Published online Mar 27, 2023. doi: 10.4254/wjh.v15.i3.410
Peer-review started: November 12, 2022
First decision: January 5, 2023
Revised: January 16, 2023
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 27, 2023
Processing time: 131 Days and 3.9 Hours
The American Association for the Study of Liver Disease recommends screening patients with cirrhosis for hepatocellular carcinoma (HCC) using imaging with or without alpha-fetoprotein every six months. Unfortunately, screening rates remain inadequate.
To assess root causes of screening failure in a subspecialty hepatology clinic.
The authors identified patients with cirrhosis seen in a subspecialty hepatology clinic and determined whether they underwent appropriate screening, defined as two cross-sectional images between five and seven months apart. The authors characterized the primary driver of screening failure. Finally, other hepatologists were surveyed to determine provider perceptions of screening failure causes.
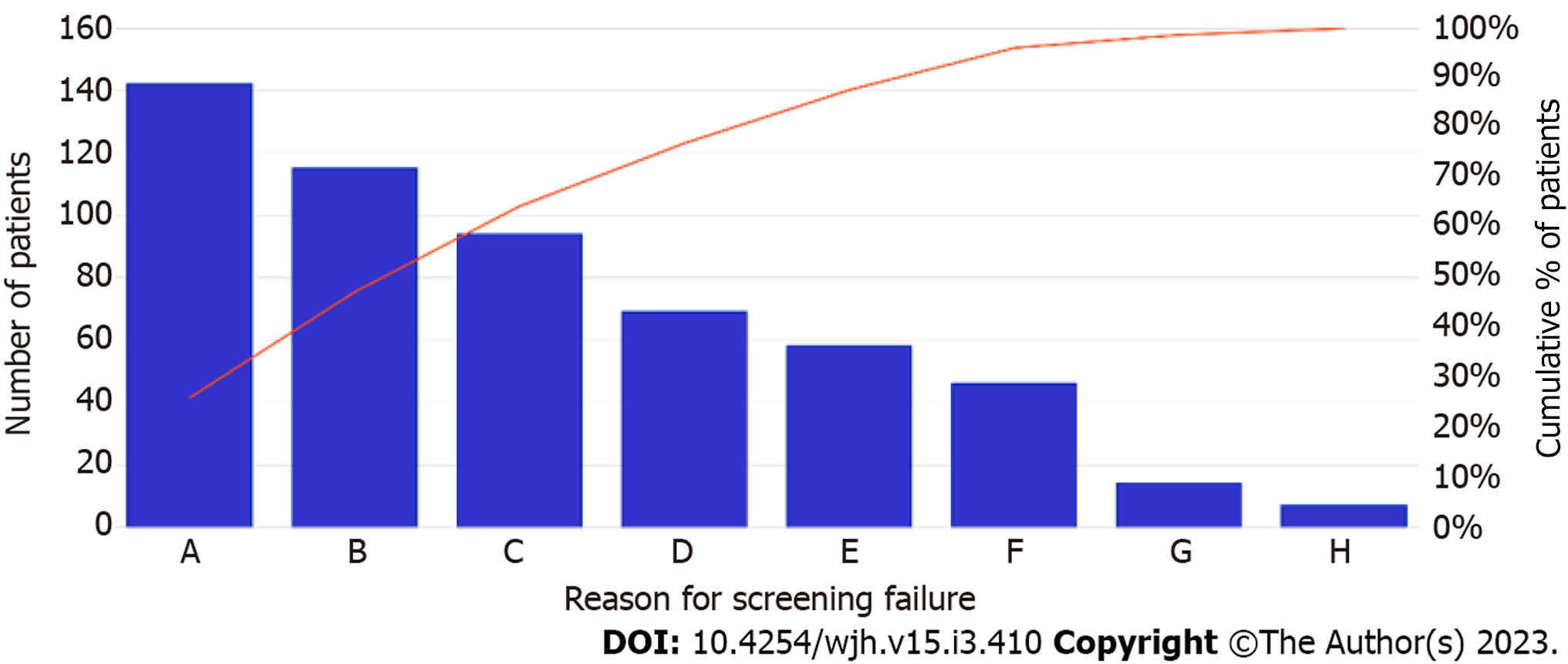
1034 patients were identified with an average age of 61 years and a mean MELD of 8.1 ± 3.8. Hepatitis C virus was the most common cirrhosis etiology. 489 (47%) underwent appropriate screening. No demographic or clinical differences were detected between those who underwent appropriate screening and those who did not. The most common etiologies of screening failure, in descending order, were: radiology unable to schedule timely imaging, provider did not order imaging, patient canceled follow up appointment, appointments scheduled too far apart, lost to follow up, no-show to radiology appointment, and provider canceled appointment. Hepatologists surveyed believed the most common cause of screening failure was no-show to radiology.
Rates of screening were poor even in a subspecialty hepatology clinic. Screening failure was mostly due to systemic factors such as radiology availability and time between hepatology appointments rather than individual error.
Core Tip: This study reinforces existing knowledge that screening rates for Hepatocellular carcinoma are woefully inadequate, even in a subspecialty hepatology clinic. Unlike previous studies, ours identifies specific failure points, showing that screening failures are driven more by systemic issues than by physician or patient error.
- Citation: King WW, Richhart R, Culpepper T, Mota M, Banerjee D, Ismael M, Chakraborty J, Ladna M, Khan W, Ruiz N, Wilson J, Altshuler E, Clark V, Cabrera R. Adherence to guideline-directed hepatocellular carcinoma screening: A single-center US experience. World J Hepatol 2023; 15(3): 410-418
- URL: https://www.wjgnet.com/1948-5182/full/v15/i3/410.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i3.410
Hepatocellular carcinoma (HCC) represents the sixth leading cause of cancer and the third leading cause of cancer death worldwide[1]. The most common and important risk factor for HCC is cirrhosis[2]. Estimates of the annual incidence of HCC among patients with cirrhosis range from 1 to 8%[3,4]. The lifetime incidence in patients with cirrhosis may be as high as 32% and is increasing in the United States[3,5-7].
The American Association for the Study of Liver Disease (AASLD) recommends screening for HCC with abdominal ultrasound, computed tomography, or magnetic resonance imaging, with or without alpha-fetoprotein, every six months[8-10]. Adherence to AASLD guidelines correlates with improved survival, as demonstrated in a French cohort study of 1671 patients at 35 centers. Patients who adhered to semi-annual screening protocols had increased lead-time adjusted survival[11]. A theoretical model by Sarasin et al[12] predicted an increase in life expectancy among patients with Child-Turcot-Pugh A cirrhosis with HCC screening if the expected incidence of HCC is at least 1.5% per year. Unfortunately, predictive algorithms to stratify patients by HCC risk have failed external clinical validation[13]. Much research now focuses on blood-based biomarkers for simple and accessible point of care screening, but these strategies are not yet ready for clinical practice[14].
Unfortunately, adherence to screening guidelines remains poor[15-17]. A 2011 retrospective cohort study of 13002 patients with cirrhosis across 128 Veterans Affairs medical centers showed that only 12% had received appropriate screening[18]. A 2012 systematic review by Singal et al[15] found the surveillance rate among all patients with cirrhosis to be only 18.4%, although it was higher (51.7% vs 16.9%) among patients followed in subspecialty gastroenterology clinics. A subsequent retrospective cohort study performed by the same group found that only 2% of patients received consistent surveillance; 33% had inconsistent surveillance, and 65% had no surveillance over 3 years[19]. A qualitative study within the Veterans Health Administration similarly found that following with a subspecialist, whether gastroenterology or infectious disease, significantly increased HCC screening rates[20]. Poor knowledge and vigilance of screening protocols among primary care providers has been well-documented[21,22]. Other factors included distance to a screening site and lead time between screening order and screening date[20]. Socioeconomic factors also contribute to screening utilization[23,24]. Primary care-based clinical reminders have also been shown to improve screening rates[25]. Singal et al[26] showed that a mailed outreach program increases HCC screening rates.
Many previous studies examined patients diagnosed with HCC to identify factors related to lack of screening[18,27,28]. Our group sought to collect data on all patients at a subspecialty hepatology clinic to retrospectively identify risk factors for screening failure among all patients with cirrhosis, not just those with HCC. We hypothesized that there may be additional factors not previously identified that contribute to screening failure.
The purpose of this study was two-fold: (1) To determine the rate of appropriate HCC screening in patients with cirrhosis in a subspecialty practice in which screening guidelines are well known; and (2) to identify barriers at an institutional and provider level as well as the patient-related factors. The data will be used to improve adherence to guideline-directed screening protocols via future quality improvement initiatives.
The electronic medical record was queried for billing codes from the 9th revision of the International Classification of Diseases (ICD-9) or ICD-10 to identify patients. Demographic, disease etiology, and laboratory data were collected. Inclusion criteria included patients with cirrhosis who were seen at least twice in the subspecialty hepatology clinic between August 2015 and August 2017. The charts were then manually reviewed to confirm that each patient was appropriate for screening based on AASLD guidelines. Exclusion criteria included prior liver transplantation and prior HCC.
Next, the authors determined whether the patients had been appropriately screened, defined as having undergone two imaging studies (abdominal ultrasonography, contrasted computed tomography, or magnetic resonance imaging) within 150 to 210 days of each other during the study period. Because the AASLD guidelines suggested an optional role for -fetoprotein, the authors did not look for -fetoprotein measurement. The charts of these patients were reviewed to determine the primary cause of screening failure. The reason for failure was categorized based on the screening barriers listed below. For patients with multifactorial screening failure, the first failed step in the screening process was counted as the primary reason for failure. For example, if a patient canceled a hepatology appointment and subsequently did not receive orders for imaging, the reason for screening failure was attributed to the clinic cancellation. The hierarchy of steps, in order, were: loss to follow-up, patient clinic appointment cancellation, physician clinic appointment cancellation, appointments more than 7 mo apart, failure to order imaging, failure to schedule imaging, or failure to present to radiology.
Finally, eight hepatologists in the clinic who were not involved in this study were anonymously surveyed on their perceptions of risk factors for screening failure.
Statistical significance was defined using α < 0.05. Continuous variables were abnormally distributed according to Shapiro-Wilk testing. Therefore, comparisons were made using the Mann-Whitney U test. Categorical variables were compared using chi-square or Fisher’s exact testing. The study protocol was reviewed and approved by our institutional IRB prior to any data collection and study procedures.
The authors identified 1276 patients who met the inclusion criteria. 242 were removed due to meeting exclusion criteria. Therefore, a total of 1034 patients were analyzed. The study population had an average age of 61 years, was 55% male, and was 83% White. Hepatitis C virus was the most common cirrhosis etiology, accounting for 51% of participants. The mean MELD score was 8.1 (SD 3.8). No statistically significant differences were detected in baseline characteristics between patients who underwent appropriate screening and those who did not (Table 1).
| Baseline patient characteristic | Met screening criteria (n = 463) | Failed screening criteria (n = 545) | P value |
| Age | 61.4 ± 10.7 | 60.2 ± 10.5 | 0.06 |
| Gender | 0.37 | ||
| Male | 261 (56) | 292 (54) | |
| Female | 202 (44) | 253 (46) | |
| Race | 0.85 | ||
| White | 385 (83) | 452 (83) | |
| African-American | 48 (10) | 55 (10) | |
| Other | 20 (4) | 30 (6) | |
| Unknown | 5 (1) | 5 (1) | |
| County of residence | 0.61 | ||
| Same county as institution | 115 (25) | 143 (26) | |
| Different county than institution | 348 (75) | 402 (74) | |
| Etiology | 0.64 | ||
| NASH | 144 (31) | 157 (29) | |
| AIH | 21 (5) | 25 (5) | |
| PBC | 34 (7) | 37 (7) | |
| PSC | 19 (4) | 13 (2) | |
| HCV | 229 (49) | 283 (52) | |
| HBV | 29 (6) | 37 (7) | |
| AALD | 44 (10) | 63 (12) | |
| MELD | 8.2 ± 3.8 | 8.1 ± 3.8 | 0.65 |
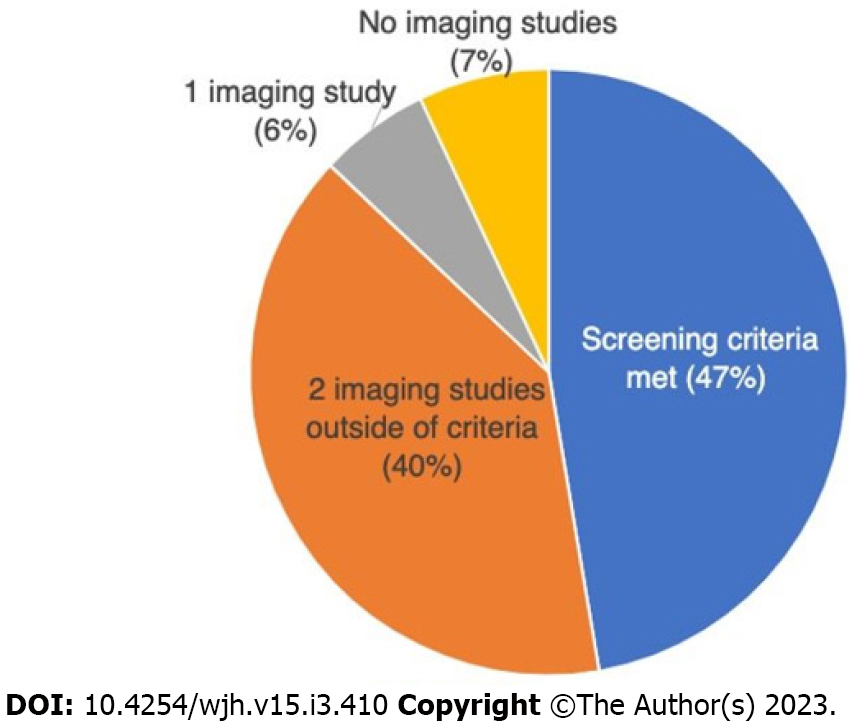
489 (47%) patients underwent appropriate screening during the study period. 410 (40%) underwent two imaging studies that were outside the time range criterion. Six percent of patients had only one imaging study, and 7% had none (Figure 1). The most common cause of HCC screening failure was delays in scheduling of imaging studies (Figure 2). Patient-centered factors, including appointment cancellations, no-shows, and loss to follow up accounted for 36% of screening failures. System failures were classified as delays in radiology and hepatology scheduling as well as physician cancellation of follow-up appointments. These accounted for 40% of screening failures. Lack of physician order accounted for 21%.
All of those who received their care exclusively within the public university medical system were referred to the radiology department within the institution. 35 patients who followed with community-based gastroenterologists and came to the institution for periodic subspecialty consultation elected to undergo HCC screening with local private radiologists.
All patients diagnosed with HCC experienced delays in screening. One was diagnosed at stage IVb and passed away due to HCC. One was lost to follow-up following discovery of a 3.1 cm nodule on an magnetic resonance imaging protocoled for liver masses. Two underwent Y-90 transarterial radioembolization and partial surgical hepatectomy. One of these patients ultimately elected to transition to hospice and passed away due to worsening hepatic decompensation; the other is still alive.
In a poll, other hepatologists at the same institution believed the most common causes of screening failure, in order, to be: failure to present to radiology, patient clinic appointment cancellation, loss to follow up, and failure to order imaging. Human error and deferral to primary care provider (PCP) were the most cited reasons for failure to order screening.
Despite guidelines that were well-known to the providers in the subspecialty hepatology practice, fewer than half of the patients in our cohort underwent appropriate screening during the study period. The findings are consistent with previous studies and add to the growing evidence that HCC screening rates are grossly insufficient.
However, our study illuminates some nuances in the reasons for screening failure. Most screening failures in our cohort were institutional rather than patient-driven or secondary to physician oversight. We were able to investigate what happened after the order was placed for screening to evaluate the system factors that contribute. Radiology scheduling failure, whether from inability to contact the patient or unavailability of timely imaging appointments, was the primary reason for lack of adherence. The multiple failure points both highlight the complexity of care coordination for cirrhosis patients in a subspecialty clinic and offer targets for intervention and improvement.
Failure to order screening was the second leading risk factor among subspecialty hepatologists in this cohort. Other investigators have demonstrated poor knowledge of screening protocols among primary care providers (PCPs), which can explain lack of adherence to guidelines. However, we do not believe a knowledge deficit was a major contributing factor in a subspecialty clinic. Many hepatologists cited deferral to PCP as a reason for not ordering screening, even though knowledge among PCPs remains poor. The authors also speculate that a busy, often overbooked clinic with competing priorities makes even the most diligent hepatologists forget to order screening. It is difficult to order abdominal imaging while counseling a patient that they will die from cirrhosis unless they overcome innumerable psychosocial barriers to abstain from alcohol for long enough to become a liver transplant candidate.
This study has several important limitations. Firstly, the window for “appropriate screening” in this study was 5 to 7 mo, which is narrower than the 4–8-mo window suggested by the AASLD, resulting in a positive bias toward ineffective screening. Secondly, patients who had two imaging studies 6 mo apart were considered “appropriately screened,” regardless of whether a third imaging study was completed on time. This data simplification may have resulted in an overestimation of the screening rate. Thirdly, the attribution of screening failure to a single step fails to capture the multifactorial nature of screening failure. For example, a patient for whom radiology did not schedule an imaging study because the physician did not order one because they missed their clinic appointment would be classified as “no show,” even though the provider could have ordered the screening even without the patient there. Finally, the logistical complexity of the screening process leaves room for interpretation variation between multiple investigators, even with rigorous standardization.
The debate over the proper length of screening is likely to continue, with many authors pointing out that longer intervals have not been studied. Some experts, including the National Cancer Institute, have opined that hepatologists ought to abandon screening protocols entirely due to a lack of survival benefit[29-32]. Furthermore, the World Gastroenterology Organization suggests that screening in low and middle-resource settings is appropriate only if the patient would have access to HCC treatments[33]. However, we contend that every effort be made to adhere to current practice guidelines when resources are available. Our findings demonstrate the need for future measures to address system and provider level improvements. We have implemented an automatic reminder in the electronic medical record for physicians and other healthcare professionals and targeted reminders via main or electronic media for patients. In addition, our findings highlight the need for serum biomarkers for HCC screening, which would eliminate the logistical delays with radiology[34].
In conclusion, the rate of appropriate HCC screening, though above the estimated national average, was inadequate in this patient population. The reasons for failure were multifactorial, but the primary driver was delays in radiology scheduling. These data immediately identify targets for future quality improvement initiatives.
The American Association for the Study of Liver Disease recommends that patients with cirrhosis be screened for hepatocellular carcinoma (HCC) every six months. Other researchers have shown that adherence to these guidelines is poor, but little is known about the causes of this failure.
The authors noted that many patients in their own subspecialty hepatology practice did not undergo appropriate screening. They studied factors contributing to screening failure in order to develop a possible quality improvement initiative.
The authors sought to identify root causes of HCC screening failure among patients with cirrhosis in their subspecialty heaptology clinic.
The authors identified patients with cirrhosis in their subspecialty hepatology clinic and determined whether they underwent appropriate screening. The authors reviewed the medical records of patients who did not undergo appropriate screening to identify the root causes of screening failure.
Among 1034 patients, only 489 underwent appropriate screening. The most common causes of screening failure, in descending order, were: radiology unable to schedule timely imaging, provider did not order imaging, patient canceled follow up appointment, appointments scheduled too far apart, lost to follow up, no-show to radiology appointment, and provider canceled appointment.
Even in a subspecialty hepatology clinic in which providers strive to follow guideline-based HCC screening, rates of screening were still poor. Most of the barriers to appropriate screening were due to systemic factors such as radiology availability, rather than to individual error.
HCC screening is vital to the comprehensive care of patients with cirrhosis, yet systemic and institutional barriers often prevent patients from receiving adequate care. The root causes identified in this article immediately suggest areas for possible quality improvement and provide guidance to those at other institutions.
The authors would like to thank Hanzhi Gao for her statistical expertise.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gatselis NK, Greece; Jin Y, China; Kumar SKY, India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64546] [Article Influence: 16136.5] [Reference Citation Analysis (176)] |
| 2. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 3. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [PubMed] [DOI] [Full Text] |
| 4. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1. [PubMed] [DOI] [Full Text] |
| 5. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1496] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 6. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1216] [Article Influence: 202.7] [Reference Citation Analysis (1)] |
| 7. | National Cancer Institute. Surveillance, Epidemiology, and End Results Program, National Cancer Institute. Available from: https://seer.cancer.gov/. |
| 8. | Kansagara D, Papak J, Pasha AS, O'Neil M, Freeman M, Relevo R, Quiñones A, Motu'apuaka M, Jou JH. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3237] [Article Influence: 462.4] [Reference Citation Analysis (1)] |
| 10. | Colombo M, Lleo A. Is there a real survival benefit of surveillance for hepatocellular carcinoma in cirrhotic patients? Hepatobiliary Surg Nutr. 2019;8:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Letouzé E, Imbeaud S, Zucman-Rossi J, Audureau E, Roudot-Thoraval F, Nahon P; ANRS CO12 CirVir Group. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology. 2018;155:431-442.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, Lee WM. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, Bian CB, Yamada S, Sun X, Venkatesh A, Goossens N, Bain G, Lauwers GY, Koh AP, El-Abtah M, Ahmad NB, Hoshida H, Erstad DJ, Gunasekaran G, Lee Y, Yu ML, Chuang WL, Dai CY, Kobayashi M, Kumada H, Beppu T, Baba H, Mahajan M, Nair VD, Lanuti M, Villanueva A, Sangiovanni A, Iavarone M, Colombo M, Llovet JM, Subramanian A, Tager AM, Friedman SL, Baumert TF, Schwarz ME, Chung RT, Tanabe KK, Zhang B, Fuchs BC, Hoshida Y; Precision Liver Cancer Prevention Consortium. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Singal AG, Yopp A, S Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Palmer LB, Kappelman MD, Sandler RS, Hayashi PH. Surveillance for hepatocellular carcinoma in a Medicaid cirrhotic population. J Clin Gastroenterol. 2013;47:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, Nehra M, Lee WM, Marrero JA, Tiro JA. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila). 2012;5:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol. 2017;51:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, Baytarian M, Fox R, Hunt K, Pedrosa M, Pocha C, Valderrama A, Kaplan DE. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | McGowan CE, Edwards TP, Luong MU, Hayashi PH. Suboptimal surveillance for and knowledge of hepatocellular carcinoma among primary care providers. Clin Gastroenterol Hepatol. 2015;13:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Simmons OL, Feng Y, Parikh ND, Singal AG. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2019;17:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Singal AG, Li X, Tiro J, Kandunoori P, Adams-Huet B, Nehra MS, Yopp A. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med. 2015;128:90.e1-90.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, Yopp AC, Parikh ND, Marrero JA, Singal AG. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Adamson B, Bishop WP, Santini NO, Halm EA. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology. 2017;152:608-615.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Marquardt P, Liu PH, Immergluck J, Olivares J, Arroyo A, Rich NE, Parikh ND, Yopp AC, Singal AG. Hepatocellular Carcinoma Screening Process Failures in Patients with Cirrhosis. Hepatol Commun. 2021;5:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Dirchwolf M, Marciano S, Ruf AE, Singal AG, D'Ercole V, Coisson P, Zerega A, Orozco F, Palazzo A, Fassio E, Arufe D, Anders M, D'Amico C, Gaite L, Thompson M, Perez D, Haddad L, Demirdjian E, Zunino M, Gadano A, Murga MD, Bermudez C, Tomatis J, Grigera N, Antinucci F, Baravalle M, Gazari MMR, Ferreiro M, Barbero M, Curia A, Demonte M, Gualano G. Failure in all steps of hepatocellular carcinoma surveillance process is frequent in daily practice. Ann Hepatol. 2021;25:100344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Liver (Hepatocellular) Cancer Screening (PDQ®): Health Professional Version. 2022 Apr 29. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002– . [PubMed] |
| 30. | Qian X, Yan X, Zhai X, Li N, Qu C, Lu F. Hepatocellular Carcinoma Surveillance and Treatment: A Way to Reduce Cancer-related Mortality in Cirrhotic Patients. J Clin Transl Hepatol. 2019;7:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, Lowy E, Berry K, Ioannou GN. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155:1128-1139.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Lidofsky SD. Screening for Hepatocellular Carcinoma in Cirrhosis: Challenges and Unresolved Issues. Gastroenterology. 2019;156:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 33. | World Gastroenterology Organization. Hepatocellular carcinoma: A global perspective. 2009 Nov. Updated Nov 2009. Available at: https://www.worldgastroenterology.org/guidelines/hepatocellular-carcinoma-hcc. |
| 34. | Parikh ND, Mehta AS, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2020;29:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |