Published online May 27, 2023. doi: 10.4254/wjh.v15.i5.666
Peer-review started: January 17, 2023
First decision: February 21, 2023
Revised: February 27, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 27, 2023
Processing time: 126 Days and 16.1 Hours
Celiac disease (CD) is a chronic inflammatory intestinal disorder mediated by the ingestion of gluten in genetically susceptible individuals. Liver involvement in CD has been widely described, and active screening for CD is recommended in patients with liver diseases, particularly in those with autoimmune disorders, fatty liver in the absence of metabolic syndrome, noncirrhotic intrahepatic portal hypertension, cryptogenic cirrhosis, and in the context of liver transplantation. Non-alcoholic fatty liver disease is estimated to affect approximately 25% of the world’s adult population and is the world’s leading cause of chronic liver disease. In view of both diseases’ global significance, and to their correlation, this study reviews the available literature on fatty liver and CD and verifies particularities of the clinical setting.
Core Tip: In view of fatty liver and celiac disease (CD) global significance, and to their correlation, this study reviews the available literature on fatty liver and CD and verifies particularities of the clinical setting.
- Citation: Narciso-Schiavon JL, Schiavon LL. Fatty liver and celiac disease: Why worry? World J Hepatol 2023; 15(5): 666-674
- URL: https://www.wjgnet.com/1948-5182/full/v15/i5/666.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i5.666
Celiac disease (CD) is a chronic inflammatory intestinal disorder mediated by the ingestion of gluten in genetically susceptible individuals. It is relatively common and affects 0.7%–1.4% of the global population[1]. Its diagnosis relies on a combination of serologic testing for anti-tissue transglutaminase, anti-endomysial, and/or anti-deamidated gliadin peptide antibodies, as well as typical findings of villous atrophy and intraepithelial lymphocytosis in duodenal biopsies[2]. The classical clinical manifestations of CD, related to the gastrointestinal tract, are seen in 50%-60% of all cases. Non-classical CD accounts for 40%-50%, and is characterized by systemic involvement including musculoskeletal, neurological, endocrine, kidney, heart, lung, and liver manifestations, concomitant with other autoimmune diseases and malignancies[3]. Liver involvement in CD has been widely described in case reports and case series in the past fifty years. Presently, active screening for CD is recommended in patients with liver diseases, particularly in those with autoimmune disorders, fatty liver in the absence of metabolic syndrome, noncirrhotic intrahepatic portal hypertension, cryptogenic cirrhosis, and in the context of liver transplantation[4]. Non-alcoholic fatty liver disease (NAFLD), estimated to affect approximately 25% of the world’s adult population, is the world’s leading cause of chronic liver disease[5]. Due to both diseases’ global significance, and to their correlation[6], this study aims to review the available literature on fatty liver and CD, and verify what has already been published on this subject in order to define the clinical particularities of their coexistence.
NAFLD refers to a spectrum of diseases of the liver ranging from simple steatosis (fatty infiltration of the liver) to nonalcoholic steatohepatitis (steatosis with inflammation and hepatocyte necrosis) to cirrhosis[7]. Massive hepatic steatosis complicating adult celiac has been described since the 1980s. These cases had a marked increase in aminotransferases, sometimes coursing with jaundice and transitory liver failure, presenting complete resolution of the liver condition after a gluten-free diet (GFD)[8-11]. A few authors have investigated the diagnosis of CD in patients with fatty liver by employing different screening methods (Table 1)[12-18]. The methodologies differ between the studies, both with regard to the diagnosis of fatty liver and the serological and histological diagnosis of CD. The prevalence of reactive celiac antibodies varies from 2% to 13%[12-16]. CD has been described as more prevalent in NAFLD patients with body mass index value < 27 kg/m2[16] and < 25 kg/m2[17].
| Ref. | Country | Patients | Ultrasound | Liver biopsy | AGA | tTG | EmA | Duodenal biopsy | Response with GFD |
| Grieco et al[12], 2001 | Italy | 30 | (+) | (+) | N/A | N/A | 4 | Negative | Complete |
| Nehra et al[13], 2001 | United States | 47 | N/A | (+) | N/A | N/A | 1 | N/A | N/A |
| Bardella et al[14], 2004 | Italy | 59 | N/A | (+) | N/A | 6 | 2 | 2/6 | Partial |
| Lo Iacono et al[15] , 2005 | Italy | 121 | N/A | (+) | N/A | 20 | 4 | 4/4 | Partial |
| Rahimi et al[16], 2011 | Iran | 316 | Either/or | Either/or | N/A | 8 | 7 | 7/8 | Complete |
| Bakhshipour et al[17], 2013 | Iran | 403 | (+) | N/A | N/A | 14 | N/A | 12/14 | Partial |
| Kamal et al[18], 2018 | Egypt | 613 | (+) | (-) | N/A | 160 | 68 | 181 | Partial |
A clinical picture of undiagnosed chronic diarrhea, bloating, refractory anemia, dermatitis herpetiformis, suboptimal body mass index (< 24) or nutritional deficiency (vitamin B12, vitamin D or folic acid) in patients with NAFLD are associated with high likelihood of CD[18]. Liver biochemistry and celiac antibodies become normal after a GFD[12,14,16]. When either abdominal ultrasound or liver biopsy are performed after treatment, steatosis resolution is observed[12,16]. Concerning this topic, controversy persists as to whether the presence of fatty liver is a hepatic manifestation of CD[19]. Some claim that the hepatic manifestation of CD would be a nonspecific chronic hepatitis[13], called by some authors celiac hepatitis[20]. Furthermore, it is not known whether liver disease associated with CD has the potential to progress to liver cirrhosis, although it has been reported that CD is associated not only with cirrhosis of various etiologies, but also with cryptogenic cirrhosis[21-24]. The association between metabolic cirrhosis and refractory CD has also been reported[25].
Zali et al[30] followed up on 98 patients with CD, and observed that 2% of the patients with CD fulfilled the diagnostic criteria for metabolic syndrome at diagnosis, while 29.5% of the patients met the criteria after 12 mo of GFD (P < 0.01; OR: 20). Agarwal et al[26] evaluated 44 naïve patients with CD, and observed that patients having fatty liver increased from 6 patients (14.3%) at baseline to 13 (29.5%) after one year of GFD (P = 0.002). Ciccone et al[27] evaluated the incidence of hepatic steatosis at diagnosis and during follow-up of 185 patients with CD. Hepatic steatosis was found by ultrasound in three patients (1.6%) at CD diagnosis. At the end of the follow-up period (median = 7 years; range 1–36), the prevalence of hepatic steatosis was significantly higher than at the time of CD diagnosis (n = 20; 11.0%) (P < 0.001). A Swedish nationwide study of over 26000 patients with CD demonstrated an increased risk of NAFLD in both children and adults with CD. The relative risk was highly increased in the first year of follow-up, but remained statistically significant even 15 years after CD diagnosis[28]. Tovoli et al[29] evaluated 202 celiac patients under a GFD and evidenced the diagnosis of NAFLD in 34.7% when compared with 21.8% in 333 controls. Curiously, in normal-weight patients the higher prevalence of NAFLD was even more evident than in the controls (20% vs 5.8%, P = 0.001). On the other hand, this difference was not observed in the overweight population (67.8% vs 55.4%, P = 0.202), suggesting that traditional metabolic risk factors may mask the effects of the GFD in these patients. Due to the above stated evidence, monitoring aminotransferases levels periodically in celiac patients under GFD is recommended, especially in patients gaining weight[30].
Long-term treatment with proton pump inhibitors (PPI) is associated with excessive weight gain[31,32]. Imperatore et al[33] evaluated 301 patients with newly diagnosed CD, where 4.3% were diagnosed with metabolic syndrome and 25.9% presented with hepatic steatosis at the time of CD diagnosis; 32.8% had long-term exposure to PPI during the study period. After one year, 23.9% of the patients had developed metabolic syndrome and 37.2% had developed hepatic steatosis. Upon multivariate analysis, HOMA-IR (OR: 9.7; P = 0.001) and PPI exposure (OR: 9.2; P = 0.001) were the only factors associated with the occurrence of hepatic steatosis in celiac patients.
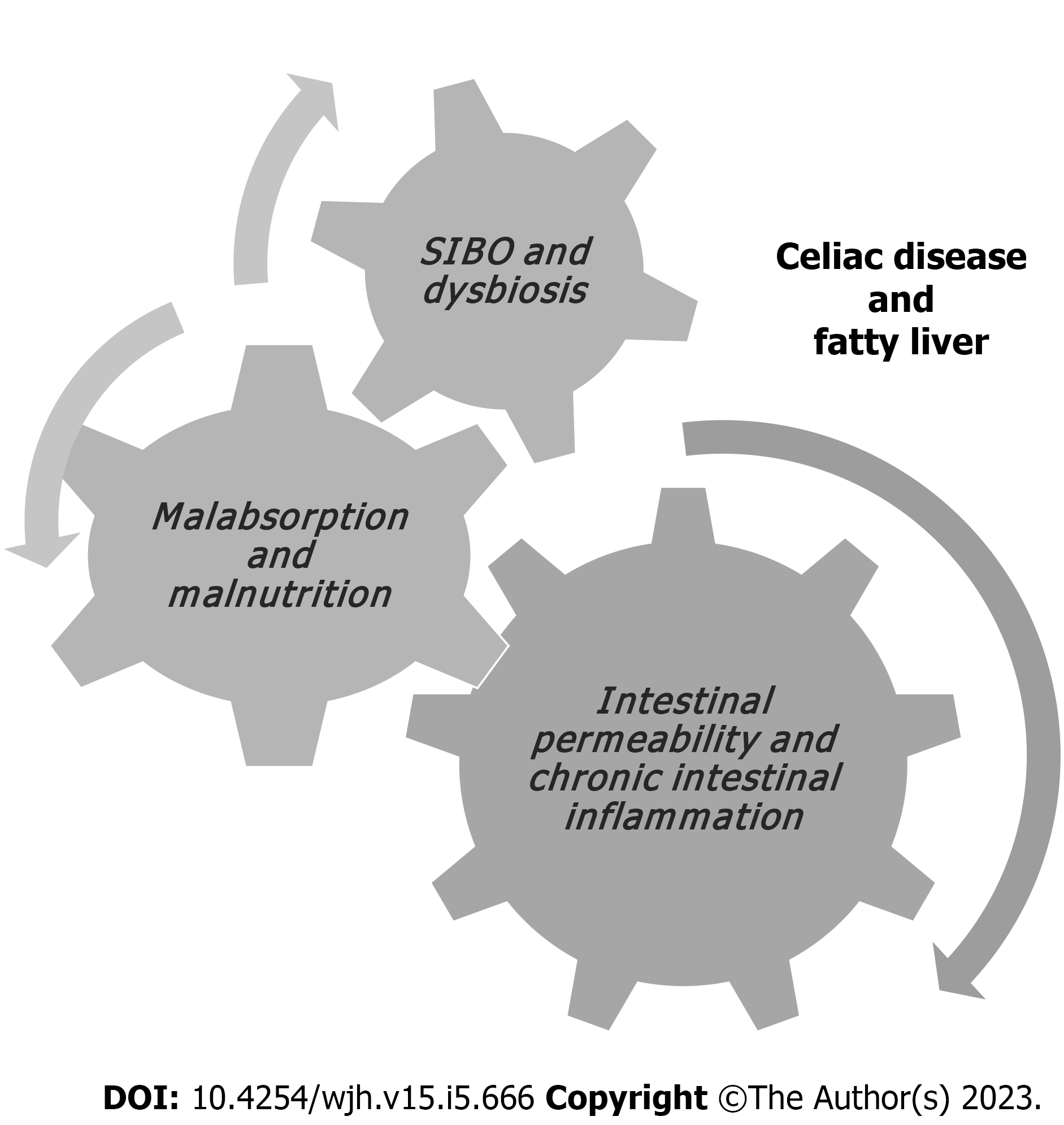
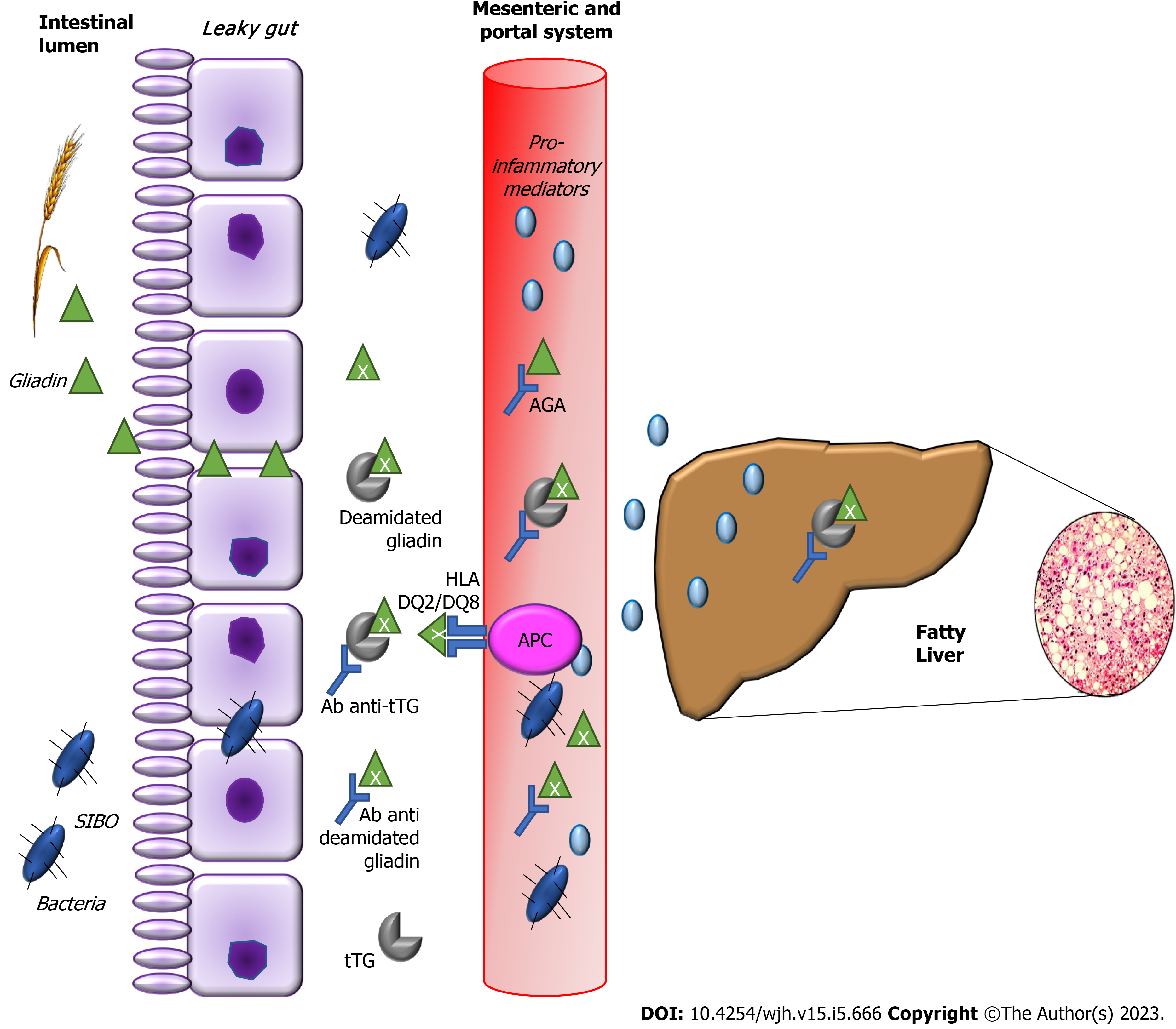
The mechanisms leading both CD and GFD to the metabolic alterations such as the increase in body weight and body mass index, blood triglyceride and cholesterol levels and blood glucose levels, as well as the development of NAFLD remain to be clarified[34]. In non-celiac patients, insulin resistance leads to fat accumulation resulting in steatosis and oxidative stress, determines lipid peroxidation and increases cytokine production, that results in inflammation and necrosis[35]. In celiac patients, malabsorption and long-standing malnutrition, increased intestinal permeability, chronic intestinal inflammation, small intestinal bacterial overgrowth and/or dysbiosis have been suggested to have possible roles in establishing celiac hepatitis in CD (Figures 1 and 2)[36,37].
At diagnosis, CD patients have lower body mass index than the general population due to malabsorption[38,39]. Kwashiorkor and dietary protein deficiency may occur associated with fatty liver on liver biopsy[40]. In patients with significantly reduced intestinal absortive surface, the ability to assimilate dietary protein may be severely reduced; intestinal malabsorption per se has been associated with hepatic steatosis after jejunoileal bypass in patients with morbid obesity[41,42] and also in patients with inflammatory bowel disease, specially after extensive intestinal resections[43]. It has been hypothesized that malabsorption in CD might lead to chronic deficiency of a lipotropic factor, and that fatty liver may occur with an associated pyridoxine deficiency[44]. In addition to malnutrition itself, qualitative and quantitative changes in the intestinal microflora occur in protein-energy malnutrition[45], however the subject of dysbiosis will be addressed below. Weight changes are common in patients suffering from CD after commencing a GFD[46,47], and the GFD dietary behavior of CD patients correlates with NAFLD[48]. A possible explanation would be the ingestion of gluten substitute products paired with the hyperphagic compensatory status that usually follows malabsorption inducing weight gain[29]. There is evidence that GFD can determine a higher intake of simple sugars, proteins and saturated fat and a lower intake of complex carbohydrates and fibers[49,50]. These changes can contribute to the development of hypertension, dyslipidemia, diabetes mellitus, metabolic syndrome and hepatic steatosis[51].
Intestinal permeability is increased in CD[52] and may favor the absorption of antigens by the intestine, via the portal circulation[53]. It is known that intestinal permeability is increased in NAFLD, as is bacterial overgrowth in the small intestine, and that these factors are associated with the severity of hepatic steatosis. The increase in intestinal permeability appears to be the key in the contribution of the gut-liver axis to the development of NAFLD[54]. The term gut–liver axis refers to a close anatomical, metabolic, and immunologic link between the gut and liver. The liver and intestine are tightly connected via the mesenteric and portal system, which supplies the liver not only with nutrients but also with gut derived food, bacterial antigens, and bacterial metabolic products. The liver portal circulation, derived from the mesenteric vessels, are the afferent part of the gut–liver axis[55]. Patients with concomitant NAFLD and CD present advanced intestinal inflammation and villous atrophy and higher levels of proinflammatory cytokines than those with CD alone, which suggests advanced intestinal injury when both diseases are present in one individual[18]. Moreover, the intestines and the liver are characterized by shared lymphocyte homing and recruitment pathways. Gut-derived T-lymphocytes may also contribute to hepato-biliary inflammation[56]. Additionally, patients with concomitant NAFLD and CD reveal higher levels of hepatic steatosis, liver stiffness, hepatic fibrosis progression rates and profibrotic mediators compared with those with either NAFLD or CD alone[18].
The diagnostic standard for small intestinal bacterial overgrowth (SIBO) is the detection of > 105 colony forming units of bacteria per ml of jejunal fluid. The difficulty in collecting jejunal fluid has led to the development of new diagnostic methods, one of them being the hydrogen breath test[57]. Wigg et al[58] observed that small intestinal bacterial overgrowth was present in 50% of patients with NAFLD and 22% of control subjects (P = 0.048), while intestinal permeability and serum endotoxin levels were similar in the two groups. For the CD patients, gliadin may impair the balance between intestinal microflora and the human body. Through digestive process, large quantity of undegraded gliadin reaches the intestines, delivers abundant substrates for different bacteria, contributes the reproduction of gliadin-degrading bacteria and breaks the steady state of intestinal microbiota[59]. Rubio-Tapia et al[60] observed a prevalence of SIBO of 9.3% diagnosed by quantitative culture of intestinal aspirate in CD patients. The association between SIBO and CD occurs mainly in patients who are newly diagnosed and beginning a GFD, and specially in those with nonresponsive CD[61,62].
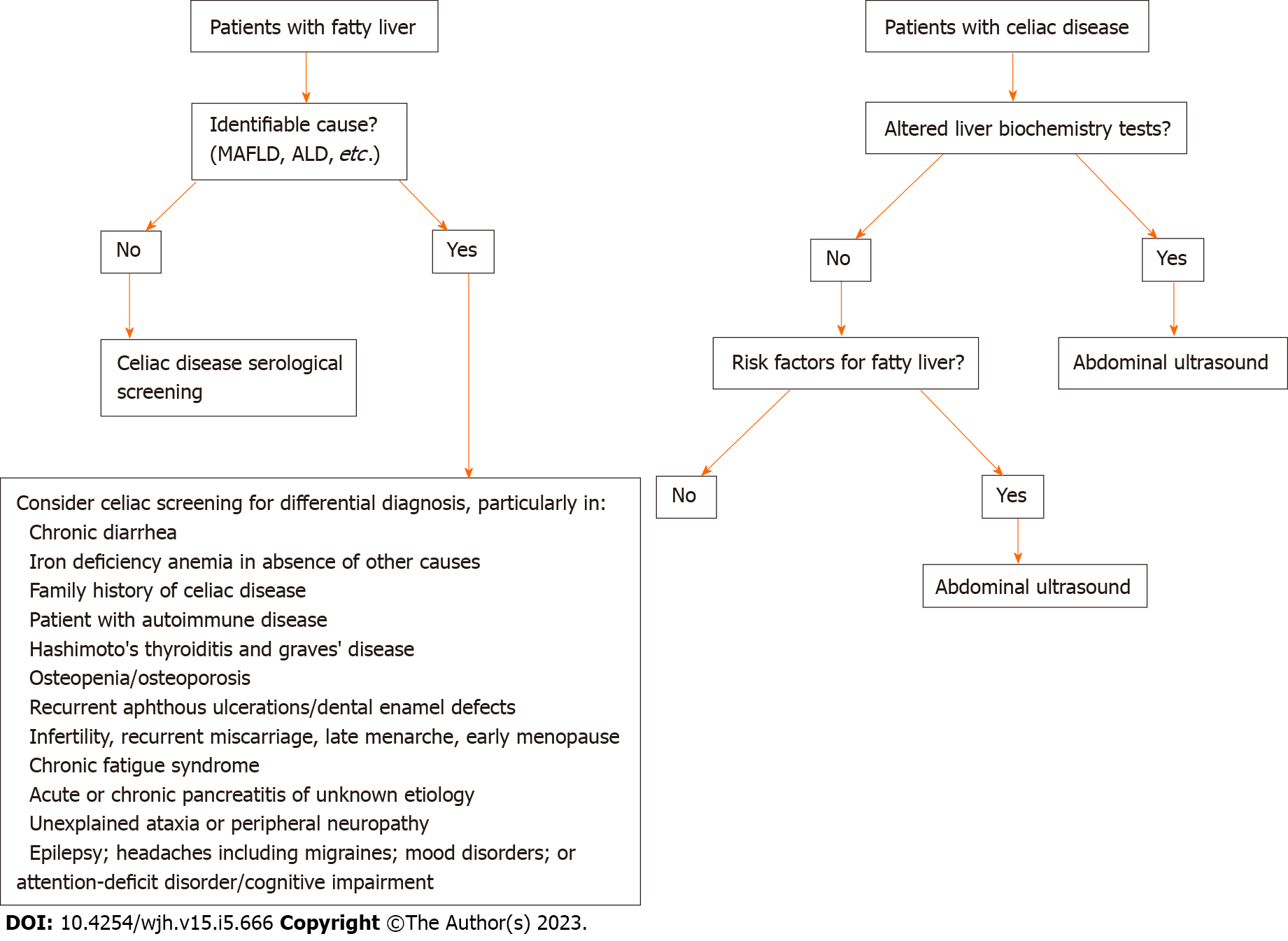
Screening all patients with NAFLD for CD is controversial. Nonetheless, clinical suspicion may arise from the presence of classical malabsorption symptoms or low body mass index, leading to active screening of celiac antibodies and early diagnosis. The GFD may improve liver tests and liver steatosis in patients with NAFLD and CD, but it remains controversial whether this effect is independent of nutritional factors. Given the biological complexity and clinical heterogeneity of NAFLD and its comorbidities, the identification of the precise drivers of such disease would aid the development of targeted therapeutics[35]. International NAFLD guidelines[63,64] recommend the investigation of other diseases that may occur with liver steatosis and that have a treatment different from NAFLD, even in the presence of metabolic risk factors. From our point of view, CD represents a disease for which diagnosis requires targeted treatment to benefit the patient. This approach not only reduces the risk of developing more severe celiac-related liver injuries, but from a systemic point of view it is known that a GFD can prevent celiac complications such as intestinal malignancies and several autoimmune diseases. Screening for CD is justified in subjects with and without known risk factors for NAFLD. Priority groups include, individuals with chronic diarrhea, iron deficiency anaemia in absence of other causes, family history of CD, patients with autoimmune disease, Hashimoto's thyroiditis and Graves' disease, osteopenia or osteoporosis, recurrent aphthous ulcerations/dental enamel defects, infertility, recurrent miscarriage, late menarche, early menopause, chronic fatigue syndrome, acute or chronic pancreatitis after excluding other known causes and neurological symptoms such as unexplained ataxia or peripheral neuropathy, epilepsy, headaches including migraines, mood disorders, or attention-deficit disorder/cognitive impairment[65]. On the other hand, it is not yet well defined whether it is necessary to investigate fatty liver in all patients with CD, as liver changes may be resolved with GFD. However, it is imperative to investigate fatty liver when liver biochemical tests persist elevated despite GFD. Figure 3 demonstrates an algorithm proposal for CD screening in patients with fatty liver and also for fatty liver screening among celiac patients.
CD and NAFLD are a common association and prompt recognition of both diseases is crucial for adequacy of treatment and to improve care. Although a direct cause-effect relationship can be clearly observed in some patients with CD that develop NAFLD as a result of malabsorption; a subtler mechanism, in which CD acts more as a cofactor capable of changing the natural history of NAFLD, has recently been suggested. Therefore, screening for CD should be strongly considered in these patients, although there are no data that exactly define the priority groups. Future investigations focusing on the pathophysiological mechanisms, particularly on the role of changes in the microbiota and intestinal permeability, may help in understanding the interference of one disease on the other. In addition, longitudinal studies evaluating the progression of these patients, particularly the impact of the GFD on NAFLD outcomes, are essential to support the clinical decision-making process.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Asociación Latinoamericana para El Estudio del Hígado; Federação Brasileira De Gastroenterologia; Sociedade Brasileira de Hepatologia; Associação Catarinense para o Estudo do
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotlyarov S, Russia; Sahin Y, Turkey S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Dieckman T, Koning F, Bouma G. Celiac disease: New therapies on the horizon. Curr Opin Pharmacol. 2022;66:102268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Green PHR, Paski S, Ko CW, Rubio-Tapia A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology. 2022;163:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Singh P, Singh AD, Ahuja V, Makharia GK. Who to screen and how to screen for celiac disease. World J Gastroenterol. 2022;28:4493-4507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 4. | Narciso-Schiavon JL, Schiavon LL. To screen or not to screen? World J Gastroenterol. 2017;23:776-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, Ekstedt M, Esmat G, George J, Marchesini G, Novak K, Ocama P, Ratziu V, Razavi H, Romero-Gómez M, Silva M, Spearman CW, Tacke F, Tsochatzis EA, Yilmaz Y, Younossi ZM, Wong VW, Zelber-Sagi S, Cortez-Pinto H, Anstee QM; NAFLD policy review collaborators. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J Hepatol. 2022;76:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 6. | Abenavoli L, Luigiano C, Larussa T, Milic N, De Lorenzo A, Stelitano L, Morace C, Consolo P, Miraglia S, Fagoonee S, Virgilio C, Luzza F, Pellicano R. Liver steatosis in celiac disease: the open door. Minerva Gastroenterol Dietol. 2013;59:89-95. [PubMed] |
| 7. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 8. | Naschitz JE, Yeshurun D, Zuckerman E, Arad E, Boss JH. Massive hepatic steatosis complicating adult celiac disease: report of a case and review of the literature. Am J Gastroenterol. 1987;82:1186-1189. [PubMed] |
| 9. | Lynch DA, Thornton JR, Axon AT. Acute fatty liver complicating coeliac disease. Eur J Gastroenterol Hepatol. 1994;6:745-747. [DOI] [Full Text] |
| 10. | Cassagnou M, Boruchowicz A, Guillemot F, Gheyssens Y, Devisme L, Cortot A, Colombel JF. Hepatic steatosis revealing celiac disease: a case complicated by transitory liver failure. Am J Gastroenterol. 1996;91:1291-1292. [PubMed] |
| 11. | Zippelius A, Diebold J. Images in hepatology. Steatosis hepatis in celiac disease. J Hepatol. 1999;30:531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Grieco A, Miele L, Pignatoro G, Pompili M, Rapaccini GL, Gasbarrini G. Is coeliac disease a confounding factor in the diagnosis of NASH? Gut. 2001;49:596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Bardella MT, Valenti L, Pagliari C, Peracchi M, Farè M, Fracanzani AL, Fargion S. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Lo Iacono O, Petta S, Venezia G, Di Marco V, Tarantino G, Barbaria F, Mineo C, De Lisi S, Almasio PL, Craxì A. Anti-tissue transglutaminase antibodies in patients with abnormal liver tests: is it always coeliac disease? Am J Gastroenterol. 2005;100:2472-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Rahimi AR, Daryani NE, Ghofrani H, Taher M, Pashaei MR, Abdollahzade S, Kalani M, Ajdarkosh H. The prevalence of celiac disease among patients with non-alcoholic fatty liver disease in Iran. Turk J Gastroenterol. 2011;22:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Bakhshipour A, Kaykhaei MA, Moulaei N, Mashhadi MA. Prevalence of coeliac disease in patients with non-alcoholic fatty liver disease. Arab J Gastroenterol. 2013;14:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kamal S, Aldossari KK, Ghoraba D, Abdelhakam SM, Kamal AH, Bedewi M, Nabegh L, Bahnasy K, Hafez T. Clinicopathological and immunological characteristics and outcome of concomitant coeliac disease and non-alcoholic fatty liver disease in adults: a large prospective longitudinal study. BMJ Open Gastroenterol. 2018;5:e000150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Marignani M, Angeletti S, Ruggeri M, Cassetta S, Delle Fave G. Coeliac disease and non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:781; author reply 781-781; author reply 782. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Rubio-Tapia A, Murray JA. The Liver and Celiac Disease. Clin Liver Dis. 2019;23:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Wakim-Fleming J, Pagadala MR, McCullough AJ, Lopez R, Bennett AE, Barnes DS, Carey WD. Prevalence of celiac disease in cirrhosis and outcome of cirrhosis on a gluten free diet: a prospective study. J Hepatol. 2014;61:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Demir H, Yüce A, Caglar M, Kale G, Kocak N, Ozen H, Gürakan F, Saltik-Temizel IN. Cirrhosis in children with celiac disease. J Clin Gastroenterol. 2005;39:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Duman AE, Oğütmen Koç D, Korkmaz U, Tohumcu A, Celebi A, Sentürk O, Hülagü S, Erçin C. Cirrhosis and intestinal B-cell lymphoma: two entities that are rarely associated with celiac disease. Turk J Gastroenterol. 2013;24:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Narciso-Schiavon JL, Schiavon LL. Celiac disease screening in patients with cryptogenic cirrhosis. World J Gastroenterol. 2023;29:410-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Hastier A, Patouraux S, Canivet CM, Lebeaupin C, Tran A, Anty R. Nonalcoholic steatohepatitis cirrhosis and type 1 refractory celiac disease: More than a fortuitous association? Clin Res Hepatol Gastroenterol. 2016;40:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Agarwal A, Singh A, Mehtab W, Gupta V, Chauhan A, Rajput MS, Singh N, Ahuja V, Makharia GK. Patients with celiac disease are at high risk of developing metabolic syndrome and fatty liver. Intest Res. 2021;19:106-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Ciccone A, Gabrieli D, Cardinale R, Di Ruscio M, Vernia F, Stefanelli G, Necozione S, Melideo D, Viscido A, Frieri G, Latella G. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion. 2019;100:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 28. | Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015;62:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Tovoli F, Negrini G, Farì R, Guidetti E, Faggiano C, Napoli L, Bolondi L, Granito A. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten-free diet: beyond traditional metabolic factors. Aliment Pharmacol Ther. 2018;48:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Zali MR, Rostami Nejad M, Rostami K, Alavian SM. Liver complications in celiac disease. Hepat Mon. 2011;11:333-341. [PubMed] |
| 31. | Syu YF, Huang HH, Chen CY. Do proton pump inhibitors contribute to weight gain? Obes Surg. 2015;25:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Yoshikawa I, Nagato M, Yamasaki M, Kume K, Otsuki M. Long-term treatment with proton pump inhibitor is associated with undesired weight gain. World J Gastroenterol. 2009;15:4794-4798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 33. | Imperatore N, Tortora R, Testa A, Gerbino N, Caporaso N, Rispo A. Proton pump inhibitors as risk factor for metabolic syndrome and hepatic steatosis in coeliac disease patients on gluten-free diet. J Gastroenterol. 2018;53:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Valvano M, Longo S, Stefanelli G, Frieri G, Viscido A, Latella G. Celiac Disease, Gluten-Free Diet, and Metabolic and Liver Disorders. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Abenavoli L, Milic N, De Lorenzo A, Luzza F. A pathogenetic link between non-alcoholic fatty liver disease and celiac disease. Endocrine. 2013;43:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Marciano F, Savoia M, Vajro P. Celiac disease-related hepatic injury: Insights into associated conditions and underlying pathomechanisms. Dig Liver Dis. 2016;48:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Sahin Y. Celiac disease in children: A review of the literature. World J Clin Pediatr. 2021;10:53-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (10)] |
| 38. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3518] [Article Influence: 390.9] [Reference Citation Analysis (0)] |
| 39. | Cheng J, Brar PS, Lee AR, Green PH. Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol. 2010;44:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Freeman HJ, Kim YS, Sleisenger MH. Protein digestion and absorption in man. Normal mechanisms and protein-energy malnutrition. Am J Med. 1979;67:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Holzbach RT. Hepatic effects of jejunoileal bypass for morbid obesity. Am J Clin Nutr. 1977;30:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Geier A, Gartung C, Theurl I, Weiss G, Lammert F, Dietrich CG, Weiskirchen R, Zoller H, Hermanns B, Matern S. Occult celiac disease prevents penetrance of hemochromatosis. World J Gastroenterol. 2005;11:3323-3326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Moxley RT 3rd, Pozefsky T, Lockwood DH. Protein nutrition and liver disease after jejunoileal bypass for morbid obesity. N Engl J Med. 1974;290:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Freeman HJ. Hepatic manifestations of celiac disease. Clin Exp Gastroenterol. 2010;3:33-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 45. | Mata LJ, Jiménez F, Cordón M, Rosales R, Prera E, Schneider RE, Viteri F. Gastrointestinal flora of children with protein--calorie malnutrition. Am J Clin Nutr. 1972;25:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, Kaukinen K. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med. 2012;23:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101:2356-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Raiteri A, Granito A, Faggiano C, Giamperoli A, Catenaro T, Negrini G, Tovoli F. Hepatic Steatosis in Patients with Celiac Disease: The Role of Packaged Gluten-Free Foods. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Mariani P, Viti MG, Montuori M, La Vecchia A, Cipolletta E, Calvani L, Bonamico M. The gluten-free diet: a nutritional risk factor for adolescents with celiac disease? J Pediatr Gastroenterol Nutr. 1998;27:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Tortora R, Capone P, De Stefano G, Imperatore N, Gerbino N, Donetto S, Monaco V, Caporaso N, Rispo A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2015;41:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 51. | West J, Logan RF, Card TR, Smith C, Hubbard R. Risk of vascular disease in adults with diagnosed coeliac disease: a population-based study. Aliment Pharmacol Ther. 2004;20:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Corazza GR, Strocchi A, Gasbarrini G. Fasting breath hydrogen in celiac disease. Gastroenterology. 1987;93:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 55. | Hoffmanová I, Sánchez D, Tučková L, Tlaskalová-Hogenová H. Celiac Disease and Liver Disorders: From Putative Pathogenesis to Clinical Implications. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 57. | Charlesworth RPG, Winter G. Small intestinal bacterial overgrowth and Celiac disease - coincidence or causation? Expert Rev Gastroenterol Hepatol. 2020;14:305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 629] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 59. | Wu X, Qian L, Liu K, Wu J, Shan Z. Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Rubio-Tapia A, Barton SH, Rosenblatt JE, Murray JA. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J Clin Gastroenterol. 2009;43:157-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Losurdo G, Marra A, Shahini E, Girardi B, Giorgio F, Amoruso A, Pisani A, Piscitelli D, Barone M, Principi M, Di Leo A, Ierardi E. Small intestinal bacterial overgrowth and celiac disease: A systematic review with pooled-data analysis. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1157] [Article Influence: 578.5] [Reference Citation Analysis (1)] |
| 64. | European Association For The Study Of The Liver. Corrigendum to 'EASL recommendations on treatment of hepatitis C: Final update of the series(') [J Hepatol 73 (2020) 1170-1218]. J Hepatol. 2023;78:452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 65. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (1)] |