Published online Mar 27, 2023. doi: 10.4254/wjh.v15.i3.419
Peer-review started: October 21, 2022
First decision: December 23, 2022
Revised: January 6, 2023
Accepted: March 1, 2023
Article in press: March 1, 2023
Published online: March 27, 2023
Processing time: 152 Days and 11.1 Hours
Non-invasive tests, such as Fibrosis-4 index and transient elastography (com
To analyze how this CDST impacted health care utilization and patient outcomes.
We performed a retrospective review of adults who had FibroScan for NAFLD indication from January 2015 to December 2017 (pre-CDST) or January 2018 to December 2020 (post-CDST). Outcomes included FibroScan result, laboratory tests, imaging studies, specialty referral, patient morbidity and mortality.
We identified 958 patients who had FibroScan, 115 before and 843 after the CDST was implemented. The percentage of FibroScans ordered by PCPs increased from 33% to 67.1%. The percentage of patients diagnosed with early F1 fibrosis, on a scale from F0 to F4, increased from 7.8% to 14.2%. Those diagnosed with ad
This CDST empowered PCPs to diagnose and manage patients with NAFLD with appropriate allocation of care towards patients with more advanced disease.
Core Tip: This was a retrospective study of nearly 1000 patients with non-alcoholic fatty liver disease who underwent FibroScan. The purpose of this study was to compare patients before and after a clinical decision support tool was implemented. This tool was designed to guide primary care providers on the management of non-alcoholic fatty liver disease. After the tool was released, we saw higher rates of early-stage fibrosis diagnosed by FibroScan. We saw appropriate allocation of care, whereby patients with advanced fibrosis had more labs, imaging studies and specialty referrals. These results suggest non-alcoholic fatty liver disease can feasibly be diagnosed and managed in the primary care setting.
- Citation: Stein L, Mittal R, Song H, Chung J, Sahota A. To scan or not to scan: Use of transient elastography in an integrated health system. World J Hepatol 2023; 15(3): 419-430
- URL: https://www.wjgnet.com/1948-5182/full/v15/i3/419.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i3.419
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease worldwide, affecting over 25% of the population[1]. In the United States alone, this translates to over 80 million individuals[2]. In its early stage, NAFLD. is reversible. However, disease progression results in irreversible fibrosis and cirrhosis and portends significant risk of hepatocellular carcinoma.
Historically, liver biopsy was the gold standard for diagnosis of NAFLD[3]. However, advancements in non-invasive testing are beginning to change the standard, with safer, cost-effective[4,5], accurate[6] and readily accessible modalities[7,8] that can be utilized in the primary care setting[9]. In the United States, the most common modality is transient elastography, often delivered by the FibroScan device (Echosens, Paris, France).
The evolution of non-invasive tests, like FibroScan, has enabled clinical pathways by which primary care physicians (PCPs) can identify patients with liver disease prior to utilization of specialty services[10,11]. Recently, professional societies[12,13] have started to embrace these diagnostic tools and clinical pathways in their recommendations. However, very few retrospective[14] and prospective[15,16] studies have assessed the effectiveness of these pathways in clinical practice. To date, no singular study has assessed management, appropriateness of care and patient outcomes for NAFLD patients who have undergone FibroScan.
In 2018, a clinical decision support tool (CDST) for NAFLD was implemented in Kaiser Permanente Los Angeles Medical Center (KPLAMC), a tertiary care center in Southern California. The goals of this CDST were to: (1) Educate and guide PCPs in identifying patients with NAFLD; (2) Risk-stratify patients via non-invasive tests; and (3) Triage patients based on risk, whereby lower risk patients were educated about lifestyle modification and higher risk patients were offered specialty referral for advanced care. We sought to determine the impact of this CDST on health care utilization, practice patterns and patient outcomes.
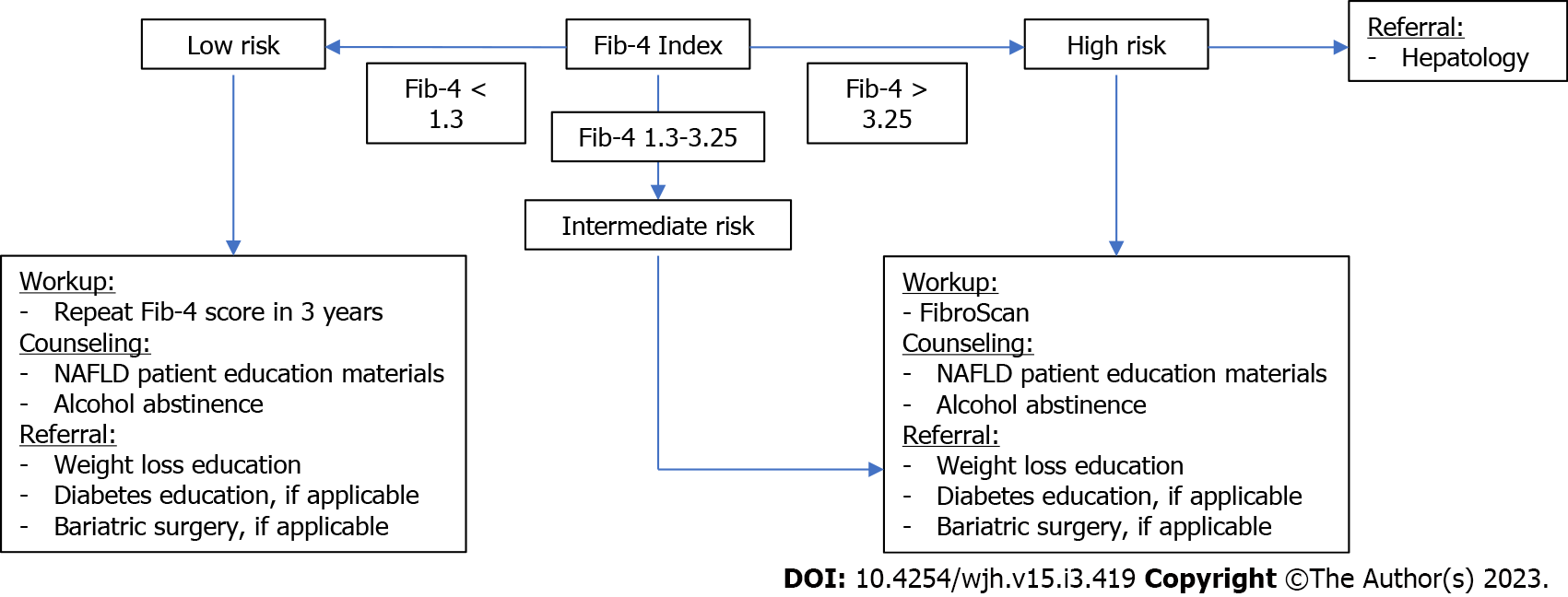
This study was centered around a CDST, part of a user-facing app, called Aura, on the electronic health record (EHR). Aura-based CDSTs populate patient clinical data to allow clinicians to calculate scores and receive recommendations. This CDST was based on the Fib-4 index, a validated calculator to predict liver fibrosis and cirrhosis[17]. If the score was below 1.3, the recommendation included lifestyle counseling and repeating the score in 3 years. If the score met a threshold of 1.3, a FibroScan was recommended. If the score was above 3.25, FibroScan and specialty referral to gastroenterology and hepatology was recommended (Figure 1).
The primary population included persons ≥ 18 years who underwent FibroScan for NAFLD indication at KPLAMC from January 1, 2015 to December 31, 2020. KPLAMC is the tertiary referral center for Kaiser Permanente Southern California (KPSC), the largest integrated health system in the state of California. KPLAMC cares for over 275000 adult members, representing about 16% of the population[18].
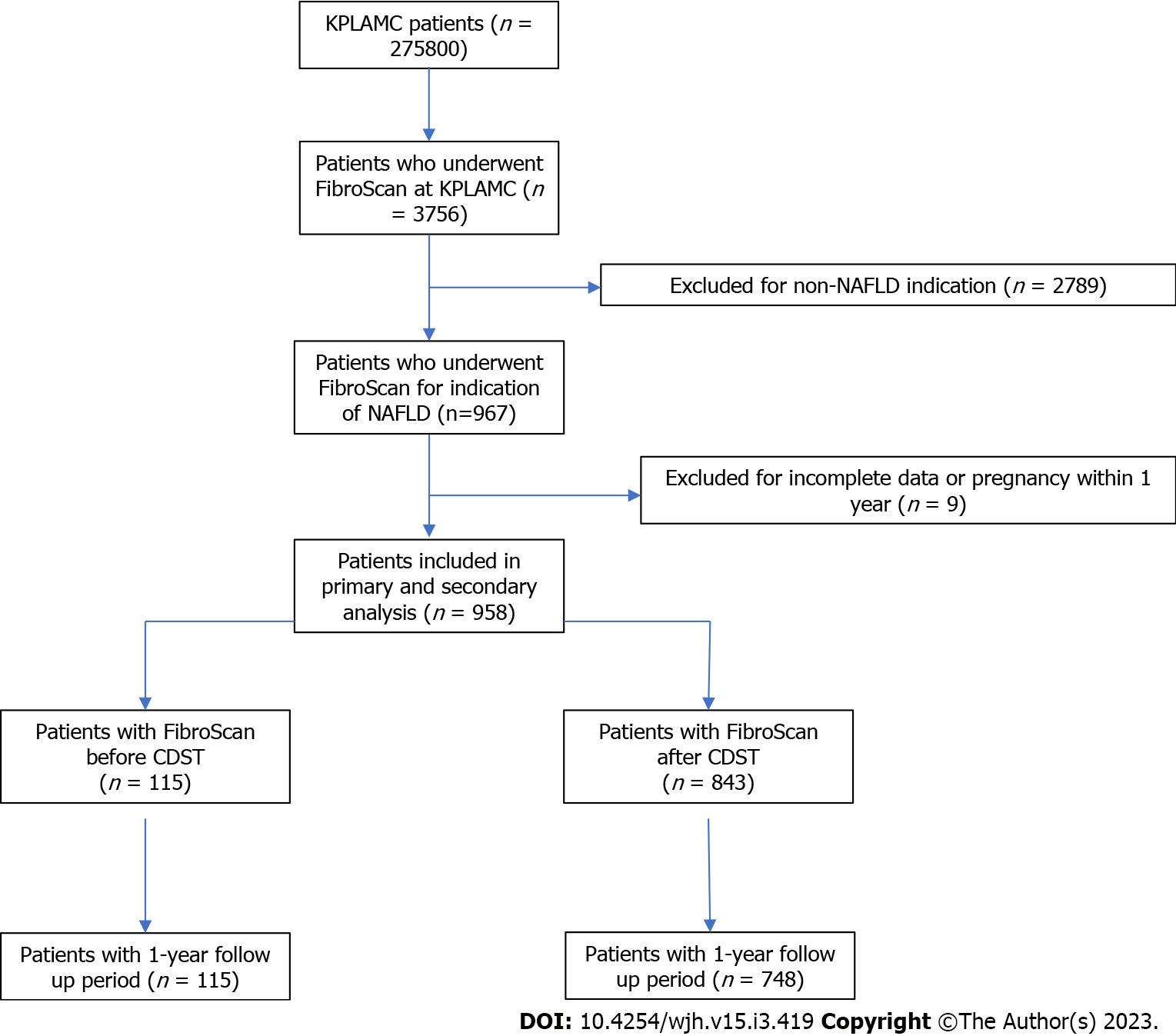
A retrospective study. The cohort was identified using an internal database of patients for whom FibroScan was performed. The population was stratified by time of FibroScan, either before (pre-CDST) or after (post-CDST) introduction of the CDST to clinical workflow. Patients were excluded from analysis if pregnant within 1 year of FibroScan. Patients with incomplete data were also excluded (Figure 2).
Data were gathered and extracted from this cohort via the KPSC Health Connect Database using International Classification of Diseases and Current Procedural Terminology codes (Supple
The primary outcome was health care utilization - who underwent FibroScan and what was the result of the scan. Variables included age, sex, body mass index (BMI), race, insurance type and medical co-morbidities such as concomitant chronic liver diseases and risk factors for metabolic syndrome. FibroScan results included fibrosis score, steatosis score, probe type used and category of physician who ordered the scan, either primary or specialty care.
The secondary outcomes included clinical management, hospitalization rate and mortality within one year of FibroScan. Clinical management was subdivided into three categories - laboratory tests, imaging studies, biopsy and specialty referral. Laboratory tests included liver function test, international normalized ratio, creatinine and complete blood count. Imaging studies included computerized tomography (CT)-4 phase liver, magnetic resonance imaging (MRI) liver, right upper quadrant ultrasound and repeat FibroScan. Specialty referral included gastroenterology, hepatology and health education, for services like diet and weight loss. Primary hospital admission diagnoses included hepatic encephalopathy, variceal bleeding, spontaneous bacterial peritonitis and liver cancer (Supp
Statistical significance was calculated by chi-square and Kruskal-Wallis for categorical and continuous variables, respectively. All P-values were determined to be significant if they were below the 0.05 threshold.
Subgroup analysis included a multivariable logistic regression to quantify the relationship between clinical management - laboratory tests, imaging studies and specialty referrals - and fibrosis score. The multicollinearity and variance inflation factor were checked and determined to be negligible. For multivariate logistic regression, the p-value was calculated by the Wald Test, with multicollinearity between variables checked with high correlation of 0.8, tolerance below 0.1 and variance inflation factor of above 10. All analyses were done using SAS 9.4 and SAS Enterprise Guide 7.15 (SAS Institute, Cary, NC, United States).
We identified 958 patients who underwent FibroScan from January 1, 2015 to December 31, 2020. Of these, 115 patients had FibroScan from January 1, 2015 to December 31, 2017 (pre-CDST) and 843 patients had FibroScan from January 1, 2018 to December 31, 2020 (post-CDST). Patient demographics and clinical characteristics are represented in Tables 1 and 2.
| Characteristic | Pre-clinical decision support tool (n = 115) | Post-clinical decision support tool (n = 843) | P value |
| Age, year | 58.3 ± 13.78 | 57.1 ± 14.02 | 0.3777 |
| Female (%) | 53.9 | 53.3 | 0.8956 |
| Body mass index | 31.6 ± 6.13 | 33.1 ± 7.10 | 0.0358 |
| Race (%) | 0.4486 | ||
| African American | 4.3 | 3.3 | |
| Asian | 19.1 | 17.4 | |
| Hispanic | 47.8 | 56.8 | |
| Non-Hispanic White | 25.2 | 19.9 | |
| Other, unknown | 3.5 | 2.5 | |
| Insurance plan type (%) | 0.1312 | ||
| Commercial, private pay | 64.3 | 64.7 | |
| Dual | 3.5 | 5.9 | |
| Medicaid | 6.1 | 4.5 | |
| Medicare | 24.3 | 24.7 | |
| Other, unknown | 1.7 | 0.2 | |
| Medical Comorbidities (%) | |||
| Chronic hepatitis B | 2.6 | 1.8 | 0.5415 |
| Chronic hepatitis C | 4.3 | 1.3 | 0.0172 |
| Diabetes mellitus | 45.2 | 42.4 | 0.5572 |
| Hepatocellular carcinoma | 0 | 0 | |
| Hyperlipidemia | 52.2 | 58.6 | 0.1891 |
| Liver transplant | 0 | 0.4 | 0.5212 |
| Obstructive sleep apnea | 14.7 | 13.3 | 0.6662 |
| Polycystic ovarian syndrome | 1.7 | 0.5 | 0.1076 |
| Primary biliary cholangitis | 0.9 | 0.4 | 0.4242 |
| Primary sclerosing cholangitis | 0 | 0 |
| Parameter | Pre-clinical decision support tool (n = 115) | Post-clinical decision support tool (n = 843) | P value |
| Physician ordering FibroScan (%) | < 0.0001 | ||
| Primary care | 33 | 67.1 | |
| Specialty care | 67 | 32.9 | |
| Exam probe used (%) | 0.9453 | ||
| Medium | 44.3 | 44 | |
| Extra large (XL) | 55.7 | 56 | |
| FibroScan result (%) | |||
| Fibrosis score | 0.0142 | ||
| F0 | 32.2 | 38.1 | |
| F1 | 7.8 | 14.2 | |
| F2 | 17.4 | 17.9 | |
| F3 | 13.9 | 13.3 | |
| F4 | 28.7 | 16.5 | |
| Steatosis score | < 0.0001 | ||
| S0 | 43.5 | 8.1 | |
| S1 | 4.3 | 10 | |
| S2 | 8.7 | 14.7 | |
| S3 | 43.5 | 67.3 |
In the pre-CDST cohort, mean age was 58.3 ± 13.78 years with over half (53.9%) being female. Mean BMI was 31.6 ± 6.13. The majority racial group was Hispanic (47.8%), followed by non-Hispanic White (25.2%) and Asian (19.1%). Most patients had commercial health insurance (64.3%) while many others had Medicare (24.3%). Patients carried comorbid diagnoses of diabetes mellitus (45.2%), hyperlipidemia (52.2%) and obstructive sleep apnea (14.7%). Very few patients had comorbid liver diseases. The post-CDST cohort had statistically similar data to the pre-CDST cohort with one exception. Mean BMI in the post-CDST cohort was 33.1 ± 7.1 (P = 0.0358).
In the pre-CDST cohort, 33% of FibroScans were ordered by PCPs. In the post-CDST cohort, 67.1% of FibroScans were ordered by PCPs. In both cohorts, a little over half (55.7%-56%) of probes used during FibroScan were XL.
Regarding FibroScan results, 9 patients, representing 7.8% of the pre-CDST cohort, had low grade F1 fibrosis. In the post-CDST cohort, this increased to 120 patients with F1 fibrosis, representing 14.2% (P = 0.0142). Additionally, 33 patients in the pre-CDST cohort had advanced F4 fibrosis, representing 28.7%. This decreased to 16.5%, a total of 139 patients, in the post-CDST cohort with F4 fibrosis (P = 0.0142). The percentage of patients with advanced steatosis S3 increased from 43.5% in the pre-CDST cohort to 67.3% in the post-CDST cohort (P ≤ 0.0001).
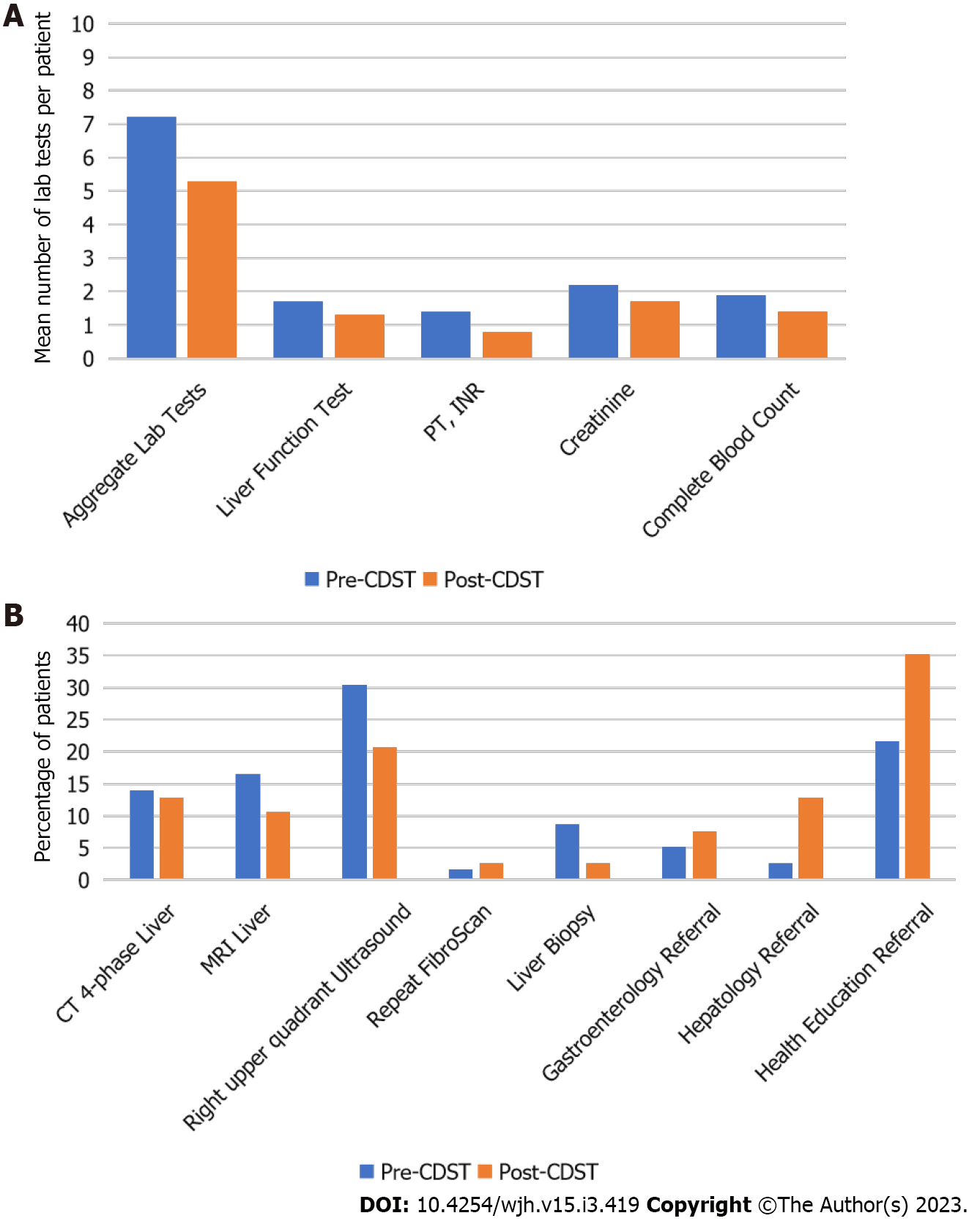
Laboratory tests: In the pre-CDST cohort, an average of 7.2 tests were performed per patient in the first year after FibroScan. This significantly decreased to 5.3 Laboratory tests in the post-CDST cohort (P < 0.0001). When subdivided by type of test, this significant decrease remained true (Figure 3A).
Imaging studies: The percentage of patients who had an MRI 4-phase liver decreased from 13.9% in the pre-CDST cohort to 12.8% in the post-CDST cohort (P = 0.7486). The percentage of patients who had an MRI liver decreased from 16.5% in the pre-CDST cohort to 10.6% in the post-CDST cohort (P = 0.0607). The percentage of patients who had a right upper quadrant ultrasound decreased significantly from 30.4% in the pre-CDST cohort to 20.7% in the post-CDST cohort (P = 0.0193). The percentage of patients who had a repeat FibroScan increased from 1.7% in the pre-CDST cohort to 2.7% in the post-CDST cohort (P = 0.5538, Figure 3B).
Biopsy: In the pre-CDST cohort, 8.7% of patients had liver biopsy within the first year. This decreased significantly to 2.7% in the post-CDST cohort (P = 0.001). The average number of months to biopsy was similar in both cohorts, 3.9 mo in the pre-CDST cohort vs 3.5 mo in the post-CDST cohort (P = 0.9822, Figure 3B).
Of those who were referred for liver biopsy, 82.8% of patients had fibrosis scores of F3 or F4 from FibroScan. Of the biopsies done, 76.9% resulted in fibrosis scores that agreed with the patient’s FibroScan result. The remaining 23.1% were discordant to the FibroScan result. In all the discordant biopsy results, Fibroscan overestimated the fibrosis score from the biopsy pathology.
Specialty referral: The percentage of patients for whom gastroenterology referral was placed increased from 5.2% in the pre-CDST cohort to 7.5% in the post-CDST cohort (P = 0.3803). The percentage of patients for whom hepatology referral was placed increased significantly from 2.6% in the pre-CDST cohort to 12.8% in the post-CDST cohort (P = 0.0014). The percentage of patients for whom health education referrals were placed increased significantly from 21.7% in the pre-CDST cohort to 35.2% in the post-CDST cohort (P = 0.0045, Figure 3B).
Morbidity: In the pre-CDST cohort, no patients were hospitalized for complications of liver disease in the first year. In the pre-CDST cohort at any time in the study time frame, 4 patients were hospitalized for hepatic encephalopathy, 1 patient was hospitalized for variceal bleeding and 1 patient was hospitalized for spontaneous bacterial peritonitis.
In the post-CDST cohort, 1 patient was hospitalized for hepatic encephalopathy and 2 patients were hospitalized for liver cancer in the first year. In the post-CDST cohort at any time in the study time frame, 4 patients were hospitalized for hepatic encephalopathy and 5 patients were hospitalized for liver cancer (Table 3).
| Variable | Pre-clinical decision support tool | Post-clinical decision support tool |
| Patients hospitalized in first year for: | ||
| Hepatic encephalopathy | 0 | 1 |
| Variceal bleeding | 0 | 0 |
| Spontaneous bacterial peritonitis | 0 | 0 |
| Liver cancer | 0 | 2 |
| Patients hospitalized at anytime for: | ||
| Hepatic encephalopathy | 4 | 4 |
| Variceal bleeding | 1 | 0 |
| Spontaneous bacterial peritonitis | 1 | 0 |
| Liver cancer | 0 | 5 |
| Patients deceased in first year | 1 | 7 |
| Patients deceased at any time | 9 | 17 |
Mortality: In the pre-CDST cohort, 1 patient died in the first year. In the pre-CDST cohort at any time, 9 patients died. In the post-CDST cohort, 7 patients died in the first year. In the post-CDST cohort at any time, 17 patients died. No patients died of complications of liver disease. Cause of death was primarily cardiovascular or complications of coronavirus disease 19 (COVID-19) (Table 3).
Multivariable analysis: The likelihood of healthcare utilization across all categories - laboratory tests, imaging studies and specialty referrals - increased with advancing fibrosis, most prominent in F4 fibrosis (Table 4). The reference group for this analysis was F0 fibrosis patients, unless otherwise specified.
| Variable | Fibrosis score | Odds ratio | 95% confidence interval | P value |
| Lab tests | F1 vs F0 | 0.955 | 0.621-1.469 | 0.8354 |
| F2 vs F0 | 1.055 | 0.711-1.566 | 0.7886 | |
| F3 vs F0 | 1.507 | 0.946-2.4 | 0.0845 | |
| F4 vs F0 | 2.477 | 1.522-3.953 | 0.0001 | |
| Imaging study | F1 vs F0 | 0.825 | 0.506-1.343 | 0.4386 |
| F2 vs F0 | 1.287 | 0.855-1.937 | 0.2259 | |
| F3 vs F0 | 4.703 | 3.064-7.218 | < 0.0001 | |
| F4 vs F0 | 7.188 | 4.793-10.78 | < 0.0001 | |
| Gastroenterology referral | F1 vs F0 | 2.362 | 0.909-6.141 | 0.0778 |
| F2 vs F0 | 1.47 | 0.549-3.939 | 0.4431 | |
| F3 vs F0 | 6.195 | 2.786-13.775 | < 0.0001 | |
| F4 vs F0 | 4.122 | 1.85-9.14 | 0.0005 | |
| Hepatology referral | F1 vs F2 | 0.181 | 0.04-0.813 | 0.0258 |
| F3 vs F2 | 4.438 | 2.253-8.739 | < 0.0001 | |
| F4 vs F2 | 4.55 | 2.385-8.681 | < 0.0001 | |
| Health education referral | F1 vs F0 | 1.415 | 0.882-2.272 | 0.1501 |
| F2 vs F0 | 1.463 | 0.957-2.236 | 0.0786 | |
| F3 vs F0 | 2.054 | 1.305-3.233 | 0.0019 | |
| F4 vs F0 | 3.589 | 2.391-5.387 | < 0.0001 |
Those with F3 fibrosis were 1.507 times as likely to have a laboratory test than those with F0 fibrosis (P = 0.0845). Those with F4 fibrosis were 2.477 times as likely to have a laboratory test than those with F0 fibrosis (P = 0.0001).
Those with F3 fibrosis were 4.703 times as likely to have an imaging study than those with F0 fibrosis (P < 0.0001). Those with F4 fibrosis were 7.188 times as likely to have an imaging study than those with F0 fibrosis (P < 0.0001).
Those with F3 fibrosis were 6.195 times as likely to have a gastroenterology referral than those with F0 fibrosis (P < 0.0001). Those with F4 fibrosis were 4.122 times as likely to have a gastroenterology referral than those with F0 fibrosis (P = 0.0005).
Due to low numbers, comparisons for hepatology referrals were made with F2 fibrosis patients rather than F0 or F1 fibrosis patients. Those with F3 fibrosis were 4.438 times as likely to have a hepatology referral than those with F2 fibrosis (P < 0.0001). Those with F4 fibrosis were 4.55 times as likely to have a hepatology referral than those with F2 fibrosis (P < 0.0001).
Those with F3 fibrosis were 2.054 times as likely to have a health education referral than those with F0 fibrosis (P = 0.0019). Those with F4 fibrosis were 3.589 times as likely to have a health education referral than those with F0 fibrosis (P < 0.0001).
This study examined the demographics, clinical management, morbidity and mortality of a cohort of patients with NAFLD who underwent FibroScan, a non-invasive test to diagnose liver fibrosis. This study was centered around a CDST designed to guide PCPs in the care of patients with NAFLD. We compared patients before and after the CDST was implemented to determine its impact on health care utilization, practice patterns and patient outcomes.
The CDST pathway, combining Fib-4 and FibroScan, was chosen in particular because of robust clinical data supporting its use in the NAFLD population. When compared head-to-head with other scoring systems, Fib-4 has a high negative predictive value[19], making it an ideal rule out test in detecting advanced fibrosis and cirrhosis[20,21]. Furthermore, FibroScan has a high positive predictive value for the measurement of liver stiffness[22], to rule in advanced fibrosis and cirrhosis, and thus risk-stratify patients. When Fib-4 and FibroScan are used in tandem, it is predicted that 87% of unnecessary further assessments may be avoided[23].
Our data revealed three important findings. First is regarding FibroScan orders. Prior to the CDST, about two-thirds of all FibroScans were ordered by specialty providers. Additionally, the overall number of scans ordered by any provider during that time was low. This indicates either poor understanding of the test’s presence or low level of confidence in the test itself. After the CDST, not only did the overall number of scans increase 7-fold, but also, the majority of scans - about two-thirds - were ordered by PCPs. This drastic shift shows that the CDST achieved its goal of educating PCPS on the utility of FibroScan and fostered a new confidence in the test, leading to higher rates of utilization.
As such, the average BMI of patients in the post-CDST cohort was statistically significantly higher than those in the pre-CDST cohort. We attribute this difference to provider education regarding risk factors for NAFLD. When the CDST was implemented, PCPs were alerted of its presence and provided educational materials in the form of EHR alerts, informational emails and formal lectures. Since obesity is a known risk factor for NAFLD, it is likely that PCPs thought to screen patients with higher BMIs.
The second important finding is regarding fibrosis score. In the pre-CDST cohort, fibrosis scores had a bi-modal distribution. About half of the patients either had no fibrosis (F0) or had advanced fibrosis (F4). Conversely, the post-CDST cohort contained almost half the number of patients with advanced fibrosis (F4) and also twice the number of patients with early fibrosis (F1). This change shows that the CDST captured patients earlier in the disease process. As we know, while early fibrosis is reversible, advanced fibrosis and cirrhosis is not. Early recognition and diagnosis are crucial.
The third important finding is regarding care utilization. In aggregate, the utilization rates of laboratory tests, imaging studies and biopsy decreased with the introduction of the CDST. In particular, there was no significant difference in gastroenterology referral for patients with early fibrosis (F0-F1). Furthermore, patients with advanced fibrosis (F3-F4) had more tests and studies done and more referrals placed. This not only represents appropriate allocation and utilization of care, but also may serve to quell providers’ worries that identification of NAFLD patients may lead to unnecessary testing, in particular endoscopies for variceal surveillance[24].
Regarding strengths and weaknesses, this study cohort is robust and diverse and can reasonably be extrapolated to the national population. To date, no singular study of a clinical pathway has assessed management, appropriateness of care and patient outcomes in the NAFLD population. Unfortunately, the study period included the COVID-19 pandemic, which is known to have resulted in decreased rates of care utilization and delivery[25].
This study is of particular importance. PCPs see more than 300 cases of NAFLD for every 1000 patient encounters[26]. The average annual cost of care per NAFLD patient with private health insurance in the United States is $7804 for a new diagnosis and $3789 for long-term management[27]. Not only is NAFLD independently associated with 17% higher annual attributable healthcare costs, but also more advanced disease, F3 and above, is associated with a 40% increase in median annual healthcare cost when compared to F2 and below[28]. The lion share of this increase in cost can be attributed to liver biopsy, imaging and hospitalizations[27].
Not only is the prevalence of NAFLD and NASH projected to increase by up to 56% in the next 10 years[29], but also high primary care workload and physician burnout[30] necessitates action and education. Early and accurate diagnosis of fibrosis in NAFLD patients, particularly those with advanced disease, is necessary to determine the patient’s prognosis and guide clinical decision making.
Workflows such as this CDST can not only help patients attain adequate, appropriate, preventative care, but also can help streamline primary care clinical practice and empower physicians beyond the liver clinic to appropriately recognize and manage high risk NAFLD. Future directions for this work include longitudinal study of this population and clinical workflow in multiple centers on a national and international scale.
Non-alcoholic fatty liver disease (NAFLD) is a growing problem, affecting over 25% of the global population. Non-invasive tests are being used more and more to risk stratify and diagnose patients with NAFLD. However, there is a paucity of data for how these tests are being used for clinical decision making in real-world practice.
We examined a clinical decision support tool (CDST) designed to guide primary care providers (PCPs) in the care of patients with NAFLD.
To evaluate health care utilization, practice patterns and patient outcomes of patients who underwent Fib
A retrospective review of 958 adult patients who underwent FibroScan. Patients were compared before and after introduction of the CDST. Univariate and multivariate logistic regression models were performed in statistical analyses.
Introduction of the CDST allowed for more patients with early fibrosis and fewer patients with advanced fibrosis to be identified. Overall, fewer labs, imaging studies and biopsies were ordered after the CDST. Providers appropriately ordered more specialty referrals for patients with more advanced fibrosis.
This CDST empowered PCPs to diagnose and manage patients with NAFLD with appropriate allocation of care towards patients with more advanced disease.
Non-alcoholic fatty liver disease can feasibly be diagnosed and managed in the primary care setting. Future research is required to streamline and refine care of this patient population.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pham TTT, Viet Nam; Taura K, Japan; Tolunay HE, Turkey S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (6)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7536] [Article Influence: 837.3] [Reference Citation Analysis (0)] |
| 3. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 4. | Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-Effectiveness Analysis: Risk Stratification of Nonalcoholic Fatty Liver Disease (NAFLD) by the Primary Care Physician Using the NAFLD Fibrosis Score. PLoS One. 2016;11:e0147237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Srivastava A, Jong S, Gola A, Gailer R, Morgan S, Sennett K, Tanwar S, Pizzo E, O'Beirne J, Tsochatzis E, Parkes J, Rosenberg W. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, Trauner M, Kersey K, Li G, Han L, Jia C, Wang L, Chen G, Subramanian GM, Myers RP, Djedjos CS, Kohli A, Bzowej N, Younes Z, Sarin S, Shiffman ML, Harrison SA, Afdhal NH, Goodman Z, Younossi ZM. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology. 2019;70:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 7. | Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol. 2015;13:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1036] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 9. | Reinson T, Byrne CD, Patel J, El-Gohary M, Moore M. Transient elastography in patients at risk of liver fibrosis in primary care: a follow-up study over 54 months. BJGP Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Rikhi R, Singh T, Modaresi Esfeh J. Work up of fatty liver by primary care physicians, review. Ann Med Surg (Lond). 2020;50:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Dokmak A, Lizaola-Mayo B, Trivedi HD. The Impact of Nonalcoholic Fatty Liver Disease in Primary Care: A Population Health Perspective. Am J Med. 2021;134:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4948] [Article Influence: 706.9] [Reference Citation Analysis (9)] |
| 13. | Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 379] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 14. | Patton H, Burchette R, Tovar S, Pio J, Shi J, Nyberg LM. Retrospective analysis of a dedicated care pathway for nonalcoholic fatty liver disease in an integrated US healthcare system demonstrates support of weight management and improved ALT. BMC Gastroenterol. 2020;20:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Grattagliano I, Ubaldi E, Napoli L, Marulli CF, Nebiacolombo C, Cottone C, Portincasa P. Utility of noninvasive methods for the characterization of nonalcoholic liver steatosis in the family practice. The "VARES" Italian multicenter study. Ann Hepatol. 2013;12:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, Suri D, Thorburn D, Sennett K, Morgan S, Tsochatzis EA, Rosenberg W. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 17. | Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, Zafarmand MH. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021;41:261-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 18. | Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 19. | Xu XL, Jiang LS, Wu CS, Pan LY, Lou ZQ, Peng CT, Dong Y, Ruan B. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J Formos Med Assoc. 2022;121:454-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 21. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 681] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 22. | Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a "FIB-4 First" Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019;3:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | de Franchis R, Krag A. Ruling out esophageal varices in NAFLD cirrhosis: Can we do without endoscopy? J Hepatol. 2018;69:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, Jones M, Kitchener E, Fox M, Johansson M, Lang E, Duggan A, Scott I, Albarqouni L. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11:e045343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 774] [Article Influence: 193.5] [Reference Citation Analysis (1)] |
| 26. | Grattagliano I, D'Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; "Steatostop Project" Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17:389-394. [PubMed] |
| 27. | Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology. 2018;68:2230-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Cotter TG, Dong L, Holmen J, Gilroy R, Krong J, Charlton M. Nonalcoholic fatty liver disease: impact on healthcare resource utilization, liver transplantation and mortality in a large, integrated healthcare system. J Gastroenterol. 2020;55:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1308] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 30. | Agarwal SD, Pabo E, Rozenblum R, Sherritt KM. Professional Dissonance and Burnout in Primary Care: A Qualitative Study. JAMA Intern Med. 2020;180:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |