Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.265
Peer-review started: December 6, 2022
First decision: January 11, 2023
Revised: January 21, 2023
Accepted: February 8, 2023
Article in press: February 8, 2023
Published online: February 27, 2023
Processing time: 79 Days and 23.7 Hours
Non-alcoholic fatty liver disease (NAFLD) is a global health concern with a prevalence of about 25% amongst United States adults. Its increased prevalence is attributed to increase in patients with obesity and metabolic syndrome, partly due to similar mechanisms of injury. Nephrotic syndrome (NS) is a clinical entity resulting from extensive proteinuria leading to hypoalbuminemia, hyperlipidemia, edema, and other complications. Given its association with hyperlipidemia, there is concern that patients with NS may be at increased risk of NAFLD.
To perform a cross-sectional population-based study to investigate the prevalence and risk factors of NAFLD in patients with NS.
A large multicenter database (Explorys Inc., Cleveland, OH, United States) was utilized for this retrospective cohort study. A cohort of 49700 patients with a diagnosis of “Non-Alcoholic fatty liver disease” using the Systematized Nomenclature of Medicine-Clinical Terms (SNOMED-CT) between 1999-2022 was identified. Inclusion criteria were age ≥ 18 years, presence of NAFLD, presence of NS. There were no specific exclusion criteria. Univariate and multivariate analysis were performed to adjust for multiple risk factors including age, gender, Caucasian race, NS, type II diabetes mellitus, hypothyroidism, dyslipidemia, obesity, metabolic syndrome and chronic kidney disease. Statistical analysis was conducted using R, and for all analyses, a 2-sided P value of < 0.05 was considered statistically significant.
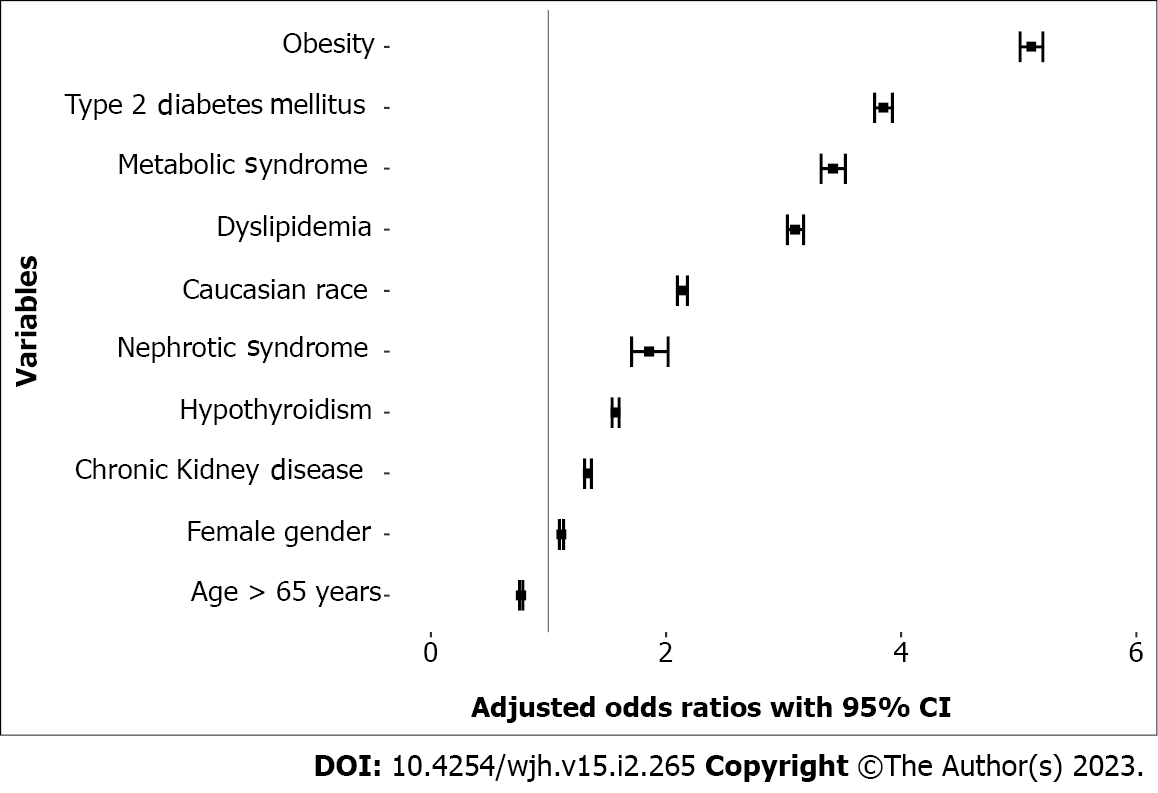
Among the 78734750 individuals screened in this database, there were a total of 49700 subjects with NAFLD. In univariate analysis, the odds of having NAFLD in patients with NS, type 2 diabetes mellitus, hypothyroidism, dyslipidemia, obesity, metabolic syndrome and chronic kidney disease were 14.84 [95% confidence interval (95%CI) 13.67-16.10], 17.05 (95%CI 16.78-17.32), 6.99 (95%CI 6.87-7.11), 13.61 (95%CI 13.38-13.84), 19.19 (95%CI 18.89-19.50), 29.09 (95%CI 28.26--29.95), and 9.05 (95%CI 8.88-9.22), respectively. In multivariate analysis, the odds of having NAFLD amongst patients with NS were increased to 1.85 (95%Cl 1.70-2.02), while the odds were also remained high in patients that have type 2 diabetes mellitus [odds ratio (OR) 3.84], hypothyroidism (OR 1.57), obesity (OR 5.10), hyperlipidemia (OR 3.09), metabolic syndrome (OR 3.42) and chronic kidney disease (OR 1.33).
Patients with NS are frequently found to have NAFLD, even when adjusting for common risk factors. Hence, clinicians should maintain a high index of suspicion regarding presence of NAFLD in patients with NS.
Core Tip: We conducted a population-based study to investigate the prevalence of non-alcoholic fatty liver disease (NAFLD) in patients with Nephrotic syndrome. We screened over 78 million individuals in a nationwide multicenter database. We performed a comprehensive multivariate analysis accounting for multiple cofounding factors including age ≥ 65 years, gender, Caucasian race, obesity, diabetes mellitus type 2, metabolic syndrome, dyslipidemia, chronic kidney disease and hypothyroidism. We found that patients with nephrotic syndrome had a higher prevalence of NAFLD. However, we could not account for certain confounders such as elevated uric acid levels, hormonal therapy, chemotherapy for tumors, and certain drugs such as corticosteroids, which are known to be risk factors for NAFLD. Further studies are required to confirm these findings and assess the utility of surveillance strategies for NAFLD in patients with nephrotic syndrome.
- Citation: Onwuzo SS, Hitawala AA, Boustany A, Kumar P, Almomani A, Onwuzo C, Monteiro JM, Asaad I. Prevalence of non-alcoholic fatty liver disease in patients with nephrotic syndrome: A population-based study. World J Hepatol 2023; 15(2): 265-273
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/265.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.265
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver disease worldwide with a prevalence of about 25% in the adult world population. It is characterized by excessive hepatic deposition of fat without any other probable explanation including alcohol, viral hepatitis, inherited liver conditions, or protracted use of steatogenic drugs[1]. NAFLD is seen to occur in a progressive manner from steatosis to nonalcoholic steatohepatitis (NASH), which may lead to fibrosis and cirrhosis[2]. Multiple studies have confirmed that NASH and cirrhosis increase the risk of hepatocellular carcinoma (HCC), which is one of the most common causes of cancer related deaths worldwide[3]. It is thus no wonder that NAFLD and NASH have been recognized as a growing public health problem. The disease burden of NAFLD is influenced by diabetes mellitus type 2, obesity, metabolic syndrome and hypothyroidism which have all been recognized as risk factors in its development as these conditions either directly or indirectly, promote fat accumulation in the liver[4-7]. Unfortunately, these conditions are not expected to decrease in the forthcoming decades. NAFLD and its related liver complications (NASH, cirrhosis and HCC) are the leading cause of chronic liver disease and the major cause of liver transplantation in the United States[8,9]. NAFLD is not only associated with liver related morbidity and mortality, clinical evidence also suggests its associations with other important extra-hepatic diseases such as cardiovascular diseases ranging from cardiomyopathy, coronary heart disease, cardiac arrhythmias to hypertension and kidney diseases such as chronic kidney disease[10-12]. These cardiovascular manifestations are recognized to be the leading cause of death in patients with NAFLD[13,14]. No wonder a tailored multistep approach involving lifestyle changes, anti-diabetic drugs and lipid lowering medications are have in been put in place for the management of NAFLD to reduce incidence of cardiovascular complication and also concomitantly treat existing comorbid conditions.
Nephrotic syndrome (NS) is a kidney disorder characterized by excessive proteinuria (urinary loss of ≥ 3 g of proteins per 24 h or, on a single spot urine sample, the presence of ≥ 2 g of protein per gram of urinary creatinine) resulting in hypoalbuminemia, dyslipidemia and oedema[15]. Dyslipidemia is known to cause premature atherosclerosis increasing the risk for acute coronary syndrome and stroke. Furthermore, there is increased risk of thrombosis in patients with nephrotic syndrome, not only from increased urinary loss of antithrombotic factors but also atherosclerosis induced platelet hyperreactivity[16]. Nutritional optimization as well as pharmacological interventions involving use of, Ace inhibitors, albumin, corticosteroid, antibiotic, anticoagulation therapy have all been proposed as measures to reduce mortality from NS.
Given their association with dyslipidemia, NAFLD and NS might have similarities in their pathophysiology. Both disease processes are associated with elevated levels of circulating free fatty acid[17-20]. In NAFLD, patients have underlying insulin resistance causing decreased inhibitory effect of insulin on peripheral lipolysis leading to increased pool of circulating free fatty acid and glycerol. As fat and triglycerides in the form of VLDL accumulates in the liver, it eventually leads to excessive production of ROS by Kupffer cells and alteration in mitochondrial DNA occurs. This demonstrates the slowed progression of hepatic steatosis to NASH, hepatocellular necroinflammation and fibrosis and lastly carcinoma[21-24]. Interestingly, patients with NS also exhibit dysregulated fatty acid metabolism with or without the presence of chronic kidney disease. In these patients, injury to podocytes stems from elevated plasma concentrations of major lipoproteins. This alteration in lipid metabolism stems from downregulation of lipoprotein lipase in peripheral tissues, suppression of hepatic lipase and increased activity of acetyl-CoA carboxylase and fatty acid synthase[17-20].
Our hypothesis is that the excess synthesized and circulating lipids in patients with NS affect fat metabolism in the liver, increasing the risk of NAFLD. It has been proven that NS might lead to chronic kidney disease (CKD), and there have been studies suggesting increased prevalence of NAFLD in patients with CKD[4,25-27]. However, there have been few studies, if any, correlating prevalence of NAFLD in patients with NS. Given the increasing prevalence of NAFLD and associated morbidity and mortality, identification of at-risk patients is essential for targeted monitoring and treatment. Since NAFLD and NASH often do not cause any symptoms, surveillance strategies for at-risk patients might aid in early diagnosis and help prevent adverse outcomes. Since both NAFLD and NS are associated with elevated circulating lipids, patients with NS might be at risk for NAFLD, especially if they have other risk factors for NAFLD such as diabetes mellitus, obesity, or steroid use. It is essential to know if NS itself can be a risk factor for NAFLD, since only then can cost-effectiveness and usefulness of any surveillance and preemptive strategies be commented on. Furthermore, if patients with NS are at increased risk of NAFLD, more aggressive approach towards controlling other NAFLD risk factors and reducing use of certain medications such as steroids might be warranted. Therefore, we conducted a study with the aim of assessing the prevalence as well as risk factors of NAFLD in patients with NS.
Our cohort’s data were obtained using a validated, multicenter and daily-updated database called Explorys (Explorys Inc, Cleveland, OH, United States) developed by IBM Corporation, Watson Health [IBM corporation]. Explorys consists of electronic health records of 26 different healthcare systems with a total of about 360 hospitals and more than 70 million patients across the United States. Explorys utilizes Systematized Nomenclature of Medicine-Clinical Terms (SNOMED-CT) for the definition of the diseases. The diagnosis is made by individual health care providers and the collected data is then uploaded into the database in the form of SNOMED-CT codes. The database pools large outpatient as well as inpatient deidentified data that can be formulated into numerous cohorts according to the clinical element being studied. Explorys does not record individual patient data such as laboratory or imaging results. Since the data is pooled from multiple organizations, different organizations, and by extension health care providers, may differ in method of diagnoses of various medical conditions. The way the database is established, assessment of the method of diagnoses is not feasible, and thus the database is largely dependent on individual organizations providing accurate data. The approval of Institutional Review Board is not required since Explorys is a Health Insurance Portability and Accountability Act (HIPAA)-compliant platform. Use of this database has been validated in multiple fields including cardiology, hematology and gastroenterology.
A cohort of patients with a SNOMED-CT diagnosis of “Non-Alcoholic Fatty Liver Disease” and “Nephrotic syndrome” between 1999 and May 2022 was identified. Inclusion criteria were age ≥ 18 years, presence of NAFLD, presence of NS. There were no specific exclusion criteria.
We collected age > 65 years, gender and Caucasian race as variables. Confounding factors associated with NAFLD and NS were also identified and collected if SNOMED-CT diagnoses were available. These were obesity, diabetes mellitus type 2, metabolic syndrome, dyslipidemia, chronic kidney disease and hypothyroidism.
To account for confounding from the covariates listed above, we conducted 1024 searches to explore every probability, with NS as one of the variables. A univariate analysis was conducted initially for all the variables, followed by multivariate analysis. Statistical analysis was performed using R and RStudio (version 1.4.1717), and for all analyses, a 2-sided P value of < 0.05 was considered statistically significant. Multivariate analysis was performed to adjust for multiple factors including age ≥ 65 years, gender, caucasian race, obesity, diabetes mellitus type 2, metabolic syndrome, dyslipidemia, chronic kidney disease and hypothyroidism. The study was reviewed by our expert biostatistician Antoine Boustany, MD, MPH, MEM.
Among the 78734750 individuals screened in this database, there were a total of 49700 subjects with NAFLD. Most subjects with NAFLD were between the age of 18-65 years, with female affected more than males. Interestingly, while majority of subjects were Caucasians, 5% were African Americans. About half the patients with NAFLD had BMI ≥ 30, with the prevalence of NAFLD rising with the increase in BMI (Table 1). In univariate analysis, the odds of having NAFLD with age ≥ 65 years was 2.18 [95% confidence interval (95%CI) 2.15-2.22], while it was also high in females [odds ratio (OR) 1.18, 95%CI 1.16-1.20], Caucasians (OR 3.62, 95%CI 3.55–3.69), subjects with NS (OR 14.84, 95%CI 13.67-16.10), type 2 diabetes mellitus (OR 17.05, 95%CI 16.78-17.32), hypothyroidism (OR 6.99, 95%CI 6.87-7.11), dyslipidemia (OR 13.61, 95%CI 13.38-13.84), obesity (OR 19.19, 95%CI 18.89-7.11), metabolic syndrome (OR 29.09, 95%CI 28.26-29.95) and CKD (OR 9.05, 95%CI 8.88-9.22). In multivariate analysis, the odds of having NAFLD amongst patients with nephrotic syndrome was 1.85 (95% Cl 1.70-2.02), while the odds also remained high in patients that have type 2 diabetes mellitus (OR 3.84), hypothyroidism (OR 1.57), obesity (OR 5.10), hyperlipidemia (OR 3.09), metabolic syndrome (OR 3.42) and CKD (OR 1.33) (Figure 1).
| Parameters | NAFLD, n (%) | No NAFLD, n (%) | P value |
| Age, yr | < 0.00001 | ||
| Adults 18-65 | 30980 (62.33) | 56486180 (71.79) | |
| Seniors > 65 | 18720 (37.67) | 22198870 (28.21) | |
| Gender | < 0.00001 | ||
| Male | 20640 (41.53) | 35921730 (45.65) | |
| Female | 29060 (58.47) | 42763320 (54.35) | |
| Race | < 0.00001 | ||
| Caucasian | 39420 (79.32) | 40569460 (51.56) | |
| African American | 2550 (5.13) | 7765730 (9.87) | |
| Hispanic/Latino | 790 (1.59) | 1037520 (1.32) | |
| Other | 6940 (13.96) | 29312340 (37.25) | |
| BMI | < 0.00001 | ||
| < 18.5 | 1180 (2.38) | 3610880 (4.59) | |
| 18.5-24.9 | 7860 (15.81) | 13727720 (17.45) | |
| 25.0-29.9 | 16810 (33.82) | 13117450 (16.67) | |
| > 30.0 | 23850 (47.99) | 48229000 (61.29) | |
| Type 2 diabetes mellitus | 24830 (49.95) | 4526510 (5.75) | < 0.00001 |
| Metabolic syndrome | 3640 (7.32) | 205830 (0.26) | < 0.00001 |
| Hyperlipidemia | 33130 (66.65) | 10,152,960 (12.90) | < 0.00001 |
| Nephrotic syndrome | 100 (0.14) | 17300 (0.02) | < 0.00001 |
| Hypothyroidism | 11930 (24.00) | 3472880 (4.41) | < 0.00001 |
| Chronic kidney disease | 13485 (27.13) | 2347230 (2.98) | < 0.00001 |
| Total | 49700 | 78685050 |
With the high prevalence of NAFLD and its associated complications, there is worldwide interest in learning more about the disease and its associations with other systemic illnesses. To date despite extensive research, we were unable to find another study reporting the prevalence of NAFLD in patients with NS. Two prospective studies conducted by Targher et al[28,29], one in patients with type 1 diabetes mellitus (T1DM) and the other in T2DM, to assess the development of CKD in patients with NAFLD did not report development of NS in any patient over a follow-up period of 5.2 years and 6.5 years, respectively. In our study, patients with NS were frequently found to have NAFLD. One explanation is that impairment in lipid metabolism in NS promotes development of NAFLD. However, further studies are needed to explore this possibility.
In contrast, there have been several studies assessing renal impairment in patients with NAFLD. The results of these studies have been contradictory. Musso et al[10] conducted a systematic review and meta-analysis of articles published through 1980 -2014 and showed that NAFLD was associated with increase in prevalence as well as incidence of CKD [odds ratio (OR) 2.12, 95%CI 1.69-2.66; and hazard ratio (HR) 1.79, 95%CI 1.65-1.95, respectively]. Furthermore, NASH was associated with a higher prevalence and incidence of CKD (OR 2.53, 95%CI 1.58-4.05; and HR 2.12, 95%CI 1.42-3.17, respectively) than simple steatosis[10]. Our study had similar results, with increased odds of having CKD in patients with NAFLD, which remained significant on multivariate analysis.
In comparison, two studies by Targher et al[29], one conducted in patients with type 2 diabetes mellitus and the other in type 1 diabetes mellitus, showed that patients with NAFLD had lower estimated glomerular filtration rate and increased incidence of CKD as compared to patients without NAFLD In contrast, a study by Sirota et al[30] conducted on the National Health And Nutrition Examination Survey III (NHANES III) data showed increased prevalence of NAFLD in patients with CKD, which was not significant after adjusting for certain risk factors. One possible explanation for these discrepancies is that the prevalence of NAFLD in CKD may be driven by race, which was adjusted for in the latter study but not the former one. In our study, the prevalence of NAFLD remained significant in patients with CKD, even on multivariate analysis and adjusting for Caucasian race. The reason for this discrepancy is unclear, although a larger sample size in our cohort might have played a role.
With regards to factors associated with NAFLD, our study concluded that patients with type 2 DM, obesity, hypothyroidism, metabolic syndrome and hyperlipidemia have higher prevalence of NAFLD, even on multivariate analysis, which is similar to studies done elsewhere[6,28,29,31]. One interesting finding was that 5% of patients with NAFLD in our cohort identified as African American, which is consistent with low prevalence of NAFLD in this population as reported in the literature[32]. In our study, the prevalence of NAFLD increased as BMI rose, with a prevalence of 48% in subjects with BMI ≥ 30 as compared to 33.82%, 15.81%, and 2.38% in patients with BMI 25.0-29.9, 18.5-24.9, and < 18.5, respectively. Similar results have been observed in literature, with one study by Loomis et al[6], demonstrating a strong and striking near-linear relationship between BMI and future risk of recorded NAFLD.
Our study has several strengths. To the best of our knowledge, this is the first study to assess the prevalence of NAFLD in patients with NS. Being a multicenter study with a large sample size derived from the United States population, our results are reliable and generalizable. We assessed several common risk factors, and our study showed that these factors were independently associated with increased prevalence of NAFLD, which have been well documented in the literature.
Limitation to our study includes its retrospective nature and inability to establish causality. Being a database study, there is always a concern regarding selection bias. Furthermore, given that this database is HIPAA-compliant and anonymous, it is not possible to verify the accuracy of the diagnoses made. Hence, further in-depth analysis is not feasible. Also, certain NAFLD risk factors such as presence of elevated uric acid levels and pharmacological interventions such as corticosteroid use, hormonal therapy, certain chemotherapeutic agents, etc. could not be assessed.
Our study demonstrates that patients with NS are frequently found to have NAFLD, even when adjusting for common risk factors including CKD. Females and subjects with age 18-65 years were most commonly affected with NAFLD, with most subjects being Caucasians and only 5% were African American. The American Association for the Study of Liver Disease still recommends against routine screening for NAFLD in any population[1]. Further studies are needed to assess the relationship between NS and NAFLD. While lipid metabolism is abnormal in both these diseases, whether these diseases develop independently of each other or through a common pathway needs to be further explored. Clinicians should be aware of the increased prevalence of NAFLD in this patient population.
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver disease worldwide, with hyperlipidemia as one of its risk factors. Nephrotic syndrome (NS) is known to cause hyperlipidemia. Since both NAFLD and NS patients are known to have abnormalities in lipid metabolism, patients with NS might be at increased risk of developing NAFLD.
Given the increasing prevalence of NAFLD and associated morbidity and mortality, assessment of risk factors for targeted surveillance is warranted. This might help in early diagnosis of NAFLD and improve outcomes. We hypothesized that the excess synthesized and circulating lipids in patients with NS affect fat metabolism in the liver, increasing the risk of NAFLD.
To conduct a cross-sectional population-based study to assess the prevalence of NAFLD in patients with NS while adjusting for common risk factors.
A large multicenter database (Explorys Inc., Cleveland, OH, United States) was utilized for this study. A cohort of patients with a diagnosis of “Non-Alcoholic fatty liver disease” was identified. Inclusion criteria were age ≥ 18 years, presence of NAFLD, presence of NS. There were no specific exclusion criteria. Univariate and multivariate analyses were performed to adjust for multiple risk factors including age, gender, Caucasian race, nephrotic syndrome, type II diabetes mellitus, hypothyroidism, dyslipidemia, obesity, metabolic syndrome and chronic kidney disease. Statistical analysis was conducted using R, and for all analyses, a 2-sided P value of < 0.05 was considered statistically significant.
In multivariate analysis, the odds of having NAFLD amongst patients with NS was 1.85 (95%Cl 1.70-2.02), while the odds also remained high in patients that have type 2 diabetes mellitus (OR 3.84), hypothyroidism (OR 1.57), obesity (OR 5.10), hyperlipidemia (OR 3.09), metabolic syndrome (OR 3.42) and chronic kidney disease (CKD) (OR 1.33).
Our study demonstrates that patients with NS are frequently found to have NAFLD, even when adjusting for common risk factors including CKD. Further studies are required to confirm these findings, investigate causality and assess the utility of surveillance strategies for NAFLD in patients with NS.
Studies assessing associations of NAFLD with other diseases can help identify at-risk populations that may benefit from routine screening. While patients with NS seem to have higher prevalence of NAFLD, further research is required to assess if routine surveillance of patients with NS is cost-effective and improves outcomes.
First of all, I would like to express my deepest appreciation to the editorial team and peer reviewers of World Journal of Hepatology (WJH) for making the manuscript process an efficient, smooth and easy one. Also, I want to thank other authors, I would have not completed this piece without their brilliant contributions, moral support and editing help. Finally, I would like to Thank God for providing me with the resilience to complete this work despite the step-backs encountered along the way.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology, No. 64278.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isac S, Romania; Wu SZ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 2. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 938] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 3. | Guo C, Guo X, Rong Y, Guo Y, Zhang L. Gene Expression Characteristics of Liver Tissue Reveal the Underlying Pathogenesis of Hepatocellular Carcinoma. Biomed Res Int. 2021;2021:9458328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 5. | Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med. 2019;286:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, Desai J, Gill JM, Welsh P, Waterworth D, Sattar N. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016;101:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2294] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 8. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1758] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 9. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1217] [Article Influence: 304.3] [Reference Citation Analysis (0)] |
| 10. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 525] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 11. | Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 13. | Zhang S, Du T, Li M, Jia J, Lu H, Lin X, Yu X. Triglyceride glucose-body mass index is effective in identifying nonalcoholic fatty liver disease in nonobese subjects. Medicine (Baltimore). 2017;96:e7041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 433] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 15. | Tapia C, Bashir K. Nephrotic Syndrome. 2022 Jun 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 16. | Jackson SP, Calkin AC. The clot thickens--oxidized lipids and thrombosis. Nat Med. 2007;13:1015-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lucero D, Miksztowicz V, Gualano G, Longo C, Landeira G, Álvarez E, Zago V, Brites F, Berg G, Fassio E, Schreier L. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin Chim Acta. 2017;473:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Liang K, Vaziri ND. Acquired VLDL receptor deficiency in experimental nephrosis. Kidney Int. 1997;51:1761-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, Xie Y, Jiang Y, Yang P, Tang R, Pan Q, Hall AR, Luong TV, Fan J, Varghese Z, Moorhead JF, Pinzani M, Chen Y, Ruan XZ. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol. 2018;69:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 21. | Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 799] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 24. | Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, Iezzoni JC, Hespenheide EE, Al-Osaimi A, Peterson TC. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Non-alcoholic Fatty Liver Disease Study Group; Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L, Targher G. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 26. | Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Targher G, Byrne CD. Diagnosis and management of nonalcoholic fatty liver disease and its hemostatic/thrombotic and vascular complications. Semin Thromb Hemost. 2013;39:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, Franchini M, Zoppini G, Muggeo M. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol. 2008;19:1564-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, Zenari L, Bonora E. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988-1994. Am J Nephrol. 2012;36:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Bano A, Chaker L, Plompen EP, Hofman A, Dehghan A, Franco OH, Janssen HL, Darwish Murad S, Peeters RP. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J Clin Endocrinol Metab. 2016;101:3204-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Bonacini M, Kassamali F, Kari S, Lopez Barrera N, Kohla M. Racial differences in prevalence and severity of non-alcoholic fatty liver disease. World J Hepatol. 2021;13:763-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (2)] |