Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.129
Peer-review started: September 18, 2022
First decision: October 30, 2022
Revised: November 13, 2022
Accepted: January 23, 2023
Article in press: January 23, 2023
Published online: February 27, 2023
Processing time: 159 Days and 3.7 Hours
Owing to its heterogeneous and highly aggressive nature, hepatocellular car
Core Tip: The current rate of recurrence after initial hepatocellular carcinoma treatment remains unsatisfactory. Repeat hepatectomy and salvage liver transplantation are the preferred options for patients who meet the criteria. However, for patients whose clinical situation do not allow these treatments, non-surgical treatment can also provide survival benefits. Additionally, adjuvant treatment strategies to prevent recurrence and proper surveillance are effective tools to improve overall patient survival. This review summarizes the existing literature to help guide clinical decision-making and provide directions for further research.
- Citation: Yang YQ, Wen ZY, Liu XY, Ma ZH, Liu YE, Cao XY, Hou L, Xie H. Current status and prospect of treatments for recurrent hepatocellular carcinoma. World J Hepatol 2023; 15(2): 129-150
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/129.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.129
Hepatocellular carcinoma (HCC), a heterogeneous disease with multiple etiologies, is the major subtype of primary malignancies of the liver, accounting for 70%-85% of primary liver cancers[1]. Globally, HCC is the third most common cause of cancer-related mortality, and its incidence is rising[2]. Treatment options for HCC have improved, but frequent recurrence after treatment is a major concern. International guidelines provide detailed treatment options for each stage of HCC, and depending on the patient’s liver function and tumor burden, treatment options vary from radical treatment options, such as resection, transplantation, ablation, and combination therapy, to palliative treatment options, such as transcatheter arterial chemoembolization (TACE), systemic therapy, and supportive care. Although hepatectomy is the preferred option for patients with HCC who meet the criteria, 67.6% of patients develop tumor recurrence or metastasis after hepatectomy[3]. Moreover, few patients can undergo radical hepatectomy due to insufficient liver function reserve, vascular invasion, extrahepatic metastases, and the size and number of lesions[4]. With the continuous development and maturation of transplantation technology, liver transplantation has become the best long-term treatment for patients with early-stage HCC. However, liver transplantation also has limitations, including a 25% risk of recurrence even if the patient meets the strict Milan criteria and a lack of donor organs, which limit the use of transplantation[5]. Ablation is another way to treat patients with small HCC who are not candidates for surgery due to comorbidities, liver dysfunction, or tumor location. However, the risk of recurrence after ablative therapy is as high as 80%; therefore, this option is limited to patients who cannot undergo surgical resection but are suitable for liver-directed therapy[6]. The combination of TACE and ablation is one of the most widespread and efficacious combination therapies. The latest version of the Barcelona Clinic Liver Cancer (BCLC) guidelines suggests that the combination of TACE and ablation as a radical treatment solution for 3-5 cm masses has the advantage of reducing heat deposition and expanding the scope of ablation compared with a single treatment option[7]. Nevertheless, 76.4% of patients undergoing TACE with ablation develop recurrence, probably because of the presence of portal vein collateral circulation and high alpha-fetoprotein (AFP) levels[8]. Finally, palliative care options mostly play a role in improving the symptoms and quality of life of patients with advanced HCC that is incurable. Given the high risk of recurrence with radical treatment regimens, refining and optimizing treatment options for recurrent liver cancer are urgent issues.
In addition to differences in recurrence risk, various treatment modalities have varying patterns of recurrence, which affects the choice of treatment options for recurrent HCC. In general, hepatectomy is mostly associated with intrahepatic recurrence, with few extrahepatic metastases, most probably due to residual minuscule lesions. Lee et al[9] observed that tumor recurrence after resection was detected in the liver in 80.1% of patients and suggested that curative therapeutic results might be achieved through repeat hepatectomy or local ablation. In addition, HCC recurrence can be classified into early and late recurrence, depending on the time of recurrence after surgery. It is generally believed that early recurrence may be associated with tiny preoperative or intraoperative metastases and the continued growth of tiny postoperative residual lesions, mostly close to surgically resected lesions. Late recurrences are mostly new tumors arising from the malignant transformation of normal liver cells due to latent cancer-causing factors in the liver, such as frequent recurrent inflammation of the liver and cirrhotic fibrosis[10]. There is no consensus on the dividing line between early and late recurrences; however, a 2-year cutoff after resection has been widely used to distinguish between the two types of HCC recurrence[11]. Treatment options for recurrent HCC after resection vary according to the type of recurrence pattern and timing. The best treatment plan should be developed by fully integrating multiple treatments and following the principle of the maximum benefit to the recipient.
Similarly, with the demarcation line being set at 2 years, HCC recurrence after liver transplantation can be divided into early and late recurrence. A higher original tumor burden and more aggressive features may account for early recurrence in patients who undergo liver transplantation[12]. A high primary tumor burden predisposes to missed or undetectable extrahepatic metastases before transplantation, leading to the recurrence of HCC. Similarly, more aggressive tumors tend to trigger the engraftment and growth of circulating HCC cell clones in the target organ after transplantation[12].
Early recurrent HCC tends to involve multiple organs and has a poor prognosis; therefore, its treatment plan should be selected carefully[13]. In contrast, late recurrence appears to be the result of transplantation of a small number of latent advanced HCC cells, and patients tend to have more favorable tumor characteristics at this time; thus, TACE and local ablation may be capable of achieving positive outcomes[5]. Radiofrequency ablation (RFA) is one of the main applications of ablation therapy, which is typically performed for unresectable solitary tumors < 3 cm in diameter and has comparatively high safety and efficacy. However, RFA is prone to leaving residual tumor cells owing to incomplete ablation, thus causing local recurrence[4]. Heat dissipation effects and tumor size are the primary limiting factors for RFA, and combination therapy may be a solution[4].
Prevention and treatment of recurrent HCC have become an urgent issue. In this review, we evaluate the available evidence on the effectiveness of adjuvant therapy, summarize the treatment options for the recurrence of primary liver cancer after treatment, and describe appropriate monitoring protocols for predictors of liver cancer recurrence to ultimately identify the optimal management strategy for patients with recurrent liver cancer.
Given the high recurrence rate after HCC treatment, adjuvant therapy has been proposed to reduce the risk of HCC recurrence and further improve the long-term survival of patients with liver cancer. Nonsurgical therapy, including antiviral therapy, TACE, systemic therapy, radiation therapy, and other strategies, may be performed preoperatively to improve liver function or postoperatively to improve patient survival outcomes.
Previous studies have shown that high hepatitis B virus (HBV) levels, HBV e-antigen positivity, and HBV reactivation are strongly associated with a high risk of recurrence of HBV-related liver cancer after resection[14]. Similarly, a recent study showed that in HCC patients with viral infection who underwent living liver transplantation, HBV recurrence tended to cause HCC recurrence, and hepatitis D virus infection was considered an independent risk factor for HBV-HCC co-occurrence after transplantation[15]. This suggests that antiviral therapy plays an essential role in the prevention of postoperative recurrence of viral hepatitis-related HCC.
Currently, the primary antiviral treatments include nucleoside analogs (NAs), interferons, and direct antiviral agents (DAAs)[16]. NAs can significantly reduce the incidence of HBV-associated HCC by lowering the patient’s HBV load. Several studies have confirmed the effectiveness of NAs in preventing liver cancer[17,18]. The Asian Pacific Association for the Study of the Liver guidelines on the mana
TACE is the standard of care for intermediate to advanced HCC and the primary method of bridging or step-down therapy before liver transplantation[27]. Many studies have shown that TACE as an adjuvant therapy has certain advantages in improving the prognosis of patients with HCC and preventing cancer recurrence. Liu et al[28] systematically analyzed the outcomes of 117 patients with HCC who underwent hepatectomy between 2010 and 2014 and received postoperative TACE and found that postoperative TACE improved the 1-year disease-free survival (DFS) compared with surgical resection only (64.5% vs 45.5%, P = 0.04). In addition, they recommended postoperative TACE for patients with tumors > 5 cm with microvascular invasion or satellite nodules[28]. Other studies also support this view and concluded that postoperative TACE is a safe intervention to prevent tumor recurrence in patients with BCLC early- and intermediate-stage HCC with microvascular invasion[29-31]. However, preoperative TACE is controversial, and a meta-analysis of randomized controlled trials based in Asia showed that preoperative TACE did not improve the long-term prognosis of patients with resectable HCC, possibly because of the risk of tumor progression or deterioration of liver function in patients undergoing TACE[32].
Because HCC is a relatively radiation-sensitive tumor, radiation therapy is one of the commonly used treatments for liver cancer. A recent systematic review evaluating the impact of different postoperative treatments on patients with HCC with microvascular invasion after radical resection revealed that postoperative radiotherapy is more effective in reducing recurrence than postoperative TACE[33]. Yoon et al[34] shared the same view and concluded that the combination of TACE and radiotherapy is a promising treatment option to alleviate symptoms in patients with HCC and portal vein tumor thrombosis. A narrow-margin (< 1 cm) hepatectomy is prone to residual microscopic lesions that can spread through intrahepatic vessels and lead to recurrence due to detailed control issues during the procedure. However, a prospective randomized study found that adjuvant radiotherapy for central HCC after narrow-margin hepatectomy is technically feasible and relatively safe. Subgroup analysis showed that adjuvant radiotherapy significantly improved recurrence-free survival (RFS) in patients with HCC ≤ 5 cm in diameter, although there was no difference in overall survival (OS)[35]. An additional prospective phase 2 study concurred with this finding and suggested that intraoperative electron radiotherapy was more beneficial for survival in patients with microvascular infiltration after resection[36].
In 1999, Lau et al[37] first proposed that adjuvant therapy with intra-arterial administration of 1850 MBq of 131I-labeled lipiodol after radical resection significantly reduced recurrence in patients with HCC and improved DFS and OS. However, Chung et al[38] found that administration of adjuvant intra-arterial 131I-labeled lipiodol after resection showed negligible improvement in controlling HCC tumor recurrence and that patients were at risk for hypothyroidism and hepatic artery dissection during angiography. Conversely, several meta-analyses have positively evaluated the efficacy of adjuvant treatment with intra-arterial 131I-lipiodol[39-41]. A systematic review including three case-control studies and two randomized controlled trials showed robust evidence that adjuvant 131I-labeled lipiodol prolongs DFS and OS by up to 5 years after resection in patients with sound liver function and low microvascular invasion[40]. Therefore, more well-designed, randomized pilot studies are required to draw solid conclusions.
Chemotherapy is the most widely administered cancer treatment. Generally speaking, chemotherapy is mostly utilized in the systemic treatment of primary liver cancer; however, with the development of modern technology, regional adjuvant chemotherapy also plays an important role in the prevention of liver cancer recurrence. However, chemotherapy has its limitations, as many drugs kill both cancer and healthy cells[42]. Therefore, chemotherapy is also utilized in combination with other therapies, such as surgery, radiotherapy, and immunotherapy, which have shown positive synergistic effects. As early as 1996, Yamamoto et al[43] systematically analyzed the efficacy of oral adjuvant chemotherapy in 67 patients with HCC who underwent radical resection between 1988 and 1990. They found that the OS and RFS were significantly higher in patients who received adjuvant oral 1-hexylcarbamoyl-5-fluorouracil than those who did not among patients with mild hepatic dysfunction, but no significant differences in survival were observed in patients with moderate hepatic dysfunction[43]. A subsequent randomized controlled trial had a different conclusion on the controversial question of whether adjuvant chemotherapy after resection can prevent recurrence of HCC. This trial showed similar relapse-free survival rates in the postoperative oral uracil-tegafur (UFT) and no adjuvant therapy groups and a significantly higher proportion of late recurrence in the UFT group than in the control group (74% vs 53%, P = 0.02)[44]. Interestingly, Ueda et al[45] discovered that adjuvant chemotherapy with UFT after TACE significantly prolonged the time to treatment failure in patients with advanced HCC, and no serious adverse events were observed with this regimen. This regimen may have adjuvant and anti-angiogenic functions in the treatment of advanced HCC. In addition, Nagano et al[46] found that adjuvant interferon-/5-fluorouracil could benefit patients with advanced HCC after palliative hepatectomy. Therefore, combining chemotherapy with another treatment may be a solution to the poor efficacy of adjuvant chemotherapy when applied alone. Similarly, adjuvant chemotherapy after liver transplantation can provide survival benefits. A systematic evaluation and meta-analysis showed that implementing adjuvant chemotherapy early after liver transplantation in patients with advanced HCC can significantly prolong patient survival and delay liver cancer recurrence[47].
Hepatic arterial infusion chemotherapy (HAIC) is a type of chemotherapy primarily administered to patients with advanced intrahepatic HCC, such as those with major portal vascular invasion and intrahepatic multinodular lesions with Child-Pugh class B liver function[48]. As patients with HCC with vascular invasion tend to have a poor prognosis after surgical resection, the postoperative administration of HAIC has been increasingly emphasized by investigators. A retrospective study that included 73 patients with HCC with visible vascular invasion found that DFS was significantly higher in the hepatic resection with HAIC group than in the control group without HAIC (33.1% vs 11.8%, P = 0.029) after 5 years of follow-up; however, there was no significant difference in OS between the two groups[49]. Hsiao et al[50] had similar findings and suggested that patients with HCC with multiple small nodules in close proximity to each other or a single large tumor with several satellite nodules could achieve greater benefit when HAIC was performed as an adjuvant treatment after resection. Preoperative HAIC can also be a means of downstaging before resection in patients with advanced HCC. Lee et al[51] showed that the median survival time and response rate of patients with advanced HCC who underwent hepatectomy after preoperative HAIC were 14 ± 1.7 mo and 26.4%, respectively.
The drug combinations for HAIC are also being continuously explored by scholars in various countries. A Japanese HAIC study compared the outcomes of 476 patients with HCC who received HAIC (5-fluorouracil and cisplatin) with 1466 patients who did not receive active treatment and showed that the median survival time was longer in patients who received chemotherapy (14.0 mo) than in those who did not receive active treatment (5.2 mo, P < 0.0001)[52]. However, several cisplatin (DDP)-based HAIC regimens are dose limited by renal, neurological, and gastrointestinal toxicity, making it difficult to achieve the desired outcomes[53]. In contrast, with the publication of the EACH study[54], oxaliplatin is coming into the limelight as a systemic chemotherapeutic agent. The study explored whether infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) as palliative chemotherapy for patients with advanced HCC provides survival benefit and efficacy compared with doxorubicin, and found that this regimen may offer some benefits for Asian patients[54]. Subsequently, Chinese scholars modified and applied the FOLFOX regimen to HAIC and achieved impressive results. In the ASCO 2021 meeting, Li et al[55] first explored the efficacy of neoadjuvant HAIC (FOLFOX regimen) and compared it with that of direct surgery in patients with HCC with ultra-Milan standard BCLC stage A/B; they found that the objective response rate (ORR) in the neoadjuvant HAIC group reached 63.6%, and the disease-control rate reached 96.0%. Furthermore, the team found that this protocol was also effective in HCC patients with microvascular invasion. The study showed that patients who received one or two cycles of postoperative adjuvant arterial perfusion chemotherapy had significantly better OS and DFS compared to patients without any adjuvant therapy (97.7% vs 78.5%; 58.7% vs 38.6%; P = 0.037 and 0.023, respectively)[56]. Thus, HAIC based on the FOLFOX regimen is gaining more and more attention in the academic community for its high ORR and surgical conversion rate.
The liver tumor microenvironment has complex immune tolerance capabilities[57]. Immunotherapy can enhance the body’s immune response, break the immune tolerance of the tumor microenvironment, and reactivate immune cells to recognize and kill tumor cells. Immunotherapies mainly include adoptive cell transfer-based therapies, tumor vaccines, and immune checkpoint inhibitors (ICIs)[58]. Adoptive cell transfer-based therapy involves isolating immunocompetent cells from the bodies of cancer patients. Through cytokine stimulation, in vitro culture, or tumor antigen loading, a large number of amplifications and functional identifications are performed in vitro, and then cells are injected back into the patient’s body. These cells are now primed to enhance the patient’s immune function and kill tumor cells. Cytokine-induced killer cells (CIKs) and genetically modified natural killer or T cells are the main immune cells used for this process in liver cancer[58]. A randomized trial published by Takayama et al[59] in 2000 first demonstrated the safety and efficacy of adoptive immunotherapy in reducing recurrence and improving patient survival after HCC resection. A study of patients with HCC undergoing curative therapy also showed that adjuvant injection of activated CIKs improved RFS and OS[60]. However, other studies have shown a limited effect of adoptive T cell therapy in solid tumors, possibly due to the poor persistence of adoptive T cells in vivo, their cytotoxicity, and other defects[61]. Tumor vaccines are immunotherapies in which the patient’s tumor antigens are infused back into the patient in various forms to enhance immunogenicity, thereby activating the patient’s immune system to attack tumor cells. This is the theoretical basis of tumor vaccine treatment for liver cancer[61]. Repáraz et al[62] indicated that tumor vaccines have significant potential in combination with ICIs for the prevention and treatment of HCC. A recent review that included 31 clinical trials worldwide held the same opinion and concluded that HBV-associated HCC may benefit more from tumor vaccines than HCV-associated HCC[63]. Currently, tumor vaccines for patients with HCC mainly include dendritic cell (DC) vaccines, AFP vaccines, and other vaccines. DC vaccines, a common tumor vaccine, can provide clinical benefits to patients with HCC by stimulating antitumor T cell responses without significantly increasing toxicity[64]. AFP vaccines are peptide-based tumor vaccines used in HCC and are characterized by low immunogenicity and tolerance to the host immune system[62]. Immune checkpoints play a protective role in the body’s immune system by preventing excessive activation of T cells from damaging the body’s tissues. Cytotoxic T lymphocyte-associated antigen-4 and programmed death 1 are the two main immune checkpoints considered in the treatment of HCC, and ICIs developed against these checkpoint molecules have been widely adopted clinically for liver cancer. Numerous studies have reported ICIs as an appropriate therapy option pre- and post-transplantation, but most of these studies were retrospective or case reports; therefore, ICIs should be administered to patients undergoing liver transplantation with caution[65]. Similarly, there is a shortage of randomized controlled trials of ICIs after HCC resection or ablation, although several relevant trials are underway, testing drugs such as pembrolizumab (KEYNOTE-937, NCT03867084), nivolumab (CheckMate 9DX, NCT03383458), and atezolizumab plus bevacizumab (IMbrave050, NCT04102098), which are expected to yield promising results.
Molecular targeted therapy is of epoch-making significance in the field of cancer treatment and is mainly based on the pathways involved in the pathogenesis of cancer. Molecular targeted therapeutics specifically cause the death of tumor cells. Sorafenib is an approved multi-target tyrosine kinase inhibitor for the treatment of patients with advanced and unresectable HCC[66]. Numerous retrospective studies have shown that adjuvant sorafenib treatment improves recurrence and prolongs survival, especially in patients at high risk of postoperative recurrence[67-69]. However, a phase 3, randomized, double-blind, placebo-controlled trial (STORM trial) evaluating the efficacy of adjuvant sorafenib after resection or ablation of HCC found no difference in the median RFS between the adjuvant sorafenib and placebo groups (33.3 mo vs 33.7 mo, P = 0.26)[70]. Sorafenib treatment in the perioperative period of liver transplantation is equally ineffective and strongly associated with a worse prognosis[71]. In contrast, lenvatinib has shown promising results as an adjuvant therapy for patients who have undergone liver transplantation. A retrospective case-control study showed that adjuvant lenvatinib can prolong DFS in patients with high-risk HBV-related HCC following liver transplantation[72]. Bevacizumab, an angiogenesis inhibitor, has shown poor results as adjuvant therapy in patients with HCC. Pinte et al[73] found that patients treated with adjuvant bevacizumab after TACE not only had no improvement in OS but also developed sepsis and vascular side effects. Consequently, for the prophylactic treatment of patients with HCC, adjuvant treatment strategies with molecular-targeted drugs should be carefully selected.
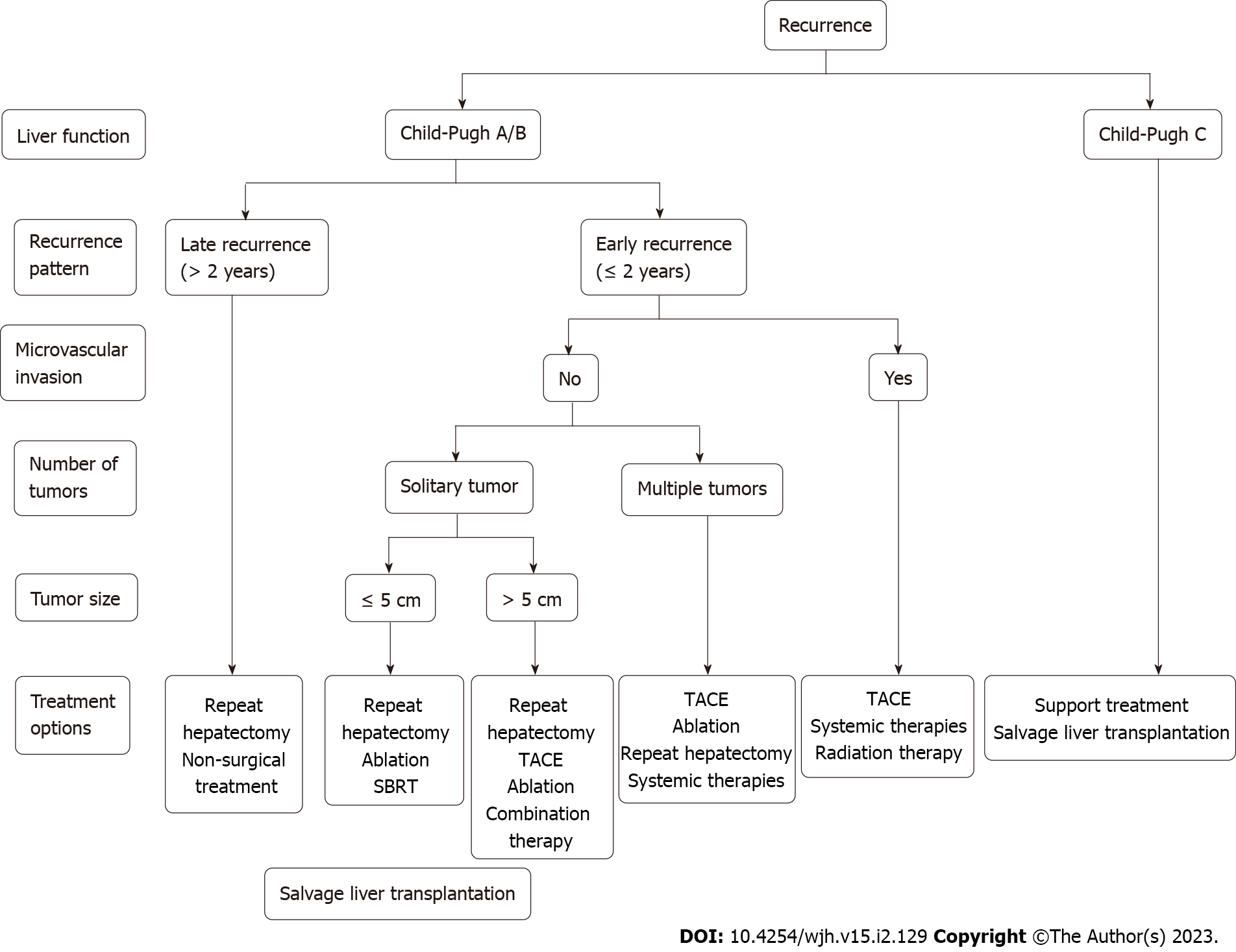
The treatment of recurrent liver cancer is mostly based on the diagnosis and treatment guidelines for primary liver cancer[74] combined with clinical experience. Multiple studies have shown that surgical resection, liver transplantation, and non-surgical treatment (such as ablation and TACE) for recurrent HCC can lead to survival benefits comparable to those of the first treatment[75-79]. However, most of these were small-sample studies at a single institution, the evidence is weak, and the results are difficult to generalize. Ideally, treatment strategies for recurrent HCC can be based on the same criteria as those for primary cancer; however, given the intratumoral heterogeneity and different clonal lineages between primary and recurrent HCC, it is still advisable to perform a comprehensive overview of the tumor before choosing the best treatment modality. In addition, patient characteristics (such as sex, age, and psychological state), conditions of the first operation (such as surgical area and main blood vessels severed during the first operation), and basic liver function status should also be comprehensively evaluated. A suggested flowchart to guide treatment decision-making in the setting of recurrent HCC is presented in Figure 1.
Hepatectomy remains a safe and effective treatment for recurrent HCC. Reoperation in patients with HCC with good liver function significantly prolongs survival, especially in patients exhibiting recurrence within 2 years and those with a primary tumor burden exceeding the Milan criteria[80,81]. Yoh et al[3] observed similar findings; in their study, 128 patients who underwent repeat surgery had better liver function and a significantly longer time to recurrence than 548 patients who did not undergo reoperation (16.5 mo vs 11.4 mo; P < 0.001). Although repeat hepatectomy is most commonly performed for patients with intrahepatic metastases, surgical resection can also provide benefits to patients with recurrent extrahepatic lesions under conditions of limited isolation of metastases, preservation of liver function, and adequate control of the primary tumor[82]. Repeat hepatectomy is also a recommended treatment option for patients with recurrent HCC occurring more than 18 mo after the initial resection, and survival rates are significantly higher for patients with multiple distant metastases than for those with intrahepatic metastases[83]. Numerous retrospective studies have suggested that appropriately selected patients undergoing partial hepatectomy can achieve long-term survival after both initial hepatectomy and liver transplantation, with 5-year OS and RFS rates ranging from 22%-84% and from 10%-43%, respectively (Table 1). Third repeat hepatectomy is also a promising technique for recurrent tumors, and it has been reported that three or more repeat hepatectomies for recurrent HCC are reasonable and safe; however, they should be performed with caution because of the high recurrence rate, long operative duration, and high patient selectivity of resection[84,85]. For recurrent HCC after liver transplantation, patients who undergo repeat hepatectomy tend to have a worse prognosis and are more susceptible to deterioration in liver function. Therefore, an alternative, less invasive laparoscopic approach can be applied for repeat hepatectomy in these patients. Recurrent HCC was previously considered a contraindication to laparoscopic surgery; however, recent studies have shown that laparoscopic surgery for recurrent HCC is reliable, and there is no significant difference in tumor recurrence or survival after laparoscopic surgery compared with open surgery[86,87]. In contrast, the advantages of laparoscopic liver resection include shorter operation time, less intraoperative bleeding, and faster recovery compared with traditional surgery; therefore, laparoscopic liver resection can be a safe alternative to open surgery.
| Ref. | Type | Year | n | 1-, 3-, and 5-yr OS | 1-, 3-, and 5-yr RFS |
| Huang et al[83] | Retrospective | 1995-2010 | 82 | 71%/41%/22% | N/A |
| Itamoto et al[168] | Retrospective | 1990-2004 | 84 | 88%/67%/50% | -/-/10% |
| Li et al[169] | Retrospective | 1997-2015 | 103 | 92%/-/54% | N/A |
| Lu et al[81] | Retrospective | 2004-2015 | 138 | 92%/82%/73% | N/A |
| Ho et al[103] | Retrospective | 2001-2007 | 54 | 90%/-/72% | N/A |
| Sun et al[170] | Retrospective | 1997-2003 | 57 | 70%/61%/31% | N/A |
| Wang et al[104] | Retrospective | 2004-2010 | 128 | 98%/84%/64% | 95%/72%/43% |
| Roayaie et al[171] | Retrospective | 1994-2009 | 35 | -/-/67% | -/55%/- |
| Faber et al[80] | Retrospective | 1990-2009 | 27 | 96%/70%/42% | 70%/46%/30% |
| Liu et al[87] | Retrospective | 2008-2015 | 30 | 97%/85%/75% | 79%/46%/30% |
| Sun et al[172] | Retrospective | 2002-2014 | 43 | 98%/83%/56% | 57%/32%/29% |
| Song et al[173] | Retrospective | 1994-2012 | 39 | 89%/89%/84% | 66%/49%/43% |
| Chan et al[93] | Retrospective | 2001-2008 | 45 | 90%/57%/35% | 41%/24%/24% |
Salvage liver transplantation (SLT) is an appropriate treatment for recurrent HCC complicated by severe cirrhosis and liver decompensation. Available studies suggest that SLT in patients with recurrence after initial hepatectomy is a highly applicable strategy with long-term survival outcomes comparable to those of early liver transplantation[88-90]. SLT is a proven curative treatment technique for patients with recurrent HCC who meet the Milan criteria, with 5-year OS and RFS rates ranging from 42%-67% and from 32%-68%, respectively (Table 2). An intention-to-treat analysis of curative SLT in patients with cirrhosis and HCC by de Haas et al[89] showed that SLT had a favorable curative potential and that a model for end-stage liver disease score > 10 and the absence of TACE were predictors of successful SLT. In addition, Lim et al[90] compared the prognosis of 77 patients with HCC who underwent SLT with that of 314 patients with HCC who underwent a second surgery. They found that the 5-year intention-to-treat OS rates calculated from the time of the first hepatectomy were similar between the two groups (SLT, 72%; second surgery, 77%; P = 0.57), and the 5-year DFS rate after transplantation was much higher than that after a second hepatectomy (SLT, 72%; second surgery, 18%; P < 0.001)[90]. However, owing to organ shortages and cancer progression while on waiting lists, SLT can provide benefit to only a limited number of patients, making it far less widely used than repeat liver resection. Therefore, secondary resection of recurrent HCC may be considered a better therapeutic option than SLT in the current context of organ shortages. Nevertheless, given adequate organ reserves, SLT remains the preferred option for patients with cirrhosis after primary HCC resection or for those who undergo inoperable resection but meet the criteria for liver transplantation. It is worth pointing out that the existing international consensus suggests that SLT is not amenable for the treatment of HCC recurrence after transplantation[91].
| Ref. | Type | Years | n | 1-, 3-, and 5-yr OS | 1-, 3-, and 5-yr RFS |
| Guerrini et al[174] | Retrospective | 2000-2011 | 28 | -/-/42% | N/A |
| Chan et al[175] | Retrospective | 2005-2017 | 776 | 96%/75%/67% | 89%/68%/68% |
| Chan et al[176] | Retrospective | 1993-2009 | 19 | -/-/50% | 68%/58%/58% |
| Bhangui et al[177] | Prospective | - | 31 | -/-/54% | -/-/48% |
| Shan et al[178] | Retrospective | 2006-2015 | 45 | 65%/53%/42% | 48%/32%/32% |
| Liu et al[179] | Retrospective | 2001-2011 | 39 | 88%/78%/61% | 14%/24%/33% |
| Hu et al[180] | Retrospective | 1999-2009 | 888 | 73%/52%/46% | N/A |
| Wang et al[181] | Prospective | 2001-2013 | 74 | 88%/79%/62% | 87%/74%/67% |
Non-surgical treatment is typically proposed for recurrent HCC in the setting of inadequately preserved liver function or advanced tumor stage. Ablation therapy, such as RFA, has also been studied in the setting of recurrent HCC, with 5-year OS rates ranging from 9%-33% and 5-year RFS rates ranging from 32%-68% (Table 3). An updated meta-analysis showed that RFA is the preferred choice for recurrent HCC meeting the Milan criteria, with OS and DFS rates being similar to those of patients undergoing resection[92,93]. One study showed that 297 patients with isolated HCC ≤ 5 cm who underwent percutaneous ultrasonography-guided RFA following the recurrence of liver cancer had a similar OS to 263 patients who underwent initial RFA during the same period[94]. Similarly, Yang et al[95] concluded that RFA is generally effective and safe for the treatment of HCC recurrence after hepatectomy and that ablation is more effective in patients who relapsed 1 year after resection. RFA is also an advantageous alternative to prolong patient survival when surgical resection is contraindicated or technically infeasible[96]. Microwave ablation (MWA) is another commonly used modality for tumor ablation. Compared with RFA, MWA can reduce the time required for ablation by 60% and is more effective in eradicating tumors 3-5 cm in size[97]. As both RFA and repeat hepatectomy are indicated for HCC tumors with similar characteristics, a randomized controlled trial compared repeat hepatectomy and RFA for recurrent HCC. After a randomized 1:1 assignment of 217 patients with the same tumor characteristics to repeat hepatectomy or percutaneous RFA, the study found no statistically significant difference in survival outcomes between the two treatment strategies for patients with early-stage recurrent HCC. However, subgroup analysis found that repeat hepatectomy may be correlated with better local disease control and long-term survival in patients with tumor diameters > 3 cm or AFP levels > 200 ng/mL. In addition, because of cirrhosis, multifocal lesions, and vascular invasion, repetitive hepatectomy for recurrent HCC is limited, and only 15%-30% of patients are eligible[98]. Ablation therapy has the advantages of less trauma, less impact on liver function, and fewer complications than surgical treatment. Therefore, RFA remains a potential treatment option for patients with recurrent HCC who are unsuitable for repeat resection or salvage transplantation. However, salvage ablation is usually only appropriate for small recurrences that are detected early.
| Ref. | Type | Years | n | 1-, 3-, and 5-yr OS | 1-, 3-, and 5-yr RFS |
| Sun et al[172] | Retrospective | 2002-2014 | 57 | 98%/77%/53% | 61%/27%/17% |
| Ho et al[103] | Retrospective | 2001-2007 | 54 | -/-/83% | N/A |
| Liang et al[182] | Retrospective | 1999-2007 | 66 | 77%/49%/40% | N/A |
| Song et al[173] | Retrospective | 1994-2012 | 178 | 99%/83% 71% | 70%/41%/30% |
| Zhang et al[183] | Retrospective | 2007-2014 | 50 | 100%/64%/64% | N/A |
| Feng et al[184] | Retrospective | 2006-2016 | 199 | 91%/69%/56% | 57%/28%/15% |
| Chan et al[93] | Retrospective | 2001-2008 | 45 | 84%/43%/29% | 32%/12%/9% |
| Koh et al[185] | Retrospective | 2002-2011 | 42 | -/-/24% | N/A |
| Chen et al[186] | Retrospective | 2009-2015 | 57 | 78%/41%/37% | 70%/38%/33% |
| Lu et al[81] | Retrospective | 2004-2015 | 194 | 94%/75%/62% | N/A |
| Wang et al[104] | Retrospective | 2004-2010 | 162 | 97%/73%/37% | 90%/54%/27% |
Most recurrent HCC cases are not amenable to curative treatment techniques, including repeat resection, transplantation, and ablation. Therefore, TACE is the most common treatment modality for recurrent HCC after primary resection. TACE exerts a combined antitumor effect by embolizing tumor vessels and increasing local drug concentrations[99-101]. Although numerous studies have shown that TACE is inferior to repeat hepatectomy and SLT[102-104], according to a prospective cohort study, TACE is more appropriate for patients with multifocal disease and early (≤ 1 year) recurrence than other treatment techniques, such as repetitive hepatectomy and RFA[105]. Similarly, it has been proposed that TACE is a more effective treatment for prolonging patient survival in patients with BCLC stage 0 or A recurrent HCC with microvascular invasion, especially those who developed recurrence < 1 year after surgical resection[106]. Furthermore, two randomized controlled trials demonstrated that TACE is the only transarterial embolization modality that offers a survival advantage over best supportive care for patients with HCC who cannot receive curative treatment techniques[107,108].
Selective internal radiotherapy with yttrium-90 is also an available solution for patients with intermediate-to-advanced HCC with portal vein thrombosis as a safe alternative to TACE[109]. However, there are no experimental data on the application of yttrium-90 in the treatment of recurrent HCC. Both regimens can be used for the treatment of recurrent tumors after liver transplantation in patients with multiple lesions[110], but there are few relevant studies, and more robust evidence is needed to demonstrate the safety and efficacy of this regimen.
Stereotactic body radiotherapy (SBRT) is an emerging treatment option for HCC, where it is mainly performed for the local control of small HCCs. A matched-pair study demonstrated that 36 patients receiving SBRT had better OS than 138 patients with relapsed HCC who received other treatments or no treatment (2-year OS, 72.6% vs 42.1%; P = 0.013)[111]. A review evaluating the efficacy and prognosis of five different strategies for the treatment of recurrent intrahepatic HCC indicated that SBRT was superior to TACE in terms of OS and DFS but less effective than curative treatment techniques. In contrast, the prognostic efficacy of SBRT was better than that of ablation and TACE among patients with tumors > 3 cm and second only to repeat hepatectomy[102]. In addition, a small, single-center, retrospective study evaluating six patients with recurrent intrahepatic HCC after liver transplantation treated with SBRT found no local progression or death in patients at a median follow-up of 15.5 mo, which may imply that SBRT is safe for use in this setting[112]. Notably, a study by Eriguchi et al[112] suggested that repeated stereotactic radiotherapy is feasible for the treatment of HCC. The 3-year OS rate of patients with HCC treated with SBRT at least twice between 2012 and 2019 was 62.8% after the second course of treatment. However, there are few prospective studies on the application of SBRT for recurrent HCC.
In recent years, systemic therapies, such as molecular targeted drug therapy and immunotherapy, have become a major focus in the treatment of intermediate and advanced liver cancer. Multiple studies have revealed that sorafenib, a representative molecular targeted therapy, prolongs the survival of patients with recurrent HCC after liver transplantation[113-116]. A case-control study showed that 15 patients with HCC treated with sorafenib had a better prognosis than 24 patients who relapsed after liver transplantation on supportive care (median survival for relapse: 21.3 mo vs 11.8 mo, P = 0.0009)[115]. Martin et al[116] also demonstrated a similar safety profile for sorafenib in patients with HCC who developed recurrence after resection. Regorafenib, another molecular targeted therapy, has gained attention as an option for the treatment of recurrent HCC after liver transplantation. In sorafenib-resistant patients who develop disease progression, the application of regorafenib for recurrent tumors after liver transplantation is safe and significantly prolongs patient OS compared with supportive therapy (13.1 mo vs 5.5 mo; P < 0.01)[117]. Regorafenib and lenvatinib are currently approved for the treatment of recurrent HCC in Japan[118]. However, many patients have de novo or acquired resistance to monotherapy; therefore, drug combinations are gradually gaining recognition among investigators. Immunotherapy, such as ICIs, has also proven to be advantageous in the treatment of recurrent HCC when combined with tyrosine kinase inhibitors. One study suggested that the combination of lenvatinib plus pembrolizumab for patients with postoperative refractory recurrent metastatic HCC resulted in partial remission and an OS of up to 60 mo after surgery[119]. Similarly, the combination of mammalian target of rapamycin target inhibitors and sorafenib is safe and effective in patients with post-transplant relapsed HCC[120]. Nevertheless, studies on systemic therapy for the treatment of recurrent HCC after resection are still insufficient, and more data are needed to confirm the therapeutic value of this strategy in the relevant populations.
A combination of nonsurgical treatments for recurrent HCC is being tested in multiple studies, with the combination of TACE and ablation being the most promising. Heat dissipation may be the reason for the poor ablation effect of RFA. Applying both RFA and TACE can block the blood supply to the tumor, expand the tumor ablation margin to destroy satellite lesions, and minimize the heat loss caused by the heat sink effect, whereas the effect of chemotherapeutic anticancer agents on cancer cells is enhanced by the heat therapy effect[121]. Song et al[122] analyzed the outcomes of 96 patients with recurrent HCC ≤ 5 cm treated with a combination regimen of TACE-RFA and found that TACE-RFA as a first-line local therapy led to better DFS than TACE alone. This was also confirmed by a prospective randomized trial in which sequential TACE-RFA was more effective than RFA alone in patients with recurrent HCC ≤ 5 cm in diameter[123]. Furthermore, the combined TACE-RFA regimen was superior in prolonging patient survival compared with sorafenib alone for advanced recurrent HCC. This study revealed that the median OS (14.0 mo vs 9.0 mo; P < 0.001) and time to progression (7.0 mo vs 4.0 mo; P < 0.001) were significantly longer in the TACE-RFA combination group than in the sorafenib group[124]. In addition to the TACE-RFA combination, the combination of sorafenib and TACE is effective in patients with recurrent intermediate-stage HCC and microvascular invasion, and this treatment strategy yields a longer survival time than TACE alone[125]. Similarly, TACE combined with camrelizumab was reported to have an acceptable safety profile, although its efficacy was comparable to that of TACE alone[126]. Hence, TACE combined with systemic therapy has outstanding potential for recurrent liver cancer, but the variety of combination therapies is relatively small. Larger prospective clinical studies are needed to optimize the treatment sequence and identify the appropriate combination therapy regimens. The strategy of ablation combined with systemic therapy for the treatment of recurrent HCC is currently being studied in different institutions, including in phase III clinical trials (ClinicalTrials.gov numbers: NCT05444478, NCT05277675, and NCT04663035).
The previous sections have highlighted the high risk of recurrence of liver cancer and the limitations of available treatments. For example, surgical resection is the most effective treatment. However, owing to the low sensitivity and specificity of resection caused by the technical level and unclear diagnosis, it is likely that some patients with early recurrence of HCC will be unable to undergo the optimal treatment[127]. Therefore, predicting and monitoring for recurrence of HCC after the initial treatment is key to prolonging survival and avoiding harm to the life and health of patients due to tumor progression. Although there are some treatment measures to prevent the recurrence of HCC, these preventive treatments are not targeted, which can easily lead to overtreatment and increase patients’ economic burden and decrease quality of life. Therefore, more accurate indicators are needed to supplement the stratification of prognostic and the risks of postoperative metastasis and tumor recurrence in patients with HCC. The use of molecular biological methods to study and identify effective molecular markers is one of the key means to assist clinical diagnosis, guide clinical intervention, and provide early warning of cancer.
Recurrence and metastasis are the main reasons for the poor prognosis of HCC. However, there is no sensitive and specific method for predicting early recurrence and metastasis of HCC. Several molecular markers or their combinations have been published or reported for the diagnosis or prediction of HCC; however, there is still a lack of molecular markers or combinations that can be used to predict HCC recurrence and metastasis.
Pathological factors: Owing to the high malignancy of HCC cells, the rapid growth of cancerous tissue, and the rich blood supply to the liver, cancer cells can easily invade the blood vessels of the liver and metastasize to other parts of the liver hematogenously. Therefore, many pathological factors associated with primary tumor characteristics and the underlying liver are intimately related to the recurrence of HCC, including the size and number of tumors, tumor capsule, portal vein tumor thrombus, stage and differentiation of the tumor, and degree of cirrhosis[128,129]. The size and number of tumors are important factors affecting recurrence after surgery. Some people regard the integrity of the tumor capsule as an indicator of tumor invasiveness; however, the capsule of liver cancer is actually a pseudocapsule (usually constructed from connective fibrous tissue) formed by squeezing the surrounding normal liver tissue during tumor growth[130-132]. Cancerous infiltrates are often found in the liver tissue outside the intact capsule, and there is little evidence of a clear relationship between capsule integrity and postoperative recurrence. However, the existence of an intact capsule has certain significance in the determination of the surgical margin during radical resection[132]. For patients with a tumor diameter > 3 cm and incomplete imaging of the tumor capsule, a wide resection margin is preferred[132]. The presence of intrahepatic portal vein tumor thrombus is another important factor associated with the postoperative recurrence of liver cancer, and intrahepatic metastasis is easily formed in patients with intrahepatic portal vein tumor thrombus[128]. It is generally believed that the stage and classification of the tumor are strongly correlated with prognosis: The lower the differentiation of a malignant tumor, the more invasive it is[128]. Therefore, primary liver cancers with poor differentiation are prone to early metastasis, resulting in incomplete resection and postoperative recurrence. Cirrhosis may affect recurrence, because it limits the size of the resection margin, thereby reducing the rate of radical resection. In addition, spleen stiffness measurements directly related to the degree of liver disease and portal hypertension, as assessed using transient elastography, appear to be the only predictors of late recurrence of HCC[129]. Finally, factors related to surgery are also strongly associated with the recurrence of HCC, including tumor margins[133], intraoperative bleeding and blood transfusion[134], and intraoperative compression of the tumor[135]. Tumor margin is the most important factor in the criteria for radical resection of liver cancer. A larger resection margin is associated with a lower detection rate of tumor thrombus and a lower recurrence rate after surgery[133]. Intraoperative bleeding and blood transfusion reflect the degree of surgical trauma, and the magnitude of intraoperative estimated blood loss is related to the biological characteristics of the tumor and the extent of surgery[134]. Moreover, estimated blood loss during HCC resection can affect the postoperative course of hepatitis and the recovery of immune function. Intraoperative compression of the tumor may cause shedding of cancer tissue or tumor cells, resulting in intrahepatic metastasis or distant dissemination and becoming an important source of postoperative recurrence[135].
Serum biomarkers: Serum AFP and albumin levels were the earliest serological markers used to assist in the diagnosis of HCC. Serum AFP ≥ 400 ng/mL is highly suggestive of HCC if pregnancy, chronic or active liver disease, gonad embryonic-derived tumors, and other gastrointestinal tumors can be ruled out. AFP L3 can be used as a prognostic indicator of HCC recurrence. Additionally, in patients with chronic HBV infection and those at a high risk of cirrhosis, AFP L3 can be an early indicator of HCC. After radical resection of HCC, a lack of obvious decrease in AFP L3 indicates the presence of metastasis or residual carcinoma[136]. In addition, given the high false-negative rate of AFP in the detection of early or small HCC, prothrombin induced by vitamin K deficiency or antagonist-II (PIVKA-II) can be used as a complement to AFP. As early as 1984, Liebman et al[137] found abnormally elevated levels of des-γ-carboxy prothrombin in patients with primary HCC and proposed its use for the laboratory diagnosis of HCC. Many have since studied this serum marker further and compared it with the traditional diagnostic marker AFP. Feng et al[138] evaluated the diagnostic efficacy of AFP and PIVKA-II when used separately and in combination in patients with primary and recurrent HCC and observed that the combination of both markers dramatically improved the diagnostic efficiency compared to either marker alone. Conversely, a recent retrospective cohort study indicated that preoperative PIVKA-II positivity, but not preoperative AFP positivity, was an independent risk factor for early recurrence of HCC[139]. This suggests that PIVKA-II is equally effective as a serum diagnostic biomarker and can be considered an alternative to AFP.
Inflammatory markers: C-reactive protein (CRP), which is synthesized by hepatocytes and regulated by interleukin-1 (IL-1) and IL-6, has important clinical value as a marker of acute and chronic inflammation. Several recent studies have found that CRP is an independent risk factor for tumor recurrence in patients with HCC who exceed the Milan criteria after liver transplantation[140,141]. Similarly, elevated postoperative serum CRP may be a prognostic indicator for patients with HCC after elective hepatectomy[142]. Further, the peripheral blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are correlated with the prognosis of malignant tumors[143,144]. Halazun et al[143] followed up 150 patients who underwent liver transplantation for HCC and found that the tumor recurrence rate of 13 patients with an NLR ≥ 5 was 62%, and the 5-year OS and DFS rates after surgery were significantly lower than those of patients with an NLR < 5. Further, multivariate analysis showed that a high NLR was a risk factor affecting the DFS rate of recipients (hazard ratio = 19.98; P = 0.005). Another study of 865 patients who underwent liver transplantation for HCC found that the risk of HCC recurrence increased 1.89 times for each logarithmic unit increase in the NLR[144]. A meta-analysis conducted by Lai et al[145] revealed that an elevated PLR was associated with an increased risk of HCC recurrence after liver transplantation (odds ratio = 3.33; 95%CI: 1.78-6.25; P < 0.001). Although these studies show the predictive potential of these inflammatory markers, there is heterogeneity and poor reproducibility. In addition, the cutoff values of inflammatory markers vary greatly between studies; therefore, the optimal cutoff requires further study, and it is difficult to use these as biomarkers widely in clinical practice.
Immunohistochemical indicators: Patients with liver cancer often have a history of HBV or HCV infection, liver cirrhosis, and other backgrounds, and the resulting inflammatory response often leads to large numbers of lymphocytes in or around the lesion. The ratio of CD4/CD8+ T cells in the tumor is associated with recurrence after liver transplantation, and more CD4+ T cell infiltration reduces the risk of recurrence after liver transplantation[146]. Further, tumor or peripheral blood regulatory T (Treg) cells are associated with tumor invasion, and Treg cells reduce the antitumor effect of effector T cells, which promotes tumor immune escape[147,148]. The imbalance between regulatory and cytotoxic T cells in HCC is also expected to be an effective prognostic factor. Clinical studies have found that Treg cells are significantly higher in HCC tissues than in non-cancerous liver tissues, suggesting that Treg cell infiltration in HCC can inhibit antitumor immunity and high Treg cell infiltration in HCC is a predictor of poor prognosis[149].
Genetic biomarkers: In the process of tumor invasion and metastasis, tumor cells need to break through the barriers of the extracellular matrix and basement membrane. Matrix metalloproteinase (MMP)-9 can degrade the extracellular matrix; therefore, tumors with high expression of MMP-9 have stronger invasion and metastasis abilities. Most patients with high MMP-9 expression in liver cancer tissues and plasma have portal vein tumor thrombus or intrahepatic metastasis[150]. The level of serum vascular endothelial growth factor (VEGF) in patients with liver cancer is significantly higher than that in patients with benign liver disease and healthy individuals. High VEGF is closely related to portal vein tumor tether, tumor size, and TNM stage[151]. VEGF plays an important role in the invasion and metastasis of liver cancer, and preoperative examination of serum VEGF levels is of great significance in predicting the invasion and metastasis of liver cancer[152,153]. AFP mRNA in circulating blood can be used to detect the presence of circulating cancer cells[154,155]. Reverse transcription-polymerase chain reaction indicated the presence of AFP mRNA in the peripheral blood of 59.7% of patients with liver cancer[154]. Therefore, the presence of disseminated HCC cells in the blood circulation can be detected before treatment is initiated. The positive rate of AFP mRNA in the peripheral blood is significantly correlated with the clinical stage and postoperative recurrence of liver cancer, and 57% of patients with postoperative recurrence have AFP mRNA in the peripheral blood[156]. Therefore, AFP mRNA expression in the systemic circulation can be used to assess the risk of recurrence and metastasis.
The establishment of prognostic models based on predictors is important in the field of HCC recurrence prevention and monitoring. Hwang et al[156] integrated three variables (tumor size > 5 cm, high AFP, and high des-γ-carboxy prothrombin) by direct multiplication and constructed the ADV score as a comprehensive proxy for predicting prognosis after isolated HCC resection. This score had a sensitivity of 73.9% and specificity of 66.7%[157]. This team then performed preoperative evaluation and postoperative follow-up of 526 patients with isolated HCC ≥ 8 cm treated by hepatectomy, which led to the development of a comprehensive, predictive surrogate marker that is equally valid in patients with very large HCC. The PPM prediction model constructed in that study is based on four factors, including AFP ≥ 100 ng/mL, hypermetabolic 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) findings, microvascular invasion, and satellite nodules, and had C-indexes of 0.66 for tumor recurrence and 0.69 for patient survival. In contrast, in the new version of the PPM prediction model constructed based on two previously studied factors, ADV7 log and FDG-PET, the C-indexes for tumor recurrence and patient survival were 0.64 and 0.70, respectively[158].
The construction of a reliable risk score for recurrence of HCC after liver transplantation could vastly improve surveillance strategies and help identify patients who may benefit from adjuvant therapy. The RETREAT score constructed by Mehta et al[157] is effective in predicting recurrence after transplantation in patients with HCC who meet the Milan criteria. The score includes three main factors: Microvascular invasion, post-transplant AFP, and the sum of the maximum diameter and number of surviving tumors. Compared with the Milan criteria, the RETREAT score improved the prediction of HCC recurrence at 1 (0.40, P = 0.001) and 5 (0.31, P < 0.001) years after liver transplant[159]. Based on the RETREAT score, Costentin et al[158] recently proposed a novel composite prediction tool, the R3-AFP score, to optimize the prediction of HCC recurrence after liver transplantation. In addition to the factors included in RETREAT, the model also incorporated pre-transplant AFP and pathological variables, which led to the classification of patients into four risk groups, with a 5-year survival rate of 77.2% for patients in the very low-risk group[160].
In addition to these traditional Cox proportional hazard prediction models based on linearity assumption, the construction of prediction models by machine learning algorithms has become an important method for predicting tumor recurrence. Given the complex, multidimensional, nonlinear relationships between clinical data, machine learning models outperform traditional regression models in predicting HCC progression[161]. The XGBoost model based on clinical data is effective in predicting the risk of early recurrence in patients after MWA, with an area under the curve of 0.75 (95%CI: 0.72-0.78)[162]. Moreover, incorporating magnetic resonance imaging (MRI) data in a machine learning model for recurrent HCC after transplantation can effectively improve the predictive performance of the model compared to incorporating clinical parameters alone[163]. Therefore, appropriate monitoring protocols can be developed to maximize the prevention of recurrence and prolong patient survival after HCC resection.
The main methods currently used for the clinical monitoring of HCC recurrence are serum AFP monitoring, regular abdominal ultrasonography, and computed tomography (CT). In addition, MRI has strong soft tissue resolution and can reflect the changes in blood flow and enhancement at the lesion site and has been widely used in clinical practice to monitor the recurrence of liver cancer. Gadoxetic acid (Gd-EOB-DTPA) is a relatively safe and well-tolerated liver-specific contrast agent that adequately combines the properties of conventional extracellular contrast agents and hepatocyte-specific magnetic resonance contrast agents with the higher soft tissue resolution of MRI. Therefore, EOB-MRI has better detection and diagnostic efficacy for HCC than CT[164]. The apparent diffusion coefficient (ADC) in magnetic resonance diffusion-weighted imaging (DWI) can quantify the overall diffusion of a lesion. Chuang et al[165] revealed that tumor recurrence after liver transplantation could be effectively predicted by analyzing the correlation between tumor recurrence, explant pathologic findings, and the ADC. Furthermore, several lines of evidence suggest that Gd-EOB-DTPA and DWI are more advantageous in detecting small liver lesions than CT[166,167]. Therefore, clinicians may be able to select more appropriate monitoring methods based on the combination of imaging and prognostic models to identify patients at high risk of recurrence and determine the optimal treatment.
In conclusion, since HCC has varied recurrence patterns and timing, the choice of treatment option after treatment for primary HCC varies. Repeat hepatectomy is the treatment of choice for recurrent HCC; laparoscopic surgery techniques are becoming increasingly sophisticated and offer a novel, safe, and effective surgical option for hepatectomy in patients with recurrent disease. However, the clinical application of repeat hepatectomy is limited due to the small number of eligible patients. Liver transplantation is preferable for patients with recurrent HCC complicated by severe cirrhosis and hepatic decompensation, and it has a better RFS than repeated hepatectomy; however, a shortage of organ donors and long wait times are two major factors that limit the utilization of SLT. In patients with recurrent HCC who are not candidates for resection or transplantation, nonsurgical treatment options are worth considering. Whether HCC recurs after resection or transplantation, ablative therapy, especially RFA, has become another treatment alternative advocated by many researchers, owing to its minimally invasive nature and convenient advantages. However, salvage ablation is recommended only for patients with early recurrence of tumors ≤ 3 cm in diameter. Although TACE does not provide the same survival benefit as repeat hepatectomy and SLT for recurrent HCC, it should be considered in patients with early recurrence with microvascular invasion or multiple lesions. Similarly, SBRT can provide good disease control and a modest survival benefit in patients with small HCC who relapse after operative treatment. Systemic therapy, including molecular targeted therapy and immunotherapy, is also gaining attention as an emerging therapeutic strategy for clinical application in recurrent liver cancer. Systemic therapy can provide benefit to patients with advanced recurrent HCC either as a single agent or in combination with other therapies. Combination therapy is a promising way to optimize therapeutic efficacy by combining different treatment options to reduce complications and prolong survival, and this may be a key research direction for the future. The flexible combination of systemic therapies and other complementary therapies may offer a breakthrough in the clinical efficacy of HCC treatment. Finally, despite the promising results of most of these studies, future prospective randomized controlled studies are still needed to provide more rigorous clinical evidence to develop and optimize treatment options for recurrent HCC.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozair A, United States; Tsoulfas G, Greece S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1844] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1219] [Article Influence: 203.2] [Reference Citation Analysis (1)] |
| 3. | Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg. 2021;273:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Hatzaras I, Bischof DA, Fahy B, Cosgrove D, Pawlik TM. Treatment options and surveillance strategies after therapy for hepatocellular carcinoma. Ann Surg Oncol. 2014;21:758-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2615] [Article Influence: 871.7] [Reference Citation Analysis (59)] |
| 8. | Sun Y, Ji S, Ji H, Liu L, Li C. Clinical efficacy analysis of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) in primary liver cancer and recurrent liver cancer. J BUON. 2019;24:1402-1407. [PubMed] |
| 9. | Lee KF, Chong CCN, Fong AKW, Fung AKY, Lok HT, Cheung YS, Wong J, Lai PBS. Pattern of disease recurrence and its implications for postoperative surveillance after curative hepatectomy for hepatocellular carcinoma: experience from a single center. Hepatobiliary Surg Nutr. 2018;7:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sasaki K, Shindoh J, Margonis GA, Nishioka Y, Andreatos N, Sekine A, Hashimoto M, Pawlik TM. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg. 2017;152:e165059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 14. | Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Baskiran A, Akbulut S, Sahin TT, Koc C, Karakas S, Ince V, Yurdaydin C, Yilmaz S. Effect of HBV-HDV co-infection on HBV-HCC co-recurrence in patients undergoing living donor liver transplantation. Hepatol Int. 2020;14:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Samuel M, Chow PK, Chan Shih-Yen E, Machin D, Soo KC. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;2009:CD001199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Urata Y, Kubo S, Takemura S, Uenishi T, Kodai S, Shinkawa H, Sakae M, Kaneda K, Ohata K, Nozawa A, Suehiro S. Effects of antiviral therapy on long-term outcome after liver resection for hepatitis B virus-related hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Chen LP, Zhao J, Du Y, Han YF, Su T, Zhang HW, Cao GW. Antiviral treatment to prevent chronic hepatitis B or C-related hepatocellular carcinoma. World J Virol. 2012;1:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 20. | von Marschall Z, Scholz A, Cramer T, Schäfer G, Schirner M, Oberg K, Wiedenmann B, Höcker M, Rosewicz S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst. 2003;95:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Chander G, Sulkowski MS, Jenckes MW, Torbenson MS, Herlong HF, Bass EB, Gebo KA. Treatment of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S135-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Xu J, Li J, Chen J, Liu ZJ. Effect of adjuvant interferon therapy on hepatitis b/c virus-related hepatocellular carcinoma after curative therapy - meta-analysis. Adv Clin Exp Med. 2015;24:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Qi WQ, Zhang Q, Wang X, Xu Y, Zhao P, Guo HH, Zhou CY, Sun Y, Liu L, Wang JB. Long-term clinical benefit of Peg-IFNα and NAs sequential anti-viral therapy on HBV related HCC. Neoplasma. 2021;68:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Qi W, Zhang Q, Xu Y, Wang X, Yu F, Zhang Y, Zhao P, Guo H, Zhou C, Wang Z, Sun Y, Liu L, Xuan W, Wang J. Peg-interferon and nucleos(t)ide analogue combination at inception of antiviral therapy improves both anti-HBV efficacy and long-term survival among HBV DNA-positive hepatocellular carcinoma patients after hepatectomy/ablation. J Viral Hepat. 2020;27:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Moran A, Ramos LF, Picado O, Pendola F, Sleeman D, Dudeja V, Merchant N, Yakoub D. Hepatocellular carcinoma: resection with adjuvant hepatic artery infusion therapy vs resection alone. A systematic review and meta-analysis. J Surg Oncol. 2019;119:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Liu C, Sun L, Xu J, Zhao Y. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol. 2016;14:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Ye JZ, Chen JZ, Li ZH, Bai T, Chen J, Zhu SL, Li LQ, Wu FX. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23:7415-7424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, Wu MC, Lau WY, Cheng SQ. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol. 2016;23:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Chan EK, Imai H, Hamel JC, Tan EM. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA transcription upstream binding factor. J Exp Med. 1991;174:1239-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Si T, Chen Y, Ma D, Gong X, Yang K, Guan R, Peng C. Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta-analysis of random controlled trials. Scand J Gastroenterol. 2016;51:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Yang J, Liang H, Hu K, Xiong Z, Cao M, Zhong Z, Yao Z, Deng M. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, Suh DJ. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Yu W, Wang W, Rong W, Wang L, Xu Q, Wu F, Liu L, Wu J. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg. 2014;218:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Wang L, Liu Y, Rong W, Wu F, Yu W, Liu K, Lin S, Zheng Y, Zhang K, Siqin T, Tao C, Liu M, Chen B, Feng Q, Wu J. The role of intraoperative electron radiotherapy in centrally located hepatocellular carcinomas treated with narrow-margin (<1 cm) hepatectomy: a prospective, phase 2 study. Hepatobiliary Surg Nutr. 2022;11:515-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Lau WY, Leung TW, Ho SK, Chan M, Machin D, Lau J, Chan AT, Yeo W, Mok TS, Yu SC, Leung NW, Johnson PJ. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 284] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Chung AY, Ooi LL, Machin D, Tan SB, Goh BK, Wong JS, Chen YM, Li PC, Gandhi M, Thng CH, Yu SW, Tan BS, Lo RH, Htoo AM, Tay KH, Sundram FX, Goh AS, Chew SP, Liau KH, Chow PK, Tan YM, Cheow PC, Ho CK, Soo KC. Adjuvant hepatic intra-arterial iodine-131-lipiodol following curative resection of hepatocellular carcinoma: a prospective randomized trial. World J Surg. 2013;37:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Gong L, Shi L, Sun J, Yuan WS, Chen JF, Liu P, Gong F, Dong JH. Comparative survival analysis of adjuvant therapy with iodine-131-labeled lipiodol to hepatic resection of primary hepatocellular carcinoma: a meta-analysis. Nucl Med Commun. 2014;35:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Furtado R, Crawford M, Sandroussi C. Systematic review and meta-analysis of adjuvant i(131) lipiodol after excision of hepatocellular carcinoma. Ann Surg Oncol. 2014;21:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Hong Y, Wu LP, Ye F, Zhou YM. Adjuvant Intrahepatic Injection Iodine-131-Lipiodol Improves Prognosis of Patients with Hepatocellular Carcinoma After Resection: a Meta-Analysis. Indian J Surg. 2015;77:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1159] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 43. | Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Ueda H, Tanaka H, Kida Y, Fukuchi H, Ichinose M. Adjuvant chemotherapy with tegafur/uracil administration after transcatheter arterial chemoembolization for advanced hepatocellular carcinoma. Oncol Rep. 2008;19:1355-1361. [PubMed] |
| 46. | Nagano H, Miyamoto A, Wada H, Ota H, Marubashi S, Takeda Y, Dono K, Umeshita K, Sakon M, Monden M. Interferon-alpha and 5-fluorouracil combination therapy after palliative hepatic resection in patients with advanced hepatocellular carcinoma, portal venous tumor thrombus in the major trunk, and multiple nodules. Cancer. 2007;110:2493-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Lin HS, Wan RH, Gao LH, Li JF, Shan RF, Shi J. Adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma: a systematic review and a meta-analysis. Hepatobiliary Pancreat Dis Int. 2015;14:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 455] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 49. | Nitta H, Beppu T, Imai K, Hayashi H, Chikamoto A, Baba H. Adjuvant hepatic arterial infusion chemotherapy after hepatic resection of hepatocellular carcinoma with macroscopic vascular invasion. World J Surg. 2013;37:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Hsiao JH, Tsai CC, Liang TJ, Chiang CL, Liang HL, Chen IS, Chen YC, Chang PM, Chou NH, Wang BW. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int J Surg. 2017;45:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Lee BH, Lee DS, Cho CW, Yun SS. Role and limitation of neoadjuvant hepatic arterial infusion chemotherapy in advanced hepatocelluar carcinoma patients with Child-Pugh class A. World J Surg Oncol. 2019;17:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Nouso K, Miyahara K, Uchida D, Kuwaki K, Izumi N, Omata M, Ichida T, Kudo M, Ku Y, Kokudo N, Sakamoto M, Nakashima O, Takayama T, Matsui O, Matsuyama Y, Yamamoto K; Liver Cancer Study Group of Japan. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Cancer. 2013;109:1904-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Osaki A, Suda T, Kamimura K, Tsuchiya A, Tamura Y, Takamura M, Igarashi M, Kawai H, Yamagiwa S, Aoyagi Y. A safe and effective dose of cisplatin in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Med. 2013;2:86-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, Lee JH, Sun Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 55. | Li S, Zhong C, Li Q, Zou J, Wang Q, Shang C, Cheng Y, Cao M, Huang H, Mei J, Lu L, Zhao R, Lin W, Wen Y, Guo Z, Ling YH, Zheng L, Wei W, Guo R. Neoadjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of resectable BCLC stage A/B hepatocellular carcinoma patients beyond Milan criteria: An interim analysis of a multi-center, phase 3, randomized, controlled clinical trial. J Clin Oncol. 2021;39:4008. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Li S, Mei J, Wang Q, Guo Z, Lu L, Ling Y, Xu L, Chen M, Zheng L, Lin W, Zou J, Wen Y, Wei W, Guo R. Postoperative Adjuvant Transarterial Infusion Chemotherapy with FOLFOX Could Improve Outcomes of Hepatocellular Carcinoma Patients with Microvascular Invasion: A Preliminary Report of a Phase III, Randomized Controlled Clinical Trial. Ann Surg Oncol. 2020;27:5183-5190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, Economou JS. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902-5908. [PubMed] |
| 58. | Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 59. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 60. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 61. | Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, Xiong W, Zeng Z. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 62. | Repáraz D, Aparicio B, Llopiz D, Hervás-Stubbs S, Sarobe P. Therapeutic Vaccines against Hepatocellular Carcinoma in the Immune Checkpoint Inhibitor Era: Time for Neoantigens? Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Han CL, Yan YC, Yan LJ, Meng GX, Yang CC, Liu H, Ding ZN, Dong ZR, Hong JG, Chen ZQ, Li T. Efficacy and security of tumor vaccines for hepatocellular carcinoma: a systemic review and meta-analysis of the last 2 decades. J Cancer Res Clin Oncol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Sun K, Wang L, Zhang Y. Dendritic cell as therapeutic vaccines against tumors and its role in therapy for hepatocellular carcinoma. Cell Mol Immunol. 2006;3:197-203. [PubMed] |
| 65. | Anugwom CM, Leventhal TM, Debes JD. Understanding immune perspectives and options for the use of checkpoint immunotherapy in HCC post liver transplant. Hepatoma Res. 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 66. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 67. | Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: A pilot study. Hepatol Res. 2014;44:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Lei J, Zhong J, Hao J, Liu Z, Zhang P, Wu L, Yan L, Zhu J, Zeng Y, Li B, Wen T, Wang W. Hepatocellular carcinoma cases with high levels of c-Raf-1 expression may benefit from postoperative adjuvant sorafenib after hepatic resection even with high risk of recurrence. Oncotarget. 2016;7:42598-42607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Li Q, Song T. Association Between Adjuvant Sorafenib and the Prognosis of Patients With Hepatocellular Carcinoma at a High Risk of Recurrence After Radical Resection. Front Oncol. 2021;11:633033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 70. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 71. | Qi HL, Zhuang BJ, Li CS, Liu QY. Peri-operative use of sorafenib in liver transplantation: a time-to-event meta-analysis. World J Gastroenterol. 2015;21:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Han B, Ding H, Zhao S, Zhang Y, Wang J, Gu J. Potential Role of Adjuvant Lenvatinib in Improving Disease-Free Survival for Patients With High-Risk Hepatitis B Virus-Related Hepatocellular Carcinoma Following Liver Transplantation: A Retrospective, Case Control Study. Front Oncol. 2020;10:562103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Pinter M, Ulbrich G, Sieghart W, Kölblinger C, Reiberger T, Li S, Ferlitsch A, Müller C, Lammer J, Peck-Radosavljevic M. Hepatocellular Carcinoma: A Phase II Randomized Controlled Double-Blind Trial of Transarterial Chemoembolization in Combination with Biweekly Intravenous Administration of Bevacizumab or a Placebo. Radiology. 2015;277:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 75. | Chok KS, Chan SC, Poon RT, Fan ST, Lo CM. Re-resection for metachronous primary hepatocellular carcinoma: is it justified? ANZ J Surg. 2012;82:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 77. | Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, Farges O, Kianmanesh R. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885-92; discussion 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 78. | Suenaga M, Sugiura H, Kokuba Y, Uehara S, Kurumiya T. Repeated hepatic resection for recurrent hepatocellular carcinoma in eighteen cases. Surgery. 1994;115:452-457. [PubMed] |
| 79. | Matsumoto M, Yanaga K, Shiba H, Wakiyama S, Sakamoto T, Futagawa Y, Gocho T, Ishida Y, Ikegami T. Treatment of intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Ann Gastroenterol Surg. 2021;5:538-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Faber W, Seehofer D, Neuhaus P, Stockmann M, Denecke T, Kalmuk S, Warnick P, Bahra M. Repeated liver resection for recurrent hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, Chen MS, Zhang YF, Guo RP. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection versus radiofrequency ablation. Cancer Med. 2020;9:2997-3005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Huang ZY, Liang BY, Xiong M, Zhan DQ, Wei S, Wang GP, Chen YF, Chen XP. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol. 2012;19:2515-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Mise Y, Hasegawa K, Shindoh J, Ishizawa T, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. The Feasibility of Third or More Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg. 2015;262:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 85. | Wu CC, Cheng SB, Yeh DC, Wang J, P'eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, Romito R, Mariani L, Mazzaferro V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 87. | Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, Tan W, Zhang L. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc. 2017;31:4790-4798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Chan DL, Alzahrani NA, Morris DL, Chua TC. Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | de Haas RJ, Lim C, Bhangui P, Salloum C, Compagnon P, Feray C, Calderaro J, Luciani A, Azoulay D. Curative salvage liver transplantation in patients with cirrhosis and hepatocellular carcinoma: An intention-to-treat analysis. Hepatology. 2018;67:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Lim C, Shinkawa H, Hasegawa K, Bhangui P, Salloum C, Gomez Gavara C, Lahat E, Omichi K, Arita J, Sakamoto Y, Compagnon P, Feray C, Kokudo N, Azoulay D. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: An intent-to-treat analysis. Liver Transpl. 2017;23:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 92. | Liu J, Zhao J, Gu HAO, Zhu Z. Repeat hepatic resection VS radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma: an updated meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, Lo CM. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 94. | Bai XM, Cui M, Yang W, Wang H, Wang S, Zhang ZY, Wu W, Chen MH, Yan K, Goldberg SN. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary versus Recurrent HCC. Radiology. 2021;300:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 95. | Yang W, Chen MH, Yin SS, Yan K, Gao W, Wang YB, Huo L, Zhang XP, Xing BC. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early- and late-phase recurrence. AJR Am J Roentgenol. 2006;186:S275-S283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Yu J, Yu XL, Han ZY, Cheng ZG, Liu FY, Zhai HY, Mu MJ, Liu YM, Liang P. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 98. | Aquina CT, Eskander MF, Pawlik TM. Liver-Directed Treatment Options Following Liver Tumor Recurrence: A Review of the Literature. Front Oncol. 2022;12:832405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 100. | Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Zheng J, Cai J, Tao L, Kirih MA, Shen Z, Xu J, Liang X. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 102. | Midorikawa Y, Takayama T, Moriguchi M, Yagi R, Yamagishi S, Nakayama H, Aramaki O, Yamazaki S, Tsuji S, Higaki T. Liver Resection Versus Embolization for Recurrent Hepatocellular Carcinoma. World J Surg. 2020;44:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 105. | Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, Shin WY. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 107. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 108. | Fidelman N, Kerlan RK Jr. Transarterial Chemoembolization and (90)Y Radioembolization for Hepatocellular Carcinoma: Review of Current Applications Beyond Intermediate-Stage Disease. AJR Am J Roentgenol. 2015;205:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, Su YF, Fan CY, Chang YW. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 111. | Au KP, Chiang CL, Chan ACY, Cheung TT, Lo CM, Chok KSH. Initial experience with stereotactic body radiotherapy for intrahepatic hepatocellular carcinoma recurrence after liver transplantation. World J Clin Cases. 2020;8:2758-2768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Eriguchi T, Tsukamoto N, Kuroiwa N, Nemoto T, Ogata T, Okubo Y, Nakano S, Sugawara A. Repeated Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Pract Radiat Oncol. 2021;11:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, Moon DB, Kang YK. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Li BCW, Chiu J, Shing K, Kwok GGW, Tang V, Leung R, Ma KW, She WH, Tsang J, Chan A, Cheung TT, Lo CM, Yau T. The Outcomes of Systemic Treatment in Recurrent Hepatocellular Carcinomas Following Liver Transplants. Adv Ther. 2021;38:3900-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 116. | Martin RC 2nd, Bruenderman E, Cohn A, Piperdi B, Miksad R, Geschwind JF, Goldenberg A, Sanyal A, Zigmont E, Babajanyan S, Foreman P, Mantry P, McGuire B, Gholam P. Sorafenib use for recurrent hepatocellular cancer after resection or transplantation: Observations from a US regional analysis of the GIDEON registry. Am J Surg. 2017;213:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, Fraile-López M, Czauderna C, Di Costanzo G, Bhoori S, Pinter M, Manini MA, Amaddeo G, Yunquera AF, Piñero F, Blanco Rodríguez MJ, Anders M, Aballay Soteras G, Villadsen GE, Yoon PD, Cesarini L, Díaz-González Á, González-Diéguez ML, Tortora R, Weinmann A, Mazzaferro V, Romero Cristóbal M, Crespo G, Regnault H, De Giorgio M, Varela M, Prince R, Scudeller L, Donato MF, Wörns MA, Bruix J, Sapisochin G, Lampertico P, Reig M. Regorafenib Efficacy After Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Retrospective Study. Liver Transpl. 2021;27:1767-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 118. | Suzuki R, Goto R, Kawamura N, Watanabe M, Ganchiku Y, Hatanaka KC, Hatanaka Y, Kamiyama T, Shimamura T, Taketomi A. Efficient multiple treatments including molecular targeting agents in a case of recurrent hepatocellular carcinoma, post-living donor liver transplantation. Clin J Gastroenterol. 2022;15:755-764. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 119. | He X, Peng Y, Zhou Z, Li W. Immune Checkpoint Inhibitor-Based Systemic Therapy Shows Remarkable Curative Effect in a Hepatocellular Carcinoma Patient With Intractable Postoperative Recurrence and Metastases: A Case Report and Literature Review. Front Oncol. 2022;12:784224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 121. | Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Kuang M. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol. 2018;28:3522-3531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 122. | Song Q, Ren W, Fan L, Zhao M, Mao L, Jiang S, Zhao C, Cui Y. Long-Term Outcomes of Transarterial Chemoembolization Combined with Radiofrequency Ablation Versus Transarterial Chemoembolization Alone for Recurrent Hepatocellular Carcinoma After Surgical Resection. Dig Dis Sci. 2020;65:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 124. | Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 125. | Peng Z, Chen S, Xiao H, Wang Y, Li J, Mei J, Chen Z, Zhou Q, Feng S, Chen M, Qian G, Peng S, Kuang M. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology. 2019;292:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 126. | Guo Y, Ren Y, Chen L, Sun T, Zhang W, Sun B, Zhu L, Xiong F, Zheng C. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 127. | Chen Y, Guo D, Li X, Xu C, Zhu Q. Predictors of Spontaneous Rupture of Hepatocellular Carcinoma and Clinical Outcomes Following Hepatectomy. Front Oncol. 2022;12:820867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 129. | Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M, Festi D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 130. | Grazioli L, Olivetti L, Fugazzola C, Benetti A, Stanga C, Dettori E, Gallo C, Matricardi L, Giacobbe A, Chiesa A. The pseudocapsule in hepatocellular carcinoma: correlation between dynamic MR imaging and pathology. Eur Radiol. 1999;9:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, Nishie A, Hirakawa M, Ushijima Y, Okamoto D, Taketomi A, Honda H. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009;250:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 132. | Chao JS, Zhu Q, Chen DS, Chen GM, Xie XQ, Liu AQ, Zhao SL, Sun HC. Combined analysis of imaging tumor capsule with imaging tumor size guides the width of resection margin for solitary hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2022;21:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 133. | Lafaro K, Grandhi MS, Herman JM, Pawlik TM. The importance of surgical margins in primary malignancies of the liver. J Surg Oncol. 2016;113:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 134. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 135. | Dong XF, Zhong JT, Liu TQ, Yang JR. [Relationship of operation manner and postoperative recurrence of hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 2021;43:635-637. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 136. | Yi X, Yu S, Bao Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta. 2013;425:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 137. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 421] [Article Influence: 10.3] [Reference Citation Analysis (16)] |
| 138. | Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 139. | Wang MD, Sun LY, Qian GJ, Li C, Gu LH, Yao LQ, Diao YK, Pawlik TM, Lau WY, Huang DS, Shen F, Yang T. Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: A retrospective cohort study. Int J Surg. 2022;105:106843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 140. | Kornberg A, Witt U, Kornberg J, Müller K, Friess H, Thrum K. Postoperative peak serum C-reactive protein is a predictor of outcome following liver transplantation for hepatocellular carcinoma. Biomarkers. 2016;21:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 141. | An HJ, Jang JW, Bae SH, Choi JY, Yoon SK, Lee MA, You YK, Kim DG, Jung ES. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Shiba H, Furukawa K, Fujiwara Y, Futagawa Y, Haruki K, Wakiyama S, Ishida Y, Misawa T, Yanaga K. Postoperative peak serum C-reactive protein predicts outcome of hepatic resection for hepatocellular carcinoma. Anticancer Res. 2013;33:705-709. [PubMed] |
| 143. | Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 144. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 145. | Lai Q, Melandro F, Larghi Laureiro Z, Giovanardi F, Ginanni Corradini S, Ferri F, Hassan R, Rossi M, Mennini G. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 146. | Li SP, Zhang JM, Chen XJ, Zhou GP, Sun J, Cui B, Zhou LX, Zhang HM, Que WT, Sun LY, Zhu ZJ. Characteristics of changes in double positive CD4(+)CD8(+) T cells in liver transplantation. Int Immunopharmacol. 2022;110:109028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 147. | Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol. 2020;38:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 724] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 148. | Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404-421.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 531] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 149. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 892] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 150. | Chen R, Cui J, Xu C, Xue T, Guo K, Gao D, Liu Y, Ye S, Ren Z. The significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence of hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2012;19 Suppl 3:S375-S384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 152. | Lacin S, Yalcin S. The Prognostic Value of Circulating VEGF-A Level in Patients With Hepatocellular Cancer. Technol Cancer Res Treat. 2020;19:1533033820971677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 153. | Jin J, Niu X, Zou L, Li L, Li S, Han J, Zhang P, Song J, Xiao F. AFP mRNA level in enriched circulating tumor cells from hepatocellular carcinoma patient blood samples is a pivotal predictive marker for metastasis. Cancer Lett. 2016;378:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 154. | Kamiyama T, Takahashi M, Nakagawa T, Nakanishi K, Kamachi H, Suzuki T, Shimamura T, Taniguchi M, Ozaki M, Matsushita M, Furukawa H, Todo S. AFP mRNA detected in bone marrow by real-time quantitative RT-PCR analysis predicts survival and recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg. 2006;244:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Lee SG. Multiplication of Tumor Volume by Two Tumor Markers Is a Post-Resection Prognostic Predictor for Solitary Hepatocellular Carcinoma. J Gastrointest Surg. 2016;20:1807-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 156. | Hwang S, Joh JW, Wang HJ, Kim DG, Kim KS, Suh KS, Kim SH, Yu HC, Cho CK, Lee YJ, Kim KH, Kim JM, Kim BW, Lee SG. Prognostic Prediction Models for Resection of Large Hepatocellular Carcinoma: A Korean Multicenter Study. World J Surg. 2018;42:2579-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 157. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 158. | Costentin C, Piñero F, Degroote H, Notarpaolo A, Boin IF, Boudjema K, Baccaro C, Podestá LG, Bachellier P, Ettorre GM, Poniachik J, Muscari F, Dibenedetto F, Duque SH, Salame E, Cillo U, Marciano S, Vanlemmens C, Fagiuoli S, Burra P, Van Vlierberghe H, Cherqui D, Lai Q, Silva M, Rubinstein F, Duvoux C; French-Italian-Belgium and Latin American collaborative group for HCC and liver transplantation. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022;4:100445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 159. | Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 160. | An C, Yang H, Yu X, Han ZY, Cheng Z, Liu F, Dou J, Li B, Li Y, Yu J, Liang P. A Machine Learning Model Based on Health Records for Predicting Recurrence After Microwave Ablation of Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2022;9:671-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 161. | Iseke S, Zeevi T, Kucukkaya AS, Raju R, Gross M, Haider SP, Petukhova-Greenstein A, Kuhn TN, Lin M, Nowak M, Cooper K, Thomas E, Weber MA, Madoff DC, Staib L, Batra R, Chapiro J. Machine Learning Models for Prediction of Posttreatment Recurrence in Early-Stage Hepatocellular Carcinoma Using Pretreatment Clinical and MRI Features: A Proof-of-Concept Study. AJR Am J Roentgenol. 2022;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 162. | Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 163. | Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 164. | Zhao QY, Liu SS, Fan MX. Prediction of early recurrence of hepatocellular carcinoma after resection based on Gd-EOB-DTPA enhanced magnetic resonance imaging: a preliminary study. J Gastrointest Oncol. 2022;13:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 165. | Chuang YH, Ou HY, Yu CY, Chen CL, Weng CC, Tsang LL, Hsu HW, Lim WX, Huang TL, Cheng YF. Diffusion-weighted imaging for identifying patients at high risk of tumor recurrence following liver transplantation. Cancer Imaging. 2019;19:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Min JH, Kim YK, Choi SY, Kang TW, Jeong WK, Kim K, Won HJ. Detection of recurrent hepatocellular carcinoma after surgical resection: Non-contrast liver MR imaging with diffusion-weighted imaging versus gadoxetic acid-enhanced MR imaging. Br J Radiol. 2018;91:20180177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 167. | Liu Z, Fan JM, He C, Li ZF, Xu YS, Li Z, Liu HF, Lei JQ. Utility of diffusion weighted imaging with the quantitative apparent diffusion coefficient in diagnosing residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: a meta-analysis. Cancer Imaging. 2020;20:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, Asahara T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 169. | Li M, Wang Z, Cao J, Han B, Zou H, Zang Y, Wu L. Risk factors and prognosis of patients with recurrent hepatocellular carcinoma who undergo liver re-resections. Eur J Surg Oncol. 2019;45:1684-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 170. | Sun HC, Tang ZY, Ma ZC, Qin LX, Wang L, Ye QH, Fan J, Wu ZQ, Zhou XD. The prognostic factor for outcome following second resection for intrahepatic recurrence of hepatocellular carcinoma with a hepatitis B virus infection background. J Cancer Res Clin Oncol. 2005;131:284-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 171. | Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol. 2011;55:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 172. | Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571-104581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 173. | Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, Paik YH, Gwak GY, Kim JM, Kwon CH, Joh JW. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology. 2015;275:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 174. | Guerrini GP, Gerunda GE, Montalti R, Ballarin R, Cautero N, De Ruvo N, Spaggiari M, Di Benedetto F. Results of salvage liver transplantation. Liver Int. 2014;34:e96-e104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 175. | Chan KM, Wu TH, Cheng CH, Lee CF, Wu TJ, Chou HS, Lee WC. Advantage of early liver transplantation whenever indicated for hepatocellular carcinoma recurrence after primary liver resection. Biomed J. 2019;42:335-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 176. | Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 177. | Bhangui P, Allard MA, Vibert E, Cherqui D, Pelletier G, Cunha AS, Guettier C, Vallee JC, Saliba F, Bismuth H, Samuel D, Castaing D, Adam R. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann Surg. 2016;264:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 178. | Shan Y, Huang L, Xia Q. Salvage Liver Transplantation Leads to Poorer Outcome in Hepatocellular Carcinoma Compared with Primary Liver Transplantation. Sci Rep. 2017;7:44652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 179. | Liu F, Wei Y, Wang W, Chen K, Yan L, Wen T, Zhao J, Xu M, Li B. Salvage liver transplantation for recurrent hepatocellular carcinoma within UCSF criteria after liver resection. PLoS One. 2012;7:e48932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 180. | Hu Z, Zhou J, Xu X, Li Z, Zhou L, Wu J, Zhang M, Zheng S. Salvage liver transplantation is a reasonable option for selected patients who have recurrent hepatocellular carcinoma after liver resection. PLoS One. 2012;7:e36587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 181. | Wang P, Li H, Shi B, Que W, Wang C, Fan J, Peng Z, Zhong L. Prognostic factors in patients with recurrent hepatocellular carcinoma treated with salvage liver transplantation: a single-center study. Oncotarget. 2016;7:35071-35083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 182. | Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 183. | Zhang X, Li C, Wen T, Yan L, Li B, Yang J, Wang W, Xu M, Lu W, Jiang L. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 184. | Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, Zhao X, Wang L, Xiao F, Lv H, Liu T, Qi J, Li J, Zhong N, Wang C, Feng H, Liang B, Ren W, Qin C, Nguyen MH, Zhu Q. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur Radiol. 2020;30:6357-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 185. | Koh PS, Chan AC, Cheung TT, Chok KS, Dai WC, Poon RT, Lo CM. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford). 2016;18:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 186. | Chen S, Peng Z, Xiao H, Lin M, Chen Z, Jiang C, Hu W, Xie X, Liu L, Peng B, Kuang M. Combined radiofrequency ablation and ethanol injection versus repeat hepatectomy for elderly patients with recurrent hepatocellular carcinoma after initial hepatic surgery. Int J Hyperthermia. 2018;34:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |