Published online Mar 27, 2023. doi: 10.4254/wjh.v15.i3.353
Peer-review started: October 28, 2022
First decision: January 3, 2023
Revised: January 14, 2023
Accepted: March 17, 2023
Article in press: March 17, 2023
Published online: March 27, 2023
Processing time: 145 Days and 8.5 Hours
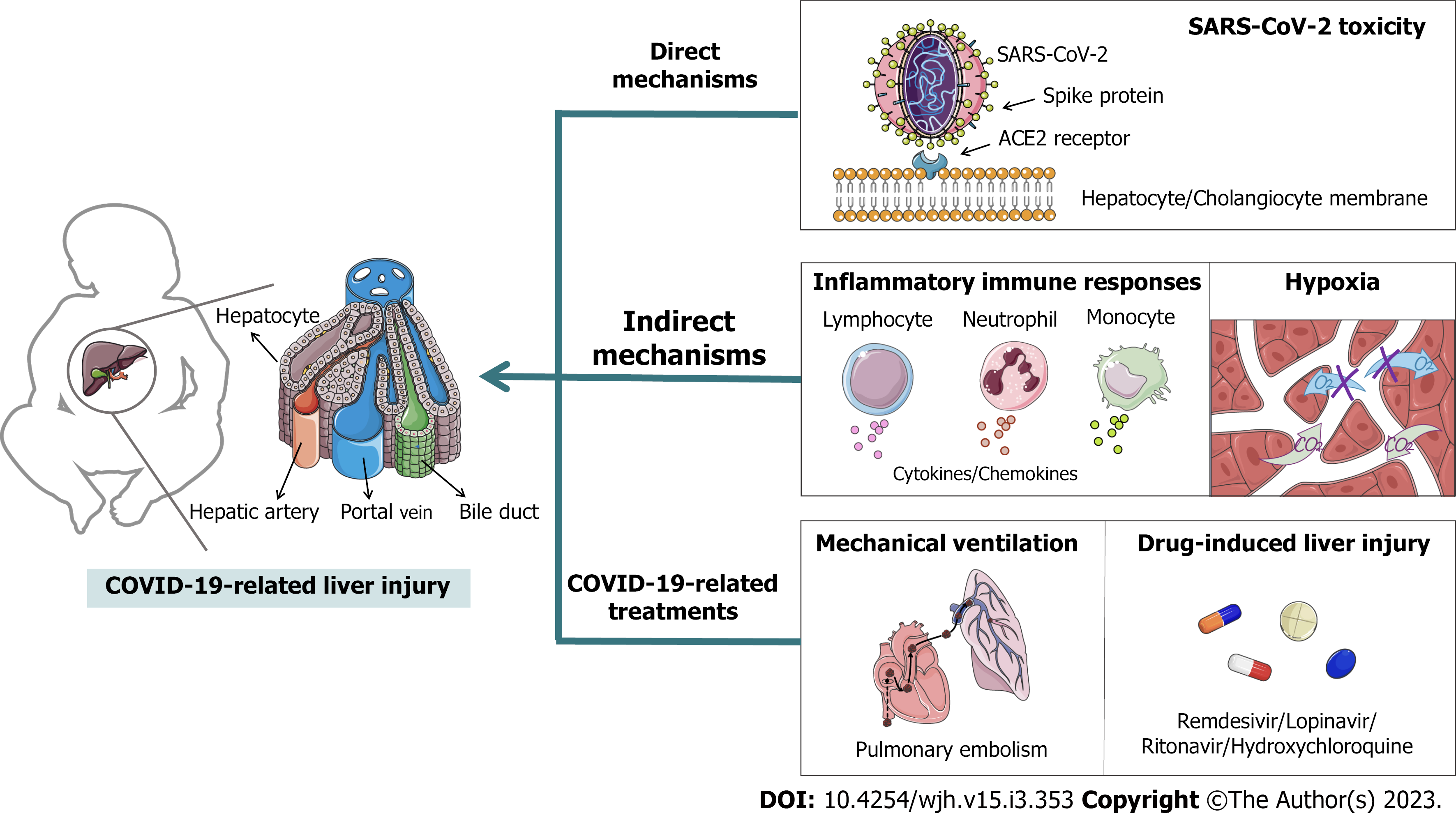
Coronavirus disease 2019 (COVID-19) poses an extremely serious global impact on public healthcare for individuals of all ages, including children. Increasing evidence has shown that liver abnormalities are commonly found in children with COVID-19, and age-related features in innate and adaptive response have been demonstrated. However, there are few reports and studies on COVID-19 related liver injury in children, and the data are scattered. So that many contradictions have arose. This situation is not only due to the serious ethical issues in studying pediatric patients with COVID-19, but also because of the short duration and wide coverage of the COVID-19 epidemic, the severity and complexity of clinical cases varied, as did the inclusion criteria for case reporting and patient outcomes. Therefore, we totaled the incidences, characteristics and pathomechanism of liver injury in children since the COVID-19 outbreak. The etiology of COVID-19-related liver injury is divided into three categories: (1) The direct mechanism involves severe acute respiratory syndrome coronavirus 2 binding to angiotensin-converting enzyme 2 in the liver or bile duct to exert direct toxicity; (2) the indirect mechanisms include an inflammatory immune response and hypoxia; and (3) COVID-19-related treatments, such as mechanical ventilation and antiviral drugs, may cause liver injury. In summary, this minireview provides fundamental insights into COVID-19 and liver dysfunction in children.
Core Tip: There are few cases of liver injury in children with coronavirus disease 2019 (COVID-19) and clinical reports are scarce. We collected reports on COVID-19-related liver injury (CRLI) in children over the last two years and divided the etiology of CRLI into three categories: (1) The direct mechanism involves severe acute respiratory syndrome coronavirus 2 binding to angiotensin-converting enzyme 2 in the liver or bile duct to exert direct toxicity; (2) the indirect mechanisms include an inflammatory immune response and hypoxia; and (3) COVID-19-related treatments, such as mechanical ventilation and antiviral drugs, may cause liver injury. We also discuss the current controversies regarding the pathophysiology of CRLI.
- Citation: Yun YF, Feng ZY, Zhang JJ. COVID-19 and liver dysfunction in children: Current views and new hypotheses. World J Hepatol 2023; 15(3): 353-363
- URL: https://www.wjgnet.com/1948-5182/full/v15/i3/353.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i3.353
Coronavirus disease 2019 (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In response to this global health crisis, governments and medical institutions have been actively working to improve epidemic prevention measures, and diagnostic and treatment methods, all of which have significantly reduced the transmission rate and mortality rate[1-3]. However, this crisis is not yet over, and the physical damage caused by COVID-19 is gradually expanding from respiratory to systemic diseases. In addition to inducing acute respiratory distress syndrome (ARDS)[4,5], COVID-19 also causes damage to organs such as the liver, gastr
COVID-19 mainly occurs in the elderly and people with potential complications[13]. The lethality of infection increases logarithmically and linearly with age in those over 30 years, but children have a lower prevalence and tend to be asymptomatic or have mild to moderate disease[14]. Therefore, currently published case reports mainly describe adult patients, resulting in a lack of details in pediatric cases. However, most infections in children originate from family contacts, they play an important role in disease transmission and have become a key target population for epidemic prevention and control measures[15]. Meta-analyses have shown that liver injury is common in children, but is often overlooked[16]. Therefore, we focused on pediatric patients with CRLI and divided the pathogenesis of CRLI in children into three categories: direct, indirect and treatment-related pathogenesis (Figure 1).
COVID-19 can cause varying severity of liver injury, as evidenced by abnormal elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), accompanied by mild elevations in alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL) and a reduction in albumin[17]. The abnormal liver enzyme levels in serum include: ALT > 40 U/L, AST > 40 U/L, GGT > 49 U/L, ALP > 135 U/L, TBIL > 17.1 μmol/L and albumin < 3 g/dL[18]. Recently, several studies have provided the results of abnormal liver tests in pediatric COVID-19. Alkan et al[19] found that 130 (44.2%) of 294 patients (age range: 14 d-18 years) with COVID-19 had abnormal liver function and most patients (33.3%) were characterized by elevated ALT, and other patients had elevated ALT (5.1%), ALP (6.6%), GGT (8.9%) and TBIL (3.8%). In addition, decreased albumin was also observed by Esmaeili et al[20] and Liu et al[21]. In their studies, the proportion of decreased albumin in pediatric patients was 16.7%[20] and 18.2%[21], respectively.
In general, the main manifestations of CRLI in children were mildly elevated ALT/AST and most research has confirmed this, for instance, Parri et al[22] reported on 130 children (age range: 0-17 years) with COVID-19 in Italy, and 8/68 (11.8%) children had elevated ALT and 11/60 (18.3%) had elevated AST. The analysis by Du et al[23] showed that ALT and AST increased in 9 (5.0%) and 24 (13.3%) of 180 subjects (age range: 0-15 years), respectively, and 11 (6.3%) of 174 subjects showed increased ALP levels. Thus, the elevation of liver enzymes in pediatric patients is not significant, which may be due to the fact that COVID-19 is mainly mild in children.
In addition, Sun et al[24] conducted a single center observational study of 8 children (age range: 2 mo-15 years) with severe COVID-19 and the results showed that ALT was increased in 4 (50.0%) cases but increased AST was not observed. It is possible that sometimes abnormally elevated ALT/AST is not a sufficient indicator of liver injury. The related studies on the features of CRLI in children are summarized in Table 1.
| Ref. | Number of patients | Age range | Abnormal liver function | E-ALT | E-AST | E-ALP | E-GGT | E-TBIL | D-albumi, n (%) |
| Alkan et al[19] | 294 | 14 d-18 years | 130 (44.2%) | 15 (5.1%) | 98 (33.3%) | 19 (6.6%) | 26 (8.9%) | 11 (3.8%) | NA |
| Esmaeili et al[20] | 18 | 3-10 years | 6 (33.3%) | 5 (27.8%) | 7 (38.9%) | 0 | NA | 3 (16.7%) | 3 (16.7%) |
| Liu et al[21] | 46 | 0-1 year | 20 (43.5%) | 11 (25.0%) | 20 (45.5%) | NA | NA | 6 (13.6%) | 8 (18.2%) |
| Parri et al[22] | 130 | 0-17 years | NA | 8/68 (11.8%) | 11/60 (18.3%) | NA | NA | NA | NA |
| Du et al[23] | 182 | 0-15 years | NA | 9/180 (5.0%) | 24/180 (13.3%) | 11/174 (6.3%) | NA | NA | NA |
| Sun et al[24] | 8 | 2 mo-15 years | 4 (50.0%) | 4 (50.0%) | 0 | NA | NA | 0 | NA |
Genome sequencing, and phylogenetic and structural analyses have confirmed that SARS-CoV-2 can bind to angiotensin-converting enzyme 2 (ACE2) of host cells depending on its spike protein, and this binding can mediate membrane fusion and viral invasion[25]. ACE2 is not only highly expressed in alveolar cells, but also distributed in various organs throughout the body, including the liver[26]. Thus, the direct pathological basis of CRLI is the viral virulence of SARS-CoV-2, and it can bind to ACE2 on liver endothelial cells and exert toxicity causing hepatocyte damage[27,28]. Unlike adults, children have milder symptoms of CRLI, possibly due to lower ACE2 expression, lower maturity, and weaker function (e.g., binding to SARS-CoV-2). However, in contrast, ACE2 expression decreases with age; thus, ACE2 Levels are higher in children than in adults[29]. Moreover, one of the functions of ACE2 is to convert angiotensin (Ang) II to Ang(1-7), which has anti-inflammatory and anti-liver fibrosis effects[30,31]. Therefore, besides the ability to mediate viral infections, the distribution of ACE2 in different age groups and the “dual action” of it on organ damage require further investigation.
Interestingly, Chai et al[32] indicated that ACE2 is highly expressed on cholangiocytes compared to hepatocytes and that SARS-CoV-2 may prefer to bind directly to ACE2 on cholangiocytes. Cholangiocytes are epithelial cells that line the intrahepatic and extrahepatic bile ducts and play an important role in liver regeneration and immune response[33]. This suggests that the liver abnormalities in COVID-19 patients may not be directly caused by hepatocyte injury and that the potential damage to cholangiocytes by SARS-CoV-2 may have a profound effect on the liver.
In addition, CRLI can be classified into three categories according to the degree of liver enzymes exceeding the upper limit of normal (ULN) (Table 2). Patients were classified as hepatocyte injury type when they had raised ALT and/or AST more than 3×ULN; patients were classified as cholangiocyte injury type when they had raised ALP and/or GGT more than 2×ULN; when the first two requirements were met simultaneously, patients were considered to have mixed injury type[18,34]. There are obvious differences in CRLI types between adults and children. Cai et al[18] found that the number of liver injuries in 318 adult COVID-19 patients with abnormal liver test results was as follows: mixed type (43.4%) > cholestatic type (29.2%) > hepatocellular type (20.8%). Furthermore, elevation of ALP, a marker of bile duct injury, is less common than abnormal liver enzymes in adults[35]. With regard to children, Alkan et al[19] found that the number of liver injuries in 130 pediatric patients was as follows: cholestatic type (71.5%) > hepatocellular type (18.5%) > mixed type (10.0%). The rate of ALP elevation in pediatric patients was slightly more than ALT (Table 1). Cholestatic liver injury is especially common in children under 5 years of age, and this may be related to ACE2 being less distributed in hepatocytes and more distributed in cholangiocytes in younger children[19]. Therefore, we should be more concerned about cholestatic liver injury in pediatric patients with COVID-19.
Inflammatory response and immune response are inseparable systemic responses at the organ, tissue, cellular and molecular levels. A moderate inflammatory immune response (IIR) plays a crucial part in protecting the body from pathological damage in the internal and external environment. However, an excessive IIR is the pathological basis for the development of multiple systemic diseases. An increasing number of studies have shown that the systemic IIR induced by SARS-CoV-2 has an intricate pathophysiological link to liver injury.
Dysfunction of innate and adaptive immune responses: Natural killer (NK) cells and natural antibodies are key components of the innate immune system and can be the first to respond to new viruses[36,37]. The adaptive immune response then comes into play and produces highly specific memory T and B cells to clear the virus and prevent reinfection[38]. However, dysfunctional innate and adaptive immune responses mediate the host damage caused by SARS-CoV-2[39].
The innate immune system detects SARS-CoV-2 mainly by two strategies: first, the presence of SARS-CoV-2 can be detected using various pattern recognition receptors, such as plasmacytoid dendritic cells detecting incoming viral genomic RNA in the intranuclear body via toll-like receptor (TLR); second, during viral replication, double-stranded RNA intermediates can be recognized by the RIG-I like receptor (RLR), a cytoplasmic RNA sensor. Following the engagement of the TLR and RLR, downstream signaling activates the transcription of interferons (IFNs) and pro-inflammatory cytokines and chemokines[40]. SARS-CoV-2 can block innate immune recognition and signal transduction using the expression of viral proteins[41]. Uncontrolled innate immune signaling may produce excess cytokines, which can trigger inflammation and worsen the condition.
With regard to the adaptive immune system, its three basic components are B cells, CD4+ T cells and CD8+ T cells. B cells can rapidly produce neutralizing antibodies after infection with SARS-CoV-2, and the antibody target is the spike protein of the virus[42]. T-cell responses were detected in almost all SARS-CoV-2 infections, and virus-specific CD4+ T cells can differentiate into Th1 cells and T follicular helper cells, which have antiviral activity through the production of IFN-γ and related cytokines[43]. The CD8+ T cells are essential for clearing virus by killing infected cells. In milder symptoms, SARS-CoV-2 specific CD4+ and CD8+ T cells can respond rapidly in the acute phase of COVID-19 and thus exert antiviral effects[44].
Recently, studies have revealed changes in the immune responses of COVID-19 pediatric patients (Table 3). Diao et al[45] retrospectively reviewed 522 patients (age range: 5 d-97 years) and demonstrated that more than 70% of the patients had a significant reduction in total T cells, CD4+ and CD8+ T cells, but the reduction was age-dependent as the younger patients had the least reduction in T cells. In addition, unlike in adults, CD4+ T cells were even increased in moderate pediatric cases[46]. Also, compared with mild cases, moderate cases had higher levels of IL-10, complement (C) 4 and NK cells, while neutrophils were significantly lower[46]. These findings suggested that the innate cells such as NK cells and neutrophils play a crucial role in the initial phase and the CD4+ T cells perform a function in the later phase of COVID-19. Studies have shown that CD4+ T cells and IL-10 Levels are positively correlated with CRLI biomarkers in pediatric patients[46], and although the IL-10 derived from CD4+ T cells plays an important anti-inflammatory role in mild patients, excess IL-10 may cause liver injury with COVID-19 progression.
| Biomarker | Severe | Moderate | Mild/asymptomatic |
| T lymphocyte[45-47] | CD3+↓; CD4+ ↓; CD8+ ↓; CD3+CD4+ ↓; CD3+CD8+ ↓ | CD3+ →; CD4+ →/↑; CD8+ → | CD3+ →; CD4+ →; CD8+ → |
| B lymphocyte[23,46] | ↓ | →/↑ | →/↑ |
| Innate cell[23,46] | Monocytes ↑; Neutrophils ↑; NK cells ↓ | Monocytes ↑; NK cells ↑; Neutrophils ↓ | Monocytes →; Neutrophils →; NK cells → |
| Immunological parameters[23,46] | IgE ↑; IgG ↓; IgA ↑; IgM ↓; C3 ↑/↓; C4 ↑/↓ | IgE →/↑; IgG →/↑; IgA →/↑; IgM →; C3 ↑/↓; C4 ↑ | IgE →/↑; IgG →/↑; IgA →/↑; IgM →; C3 ↑/↓; C4 → |
| Inflammatory cytokine[23,46,47] | IL-2 ↑; IL-4 ↑; IL-6 ↑; IL-10 ↑; IFN-γ ↑; TNF-α ↑ | IL-2 →; IL-4 →; IL-6 →; IL-10 ↑; IFN-γ →; TNF-α → | IL-2 →; IL-4 →; IL-6 →; IL-10 →; IFN-γ →; TNF-α → |
| Chemokines[49] | CXCL10 →; CXCL8 →; CCL2 → | CXCL10 →; CXCL8 →; CCL2 → | CXCL10 →; CXCL8 →; CCL2 → |
Furthermore, Li et al[47] showed that in children with severe COVID-19, CD3+, CD3+CD4+ (helper T cells), and CD3+CD8+ (memory T cells) counts were decreased, and the pro-inflammatory cytokine IL-6 and immune regulatory cytokine IL-10 were increased. Other inflammatory cytokines such as IL-2, IL-4, IL-10, TNF-α and IFN-γ were also detected[47]. However, the inflammatory cytokines IL-2, IL-4, IL-6, TNF-α and IFN-γ were rarely increased in mild and moderate pediatric patients[46]. These results suggested that although the adaptive immunity of children is relatively weak, their innate immunity is less likely to be disordered after SARS-CoV-2 infection and the cytokine storm associated with inflammation is not severe in most pediatric patients. Therefore, the direct liver injury caused by cytokine storms in children may not be as severe as in adults. However, recent research found that CRLI is most common in children under the age of five years[19]. This illustrated that SARS-CoV-2 infection can still trigger a series of damages once it exceeds the threshold due to the weak adaptive immunity of younger children.
Chemokines are small molecule proteins that have the ability to induce targeted chemotaxis of immune cells during inflammation and they also play an important role in dealing with viral infections[48]. Several studies have shown that the main pro-inflammatory chemokines such as CXCL [chemokine (C-X-C motif) ligand] 8, CXCL9, CXCL10, CCL (CC chemokine ligand) 2, CCL3 and CCL5 were increased in patients with aggravation of SARS-CoV-2 infection[48]. However, the levels of CXCL8, CXCL10, and CCL2 were unchanged in pediatric COVID-19 according to Warner et al[49]. These findings also suggested that the IIR in pediatric patients with COVID-19 is less severe than in adults.
B cells play a key role in immune regulation and antibody secretion. Previous studies have indicated that total B cells in COVID-19 patients were induced[23,48]. Du et al[23] conducted an analysis of 182 pediatric COVID-19 patients with different severity and showed that the levels of immunoglobulin (Ig) E, IgG and IgA were generally in the normal range or were elevated in isolated cases among mild or moderate patients. However, IgG and IgM counts were induced in severe COVID-19 patients[23]. Wu et al[46] noted that Igs including IgG, IgA and IgM were negatively correlated with biomarkers in the liver of pediatric patients. This may be the reason why CRLI is more common in severe patients and AST is only slightly elevated in mild patients.
Multisystem inflammatory syndrome in children-mediated liver injury: Recently, clinical reports have shown that children infected with SARS-CoV-2 for several weeks may develop a characteristic complication: multisystem inflammatory syndrome in children (MIS-C). A national study initiated in 2020 at Boston Children’s Hospital, Boston, MA, United States, with real-time monitoring of approximately 35 United States children’s hospitals, reported that of 186 MIS-C cases, 131 were positive for SARS-CoV-2[50]. MIS-C presents with persistent acute fever, abdominal pain, diarrhea, rash, lymphadenopathy, and in severe cases appendicitis and peritonitis, which may progress to multiorgan dysfunction[51,52]. Different types of liver injury mediated by MIS-C are being reported successively. Giannattasio et al[53] reviewed 55 pediatric patients (mean age 6.5 ± 3.7 years) with MIS-C and showed that 16 patients had acute liver injury (ALI) at admission and 10 more patients developed ALI during observation, ALI was defined by the presence of ALT elevation > 40 U/L. Furthermore, a 14-year-old boy developed MIS-C after SARS-COV-2 infection which was followed by hepatic steatosis, and the researchers also found elevated levels of ALT, AST and indices of cholestasis[54]. Another 10-month-old boy developed fulminant acute liver failure due to MIS-C[55]. The pathophysiology of MIS-C-mediated liver injury may also be related to the IIR as described previously.
Complement dysfunction-mediated liver injury: A new pathological mechanism of CRLI is dysfunction of the complement system. Complement is also part of the immune system which provides innate defense against pathogens and mediates inflammatory reactions. However, during SARS-CoV-2 infection, an overactive complement response leads to systemic inflammation, and a negative complement response promotes viral replication and infection, thereby exacerbating disease and inducing damage to other organs[56]. Du et al[23] published a report on 183 pediatric patients with COVID-19, C3 was elevated and decreased in 12.4% and 18.6% of severe patients, respectively, and C4 was elevated and decreased in 3.7% and 4.3% of severe patients, respectively (Table 3). In addition, it has been shown that complement correlates with the coagulation cascade and dysregulated complement activation may also contribute to the hypercoagulable state in severe COVID-19 patients[57]. For example, a report by Antala et al[58] showed that of four children with CRLI, two had complement dysfunction and resulted in microangiopathy, one of which showed rapid improvement in liver function after treatment with eculizumab. All of these findings demonstrate that severe CRLI may be associated with complement dysfunction and microangiopathy features.
The liver normally consumes 20% of whole body oxygen due to its dual blood flow system in the hepatic artery and portal system. In addition, the liver is able to extract 95% of blood-oxygen in order to maintain oxygen uptake[59]. It is well-known that ARDS is the most significant complication of COVID-19, which usually presents with respiratory distress, hypoxemia and acute respiratory failure[4,5]. All of these are important risk factors contributing to hypoxic hepatitis (HH). HH is characterized by a large and rapid increase in serum transaminases due to a decrease in oxygen delivery to the liver[60]. Furthermore, inflammatory cytokines may reduce the ability of hepatocytes to extract oxygen from the blood leading to hepatocyte death[61]. Thus, IIR caused by SARS-CoV-2 infection may promote the development of HH. Current studies suggest that HH is uncommon in patients with COVID-19, but has a very high mortality rate. For instance, Wu et al[62] identified 8 adult cases with HH among 3041 COVID-19 patients, and only 1 (12.5%) patient was discharged, and 7 (87.5%) died. Despite the lack of related reports on HH in pediatric patients, it is also a warning signal that we should be more concerned about the possibility of HH in children.
Approximately 23% of patients with SARS-CoV-2 infection developed pulmonary embolism[63]; therefore, some form of ventilation support, such as a high-flow nasal cannula, non-invasive and invasive mechanical ventilation, is required to prevent hemodynamic instability[64]. Woodruff et al[65] investigated COVID-19 -associated hospitalization surveillance network of 14 states in United States, they found that 691 (30.1%) patients required ICU admission and 122 (5.3%) patients needed invasive mechanical ventilation among 2293 hospitalized children (aged < 18 years). Moreover, other several researches also have showed 6% to 18% pediatric patients of COVID-19 required mechanical ventilation and 3% have died[66-71]. Current pediatric ventilation strategies are usually based on adult reports, which may lead to increased pulmonary vascular resistance and thus reduced right ventricular (RV) activity[72]. RV dysfunction is a good predictor of heart failure[73]. As the liver is the largest visceral organ in the human body and receives up to 25% of the entire cardiac output, RV failure can not only aggravate liver injury by liver congestion attributed to elevated central venous pressure, but also ischemic hepatitis[59]. Additionally, a multivariate regression analysis showed a significant increase in the severity of COVID-19 among pediatric patients receiving mechanical ventilation[74]. Therefore, physicians should pay attention to the changes in cardiac function and the possibility of subsequent liver injury when mechanical ventilation is given to pediatric patients.
Drugs are mainly metabolized by the liver. Drug-related liver injury (DRLI) remains an important focus in the monitoring of new drugs and drug repurposing. At present, the use of anti-SARS-CoV-2 drugs in pediatric patients is dependent on the evidence from adult clinical cases due to the emergency of COVID-19. The Italian Society of Infectious Pediatric Diseases recommends the use of remdesivir in pediatric patients with severe COVID-19 in whom renal and liver functions are normal, lopinavir/ritonavir should only be considered if remdesivir is incompatible or unavailable, dexamethasone and tocilizumab can be administered in patients with ARDS or MIS-C[75]. A medication guidance from a North American institution suggested using hydroxychloroquine as first-line treatment in children under 12 years and as second-line treatment in children above 12 years[76]. DRLI in pediatric cases is predominantly characterized by elevated liver enzymes as described by Goldman et al[77] in 77 children with severe COVID-19 treated with remdesivir, where 3 patients discontinued remdesivir due to elevated liver enzyme levels. The evaluation of other antiviral drugs in the pediatric population is uncommon. Although there are fewer pediatric patients with severe COVID-19, the use of antiviral drugs still deserves a separate discussion to develop a more appropriate therapy for children.
The above conclusions are drawn from a limited number of pediatric cases and there are serious ethical questions about research on children with COVID-19. Therefore, many conflicting views remain to be further explored, for example: (1) “SARS-CoV-2 binds to ACE2 and exerts direct liver injury” vs “Ang(1-7) produced by ACE2 hydrolysis of Ang II has anti-inflammatory and anti-fibrotic effects on the liver”; (2) “The expression and function of ACE2 are weaker in children” vs “The expression of ACE2 decreases with age”; (3) “Cholestatic liver injury is more common in children” vs “The elevation of biliary injury marker ALP was not significant”; (4) “Cytokine storm can lead to inflammation and liver injury” vs “Cytokine storm is mild in pediatric patients”; and (5) “Inhibition of the complement system may aggravate viral infection and cause liver injury” vs “Excessive activation of the complement system may induce inflammation and cause liver injury”.
It is normal for these contradictions to emerge. As the short duration and wide coverage of the COVID-19 epidemic, the severity and complexity of clinical cases vary, and the criteria for inclusion and the outcome of patients are also different among case reports. For the longer term future, we should continue to focus on CRLI to address these issues.
With the continuous progress of COVID-19, liver injury is becoming a research focus. We have divided the etiology of CRLI in children into three categories, and the possible pathophysiological mechanisms are discussed separately. Of these, the direct mechanism involves SARS-CoV-2 binding to ACE2 in the liver or bile duct to exert direct toxicity, the indirect mechanism includes IIR and hypoxia, and COVID-19-related treatments may also cause liver injury under some circumstances, such as the use of mechanical ventilation and antiviral drugs. In summary, children are characterized by strong innate immunity but weak adaptive immunity, and the IIR resulting from SARS-CoV-2 is still the main cause of liver injury. The evaluation of liver injury in pediatric patients with severe COVID-19, especially those with MIS-C, should be a focus. Another focus is the toxicity of SARS-CoV-2 to cholangiocytes, as children more commonly have cholestatic liver injury. In addition, hypoxia may promote liver injury due to the high incidence of ARDS complications. Finally, liver injury induced during COVID-19 treatment is often overlooked. Mechanical ventilation in children with respiratory distress can lead to the risk of RV dysfunction and subsequent liver injury, and the use of antiviral drugs in children may also lead to DRLI. In order to reach a consensus on the etiology of CRLI, more pediatric case reports, more detailed classifications and more in-depth studies are pending.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ulasoglu C, Turkey; Wishahi M S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1055] [Cited by in RCA: 848] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 2. | Rahman S, Montero MTV, Rowe K, Kirton R, Kunik F Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: a review of current evidence. Expert Rev Clin Pharmacol. 2021;14:601-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Wang J, Yang W, Pan L, Ji JS, Shen J, Zhao K, Ying B, Wang X, Zhang L, Wang L, Shi X. Prevention and control of COVID-19 in nursing homes, orphanages, and prisons. Environ Pollut. 2020;266:115161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398:622-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 670] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 5. | Swenson KE, Swenson ER. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit Care Clin. 2021;37:749-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 6. | Skok K, Stelzl E, Trauner M, Kessler HH, Lax SF. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Arch. 2021;478:343-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, Michael BD, Winkler AS, Beghi E, Guekht A, Pardo CA, Wood GK, Hsiang-Yi Chou S, Fink EL, Schmutzhard E, Kheradmand A, Hoo FK, Kumar A, Das A, Srivastava AK, Agarwal A, Dua T, Prasad K. Frequency of Neurologic Manifestations in COVID-19: A Systematic Review and Meta-analysis. Neurology. 2021;97:e2269-e2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 9. | Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, Johnston TP, Sahebkar A. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2021;19:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 10. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 900] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 11. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 13. | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2083] [Cited by in RCA: 3171] [Article Influence: 792.8] [Reference Citation Analysis (0)] |
| 14. | O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, Fontanet A, Cauchemez S, Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 751] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 15. | Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 789] [Article Influence: 157.8] [Reference Citation Analysis (0)] |
| 16. | Wang J, Yuan X. Digestive system symptoms and function in children with COVID-19: A meta-analysis. Medicine (Baltimore). 2021;100:e24897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 19. | Alkan G, Emiroğlu M, Tüter Öz SK, Emiroğlu HH, Türk Dağı H, Körez MK. Gastrointestinal and Liver Manifestations in Children with COVID-19 and Their Relationship to Clinical Course. Turk Arch Pediatr. 2022;57:413-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Esmaeili Dooki M, Mehrabani S, Sorkhi H, Nikpour M, Tabatabaie M, Mohammadi M, Kiani M. COVID-19 and Digestive System in Children: A Retrospective Study. Arch Iran Med. 2020;23:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Liu X, Tang J, Xie R, Li W, Chen J, Guo Y, Zhang B, Zhang Y, Wang J, Peng C, Lei X, Luo Q, Zhang Q, Li Y. Clinical and Epidemiological Features of 46 Children <1 Year Old With Coronavirus Disease 2019 in Wuhan, China: A Descriptive Study. J Infect Dis. 2020;222:1293-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Parri N, Magistà AM, Marchetti F, Cantoni B, Arrighini A, Romanengo M, Felici E, Urbino A, Da Dalt L, Verdoni L, Armocida B, Covi B, Mariani I, Giacchero R, Musolino AM, Binotti M, Biban P, Fasoli S, Pilotto C, Nicoloso F, Raggi M, Miorin E, Buonsenso D, Chiossi M, Agostiniani R, Plebani A, Barbieri MA, Lanari M, Arrigo S, Zoia E, Lenge M, Masi S, Barbi E, Lazzerini M; CONFIDENCE and COVID-19 Italian Pediatric Study Networks. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020;179:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 23. | Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, Chen HW, Li Y, Liu GH, Akdis CA, Lu XX, Gao YD. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021;76:510-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 24. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 25. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14131] [Article Influence: 2826.2] [Reference Citation Analysis (1)] |
| 26. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 28. | Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han JJ. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 30. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 739] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 31. | Amirfakhryan H. Kawasaki-like disease in children with COVID-19: A hypothesis. Med Hypotheses. 2020;143:110117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: BioRxiv:931766. [DOI] [Full Text] |
| 33. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (1)] |
| 34. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 35. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 36. | Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol. 2018;9:1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 769] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 37. | Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 378] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 38. | Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1118] [Article Influence: 223.6] [Reference Citation Analysis (0)] |
| 40. | Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 499] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 41. | Kasuga Y, Zhu B, Jang KJ, Yoo JS. Innate immune sensing of coronavirus and viral evasion strategies. Exp Mol Med. 2021;53:723-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 42. | Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, Agudelo M, Barnes CO, Gazumyan A, Finkin S, Hägglöf T, Oliveira TY, Viant C, Hurley A, Hoffmann HH, Millard KG, Kost RG, Cipolla M, Gordon K, Bianchini F, Chen ST, Ramos V, Patel R, Dizon J, Shimeliovich I, Mendoza P, Hartweger H, Nogueira L, Pack M, Horowitz J, Schmidt F, Weisblum Y, Michailidis E, Ashbrook AW, Waltari E, Pak JE, Huey-Tubman KE, Koranda N, Hoffman PR, West AP Jr, Rice CM, Hatziioannou T, Bjorkman PJ, Bieniasz PD, Caskey M, Nussenzweig MC. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1673] [Cited by in RCA: 1525] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 43. | Crotty S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. 2019;50:1132-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1066] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 44. | Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, Crotty S. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996-1012.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1541] [Cited by in RCA: 1412] [Article Influence: 282.4] [Reference Citation Analysis (0)] |
| 45. | Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1687] [Cited by in RCA: 1758] [Article Influence: 351.6] [Reference Citation Analysis (0)] |
| 46. | Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, Wang J, Shao J, Xiang Y. Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 47. | Li H, Chen K, Liu M, Xu H, Xu Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect. 2020;81:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 48. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 939] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 49. | Warner S, Richter A, Stamataki Z, Kelly D. Understanding COVID-19: are children the key? BMJ Paediatr Open. 2021;5:e001063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1856] [Article Influence: 371.2] [Reference Citation Analysis (0)] |
| 51. | Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1668] [Article Influence: 333.6] [Reference Citation Analysis (0)] |
| 52. | Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020;142:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 864] [Article Influence: 172.8] [Reference Citation Analysis (1)] |
| 53. | Giannattasio A, Maglione M, D'Anna C, Muzzica S, Pappacoda S, Lenta S, Di Mita O, Ranucci G, Mandato C, Tipo V. Liver and Pancreatic Involvement in Children with Multisystem Inflammatory Syndrome Related to SARS-CoV-2: A Monocentric Study. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 54. | Sica R, Pennoni S, Penta L, Di Cara G, Verrotti A. New Onset of Hepatic Steatosis Post-Severe Multisystem Inflammatory Syndrome in Children (MIS-C): A Case Report. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Bonilla Gonzalez C, Hincapié Echeverría M, Plazas Pachón R, Mora Umaña P, Diaz Gómez BL, Gualdron Barreto N. Case Report: Fatal Acute Liver Failure With Giant Cell Transformation in a Pediatric Patient Associated With MIS-C. Front Pediatr. 2021;9:780258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Vitiello A, La Porta R, D'Aiuto V, Ferrara F. Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Hum Immunol. 2021;82:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 387] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 58. | Antala S, Diamond T, Kociolek LK, Shah AA, Chapin CA. Severe Hepatitis in Pediatric Coronavirus Disease 2019. J Pediatr Gastroenterol Nutr. 2022;74:631-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Fuhrmann V, Jäger B, Zubkova A, Drolz A. Hypoxic hepatitis - epidemiology, pathophysiology and clinical management. Wien Klin Wochenschr. 2010;122:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Ucgun I, Ozakyol A, Metintas M, Moral H, Orman A, Bal C, Yildirim H. Relationship between hypoxic hepatitis and cor pulmonale in patients treated in the respiratory ICU. Int J Clin Pract. 2005;59:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Wu Y, Ma Z, Guo X, Li H, Tang Y, Meng H, Yu H, Peng C, Chu G, Wang X, Teng Y, Zhang Q, Zhu T, Wang B, Tong Z, Zhao H, Lu H, Qi X. Clinical characteristics and outcomes of COVID-19 patients with hypoxic hepatitis. Clin Res Hepatol Gastroenterol. 2021;45:101665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296:E186-E188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 64. | Qiu H, Tong Z, Ma P, Hu M, Peng Z, Wu W, Du B; China Critical Care Clinical Trials Group (CCCCTG). Intensive care during the coronavirus epidemic. Intensive Care Med. 2020;46:576-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 65. | Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, Anderson EJ, Weigel A, Monroe ML, Reeg L, Bye E, Sosin DM, Muse A, Bennett NM, Billing LM, Sutton M, Talbot HK, McCaffrey K, Pham H, Patel K, Whitaker M, L McMorrow M, P Havers F. Risk Factors for Severe COVID-19 in Children. Pediatrics. 2022;149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 66. | Kim L, Whitaker M, O'Halloran A, Kambhampati A, Chai SJ, Reingold A, Armistead I, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, Openo KP, Weigel A, Ryan P, Monroe ML, Fox K, Kim S, Lynfield R, Bye E, Shrum Davis S, Smelser C, Barney G, Spina NL, Bennett NM, Felsen CB, Billing LM, Shiltz J, Sutton M, West N, Talbot HK, Schaffner W, Risk I, Price A, Brammer L, Fry AM, Hall AJ, Langley GE, Garg S; COVID-NET Surveillance Team. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 67. | Fernandes DM, Oliveira CR, Guerguis S, Eisenberg R, Choi J, Kim M, Abdelhemid A, Agha R, Agarwal S, Aschner JL, Avner JR, Ballance C, Bock J, Bhavsar SM, Campbell M, Clouser KN, Gesner M, Goldman DL, Hammerschlag MR, Hymes S, Howard A, Jung HJ, Kohlhoff S, Kojaoghlanian T, Lewis R, Nachman S, Naganathan S, Paintsil E, Pall H, Sy S, Wadowski S, Zirinsky E, Cabana MD, Herold BC; Tri-State Pediatric COVID-19 Research Consortium. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. J Pediatr. 2021;230:23-31.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 68. | Fisler G, Izard SM, Shah S, Lewis D, Kainth MK, Hagmann SHF, Belfer JA, Feld LM, Mastroianni F, Kvasnovsky CL, Capone CA, Schneider J, Sweberg T, Schleien C, Taylor MD; Northwell COVID-19 Research Consortium. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Kainth MK, Goenka PK, Williamson KA, Fishbein JS, Subramony A, Barone S, Belfer JA, Feld LM, Krief WI, Palumbo N, Rajan S, Rocker J, Scotto T, Sharma S, Sokoloff WC, Schleien C, Rubin LG; NORTHWELL HEALTH COVID-19 RESEARCH CONSORTIUM. Early Experience of COVID-19 in a US Children's Hospital. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 70. | Verma S, Lumba R, Dapul HM, Gold-von Simson G, Phoon CK, Lighter JL, Farkas JS, Vinci A, Noor A, Raabe VN, Rhee D, Rigaud M, Mally PV, Randis TM, Dreyer B, Ratner AJ, Manno CS, Chopra A. Characteristics of Hospitalized Children With SARS-CoV-2 in the New York City Metropolitan Area. Hosp Pediatr. 2021;11:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, Sewell TB, Schweickert AJ, Babineau JR, Carter RC, Fenster DB, Orange JS, McCann TA, Kernie SG, Saiman L; Columbia Pediatric COVID-19 Management Group. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 72. | Shahrbaf MA, Tabary M, Khaheshi I. The right ventricle in COVID-19 patients. Eur Heart J. 2021;42:559-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 74. | Saleh NY, Aboelghar HM, Salem SS, Ibrahem RA, Khalil FO, Abdelgawad AS, Mahmoud AA. The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 2021;21:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Venturini E, Montagnani C, Garazzino S, Donà D, Pierantoni L, Lo Vecchio A, Nicolini G, Bianchini S, Krzysztofiak A, Galli L, Villani A, Castelli-Gattinara G; Italian SITIP-SIP SARS-Cov-2 pediatric infection study group. Treatment of children with COVID-19: position paper of the Italian Society of Pediatric Infectious Disease. Ital J Pediatr. 2020;46:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO, Downes KJ, Timberlake K, Young J, Orscheln RC, Tamma PD, Schwenk HT, Zachariah P, Aldrich M, Goldman DL, Groves HE, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ, Newland JG, Nakamura MM. Multicenter Initial Guidance on Use of Antivirals for Children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc. 2020;9:701-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 77. | Goldman DL, Aldrich ML, Hagmann SHF, Bamford A, Camacho-Gonzalez A, Lapadula G, Lee P, Bonfanti P, Carter CC, Zhao Y, Telep L, Pikora C, Naik S, Marshall N, Katsarolis I, Das M, DeZure A, Desai P, Cao H, Chokkalingam AP, Osinusi A, Brainard DM, Méndez-Echevarría A. Compassionate Use of Remdesivir in Children With Severe COVID-19. Pediatrics. 2021;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |