal bleed | 42, 17.50 | 31, 19.50 | 11, 13.58 | 0.29 |
| Lower GI bleed | 19, 7.92 | 11, 6.92 | 8, 9.88 | 0.45 |
| SBP | 21, 8.75 | 14, 8.81 | 7, 8.64 | 1.00 |
| Complications of cirrhosis during admission1 | ||||
| Presence of AKI | 80, 33.33 | 50, 31.45 | 30, 37.04 | 0.39 |
| HE | 49, 20.42 | 31, 19.50 | 18, 22.22 | 0.62 |
| Ascites | 139, 57.92 | 95, 59.75 | 44, 54.32 | 0.49 |
| Variceal bleeding | 37, 15.42 | 26, 16.35 | 11, 13.58 | 0.71 |
| SBP | 16, 6.67 | 12, 7.55 | 4, 4.94 | 0.59 |
| HRS | 14, 5.83 | 8, 5.03 | 6, 7.41 | 0.56 |
| Coagulopathy | 56, 23.33 | 36, 22.64 | 20, 24.69 | 0.75 |
| Portal hypertension | 46, 19.17 | 34, 21.38 | 12, 14.81 | 0.30 |
| HPS | 15, 6.25 | 8, 5.03 | 7, 8.64 | 0.27 |
| HCC | 11, 4.58 | 6, 3.77 | 5, 6.17 | 0.51 |
| Procedures performed during admission1 | ||||
| EGD | 136, 56.67 | 92, 57.86 | 44, 54.32 | 0.68 |
| Paracentesis | 115, 47.92 | 73, 45.91 | 42, 51.85 | 0.41 |
| Emergent TIPS | 10, 4.17 | 9, 5.66 | 1, 1.23 | 0.17 |
| HD | 15, 6.25 | 7, 4.40 | 8, 9.88 | 0.16 |
| Colonoscopy/flex sig | 24, 10.00 | 18, 11.32 | 6, 7.41 | 0.37 |
| Disposition1 | ||||
| Home | 159, 66.25 | 107, 67.30 | 52, 64.20 | 0.66 |
| Home with Home Health Newly Arranged | 39, 16.25 | 24, 15.09 | 15, 18.52 | |
| Home with Home Health Previously Arranged | 3, 1.25 | 2, 1.26 | 1, 1.23 | |
| SNF newly Arranged | 21, 8.75 | 16, 10.06 | 5, 6.17 | |
| SNF Previously Arranged | 11, 4.58 | 5, 3.14 | 6, 7.41 | |
| Left Against Medical Advice | 2, 0.83 | 1, 0.63 | 1, 1.23 | |
| Transfer (long term acute care hospital) | 3, 1.25 | 2, 1.26 | 1, 1.23 | |
| Homeless | 2, 0.83 | 2, 1.26 | 0, 0.00 |
Overall, 81 (33.75%) patients were readmitted within 30 d of discharge. The major reasons for first readmission included hepatic encephalopathy (n = 26, 32.10%) followed by volume overload (n = 22, 27.16%), AKI (n = 16, 19.75%), variceal bleed (n = 12, 14.82%) and hyponatremia (n = 10, 12.35%). 14 patients were readmitted twice, 3 admitted thrice and one admitted 5 times within 30 d. The mean time to first readmission was 12.65 ± 7.55 d (median 12 d, range 1-30 d). The mean LOS of first readmission was 8.11 ± 8.98 days. The mean cost of stay of first readmission was $55548.29 ± $65164.91 (Table 2). Those readmitted had a higher MELD-Na score on index admission (23.54 ± 7.80 vs 21.05 ± 8.03, P = 0.02) and index discharge (21.67 ± 7.95 vs 19.39 ± 6.89, P = 0.03) than those not readmitted. Similarly, those readmitted had a higher index admission creatinine (1.80 ± 1.53 vs 1.39 ± 1.16, P = 0.03), index discharge creatinine (1.61 ± 1.34 vs 1.20 ± 0.97, P = 0.02), and higher index admission INR (1.80 ± 0.64 vs 1.63 ± 0.50, P = 0.05) than those not readmitted.
| Readmission status | n | % |
| No | 159 | 66.25 |
| Yes | 81 | 33.75 |
| Number of readmissions within 30 d | ||
| 0 | 159 | 66.25 |
| 1 | 63 | 26.25 |
| 2 | 14 | 5.83 |
| 3 | 3 | 1.25 |
| 5 | 1 | 0.42 |
| Location of 1st readmission | ||
| OSUWMC | 59 | 72.84 |
| Outside hospital | 22 | 27.16 |
| Reason for 1st readmission1 | ||
| Hepatic encephalopathy | 26 | 32.10 |
| Volume overload | 22 | 27.16 |
| Acute kidney injury | 16 | 19.75 |
| Variceal bleed | 12 | 14.82 |
| Hyponatremia | 10 | 12.35 |
| Lower GI bleed | 4 | 4.94 |
| Spontaneous Bacterial Peritonitis | 3 | 3.70 |
| LOS of first readmission (n = 81, mean ± SD), median = 5, range = 1 to 69 | 8.11 ± 8.98 | |
| LOS of all readmissions (n = 105, mean ± SD), median = 4, range = 0 to 124 | 9.03 ± 14.42 | |
| Cost of first readmission (n = 45, mean ± SD), median= $31848.95, range $765-325656.38 | $55548.29 ± 65164.91 | |
| Waiting time for first readmission (n = 81, mean ± SD), median = 12, range = 1-30 | 12.65 ± 7.55 |
Demographics including age, race, ethnicity, income, and insurance were comparable in two groups, as well as etiology of cirrhosis, MELD-Na score, CTP score, status of evaluation for liver transplant. There were majority females in the INT arm (60/120, 50% vs 45/120, 32.50%) and males in SOC arm (75/120, 62.50% vs 60/120, 50%, P = 0.03, Table 3). Index admission characteristics, disposition and index admission were also comparative in two arms (Tables 4 and 5).
| Intervention (n = 120) | Standard of care (n = 120) | P value | |
| Patient demographics | |||
| Age (mean ± SD) | 56.54 ± 11.21 | 56.14 ± 11.21 | 0.78 |
| Age group | |||
| 65+ | 32, 26.67 | 28, 23.33 | 0.79 |
| 40-64 | 75, 62.50 | 80, 66.67 | |
| 18-39 | 13, 10.83 | 12, 10.00 | |
| Gender | |||
| Male | 60, 50.00 | 75, 62.50 | 0.03 |
| Female | 60, 50.00 | 45, 32.50 | |
| Race | |||
| White | 105, 87.50 | 97, 80.83 | 0.22 |
| Other | 15, 12.50 | 23, 19.17 | |
| Ethnicity | |||
| Not Hispanic or latino | 113, 94.17 | 114, 95.00 | 0.81 |
| Hispanic or latino | 3, 2.50 | 1, 0.83 | |
| Unknown / Not reported | 4, 3.33 | 5, 4.17 | |
| Zip code income (mean ± SD) | $68045 ± $21370 | $68455 ± $21651 | 0.88 |
| Employment status | |||
| Unemployed | 33, 27.50 | 30, 25.00 | 0.78 |
| Disabled | 24, 20.00 | 24, 20.00 | |
| Retired | 26, 21.67 | 30, 20.00 | |
| Employed, part time | 5, 4.17 | 3, 2.50 | |
| Employed, full time | 23, 19.17 | 28, 23.33 | |
| Other / Unknown | 9, 7.50 | 14, 11.67 | |
| Insurance type | |||
| Self-pay | 4, 3.33 | 3, 2.50 | 0.54 |
| No Charge / Other / Unknown | 7, 5.83 | 7, 5.83 | |
| Private insurance | 38, 31.67 | 35, 29.17 | |
| Medicare | 32, 26.67 | 44, 36.67 | |
| Medicaid | 39, 32.50 | 31, 25.83 | |
| Number of admissions at OSU for DC in last 1 year (mean ± SD) | 1.99 ± 1.61 | 1.84 ± 1.48 | 0.45 |
| MELD-Na score admit (mean ± SD) | 21.32 ± 8.19 | 22.47 ± 7.85 | 0.27 |
| MELD-Na score discharge (mean ± SD, n = 117+118) | 20.07 ± 7.74 | 20.25 ± 6.93 | 0.84 |
| CTP score admit (mean ± SD) | 9.31 ± 2.02 | 9.41 ± 1.89 | 0.69 |
| CTP score discharge (mean ± SD) | 8.44 ± 1.86 | 8.73 ± 1.89 | 0.24 |
| Etiology of cirrhosis (Index admission1) | |||
| Alcoholic | 61, 50.83 | 60, 50.00 | 1.00 |
| Non-alcoholic fatty liver | 42, 35.00 | 37, 30.83 | 0.58 |
| Viral | 21, 17.50 | 22, 18.33 | 1.00 |
| Hep B | 1, 4.76 | 3, 13.64 | 0.80 |
| Hep C | 19, 90.48 | 18, 81.82 | |
| Hep B and C | 1, 4.76 | 1, 4.55 | |
| Cryptogenic | 6, 5.00 | 7, 5.83 | 1.00 |
| Autoimmune | 1, 0.83 | 1, 0.83 | 1.00 |
| Primary sclerosing cholangitis | 2, 1.67 | 2, 1.67 | 1.00 |
| Hemochromatosis | 0, 0.0 | 3, 2.5 | 0.25 |
| Alpha 1 anti-trypsin deficiency | 3, 2.5 | 0, 0.0 | 0.25 |
| Under evaluation for liver transplant | |||
| No | 45, 37.50 | 61, 50.83 | 0.08 |
| Yes | 63, 52.50 | 53, 44.17 | |
| Unknown | 12, 10.00 | 6, 5.00 |
| Index admission characteristics | Intervention (n = 120) | Standard of care (n = 120) | P value |
| Reasons for admission1 | |||
| Acute kidney injury | 30, 25.00 | 35, 29.17 | 0.56 |
| Hyponatremia | 10, 8.33 | 6, 5.00 | 0.44 |
| Hepatic encephalopathy | 22, 18.33 | 23, 19.17 | 1 |
| Volume overload | 59, 49.17 | 52, 43.33 | 0.44 |
| Variceal bleed | 21, 17.50 | 21, 17.50 | 1 |
| Lower GI bleed | 8, 6.67 | 11, 9.17 | 0.63 |
| SBP | 9, 7.50 | 12, 10.00 | 0.65 |
| Complications of cirrhosis during admission1 | |||
| Presence of AKI | 39, 32.50 | 41, 34.17 | 0.89 |
| HE | 25, 20.83 | 24, 20.00 | 1 |
| Ascites | 70, 58.33 | 69, 57.50 | 1 |
| Variceal bleeding | 21, 17.50 | 16, 13.33 | 0.48 |
| SBP | 10, 8.33 | 6, 5.00 | 0.44 |
| HRS | 7, 5.83 | 7, 5.83 | 1 |
| Coagulopathy | 32, 26.67 | 24, 20.00 | 0.29 |
| Portal hypertension | 19, 15.83 | 27, 22.50 | 0.25 |
| HPS | 10, 8.33 | 5, 4.17 | 0.29 |
| HCC | 6, 5.00 | 5, 4.17 | 1 |
| Procedures performed during admission1 | |||
| EGD | 68, 56.67 | 68, 56.67 | 1 |
| Paracentesis | 60, 50.00 | 55, 45.83 | 0.61 |
| TIPS | 7, 5.83 | 3, 2.50 | 0.33 |
| HD | 5, 4.17 | 10, 8.33 | 0.29 |
| Colonoscopy/flex sig | 13, 10.83 | 11, 9.17 | 0.83 |
| Disposition | |||
| Home | 83, 69.17 | 76, 63.33 | 0.44 |
| Home with home health newly arranged | 17, 14.17 | 22, 18.33 | |
| Home with home health previously arranged | 2, 1.67 | 1, 0.83 | |
| SNF newly arranged | 7, 5.83 | 14, 11.67 | |
| SNF previously arranged | 6, 5.00 | 5, 4.17 | |
| Left against medical advice | 1, 0.83 | 1, 0.83 | |
| Transfer (Long term acute care hospital) | 3, 2.50 | 0, 0.00 | |
| Homeless | 1, 0.83 | 1, 0.83 |
| Intervention (n = 120) | Standard of care (n = 120) | P value | |
| Index admission labs (mean ± SD) | |||
| Sodium | 132.59 ± 5.58 | 132.28 ± 6.28 | 0.68 |
| Serum creatinine (mg/dL) | 1.42 ± 1.11 | 1.64 ± 1.47 | 0.19 |
| Total bilirubin (mg/dL) | 5.90 ± 9.10 | 6.19 ± 7.80 | 0.79 |
| Albumin (g/dL) | 2.83 ± 0.59 | 2.85 ± 0.55 | 0.72 |
| INR | 1.68 ± 0.52 | 1.70 ± 0.59 | 0.80 |
| Hemoglobin (g/dL) | 10.22 ± 2.34 | 10.02 ± 2.04 | 0.48 |
| Ascites | |||
| Absent | 35, 29.17 | 35, 29.17 | 0.44 |
| Slight | 26, 21.67 | 34, 28.33 | |
| Moderate | 59, 49.17 | 51, 42.50 | |
| Encephalopathy | |||
| None | 91, 75.83 | 96, 80.00 | 0.78 |
| Grade 1-2 | 22, 18.33 | 18, 15.00 | |
| Grade 3-4 | 7, 5.83 | 6, 5.00 | |
| Dialysis at least twice in last week | |||
| No | 117, 97.50 | 115, 95.83 | 0.72 |
| Yes | 3, 2.50 | 5, 4.17 | |
| Index admission discharge labs (mean ± SD) | |||
| Sodium (mmol/L) | 134.72 ± 4.14 | 134.95 ± 3.57 | 0.64 |
| Serum creatinine (mg/dL) | 1.31 ± 1.06 | 1.37 ± 1.18 | 0.69 |
| Total bilirubin (mg/dL, n = 237) | 5.50 ± 8.80 | 5.39 ± 6.96 | 0.92 |
| Albumin (g/dL, n = 237) | 2.98 ± 0.64 | 2.94 ± 0.61 | 0.65 |
| INR (n = 238) | 1.71 ± 0.49 | 1.69 ± 0.45 | 0.65 |
| Hemoglobin (g/dL) | 9.30 ± 1.69 | 9.21 ± 1.68 | 0.68 |
| Ascites | |||
| Absent | 42, 35.00 | 39, 32.50 | 0.35 |
| Slight | 56, 46.67 | 66, 55.00 | |
| Moderate | 22, 18.33 | 15, 12.50 | |
| Encephalopathy | |||
| None | 117, 97.50 | 112. 93.33 | 0.10 |
| Grade 1-2 | 2, 1.67 | 8, 6.67 | |
| Grade 3-4 | 1, 0.83 | 0, 0.00 | |
| Dialysis at least twice in last week | |||
| No | 114, 95.00 | 110, 91.67 | 0.44 |
| Yes | 6, 5.00 | 10, 8.33 |
There was no difference in the readmission rates for patients in the INT (n = 4, 35.83%) vs SOC arm (n = 38, 31.67%, P = 0.59, Table 6). Other outcomes including number of readmissions within 30 d (P = 0.65), index admission cost (P = 0.49), index admission LOS (P = 0.63), 1st readmission LOS (P = 0.58), all readmissions’ LOS (P = 0.82) and waiting time for 1st readmission (P = 0.06) were comparable in two arms.
| Intervention (n = 120) | Standard of care (n = 120) | P value | |
| Readmission | |||
| No | 77, 64.17 | 82, 68.33 | 0.59 |
| Yes | 43, 35.83 | 38, 31.67 | |
| Number of readmissions within 30 d | |||
| 0 | 77, 64.17 | 82, 68.33 | 0.65 |
| 1 | 31, 25.83 | 32, 26.67 | |
| 2 | 9, 7.50 | 5, 4.17 | |
| 3 | 2, 1.67 | 1, 0.83 | |
| 5 | 1, 0.83 | 0, 0.00 | |
| Location of 1st readmission | |||
| Our institution | 36, 83.72 | 23, 60.53 | 0.03 |
| Outside hospital | 7, 16.28 | 15, 39.47 | |
| Reason for 1st readmission1 | |||
| Acute kidney injury | 10, 23.26 | 6, 15.79 | 0.58 |
| Hyponatremia | 4, 9.30 | 6, 15.79 | 0.50 |
| Hepatic encephalopathy | 9, 20.93 | 17, 44.74 | 0.03 |
| Volume overload | 13, 30.23 | 9, 23.68 | 0.62 |
| Variceal bleed | 6, 13.95 | 6, 15.79 | 1.00 |
| Lower GI bleed | 1, 2.33 | 3, 7.89 | 0.34 |
| Spontaneous bacterial peritonitis | 2, 4.65 | 1, 2.63 | 1.00 |
| Other | 20, 46.51 | 22, 57.89 | 0.37 |
| Index admission cost (mean ± SD, n = 116 + 109) | 61581 ± 47825 | 59547 ± 46669 | 0.46 |
| Index admission LOS (mean ± SD) | 8.17 ± 5.56 | 8.08 ± 6.11 | 0.63 |
| First readmission LOS (n = 43 + 38, mean ± SD) | 7.58 ± 7.57 | 8.71 ± 10.41 | 0.58 |
| All readmissions LOS (n = 60 + 45, mean ± SD) | 9.28 ± 16.88 | 8.69 ± 10.44 | 0.82 |
| Waiting time for first readmission (n = 43 + 38, mean ± SD) | 11.16 ± 7.10 | 14.34 ± 7.77 | 0.06 |
Statistically significant differences were noticed in INT arm in location of 1st readmission (n = 36, 83.72% at OSU as compared to n = 23, 60.5% outside hospital, P = 0.03), and lesser 1st readmission with HE in the INT arm (n = 9, 20.9%) vs SOC (n = 17, 44.7%, P = 0.03). Finally, contingency analysis of readmission data showed fewer readmissions in patients who attended outpatient follow-up within 30 days of discharge from index admission (n = 17, 23.61% vs n = 55, 76.39%, P = 0.04).
At the end of our study, 47 (19.58%) patients received a liver transplant and 62 (25.83%) died; among those who died, 5 patients were post-transplant and 22 died in hospice. Due to the COVID-19 pandemic we were unable to achieve the anticipated sample size. Therefore, multivariate analysis was not performed.
This prospective randomized study investigated early readmission rates and healthcare utilization in patients with DC. Our readmission rate of 33.75% is higher than the United States national average (27%). While our nurse CM interventions did not reduce told readmissions, we found that HE was the top reason for readmission and such interventions were helpful in reducing early readmissions in patients with HE. This is an important lesson learned given increased burden of HE on hospitalizations, falls, mortality, impaired quality of life and caregiver burden[21]. In the validation of readmission using the liver-renal-risk score or “LIRER score”, Freitas et al[22] showed that HE was not only a predictor of 30 d readmission independent of MELD score, index, first-year, two-years and overall mortality, but also HE at admission had significantly higher mean LIRER scores. Furthermore HE patients on Medicare and geographically from the South or Midwest have higher in-hospital mortality[23]. Considerable research has been done to address HE readmissions. Bajaj et al[24] found that efforts to reduce medication-precipitated HE, prevent aspiration pneumonia and optimize HE medications on hospital discharge should be areas of focus to decrease HE readmissions. Tapper et al[25] demonstrated that development of a checklist for HE protocols integrated into the EMR and order entry system reduced odds of 30-d readmission for patients with HE (from 39.2% to 27.6%). Thus, our results are congruent with existing evidence that interventions should be invested in post-discharge education and communication for all patients with cirrhosis, especially with HE.
One of the components of intervention in our study was to arrange appointment of patients in the clinic within a week with their hepatologist. Patients with DC who attended their follow up appoint
One of the major limitations of our study was inability to enroll patients according to the proposed sample size due to the COVID-19 pandemic. Our study was underpowered to perform multiple regression analysis to detect differences in readmission rates in INT vs SOC arm. From March 2020 to July 2020 our recruitment process was put on hold due to hospital regulations to reduce patient and staff exposure. Despite this major limitation, we were able to enroll 80.17% (279 consented out of 348 approached) of patients in our study.
This study was also performed in the setting of a large academic medical center and a high-volume liver transplant center. While our methods and results may be applicable to the clinical practice of other such centers, the same impact may not be appreciated by smaller, community hospitals that are not liver transplant centers.
Future work in patients with DC should continue to focus on prospective intervention strategies to reduce early readmissions and educate patients and providers. To achieve desired sample size, we would suggest collaborations with various centers to identify and recruit patients with DC into a multicenter prospective cohort. Given our finding that there were fewer readmissions in patients with follow-up within 30 d, studies should evaluate the use of telehealth visits for follows up, especially in the COVID19 era, as outlined by Stotts et al[29].
In conclusion, this prospective randomized study investigated the impact of various pragmatic interventions to reduce early readmission and healthcare utilization in patients with DC. Our study was underpowered to detect statistically significant differences in readmission rates in INT vs SOC arm. We reported that readmission rate of our medical center was 33.75% and HE was the top reason for readmission. We found a reduction in early readmission in patients with HE in the INT arm and those who attended their follow up appointment within 30 d of discharge from index admission. We demonstrated that simple interventions in patients with DC are pragmatic and there is need for more prospective multicenter trials in this area of research.
Decompensated cirrhosis (DC) is a leading cause of morbidity and mortality in the United States often requiring multiple hospitalizations to manage. Studies show 20%-37% of patients with DC are readmitted within 30 d of index admission, which has significant burden on patients, their families and the healthcare system.
We were motivated to study and reduce readmissions as we see the physical, mental and emotional toll repeated hospitalizations for DC take on our patients and their families.
We sought to enroll patients in a randomized trial seeing if a nurse case manager (CM) ensuring early outpatient follow up, medication compliance and outpatient paracentesis if needed reduced readmissions in patients with DC.
We sought to enroll patients in a randomized trial seeing if a nurse CM ensuring early outpatient follow up, medication compliance and outpatient paracentesis if needed reduced readmissions in patients with DC.
While our calculated sample size was not achieved due to the coronavirus disease 2019 pandemic, we found a 33.75% 30 d readmission rate in our patients admitted with DC. There was no difference in readmission between intervention and standard of care arms. Most patients were re-admitted with hepatic encephalopathy. There was a lower 30 d readmission rate in patients with hepatic encephalopathy in the intervention arm and those who attended early outpatient follow up.
Our 30 d readmission was higher than the national rate. Further efforts should explore the positive impact of a nurse CM and early outpatient follow up in reducing readmissions for patients with cirrhosis, especially with hepatic encephalopathy.
Further development of strategies to predict and reduce readmissions for patients with DC should be done.
This research was supported by the Clinical Research Center/Center for Clinical Research Management of The Ohio State University Wexner Medical Center and The Ohio State University College of Medicine in Columbus, Ohio. The project was entitled “GASTR29: Prospective validation of readmission risk score and interventions to prevent readmission in patients with decompensated cirrhosis (CCTS ID#: 6018)”. This project was funded by the Ohio State University Self Insurance Program and supported by NIH Award Number UL1TROO2733 from the National Center for Advancing Translational Science. We also give a special thanks to our nurse case managers from The Ohio State University Wexner Medical Center Clinical Research Center for their work in the weekly patient calls: Holly Bookless, RN, Elizabeth Cassandra, RN and Dina McGowan, RN.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M, India; Zeng QL, China S-Editor: Liu XF L-Editor: A P-Editor: Yuan YY
| 1. | Blackwell DL VM. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2016. Hyattsville, United States of America: National Center for Health Statistics, Centers for Disease Control and Prevention, 2018. |
| 2. | Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1-75. [PubMed] |
| 3. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1074] [Article Influence: 179.0] [Reference Citation Analysis (1)] |
| 4. | Talwalkar JA. Prophylaxis with beta blockers as a performance measure of quality health care in cirrhosis. Gastroenterology. 2006;130:1005-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Berman K, Tandra S, Forssell K, Vuppalanchi R, Burton JR Jr, Nguyen J, Mullis D, Kwo P, Chalasani N. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Agrawal K, Kumar P, Markert R, Agrawal S. Risk Factors for 30-Day Readmissions of Individuals with Decompensated Cirrhosis. South Med J. 2015;108:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas H, Thacker LR, O'Leary JG; North American Consortium for the Study of End-Stage Liver Disease. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol. 2016;14:1181-1188.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Singal AG, Rahimi RS, Clark C, Ma Y, Cuthbert JA, Rockey DC, Amarasingham R. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11:1335-1341.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Orman ES, Ghabril M, Emmett TW, Chalasani N. Hospital Readmissions in Patients with Cirrhosis: A Systematic Review. J Hosp Med. 2018;13:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Morales BP, Planas R, Bartoli R, Morillas RM, Sala M, Cabré E, Casas I, Masnou H. Early hospital readmission in decompensated cirrhosis: Incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017;49:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Mumtaz K, Issak A, Porter K, Kelly S, Hanje J, Michaels AJ, Conteh LF, El-Hinnawi A, Black SM, Abougergi MS. Validation of Risk Score in Predicting Early Readmissions in Decompensated Cirrhotic Patients: A Model Based on the Administrative Database. Hepatology. 2019;70:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Sobotka LA, Modi RM, Vijayaraman A, Hanje AJ, Michaels AJ, Conteh LF, Hinton A, El-Hinnawi A, Mumtaz K. Paracentesis in cirrhotics is associated with increased risk of 30-day readmission. World J Hepatol. 2018;10:425-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kruger AJ, Aberra F, Black SM, Hinton A, Hanje J, Conteh LF, Michaels AJ, Krishna SG, Mumtaz K. A validated risk model for prediction of early readmission in patients with hepatic encephalopathy. Ann Hepatol. 2019;18:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Morales BP, Planas R, Bartoli R, Morillas RM, Sala M, Casas I, Armengol C, Masnou H. HEPACONTROL. A program that reduces early readmissions, mortality at 60 days, and healthcare costs in decompensated cirrhosis. Dig Liver Dis. 2018;50:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Morando F, Maresio G, Piano S, Fasolato S, Cavallin M, Romano A, Rosi S, Gola E, Frigo AC, Stanco M, Destro C, Rupolo G, Mantoan D, Gatta A, Angeli P. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 14596] [Article Influence: 2432.7] [Reference Citation Analysis (0)] |
| 20. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36692] [Article Influence: 2293.3] [Reference Citation Analysis (0)] |
| 21. | Frenette CT, Levy C, Saab S. Hepatic Encephalopathy-Related Hospitalizations in Cirrhosis: Transition of Care and Closing the Revolving Door. Dig Dis Sci. 2022;67:1994-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 22. | Freitas M, Xavier S, Magalhães R, Magalhães J, Marinho C, Cotter J. LIRER score - a valuable tool to predict medium-long-term outcomes in hepatic cirrhosis decompensation. Scand J Gastroenterol. 2020;55:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Trieu H, Patel A, Wells C, Saab S, Lee EW. Disparities in Mortality and Health Care Utilization for 460,851 Hospitalized Patients with Cirrhosis and Hepatic Encephalopathy. Dig Dis Sci. 2021;66:2595-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Bajaj JS, O'Leary JG, Tandon P, Wong F, Kamath PS, Biggins SW, Garcia-Tsao G, Lai J, Fallon MB, Thuluvath PJ, Vargas HE, Maliakkal B, Subramanian RM, Thacker LR, Reddy KR. Targets to improve quality of care for patients with hepatic encephalopathy: data from a multi-centre cohort. Aliment Pharmacol Ther. 2019;49:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Serper M, Kaplan DE, Shults J, Reese PP, Beste LA, Taddei TH, Werner RM. Quality Measures, All-Cause Mortality, and Health Care Use in a National Cohort of Veterans With Cirrhosis. Hepatology. 2019;70:2062-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Chirapongsathorn S, Talwalkar JA, Kamath PS. Strategies to Reduce Hospital Readmissions. Semin Liver Dis. 2016;36:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Tapper EB, Volk M. Strategies to Reduce 30-Day Readmissions in Patients with Cirrhosis. Curr Gastroenterol Rep. 2017;19:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Stotts MJ, Grischkan JA, Khungar V. Improving cirrhosis care: The potential for telemedicine and mobile health technologies. World J Gastroenterol. 2019;25:3849-3856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (3)] |