Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.225
Peer-review started: June 27, 2022
First decision: July 25, 2022
Revised: August 2, 2022
Accepted: January 13, 2023
Article in press: January 13, 2023
Published online: February 27, 2023
Processing time: 241 Days and 19.7 Hours
Cirrhosis and its complications develop in a subgroup of patients with non-alcoholic fatty liver disease (NASH). Early detection of liver fibrosis represents an important goal of clinical care.
To test the hypothesis that the development of cirrhosis in nonalcoholic fatty liver disease patients is preceded by the long-term trends of platelet counts and Fib-4 scores.
We identified all patients in our healthcare system who had undergone fibrosis staging by liver biopsy or magnetic resonance elastography (MRE) for non-alcoholic fatty liver disease during the past decade (n = 310). Platelet counts, serum glutamic-pyruvic transaminase and serum glutamic oxalacetic transaminase values preceding the staging tests were extracted from the electronic medical record system, and Fib-4 scores were calculated. Potential predictors of advanced fibrosis were evaluated using multivariate regression analysis.
Significant decreases in platelet counts and increases in Fib-4 scores were observed in all fibrosis stages, particularly in patients with cirrhosis. In the liver biopsy group, the presence of cirrhosis was best predicted by the combination of the Fib-4 score at the time closest to staging (P < 0.0001), the presence of diabetes (P = 0.0001), and the correlation coefficient of the preceding time-dependent drop in platelet count (P = 0.044). In the MRE group, Fib4 score (P = 0.0025) and platelet drop (P = 0.0373) were significant predictors. In comparison, the time-dependent rise of the Fib-4 score did not contribute in a statistically significant way.
Time-dependent changes in platelet counts and Fib-4 scores contribute to the prediction of cirrhosis in NASH patients with biopsy- or MRE-staged fibrosis. Their incorporation into predictive algorithms may assist in the earlier identification of high-risk patients.
Core Tip: Our study is based on the well-known phenomenon of declining platelet counts in patients who develop cirrhosis, including those with underlying non-alcoholic fatty liver disease (NASH). This phenomenon has resulted in several recent publications using large health registries to show that progressive changes in non-invasive fibrosis scores preceded the ICD 9-based diagnoses of cirrhosis. These studies raised the issue of “predictability” of cirrhosis development. Our analysis extends these studies by examining a smaller, well-defined NASH patient population. Unlike previous studies, we included ALL fibrosis stages, provided that patients had undergone definitive staging by liver biopsy or magnetic resonance elastography. Our data unequivocally confirm that progressive thrombocytopenia and an increase in the Fib-4 scores precedes the diagnosis of cirrhosis. Moreover, the kinetics of the platelet drop add to the prediction of cirrhosis, suggesting that the time-dependent decrease in platelet counts may have true predictive power.
- Citation: Zijlstra MK, Gampa A, Joseph N, Sonnenberg A, Fimmel CJ. Progressive changes in platelet counts and Fib-4 scores precede the diagnosis of advanced fibrosis in NASH patients. World J Hepatol 2023; 15(2): 225-236
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/225.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.225
The incidence of non-alcoholic fatty liver disease (NAFLD) induced cirrhosis and its complications are rising in the US and worldwide[1,2]. Due to its indolent clinical course, advanced fibrosis and cirrhosis may go undiagnosed for years or even decades[3]. Frequently, patients are first referred to a hepatologist when they already present with signs and symptoms of decompensation, including portal hypertension, synthetic dysfunction, or hepatocellular cancer. At such stage, patients are typically older and suffer from multisystem comorbidities[4], resulting in their ineligibility for liver transplantation or aggressive cancer treatment regimens with poor outcomes[5]. An early diagnosis of liver fibrosis would allow for appropriate disease surveillance, timely interventions for complications, and improved long-term survival[6].
Due to the advent of electronic medical record (EMR) systems, physicians can easily analyze long-term trends of patients’ demographic, clinical, and laboratory data. Automated machine learning methods are being developed to predict and monitor progression of a wide range of disease states[7]. Recent reports suggest that time-dependent trends of platelet count and Fib-4 scores – extracted from the patients’ electronic medical record system - might help predict the occurrence of advanced liver fibrosis and cirrhosis[8-10]. We tested our hypothesis in a long-term follow-up study of patients in our healthcare system prior to undergoing a liver biopsy or magnetic resonance elastography for staging of their non-alcoholic fatty liver disease.
The study protocol (EH 21-163) conformed with the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Institutional Review Board of NorthShore University Health System. Informed consent and HIPAA authorization requirements were waived.
We searched the NorthShore EPIC patient database to identify all patients who had undergone a liver biopsy or magnetic resonance elastography for the assessment of non-alcoholic fatty liver disease during the time period between February of 2010 and October of 2020. A chart review was performed for each patient to ascertain the correct diagnosis, and to extract clinical, demographic, laboratory, liver biopsy, and magnetic resonance elastography (MRE) data. When necessary, liver biopsies were reviewed by a trained hepato-pathologist (N.J.) to determine the fibrosis stage, using the NASH Clinical Research Network criteria[11]. Samples subclassified as fibrosis stages 1A, 1B, or 1C were combined under “stage 1”. The MRE measurements were performed using a Siemens Magnetom Aera 1.5T scanner. MRE liver stiffness measurements were stratified into five groups (0-2.9 kPa, 2.9-3.5 kPa, 3.5-4.0 kPa, 4.0-5.0 kPa, and >5.0 kPa), following published guidelines[12].
Time-dependent changes in platelet counts and Fib-4 scores were analyzed in subgroups of patients in whom data were available for a minimum time period of five years (biopsy group: n = 120 for platelet count, n = 105 for Fib-4, MRE group: n = 79 for platelet count, n = 75 for Fib-4, respectively). Representative values were calculated as the mean of all available measurements for each year. No attempts were made to replace missing data.
In the statistical analysis, liver fibrosis scores constituted the primary outcome variable. In two separate groups of patients, liver fibrosis was ranked 0 through 4 based on histopathology of liver biopsy specimens, or on numeric scores obtained through magnetic resonance elastography (MRE). The presence or absence of categorical variables, such as gender or hypertension, in patients with different fibrosis scores were compared using chi-squared tests. In the final analysis, smoking was also entered as dichotomous variable, with former and current smokers being grouped together. Differences in body mass index (BMI) or laboratory values between two patient subgroups were compared using t-tests. For each individual patient, linear regression analysis was used to calculate the correlation coefficients between passage of time and consecutive platelet counts or Fib-4 scores, respectively. Multivariable least-squares linear regression analyses were used to test the joint influence of multiple predictor variables on the occurrence of the outcome variable (liver fibrosis). The list of predictor variables included the last Fib-4 score, BMI, sex, two individual correlation coefficients associated with time-dependent changes of platelet counts or Fib-4 scores, presence or absence of diabetes mellitus, hypertension, and smoking. Patients with follow-up periods shorter than one year or with less than 3 consecutive platelet counts or Fib-4 scores were excluded from the time trend analyses.
A total of 317 patients were identified in the initial search of the patient data file. Seven patients assigned to the liver biopsy group were excluded from the analysis, due to inadequate tissue sampling (n = 3) or lack of procedural documentation (n = 4).
The remaining 310 patients were entered into the analysis. Between February 2010 and October 2020, 203 patients underwent liver biopsy for a diagnosis of non-alcoholic fatty liver disease. Between April 2015 and May 2021, 107 patients underwent MRE for the same indication. In 165/203 (81%) of patients with liver biopsy, the overall length of follow-up within the health system was longer than one year, with an average (SD) of 7.9 ± 3.9 years. In 94/107 (88%) of patients with MRE, the overall length of follow-up was longer than one year, with an average of 10.2 ± 4.6 years, resulting in a final combined sample size of 259.
Patients undergoing liver biopsies were stratified by their fibrosis scores and their demographic and clinical characteristics (Table 1). Except for random fluctuations, the five subgroups did not differ with respect to gender, ethnicity, and smoking habits. Patient ages tended to increase with fibrosis stages. The frequency of diabetes mellitus and hypertension appeared to increase with rising fibrosis stage. No obvious pattern was revealed with respect to BMI, serum glutamic oxalacetic transaminase (SGOT), or serum glutamic-pyruvic transaminase (SGPT). Average platelet counts decreased, and average Fib-4 scores increased, respectively, with increasing fibrosis stages. The average correlation coefficient for the time dependent changes in platelet counts of individual patients was negative in all five subgroups, consistent with an overall drop in the platelet counts. In absolute terms, this drop was most pronounced in patients with stage 4 fibrosis. The average correlation coefficient for the time-dependent changes in Fib-4 scores was positive in all five subgroups, consistent with an overall rise in the Fib-4 scores.
| Fibrosis 0 | Fibrosis 1 | Fibrosis 2 | Fibrosis 3 | Fibrosis 4 | ||||||
| (% or SD) | (% or SD) | (% or SD) | (% or SD) | (% or SD) | ||||||
| Total (N) | 36 | (100) | 41 | (100) | 27 | (100) | 26 | (100) | 73 | (100) |
| Follow-up > 1 yr | 29 | (81) | 32 | (78) | 22 | (81%) | 23 | (88%) | 59 | (81%) |
| Follow-up (mean, yr) | 7.1 | (4.32) | 7.3 | (4.03) | 8.6 | (3.61) | 8.4 | (3.36) | 8.2 | (3.99) |
| Age (mean, yr) | 44.6 | (15.7) | 47.6 | (14.7) | 55.9 | (10.6) | 55.6 | (13.2) | 58.0 | (11.7) |
| Sex | ||||||||||
| Male (N) | 16 | (44) | 25 | (61) | 13 | (48) | 12 | (46) | 45 | (62) |
| Female (N) | 20 | (56) | 16 | (39) | 14 | (52) | 14 | (54) | 28 | (38) |
| Ethnicity | ||||||||||
| White (N) | 23 | (64) | 22 | (54) | 15 | (56) | 20 | (77) | 54 | (74) |
| African American (N) | 0 | (0) | 0 | (0) | 1 | (4) | 0 | (0) | 0 | (0) |
| Hispanic (N) | 6 | (17) | 6 | (15) | 4 | (15) | 3 | (12) | 9 | (12) |
| Asian American (N) | 5 | (14) | 11 | (27) | 5 | (19) | 3 | (12) | 9 | (12) |
| Other (N) | 2 | (6%) | 2 | (5) | 2 | (7) | 0 | (0) | 1 | (1) |
| Smoker | ||||||||||
| Yes (N) | 5 | (14) | 1 | (2) | 5 | (19) | 0 | (0) | 6 | (8) |
| No (N) | 26 | (72) | 30 | (73) | 16 | (59) | 20 | (77) | 38 | (52) |
| Former (N) | 5 | (14) | 10 | (24) | 6 | (22) | 6 | (23) | 29 | (40) |
| Diabetes mellitus | ||||||||||
| Yes (N) | 8 | (22) | 14 | (34) | 12 | (44) | 10 | (38) | 42 | (58) |
| No (N) | 28 | (78) | 27 | (66) | 15 | (56) | 16 | (62) | 31 | (42) |
| Hypertension | ||||||||||
| Yes (N) | 14 | (39) | 20 | (49) | 16 | (59) | 15 | (58) | 49 | (67) |
| No (N) | 22 | (61) | 21 | (51) | 11 | (41) | 11 | (42) | 24 | (33) |
| BMI (last) | 30 | (7) | 32 | (7) | 31 | (7) | 31 | (7) | 33 | (7) |
| Laboratory results | ||||||||||
| SGOT (last, IU/L) | 90 | (82) | 78 | (39) | 69 | (36) | 88 | (51) | 80 | (70) |
| SGPT (last, IU/L) | 126 | (86) | 124 | (57) | 89 | (52) | 113 | (87) | 79 | (68) |
| Platelets (last, 1000/mL) | 259 | (87) | 242 | (64) | 229 | (75) | 206 | (73) | 167 | (65) |
| Fib-4 score (last) | 1.7 | (1.8) | 1.7 | (1.1) | 2.4 | (2.4) | 2.5 | (1.1) | 3.7 | (2.8) |
| Correlation-plts (mean) | -0.10 | (0.48) | -0.22 | (0.66) | -0.31 | (0.61) | -0.18 | (0.52) | -0.56 | (0.42) |
| Correlation-Fib-4 (mean) | 0.43 | (0.50) | 0.36 | (0.46) | 0.49 | (0.45) | 0.42 | (0.47) | 0.43 | (0.45) |
Patient undergoing fibrosis staging by MRE were stratified by their liver stiffness and demographic and clinical characteristics (Table 2). No obvious pattern was discernible among the five subgroups with respect to their demographic and clinical characteristics. The average platelet counts decreased, and the average Fib-4 scores increased with increasing liver stiffness scores. The average correlation coefficient for the time dependent changes in platelet counts of individual patients was negative in all five subgroups, consistent with an overall drop in the platelet counts. In absolute terms, this drop became more pronounced with increasing MRE fibrosis scores. The average correlation coefficient for the time-dependent changes in Fib-4 scores was positive in all five subgroups, indicating an overall rise in the Fib-4 scores. The rise magnitude of the rise was more pronounced in the subgroups with high than low MRE fibrosis scores.
| MRE 0-2.9 | MRE 2.9-3.5 | MRE 3.5-4 | MRE 4-5 | MRE 5+ | ||||||
| (% or SD) | (% or SD) | (% or SD) | (% or SD) | (% or SD) | ||||||
| Total (N) | 48 | (100%) | 15 | (100%) | 6 | (100%) | 13 | (100%) | 24 | (100%) |
| Follow-up > 1 yr (N) | 42 | (88%) | 12 | (80%) | 6 | (100%) | 10 | (77%) | 23 | (96%) |
| Follow-up (mean, yr) | 9.8 | (4.87) | 9.4 | (4.87) | 12.4 | (4.87) | 10.7 | (4.87) | 10.6 | (4.87) |
| Age (mean, yr) | 57.8 | (15.4) | 61.6 | (13.5) | 63.0 | (9.0) | 61.5 | (8.3) | 60.6 | (13.4) |
| Sex | ||||||||||
| Male (N) | 17 | (35%) | 5 | (33%) | 5 | (83%) | 6 | (46%) | 11 | (46%) |
| Female (N) | 31 | (65%) | 10 | (67%) | 1 | (17%) | 7 | (54%) | 13 | (54%) |
| Ethnicity | ||||||||||
| White (N) | 30 | (63%) | 8 | (53%) | 4 | (67%) | 10 | (77%) | 15 | (63%) |
| African American (N) | 2 | (4%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 1 | (4%) |
| Hispanic (N) | 6 | (13) | 3 | (20) | 1 | (17) | 1 | (8) | 5 | (21) |
| Asian American (N) | 9 | (19) | 1 | (7) | 0 | (0) | 1 | (8) | 1 | (4) |
| Other (N) | 1 | (2) | 3 | (20) | 1 | (17) | 1 | (8) | 2 | (8) |
| Smoker | ||||||||||
| Yes (N) | 2 | (4) | 1 | (7) | 0 | (0) | 1 | (8) | 1 | (4) |
| No (N) | 28 | (58) | 11 | (73) | 6 | (100) | 5 | (38) | 12 | (50) |
| Former (N) | 18 | (38) | 3 | (20) | 0 | (0) | 7 | (54) | 11 | (46) |
| Diabetes mellitus | ||||||||||
| Yes (N) | 14 | (29) | 9 | (60) | 4 | (67) | 9 | (69) | 15 | (63) |
| No (N) | 34 | (71) | 6 | (40) | 2 | (33) | 4 | (31) | 9 | (38) |
| Hypertension | ||||||||||
| Yes (N) | 29 | (60) | 12 | (80) | 5 | (83) | 8 | (62) | 16 | (67) |
| No (N) | 19 | (40) | 3 | (20) | 1 | (17) | 5 | (38) | 8 | (33) |
| BMI (mean, last) | 33 | (7) | 32 | (8) | 38 | (7) | 35 | (6) | 33 | (6) |
| Laboratory results | ||||||||||
| SGOT (last, IU/L) | 36 | (18) | 52 | (41) | 63 | (32) | 91 | (92) | 60 | (40) |
| SGPT (last, IU/L) | 48 | (29) | 57 | (48) | 79 | (34) | 101 | (88) | 59 | (43) |
| Platelets (last, 1000/mL) | 244 | (60) | 231 | (62) | 205 | (39) | 201 | (74) | 182 | (75) |
| Fib-4 score (last) | 1.4 | (0.9) | 1.8 | (1.0) | 2.6 | (1.5) | 2.6 | (1.1) | 3.5 | (2.0) |
| MRE fibrosis score (mean) | 2.5 | (0.3) | 3.2 | (0.2) | 3.7 | (0.1) | 4.3 | (0.2) | 8.2 | (3.5) |
| Correlation-plts (mean) | -0.06 | (0.43) | -0.06 | (0.43) | -0.28 | (0.45) | -0.44 | (0.33) | -0.64 | (0.35) |
| Correlation-Fib-4 (mean) | 0.44 | (0.43) | 0.44 | (0.43) | 0.66 | (0.19) | 0.61 | (0.35) | 0.68 | (0.32) |
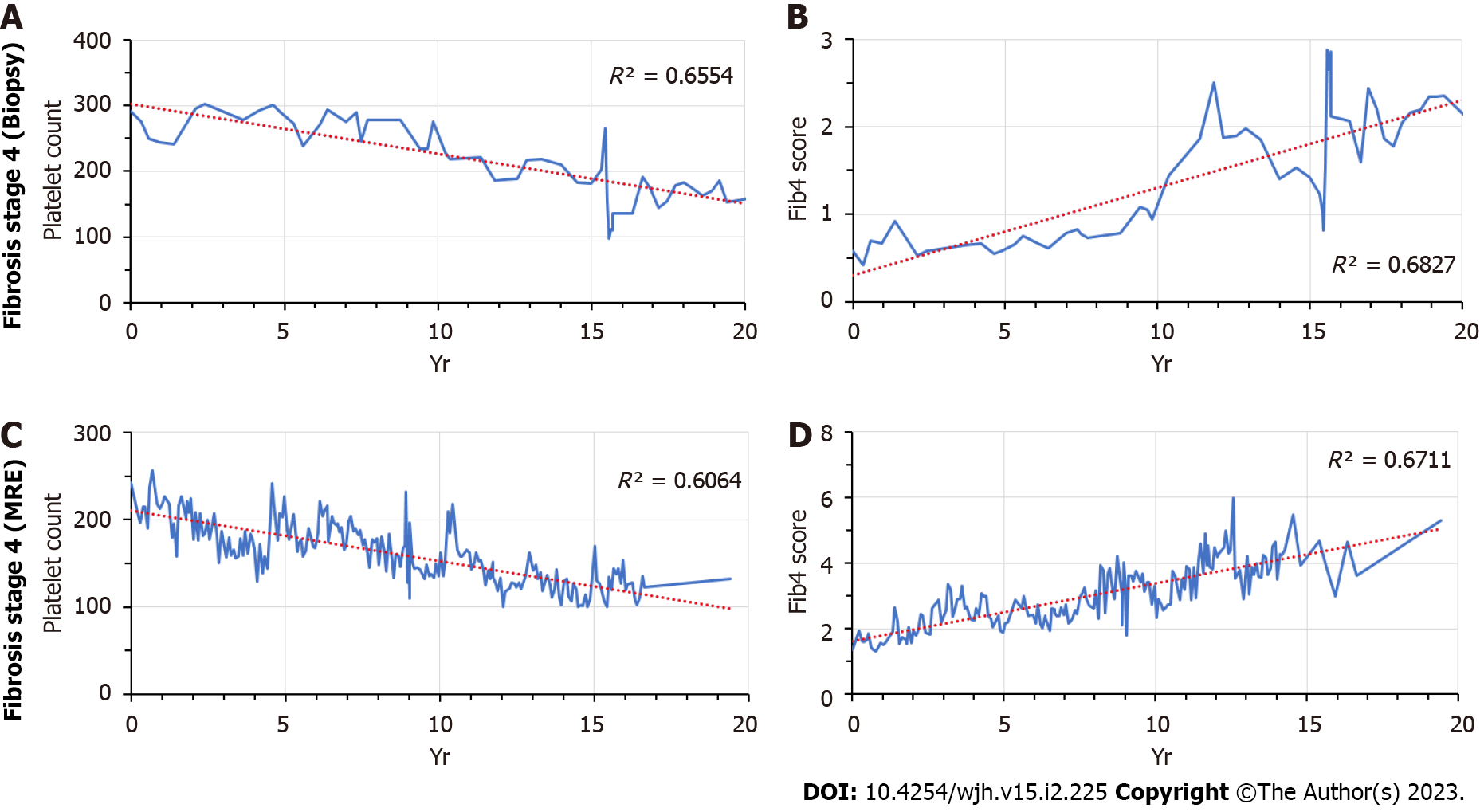
We identified two patients (one from each cohort, respectively) for whom longitudinal data were available over a 20-year time period. In both patients alike, platelet counts started to fall, and Fib-4 scores started to rise several years prior to the clinical diagnosis of cirrhosis (Figure 1).
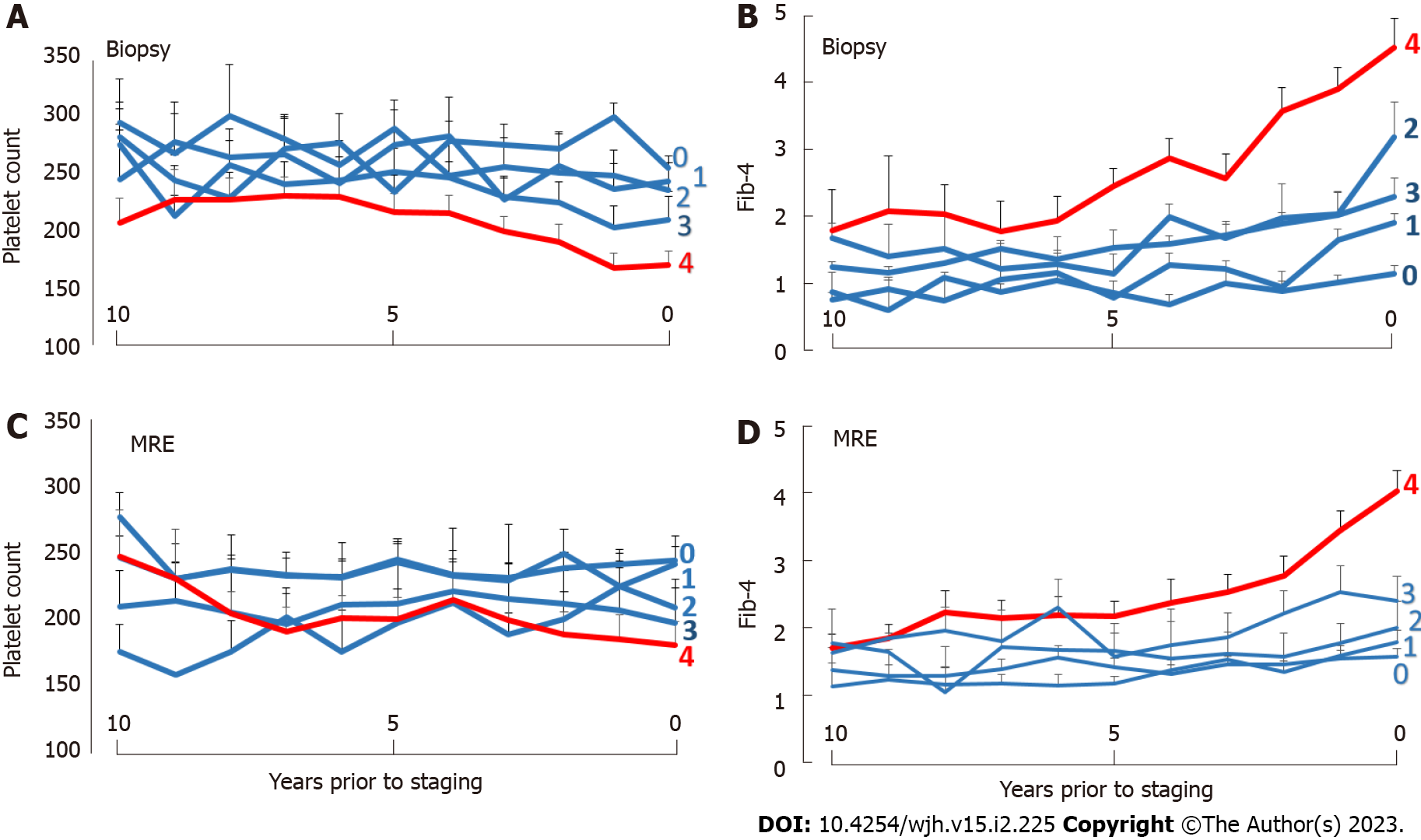
The temporal changes of platelet counts and Fib-4 scores according to biopsy- or MRE-determined fibrosis stages were analyzed for the ten-year time period prior to staging. Progressive decreases in platelet counts and increases in Fib-4-scores were apparent in patients with the biopsy-documented stage 4 fibrosis or a liver stiffness of > 5.0 kPa on MRE, respectively (Figure 2).
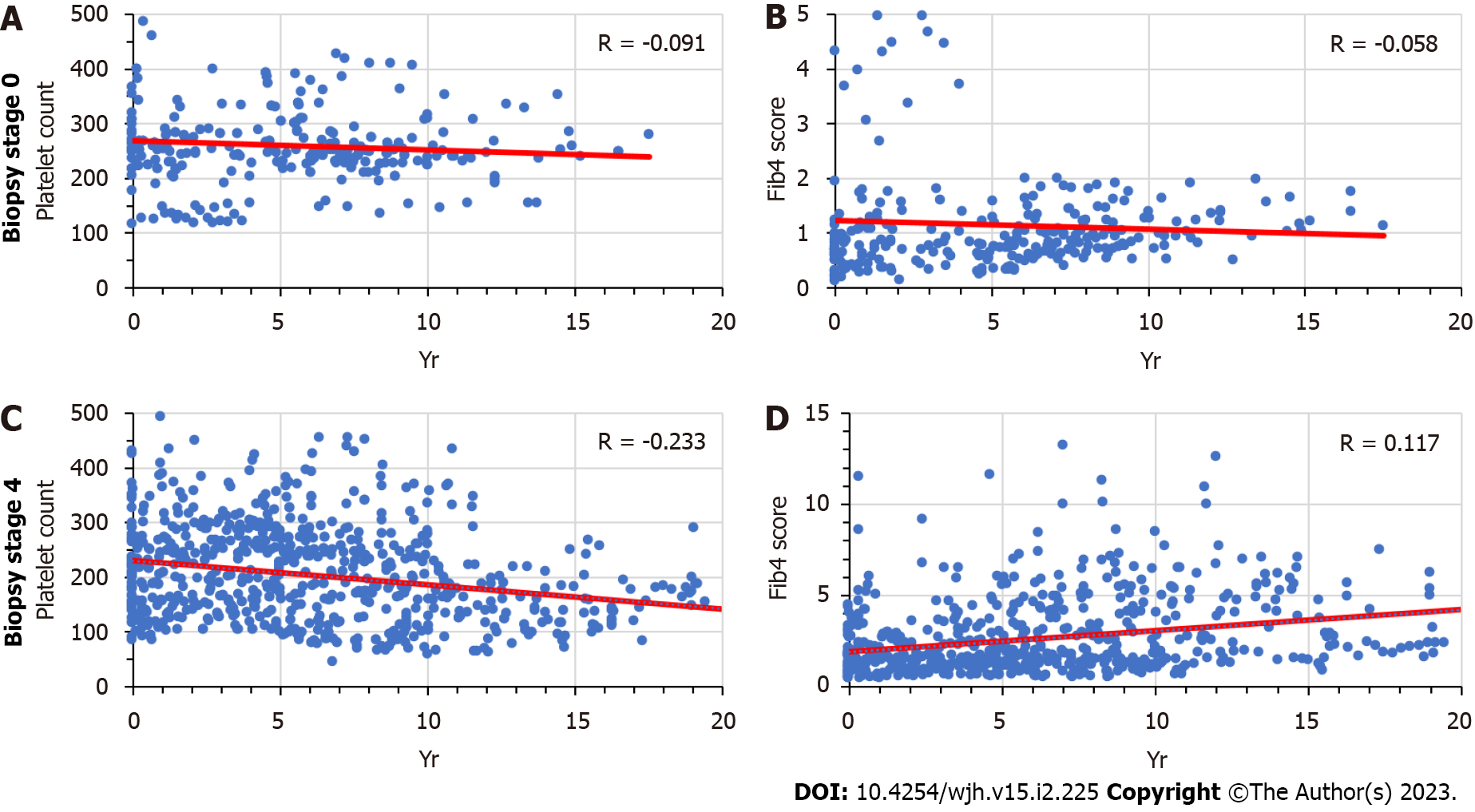
We analyzed the time trends of platelet counts and Fib-4 sores in patients with biopsy-confirmed stage 0 or stage 4 fibrosis who had been followed for a minimum of one year (Figure 3). The overall trends show no significant correlations in stage 0 liver fibrosis, as compared to a significant time-dependent decline of platelet counts in stage 4 liver fibrosis, as well as a significant time-dependent rise of Fib-4 scores. The correlation coefficients shown in the graph vary from those shown in Table 1, because they were based on all patient data analyzed jointly in a single regression analysis, whereas the average values in Table 1 were calculated as an average of many individual correlation coefficients. Based on the varying time intervals of pre-staging testing and the inter-patient variability in time-dependent increases or decreases, the trends of individual patients may mask or cancel each other out in the joint analysis. The results for patients with stage 2 or 3 were similar (data not shown). Their individual data fell within the range outlined by the two extremes shown in Figure 2, as the slopes of the two regression lines for platelet counts and Fib-4 scores tended to become increasingly steeper with increasing fibrosis stage. This pattern supports the conclusion that the occurrence of liver fibrosis was preceded by a long-term gradual fall in platelet counts.
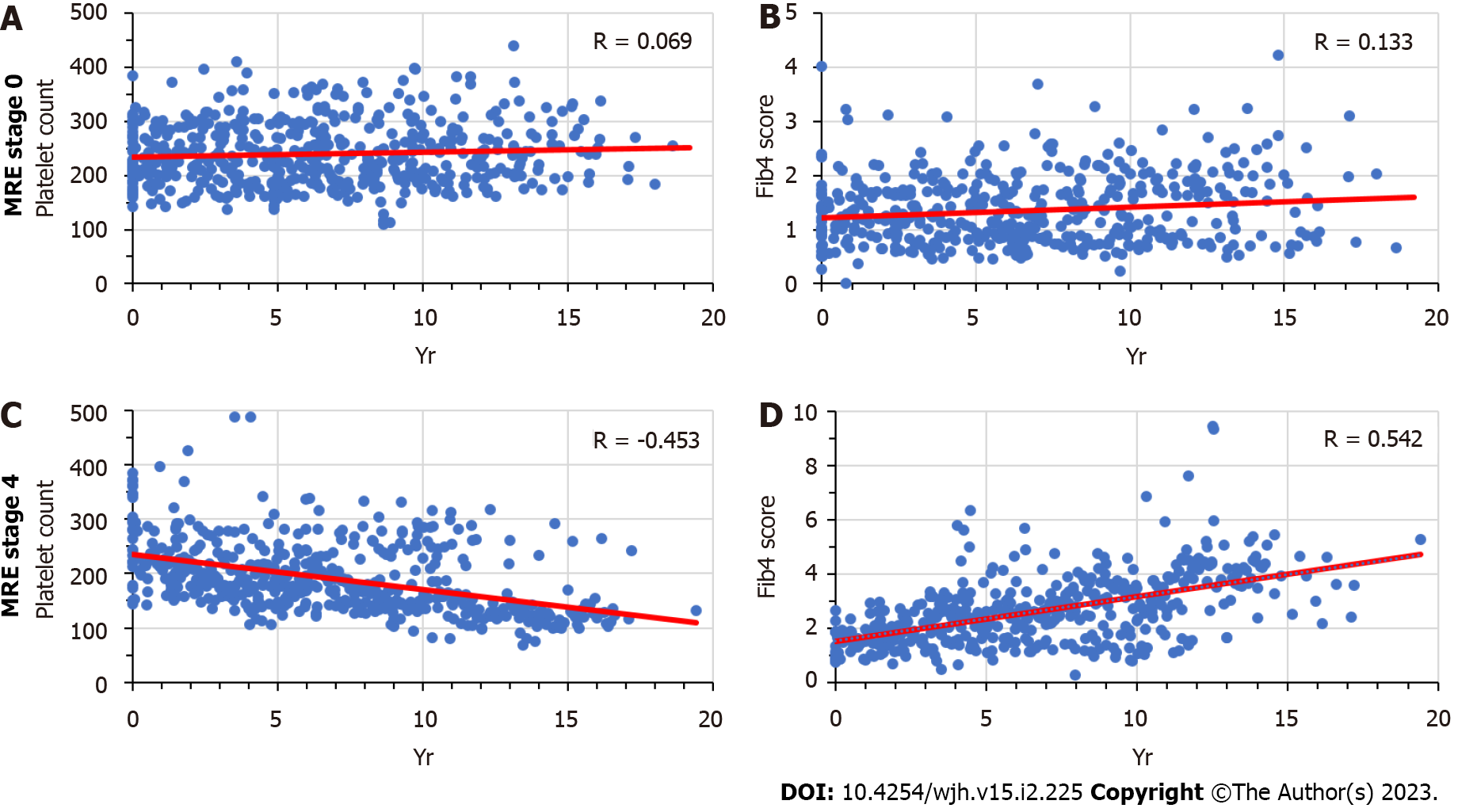
We analyzed the time trends of platelet counts and Fib-4 sores in patients with MRE-determined fibrosis stage 0 (0-2.9 kPa) or stage 4 (> 5.0 kPa) who had been followed for a minimum of one year (Figure 4). The overall trends show no significant correlations associated with fibrosis stage 0, as opposed to a significant decline of platelet counts and a significant rise of Fib-4 scores over time among patients with fibrosis stage 4. The caveats stated above with respect to Figure 3 also apply to Figure 4. Similar results were obtained for MRE fibrosis scores between 2.9 and 5.0 (data not shown). The regression patterns for these patients fell within the range outlined by the two extremes in Figure 4, as the slopes of the two regression lines for platelet counts and Fib-4 scores tended to become increasingly steeper with increasing MRE scores. Similar to the data obtained in the liver biopsy cohort, this pattern supports the impression that in patients with elevated MRE fibrosis scores the occurrence of fibrosis was preceded by a long-term gradual fall in platelet counts.
Table 3 contains the results of two separate multivariable regression analyses. Due to the exclusion of patients in whom pre-staging data were available for a time period of less than one year, only 146 and 89 patients were included in the two separate analyses. The outcome variables were fibrosis stage on biopsy or MRE score, respectively. The same set of predictor variables was used in both analyses. In both analyses, the last Fib-4 value and the correlation coefficient of the time-dependent drop in platelet counts contributed in a statistically significant fashion to the overall prediction of cirrhosis. In patients who underwent liver biopsy, the presence of diabetes mellitus was an additional independent and significant predictor. This result supports the hypothesis that a progressive long-term drop in platelet is a feature of progressive liver fibrosis.
| Predicator variable | Estimate | Std error | t value | Prob >|t| |
| Outcome: Fibrosis score on biopsy | ||||
| Fib-4 (last score) | 0.214 | 0.048 | 4.43 | < 0.0001 |
| Correlation-platelets | -0.461 | 0.227 | -2.03 | 0.044 |
| Diabetes mellitus | -0.436 | 0.111 | -3.94 | 0.0001 |
| Hypertension | -0.181 | 0.112 | -1.63 | 0.1062 |
| BMI | 0.02 | 0.016 | 1.28 | 0.2016 |
| Smoking | -0.173 | 0.113 | -1.53 | 0.1275 |
| Sex | -0.028 | 0.11 | -0.26 | 0.7988 |
| R2 = 0.35, N = 146, P < 0.0001 | ||||
| Outcome: Fibrosis score on MRE | ||||
| Fib-4 (last score) | 0.746 | 0.239 | 3.12 | 0.0025 |
| Correlation-platelets | -1.582 | 0.747 | -2.12 | 0.0373 |
| Diabetes mellitus | -0.301 | 0.365 | -0.82 | 0.4133 |
| Hypertension | 0.441 | 0.395 | 1.12 | 0.2674 |
| BMI | -0.019 | 0.039 | -0.47 | 0.6368 |
| Smoking | 0.226 | 0.333 | 0.68 | 0.5001 |
| Sex | 0.016 | 0.36 | 0.04 | 0.9647 |
| R2 = 0.24, N = 89, P < 0.002 | ||||
In a separate set of multivariable regression analyses, instead of the correlation coefficient associated with platelet counts, the correlation coefficient of the time-dependent rise in the Fib-4 scores was used as predictor variable. The calculation of the Fib-4 requires the simultaneous measurement of SGOT, SGPT, and platelet counts[13]. Because of the requirement for three simultaneous laboratory tests, fewer time points were available for this analysis. Overall, the correlation coefficient of the time-dependent rise in Fib-4 scores failed to function as an independently significant predictor for liver fibrosis in the two patient populations (data not shown).
The results of the present illustrate the long-term nature and gradual decline in platelet counts among patients with non-alcoholic fatty liver disease, who subsequently become diagnosed with liver fibrosis or cirrhosis. Such decline in platelet count is also associated with a gradual rise of the Fib-4 scores in the same patient population. The consistency of these patterns is confirmed by their similar occurrence among the two separate subgroups of patients included in the present analysis, that is, those who were diagnosed by liver biopsy vs magnetic resonance elastography. In addition to the last Fib-4 score preceding the ultimate diagnosis of liver fibrosis, the preceding decline in platelet count itself was also an independent and statistically significant predictor for the occurrence of advanced liver fibrosis.
The development of thrombocytopenia in patients with chronic liver disease – regardless of its underlying disease etiology – is well known to hepatologists. Several mechanisms have been implicated for this phenomenon, including portal hypertension, and decreases in hepatic thrombopoietin production[14]. The occurrence of progressive thrombocytopenia in NASH patients had previously been described by Liu et al[15] in a community-based, cross-sectional study. Interestingly, the authors did not attempt to relate this finding to the patients’ disease stage. The first explicit connection between progressive thrombocytopenia and cirrhosis was recently described in a study by Gotlieb et al[9]. Using a large computerized data base, the authors identified 5300 cases with an EMR diagnosis of liver cirrhosis of any etiology and compared them to 15700 control subjects. In their retrospective analysis, they noted a significant and progressive drop in platelet counts from 240000 to 190000 up to 15 years prior to diagnosis. The drop became detectable at a time when the patient’s platelet counts were still within normal range, preceding the occurrence of abnormal laboratory tests by several years. In a multivariate analysis, the odds of cirrhosis increased 1.3 times for every decrease in platelet counts by 50K. The authors suggested that it might be possible to predict the development of advanced fibrosis or cirrhosis at a much earlier preclinical stage. Similar data were recently reported by Hagstrom and colleagues in a large-scale analysis of a Swedish health care registry[16]. The authors examined the ability of several serum-based fibrosis scoring systems to predict the development of significant liver disease and its complications, as defined by ICD-9 coding. Their analysis included a predefined NAFLD group. In general, high scores (including Fib-4[10]) were associated with an increased risk of cirrhosis development over time, although the positive predictive values were modest.
Our results confirm these previous findings, although our analysis differed in several respects. Our study was smaller in scope as we confined our analysis to a single regional healthcare system. We specifically focused on non-alcoholic fatty liver disease in order to avoid possible confounding effects of different disease etiologies and their potentially unique rates of disease progression. More importantly, we included all fibrosis stages in order to evaluate the specificity of the platelet count changes for “advanced fibrosis”, and we only included patients in whom the fibrosis stage had been determined by liver biopsy or MR elastography, the two modalities with the best performance characteristics.
Our analysis demonstrates a significant drop in platelet counts and corresponding rise in Fib-4 scores for all patient groups, including those without or with early-stage fibrosis. This might be partly due to the previously described age-dependent reduction in platelet counts[17] and SGPT[18], or to the onset of fibrogenesis during the early stages of the disease. The most pronounced changes occurred in patients with the highest fibrosis stages, suggesting an acceleration of the underlying disease process.
In multivariate analyses separately performed for the biopsy and MRE cohorts, the last Fib-4 value prior to staging and the correlation coefficient for the time-dependent drop in platelet counts were both predictive for the severity of liver fibrosis. In addition, a significant association with a diagnosis of diabetes was observed, as previously reported by others[4]. In contrast, the inclusion of the correlation coefficients for the time-dependent increase in Fib-4 scores did not significantly contribute to the overall regression model. Besides the platelet count, serum levels of SGOT and SGPT also serve as separate variables in the formula for calculating the Fib-4 index. Because of the requirement for three simultaneously measured laboratory values, overall, fewer time points could be generated for the Fib-4 scores than for the platelet counts in individual patients. This might explain the lack of statistical significance for the Fib-4 scores. None of the other variables (hypertension, BMI, smoking, or sex) were statistically associated with advanced fibrosis, regardless of how the fibrosis stage was diagnosed.
The diagnostic utility of low platelet counts or increased Fib-4 scores for the diagnosis of advanced fibrosis has been extensively examined by multiple studies. The main strength of these measurements lies in their excellent negative predictive value, whereas their positive predictive value has generally been less impressive[4]. Because of these performance characteristics, secondary follow-up testing with elastography or liver biopsy is necessary for patients with abnormal Fib-4 scores.
In addition to confirming their diagnostic utility, our data indicate that the dynamic changes in serum fibrosis markers may also harbor some prognostic power. As shown by the two exemplary case patients of Figure 1, a steadily progressive decline in platelet counts may precede the formal staging test by 20 years or more. The consistency of such decline was captured by the magnitude of the correlation coefficient and its impact on predicting the final outcome. In this regard, the statistical analysis supported the underlying hypothesis of the study generated from inspecting the data of individual patients.
A review of trends in all individual patients revealed a high degree of variability within the patient population. In our study, we included all available laboratory data, regardless of the patient’s in- or outpatient status, comorbid disease conditions, surgical or other interventions, and medications. Such confounding factors may have contributed to the noticeable fluctuation in our data. However, our approach facilitated automatic data extraction and lends itself to be tested (and utilized) in future prospective studies among high-risk populations. In the future, a potential limitation of the long-term platelet counts may arise from to their overall weaker statistical impact. As shown in Table 3, the significance level for the correlation coefficient of the declining platelet counts was markedly lower than that of the “last Fib-4” or presence of diabetes mellitus.
Eventually, prospective clinical studies in carefully defined populations of patients with non-alcoholic fatty liver disease will be necessary to determine whether the inclusion of “platelet dynamics” can contribute to the prediction of advanced fibrosis. Such studies could be performed in the primary care setting by extracting EMR data from at-risk patients, followed by subsequent fibrosis staging through MRE or liver biopsy.
Time-dependent changes in platelet counts and Fib-4 scores contribute to the prediction of cirrhosis in NASH patients with biopsy- or MRE-staged fibrosis. Their incorporation into predictive algorithms may assist in the earlier identification of high-risk patients.
Our study was prompted by the growing public health challenges of non-alcoholic fatty liver disease. One of the key issues is the early detection of significant liver fibrosis. Our study focuses on the use of non-invasive predictors of cirrhosis, using data that can be easily extracted from patients' medical records.
We focused our attention on longitudinal changes in platelet count and Fib-4 scores that precede the development of progressive liver fibrosis. Our results suggest that such changes become apparent several years prior to the establishment of a clinical diagnosis of cirrhosis. Future research should include the prospective evaluation of our predictive algorithms.
The main objective was to determine whether longitudinal changes in platelet count and Fib-4 scores precede the establishment of cirrhosis by liver biopsy or magnetic resonance elastography (MRE). We found that such changes occur, regardless of the method of fibrosis staging. Our results suggest that longitudinal analyses such as those described in our study may have clinical utility for earlier disease detection.
We extracted clinical and demographic data from the patients' electronic medical records, and related them to the biopsy- or MRE-documented fibrosis stages. Regression and multivariable analyses were performed to detect significant trends related to fibrosis stage.
We found that the time-dependent changes in platelet counts and Fib4-scores are correlated with successive fibrosis stages. Our data contribute to the growing field of non-invasive fibrosis prediction in chronic liver disease.
Our study suggests that longterm, progressive changes in platelet counts and Fib-4 scores occur during the progression of non-alcoholic fatty liver disease (NASH)-related fibrosis. Our results could be incorporated into new predictive algorithms, which in turn would need to be validated prospectively. If successful, our approach might lead to a significantly earlier diagnosis of advanced liver fibrosis in NASH patients.
The next step in our work will be the prospective evaluation of platelet count and Fib-4 changes to detect stage 4 fibrosis prior to the clinical establishment of the diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du Y, China; Li Z, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Povsic M, Wong OY, Perry R, Bottomley J. A Structured Literature Review of the Epidemiology and Disease Burden of Non-Alcoholic Steatohepatitis (NASH). Adv Ther. 2019;36:1574-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1014] [Article Influence: 202.8] [Reference Citation Analysis (4)] |
| 3. | Armstrong MJ, Hazlehurst JM, Parker R, Koushiappi E, Mann J, Khan S, Philips A, Chandler L, Johnson J, Round M, Haydon G, Karamat MA, Newsome PN, Tomlinson JW. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077-11087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (3)] |
| 5. | Guss D, Sherigar J, Mohanty SR. Missed Diagnosis of Liver Cirrhosis Leads to Disparities in Care for Older Patients. Gastroenterology Res. 2018;11:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol. 2013;11:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 7. | Steele AJ, Denaxas SC, Shah AD, Hemingway H, Luscombe NM. Machine learning models in electronic health records can outperform conventional survival models for predicting patient mortality in coronary artery disease. PLoS One. 2018;13:e0202344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Miyata H, Miyata S. Speculation of the Time-Dependent Change of FIB4 Index in Patients with Nonalcoholic Fatty Liver Disease: A Retrospective Study. Can J Gastroenterol Hepatol. 2018;2018:5323061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Gotlieb N, Schwartz N, Zelber-Sagi S, Chodick G, Shalev V, Shibolet O. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis. World J Gastroenterol. 2020;26:5849-5862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 10. | Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 11. | Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: Definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, Yin M, Miller FH, Low RN, Hassanein T, Godfrey EM, Asbach P, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 14. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Liu F, Zhou H, Cao L, Guo Z, Dong C, Yu L, Wang Y, Liu C, Qiu J, Xue Y, Liu X, Xu Y. Risk of reduced platelet counts in patients with nonalcoholic fatty liver disease (NAFLD): a prospective cohort study. Lipids Health Dis. 2018;17:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Ability of Noninvasive Scoring Systems to Identify Individuals in the Population at Risk for Severe Liver Disease. Gastroenterology. 2020;158:200-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 17. | Balduini CL, Noris P. Platelet count and aging. Haematologica. 2014;99:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |