Published online Mar 27, 2023. doi: 10.4254/wjh.v15.i3.386
Peer-review started: December 26, 2022
First decision: February 1, 2023
Revised: February 20, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: March 27, 2023
Processing time: 86 Days and 5.8 Hours
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver dis
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is the fastest growing chronic disease in the world. As the disease progresses, NAFLD can lead to liver fibrosis, cirrhosis and even liver cancer. However, a well-established treatment for NAFLD has yet to be identified. Exosomes are small extracellular vesicles secreted by cells. Owing to their high delivery efficiency and biocompatibility, exosomes are expected to become a new means of drug delivery and precise treatment for a variety of diseases, including NAFLD.
- Citation: Ding J, Xu C, Xu M, He XY, Li WN, He F. Emerging role of engineered exosomes in nonalcoholic fatty liver disease. World J Hepatol 2023; 15(3): 386-392
- URL: https://www.wjgnet.com/1948-5182/full/v15/i3/386.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i3.386
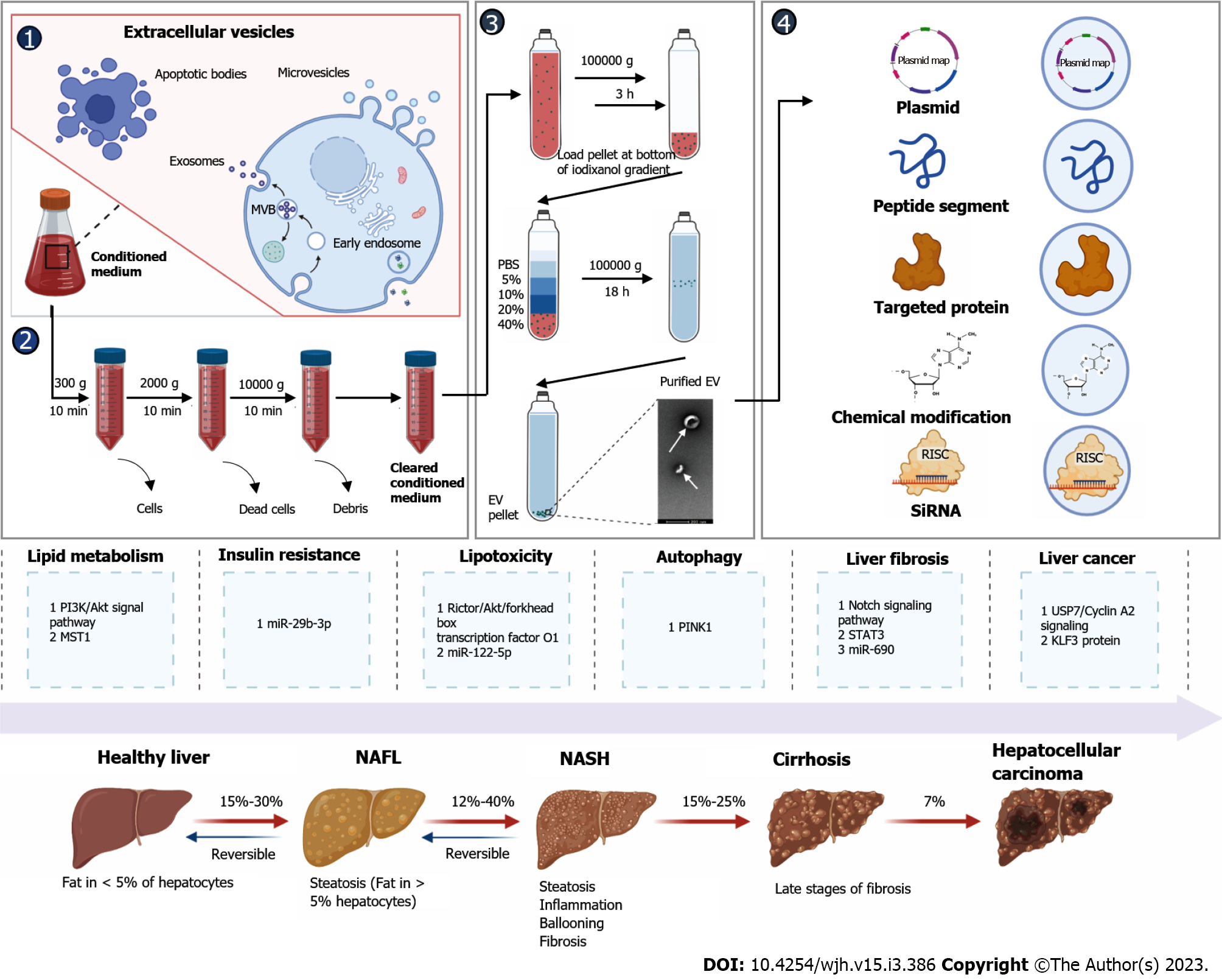
Nonalcoholic fatty liver disease (NAFLD) is a metabolic disease that is prevalent worldwide affecting at least a quarter of the population[1]. NAFLD is a continuum of liver abnormalities from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) that can even lead to cirrhosis and liver cancer. NAFL is reversible, whereas NASH with cirrhosis is difficult to reverse[2]. Therefore, it is critical to explore the pathogenesis of NAFLD and identify therapeutic targets to treat or prevent its development. Exosomes are extracellular vesicles with a particle size of 30-150 nm that play a crucial role in communication between cells[3]. Some macromolecules such as RNA or proteins in exosomes are associated with the occurrence and development of liver-related diseases and can be used as potential molecular markers in the diagnosis of NAFLD[4]. Processed and modified exosomes (known as engineered exosomes) may also facilitate the study of NAFLD and the development of new therapeutic strategies[5]. In this review, the mechanism and function of engineered exosomes in the development of NAFLD are reviewed (Figure 1).
The liver is the largest metabolic organ and a hub of lipid metabolism. Abnormal changes in lipid metabolism in the liver lead to the development of metabolic diseases[6]. A research team found that the release of exosomes in cultured astrocytes from apolipoprotein E knockout mice was significantly reduced compared to wild-type controls, and a PI3K inhibitor (LY294002) rescued the release of exosomes. They confirmed that the release of exosomes was regulated by cellular cholesterol through stimulation of the PI3K/Akt signalling pathway[7].
Li et al[8] systematically screened for microRNA expression using high-throughput small RNA sequencing and found that miR-199a-5p was significantly upregulated in adipose tissue in a mouse model of high-fat diet (HFD). Further studies confirmed that exosomal miR-199a-5p promoted lipid accumulation in the liver through induction of macrophage stimulating 1 (MST1) expression and fatty acid metabolism. Cheng et al[9] found that exosomal miR-627-5p reversed insulin resistance, prevented liver injury, normalized glucose and lipid metabolism and reduced lipid deposition in a rat model of NAFLD.
Brown adipose tissue (BAT) strongly promotes energy expenditure and shows good potential in the treatment of obesity. Zhou et al[10] treated HFD-fed mice with engineered exosomes derived from the serum of young healthy mice or from BAT. They found that treatment with BAT exosomes significantly promoted oxygen consumption in recipient cells, thus alleviating metabolic syndrome in HFD-fed mice.
Li et al[11] used a low-density lipoprotein receptor-deficient mouse (Ldlr mouse) as a model for hypercholesterolemia. Ldlr mRNA was encapsulated into exosomes by overexpression of Ldlr in donor AML12 mouse hepatocytes. The authors found that engineered exosomes loaded with Ldlr mRNA could restore the expression of Ldlr in the livers of Ldlr-deficient mice and rescue hypercholesterolemia. This study suggests that engineered exosomes may be an effective therapy for patients with hypercholesterolemia.
Insulin resistance is now believed to play a key role in the onset and progression of NAFLD[12]. A HFD reduces insulin sensitivity. Kumar et al[13] found that feeding a HFD changed the lipid composition of intestinal exosomes. These exosomes were found to be absorbed by macrophages and hepatocytes, resulting in inhibition of the insulin signalling pathway. Castaño et al[14] found that obesity can alter the expression and composition of miRNAs in mouse plasma exosomes. Ying et al[15] found that miR-690, an exosome-derived miRNA from M2-polarized macrophages, improved insulin sensitivity in obese mice. Su et al[16] found that exosomes derived from the bone marrow mesenchymal stem cells (BM-MSCs) of aged mice could be ingested by fat, muscle and liver cells, leading to insulin resistance in vivo and in vitro. The authors found that the amount of miR-29b-3p in exosomes released by BM-MSCs was significantly increased in aged mice. Furthermore, they found that inhibition of miR-29b-3p with an aptamer-mediated nanocomposite delivery system improved insulin resistance in aged mice.
Lipotoxicity promotes proinflammatory M1 polarization of liver macrophages during the development of NAFLD[17,18]. Liu et al[19] found that miR-192-5p-rich hepatocyte-exosomes induced by lipotoxic injury promoted macrophage M1 polarization and liver inflammation through Rictor/Akt/forkhead box transcription factor O1 signalling. Zhao et al[20] found that cholesterol-induced lysosomal dysfunction increased exosome release from hepatocytes, leading to M1 polarization and macrophage-induced inflammation in a miR-122-5p-dependent manner. Human umbilical cord mesenchymal stem cells (HUC-MSCs) are increasingly being studied in clinical trials of end-stage liver disease due to their excellent tissue repair and anti-inflammatory effects. Shi et al[21] found that HUC-MSC-derived exosomes could protect against methionine- and choline-deficient L-amino acid diet (MCD)-induced NASH.
Lipotoxicity can damage mitochondria and induce oxidative stress during the progression of NAFLD[22,23]. Studies have shown that adipocytes respond to mitochondrial stress by rapidly and vigorously releasing exosomes[24]. Similarly, exosomes derived from chemically induced human hepatic progenitors inhibit cell death induced by oxidative stress[25].
Autophagy is a process in which cells degrade and metabolize their own damaged organelles or protein aggregates that plays a key role in maintaining liver homeostasis[26]. Increasing evidence suggests that autophagy plays a very important role in lipid metabolism. Autophagy mainly protects cells and regulates inflammation in NAFLD[26]. Because autophagy and exosomal biogenesis share common elements, some studies have found that plasma exosomal levels are higher in NAFLD patients than in healthy controls[27]. Luo et al[28] found that miR-27a inhibited mitochondrial autophagy and promoted NAFLD-associated liver fibrosis by negatively regulating PINK1 expression via lipotoxic hepatocyte exosomes. A research team established a model of hepatocyte injury and apoptosis induced by D-galactosamine and lipopolysaccharide (D-GalN/LPS) to study the protective effect of bone marrow mesenchymal stem cell (BMSC)-derived exosomes on liver injury. They found that BMSC-derived exosomes attenuated D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro[29]. Similar studies have shown that upregulation of miR-96-5p in BMSCs and their exosomes ameliorated NASH via caspase-2[30].
It is generally believed that during the development of NAFLD, liver-related cells are replaced by fibrotic scar tissue, giving rise to liver fibrosis or cirrhosis, which are associated with poor prognosis and mortality in patients with NASH[2]. The Notch signalling pathway is a key mediator of cellular differentiation, proliferation and apoptosis[31]. We designed hairpin-type decoy oligodeoxynucleotides (ODNs) for RBP-J to inhibit the activation of Notch signalling. ODNs were loaded into HEK293T-derived exosomes by electroporation. Furthermore, we observed that tail vein-injected exosomes were mainly taken up by hepatic macrophages in mice with hepatic fibrosis. RBP-J decoy ODNs delivered by exosomes efficiently inhibited Notch signalling in macrophages and ameliorated liver fibrosis in mice[32].
Hou et al[33] found that myeloid cell-specific IL-6 signalling promoted miR-223-enriched exosome production and attenuated NAFLD-associated fibrosis. Tang et al[34] found that exosomes embedded with siRNAs or antisense oligonucleotides targeting signal transducer and activator of transcription 3 (STAT3) could attenuate liver fibrosis. Gao et al[35] showed that Kupffer cells produced endogenous miR-690 and shuttled this miRNA to other hepatocytes through exosomal secretion. Treatment with miR-690 inhibitors reduced fibrosis and steatosis in a NASH model. Wang et al[36] found that miR-6766-3p-rich 3D human embryonic stem cell (hESC) exosomes could ameliorate liver fibrosis by targeting the TGFβ RII-SMADS pathway in hepatic stellate cells. Ji et al[37] developed an exosome-liposome hybrid loaded with clodronate-nintedanib that impaired hepatic fibrosis by reducing the activation of Kupffer cells.
CRISPR-Cas9 gene editing has become a powerful therapeutic technology. However, there is a lack of safe and effective in vivo delivery systems for CRISPR-Cas9, especially for tissue-specific vectors[38]. Luo et al[39] used exosome-mediated CRISPR/dCas9-VP64 delivery to reprogram hepatic stellate cells to construct engineered exosomes for the treatment of liver fibrosis. Similarly, Wan et al[40] delivered exosome-mediated Cas9 ribonucleoprotein complexes for tissue-specific gene therapy in liver disease.
Without timely intervention, NAFLD inevitably results in liver cancer[41]. Liver cancer is the fourth leading cause of cancer-related death worldwide and occurs in patients with various chronic liver diseases[42]. To date, the exact pathogenesis of NAFLD-induced liver cancer is not fully understood, but may involve DNA damage responses, inflammation, autophagy, and disruption of the gut microbiota[41].
Adipose tissue is known to play a role in energy storage and metabolic regulation by secreting adipokines[43]. Studies have demonstrated that exosomal circRNA secreted by adipocytes promotes tumour growth by inhibiting miR-34a and activating the USP7/Cyclin A2 signalling pathway[44].
An acidic microenvironment has been shown to promote the release of exosomes, which are considered to be cell-to-cell communication agents involved in cancer progression and metastasis[45]. Tian et al[46] found that exosomal miR-21 and miR-10b induced by the acidic microenvironment in liver cancer could promote cancer cell proliferation and metastasis and be used as prognostic molecular markers and therapeutic targets for liver cancer.
Macrophage-derived exosomes play multiple roles in cancer initiation and progression[47]. Zhang et al[48] found that exosomes derived from RBP-J overexpressing macrophages inhibited the progression of liver cancer by miR-499b-5p/JAM3. M2 macrophages can influence tumour development by secreting various cytokines, including exosomes. Some studies suggest that M2 macrophage-derived exosomes modified by miR-660-5p-related oligonucleotides enhanced the development of hepatocellular carcinoma by regulating KLF3[49].
Exosomes can be derived from healthy and stressed cells to provide a snapshot of the cell of origin under physiological and pathological conditions. Hepatocyte-derived exosomes released from stressed/injured hepatocytes have been identified as a partial cause of liver disease progression and liver injury, so circulating exosomes may serve as biomarkers of NAFLD. Nanopasmon-enhanced scattering of gold nanoparticles coupled with hepatocyte-specific antibodies was used to identify hepatocyte-derived exosomes[50]. Furthermore, microarray analysis of exosomal miRNAs isolated from the serum of 41 patients with NAFLD (diagnosed using liver biopsy) suggested that serum exosomal miRNAs could be used to assess the severity of NAFLD and identify potential targets for NAFLD treatment[33]. One of the determinants of liver degeneration in the progression of NAFLD is Wnt/frizzled (FZD) signalling; for example, FZD7 delivered by plasma-derived exosomes is a good candidate for a novel and effective biomarker for the diagnosis and prognosis of NAFLD[51].
The incidence of NAFLD is rapidly increasing with changes in lifestyle and dietary habits[1]. Exosomes not only mediate communication between cells but can also be engineered to deliver specific substances. Engineered exosomes have shown some effects on NAFLD in animal experiments. Owing to their low immunogenicity and liver targeting[52,53], engineered exosomes have great potential to treat NAFLD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Forlano R, United Kingdom; Thandassery RB, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3788] [Article Influence: 541.1] [Reference Citation Analysis (2)] |
| 2. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1680] [Article Influence: 420.0] [Reference Citation Analysis (33)] |
| 3. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6553] [Article Influence: 1310.6] [Reference Citation Analysis (0)] |
| 4. | Wang W, Zhu N, Yan T, Shi YN, Chen J, Zhang CJ, Xie XJ, Liao DF, Qin L. The crosstalk: exosomes and lipid metabolism. Cell Commun Signal. 2020;18:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Nakao Y, Amrollahi P, Parthasarathy G, Mauer AS, Sehrawat TS, Vanderboom P, Nair KS, Nakao K, Allen AM, Hu TY, Malhi H. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine. 2021;36:102430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Kojta I, Chacińska M, Błachnio-Zabielska A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 7. | Abdullah M, Nakamura T, Ferdous T, Gao Y, Chen Y, Zou K, Michikawa M. Cholesterol Regulates Exosome Release in Cultured Astrocytes. Front Immunol. 2021;12:722581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Li Y, Luan Y, Li J, Song H, Li Y, Qi H, Sun B, Zhang P, Wu X, Liu X, Yang Y, Tao W, Cai L, Yang Z. Exosomal miR-199a-5p promotes hepatic lipid accumulation by modulating MST1 expression and fatty acid metabolism. Hepatol Int. 2020;14:1057-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Cheng D, Chai J, Wang H, Fu L, Peng S, Ni X. Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver Int. 2021;41:2279-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 10. | Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G, Yuan L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10:8197-8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Li Z, Zhao P, Zhang Y, Wang J, Wang C, Liu Y, Yang G, Yuan L. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11:2953-2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40:1367-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (2)] |
| 13. | Kumar A, Sundaram K, Mu J, Dryden GW, Sriwastva MK, Lei C, Zhang L, Qiu X, Xu F, Yan J, Zhang X, Park JW, Merchant ML, Bohler HCL, Wang B, Zhang S, Qin C, Xu Z, Han X, McClain CJ, Teng Y, Zhang HG. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 14. | Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158-12163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 293] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 15. | Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C, Olefsky JM. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021;33:781-790.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 16. | Su T, Xiao Y, Guo Q, Li C, Huang Y, Deng Q, Wen J, Zhou F, Luo XH. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano. 2019;13:2450-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 661] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 18. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 19. | Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H, Fan JG. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;72:454-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (1)] |
| 20. | Zhao Z, Zhong L, Li P, He K, Qiu C, Zhao L, Gong J. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res. 2020;387:111738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Shi Y, Yang X, Wang S, Wu Y, Zheng L, Tang Y, Gao Y, Niu J. Human umbilical cord mesenchymal stromal cell-derived exosomes protect against MCD-induced NASH in a mouse model. Stem Cell Res Ther. 2022;13:517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 22. | Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205-14218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 362] [Cited by in RCA: 372] [Article Influence: 33.8] [Reference Citation Analysis (2)] |
| 23. | Dabravolski SA, Bezsonov EE, Orekhov AN. The role of mitochondria dysfunction and hepatic senescence in NAFLD development and progression. Biomed Pharmacother. 2021;142:112041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Crewe C, Funcke JB, Li S, Joffin N, Gliniak CM, Ghaben AL, An YA, Sadek HA, Gordillo R, Akgul Y, Chen S, Samovski D, Fischer-Posovszky P, Kusminski CM, Klein S, Scherer PE. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33:1853-1868.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 25. | Hyung S, Jeong J, Shin K, Kim JY, Yim JH, Yu CJ, Jung HS, Hwang KG, Choi D, Hong JW. Exosomes derived from chemically induced human hepatic progenitors inhibit oxidative stress induced cell death. Biotechnol Bioeng. 2020;117:2658-2667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, Proikas-Cezanne T, Reggiori F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Tan J, Wang M, Wang Y, Dong M, Ma X, Sun B, Liu S, Zhao Z, Chen L, Liu K, Xin Y, Zhuang L. Lipid-induced DRAM recruits STOM to lysosomes and induces LMP to promote exosome release from hepatocytes in NAFLD. Sci Adv. 2021;7:eabh1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Luo X, Xu ZX, Wu JC, Luo SZ, Xu MY. Hepatocyte-derived exosomal miR-27a activateshepatic stellate cells through the inhibitionof PINK1-mediated mitophagy in MAFLD. Mol Ther Nucleic Acids. 2021;26:1241-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 29. | Zhao S, Liu Y, Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887-2897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | El-Derany MO, AbdelHamid SG. Upregulation of miR-96-5p by bone marrow mesenchymal stem cells and their exosomes alleviate non-alcoholic steatohepatitis: Emphasis on caspase-2 signaling inhibition. Biochem Pharmacol. 2021;190:114624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Xu H, Wang L. The Role of Notch Signaling Pathway in Non-Alcoholic Fatty Liver Disease. Front Mol Biosci. 2021;8:792667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | He F, Li WN, Li XX, Yue KY, Duan JL, Ruan B, Liu JJ, Song P, Yue ZS, Tao KS, Wang L. Exosome-mediated delivery of RBP-J decoy oligodeoxynucleotides ameliorates hepatic fibrosis in mice. Theranostics. 2022;12:1816-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, Li Y, Zheng MH, Kunos G, Gao B, Wang H. Myeloid-Cell-Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology. 2021;74:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 34. | Tang M, Chen Y, Li B, Sugimoto H, Yang S, Yang C, LeBleu VS, McAndrews KM, Kalluri R. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. 2021;35:e21557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 35. | Gao H, Jin Z, Bandyopadhyay G, Cunha E Rocha K, Liu X, Zhao H, Zhang D, Jouihan H, Pourshahian S, Kisseleva T, Brenner DA, Ying W, Olefsky JM. MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab. 2022;34:978-990.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 36. | Wang N, Li X, Zhong Z, Qiu Y, Liu S, Wu H, Tang X, Chen C, Fu Y, Chen Q, Guo T, Li J, Zhang S, Zern MA, Ma K, Wang B, Ou Y, Gu W, Cao J, Chen H, Duan Y. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J Nanobiotechnology. 2021;19:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Ji K, Fan M, Huang D, Sun L, Li B, Xu R, Zhang J, Shao X, Chen Y. Clodronate-nintedanib-loaded exosome-liposome hybridization enhances the liver fibrosis therapy by inhibiting Kupffer cell activity. Biomater Sci. 2022;10:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Sharma G, Sharma AR, Bhattacharya M, Lee SS, Chakraborty C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol Ther. 2021;29:571-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 39. | Luo N, Li J, Chen Y, Xu Y, Wei Y, Lu J, Dong R. Hepatic stellate cell reprogramming via exosome-mediated CRISPR/dCas9-VP64 delivery. Drug Deliv. 2021;28:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8:eabp9435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 164] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 41. | Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 42. | Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 898] [Article Influence: 179.6] [Reference Citation Analysis (2)] |
| 43. | Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 44. | Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, Li H, Sun W, Ying G, Ba Y. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38:2844-2859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 45. | Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. 2019;18:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 46. | Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics. 2019;9:1965-1979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 47. | Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv Mater. 2020;32:e2002054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 48. | Zhang L, Zhang J, Li P, Li T, Zhou Z, Wu H. Exosomal hsa_circ_0004658 derived from RBPJ overexpressed-macrophages inhibits hepatocellular carcinoma progression via miR-499b-5p/JAM3. Cell Death Dis. 2022;13:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 49. | Tian B, Zhou L, Wang J, Yang P. miR-660-5p-loaded M2 macrophages-derived exosomes augment hepatocellular carcinoma development through regulating KLF3. Int Immunopharmacol. 2021;101:108157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Nguyen HQ, Lee D, Kim Y, Bang G, Cho K, Lee YS, Yeon JE, Lubman DM, Kim J. Label-free quantitative proteomic analysis of serum extracellular vesicles differentiating patients of alcoholic and nonalcoholic fatty liver diseases. J Proteomics. 2021;245:104278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Scavo MP, Depalo N, Rizzi F, Carrieri L, Serino G, Franco I, Bonfiglio C, Pesole PL, Cozzolongo R, Gianuzzi V, Curri ML, Osella AR, Giannelli G. Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 795] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 53. | Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G, Song Z, Peng Y, Shen Y, Wang J, Cai Z. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles. 2020;10:e12030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |