Published online Dec 27, 2023. doi: 10.4254/wjh.v15.i12.1333
Peer-review started: July 16, 2023
First decision: August 14, 2023
Revised: September 14, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 27, 2023
Processing time: 161 Days and 9.7 Hours
The surge in traditional herbal dietary supplement (HDS) popularity has led to increased drug-induced liver injuries (DILI). Despite lacking evidence of efficacy and being prohibited from making medical claims, their acceptance has risen over sevenfold in the last two decades, with roughly 25% of United States (US) adults using these supplements monthly. An estimated 23000 emergency room visits annually in the US are linked to HDS side effects. NIH-funded research suggests HDS contribute to 7-20% of DILI cases, with similar trends in Europe—Spain reporting 2% and Iceland up to 16%. Patients with acute liver failure from HDS undergo liver transplantation more frequently than those from prescription medicines. Here we describe a case of drug-induced autoimmune hepatitis due to Skullcap supplements, this association appears to be the first documented instance in literature.
A middle-aged Caucasian woman, previously healthy, presented with sudden jaundice. Four months earlier, her liver enzymes were normal. She mentioned recent use of Skullcap mushroom supplements. Tests for chronic liver disease were negative. The first liver biopsy indicated severe resolving drug-induced liver injury. Despite treatment, she was readmitted due to worsening jaundice. Follow-up tests raised concerns about autoimmune hepatitis. A subsequent biopsy confirmed this diagnosis. The patient responded as expected to stopping the medication with improvement in liver enzymes.
This scenario highlights an uncommon instance of DILI caused by Skullcap supplements. It's crucial for hepatologists to recognize this connection due to the increasing prevalence of herbal supplements.
Core Tip: This case report highlights a rare presentation of drug induced liver injury from Skullcap supplement usage. While Chinese Skullcap (Scutellaria baicalensis) has been associated with a mixed hepatocellular and cholestatic picture of drug-induced liver injuries, the association of North American Skullcap is not as robust. Here we present a case of drug induced autoimmune hepatitis from North American Skullcap supplement use. Hepatologists must be aware of this association of rare histopathological presentation.
- Citation: Thakral N, Konjeti VR, Salama FW. Drug induced autoimmune hepatitis: An unfortunate case of herbal toxicity from Skullcap supplement: A case report. World J Hepatol 2023; 15(12): 1333-1337
- URL: https://www.wjgnet.com/1948-5182/full/v15/i12/1333.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i12.1333
The rise in popularity of traditional herbal dietary supplements (HDS) has caused an increase in incidence of drug induced liver injury (DILI). Herbs and botanicals along with their extracts and metabolites fall under the umbrella term “dietary supplements” in the United States[1]. Even though these agents generally lack proof of efficacy and that their manufacturers are not permitted to make medical claims, their acceptance in the society has increased over 7-fold in the last two decades[2]. Approximately, 50% of the adult population in the United States admits to having used a “dietary supplement” in the last month[3]. It is estimated that there are 23000 emergency room visits in the United States each year are secondary to the side effects of HDS[4]. The true incidence of liver injury secondary in the United States to HDS is difficult to estimate. The NIH funded Drug Induced Liver Injury Network estimates that approximately 7%-20% all cases of DILI are secondary to HDS[5]. Which is akin to the data from Europe, with Spain reporting 2% of all DILI cases as secondary to HDS and Iceland reporting the rate as high as 16%[6,7]. In addition, patients presenting with acute liver failure secondary to HDS have been found to undergo liver transplantation more frequently than those with DILI from prescription medicines, (56.1 vs 31.9%, P < 0.005)[8]. As such, awareness about the side effects of these medications and a comprehensive understanding of their outcomes is paramount. Here we describe a case of drug induced autoimmune hepatitis (DIAIH) resulting from Skullcap supplements. To our understanding, this is the first such association described in literature
New onset Jaundice.
A 62-year-old Caucasian female, with Sjogren’s disease presented with generalized fatigue, arthralgias, pruritus and new onset jaundice. She denied any prior history of liver disease, alcohol intake, intravenous or intranasal drug use, blood transfusions or needlestick injuries. She did however endorse taking Skullcap supplements over for 1-2 months due to long standing history of anxiety and insomnia.
Sjogren’s syndrome; Anxiety; Insomnia.
Nonsmoker, No alcohol or illicit Drug use. No gastrointestinal (GI) related malignancy or Liver disease in the family.
General: Age appropriate female in no distress. HEENT: Atraumatic, normocephalic; scleral icterus present, moist mucous membranes. Cyclic vomiting syndrome: S1,S2+ RRR. Lungs: Symmetric chest rise seen. Abdomen: Soft, non-distended, non-tender, baroreceptor sensitivity present, no palpable hepatomegaly appreciated. Extremities: No edema or clubbing. Skin: Jaundiced. Neuro: Alert, Awake, oriented × 3. No gross neurological deficits appreciated.
The initial laboratory results on presentation that were pertinent: International normalized ratio (INR) 2.4, Alkaline Phosphatase (Alk Phos) 164 IU/L, aspartate transaminase (AST) 1091 IU/L, alanine transaminase (ALT) 980 IU/L, total bilirubin (T bili) 9.5 mg/dL. The R factor on initial calculation was 20.5, indicative of a primary hepatocellular injury pattern. Immunoglobulin G was elevated at 2573 mg/dL. Testing for Viral Hepatitis including - Hepatitis A, B, and C, cytomegalovirus, Epstein-Barr virus, and herpes simplex virus were all negative including serology and quantitative testing. ANA was positive given her history of Sjogren’s disease. Her baseline AST, ALT, Alk phos and bilirubin were within normal range 4 months before presentation.
Magnetic resonance cholangiopancreatography done at admission did not show any signs obstruction or primary hepatic pathology.
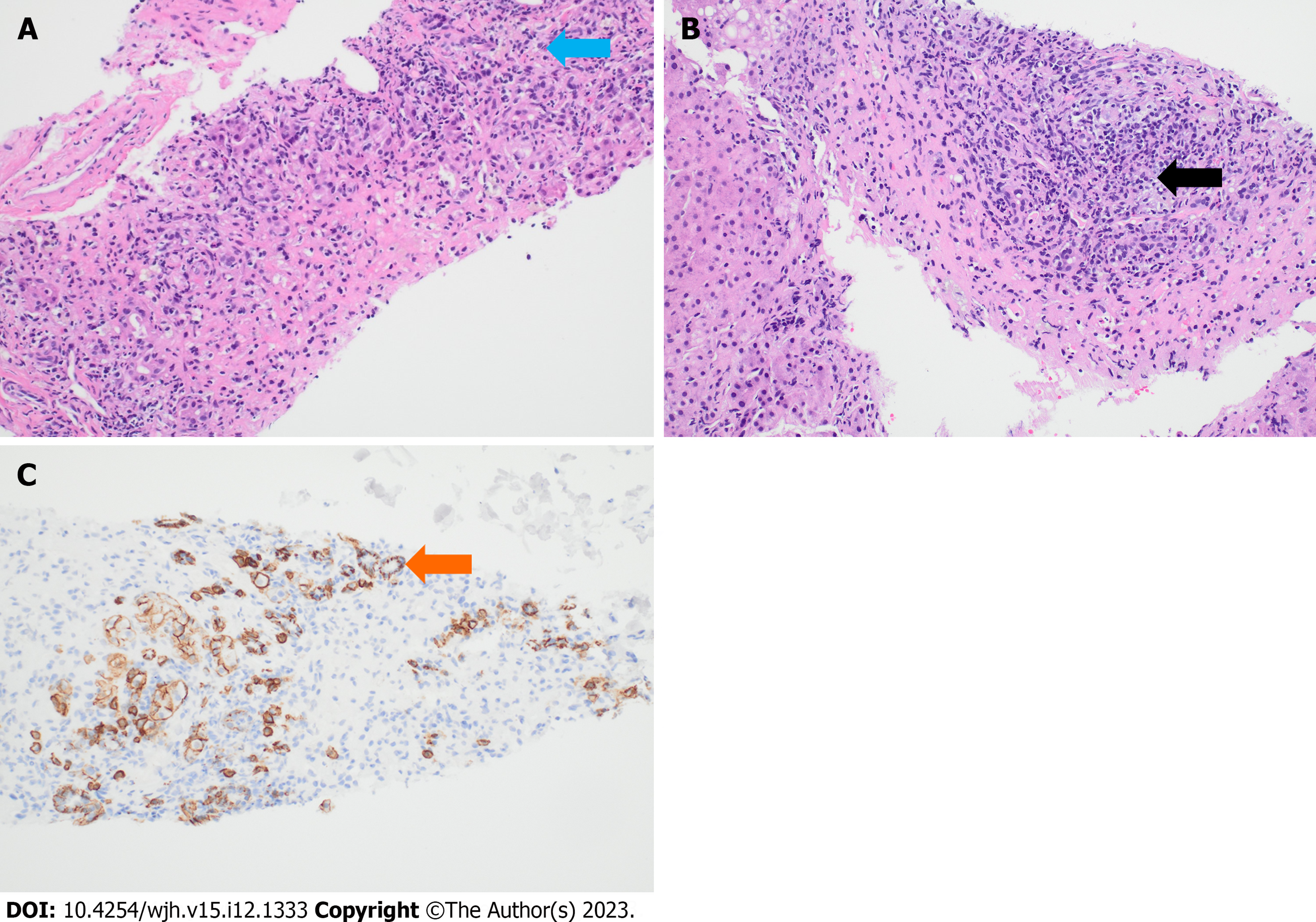
Given her prior history of autoimmune disease and new onset hypergammaglobulinemia in the context of suspected DILI, a liver biopsy was pursued. Her initial liver biopsy revealed resolving centrilobular necrosis with predominant eosinophilic inflammation. Over the next 72 h, the liver enzymes showed a strong downward trend. As such, the patient was discharged with outpatient follow-up. However, the patient was re-admitted a month later with worsening jaundice, acute kidney injury, and new onset ascites concerning for subacute liver failure. Her LFTs were Alk Phos 619 IU/L, AST 1222 IU/L, ALT 540 IU/L, and T bili 6.6 mg/dL. Infectious workup was unremarkable. In the next few days, the transaminases showed a downtrend. However, the T bili continued to rise, reaching its peak at 11.8 gm/dl. Paracentesis showed a serum ascites albumin gradient of 1.4 which was consistent with portal hypertension. A repeat liver biopsy was pursued which revealed extensive plasma cells consistent with new onset autoimmune hepatitis resulting from previous DILI (Figure 1). Unfortunately, the patient’s course was complicated by the development of spontaneous bacterial peritonitis, GI bleed and acute tubular necrosis necessitating dialysis. As such, she was never challenged with steroids. Due to her worsening status, she was listed for a simultaneous liver kidney transplantation. However, the patient finally did improve following a long and protracted course with resolution of jaundice but remained on hemodialysis.
DILI causing autoimmune Hepatitis.
Cessation of culprit drug.
Resolution of Jaundice.
The last three decades have heralded an increased use of herbal medicinal products with up to 25% of the adult American population admitting to their use at some point[9]. These products are not regulated by the Food and Drug Administration and their side effect profile remains largely unknown. Skullcap is a plant native to North America (Scutellaria lateriflora), which has been used for centuries to treat anxiety, digestive disorders, and menstrual disorders. Skullcap extracts contain large quantities of flavonoids like scutellarin and baicalin which account for its sedative and antispasmodic activities[10]. However, Skullcap has been associated with a mixed hepatocellular and cholestatic pattern of liver injury. Additionally, majority of cases of DILI from Skullcap are attributed to Chinese Skullcap (Scutellaria baicalensis), while the association of North American Skullcap with DILI is not as robust[11]. In our case, as is evident form the biopsy and the corresponding serology, the patient did have DIAIH.
Castiella et al[12] have postulated a-5-fold classification for drug induced autoimmune liver disease. Type 1, autoimmune hepatitis (AIH) with DILI: Reactivation of pre-existing AIH after the introduction of a new drug. Type 2, Drug induced Autoimmune Hepatitis (DIAIH): New onset AIH resulting from DILI. This results from an immune mediated reaction in a genetically primed individual, resulting in the necessitation of immunosuppressive treatment. Type 3, Immune Mediated DILI (IMDILI): acute or chronic liver injury that resolves upon cessation of the drug. DILI is often accompanied by a myriad of other features including fever, eosinophilia, lymphadenopathy, and rash. Individuals usually respond well to treatment and achieve sustained remission without relapse. Type 4, mixed autoimmune type: Mixed features of DI-AIH and IM-DILI. Individuals exhibit a full response to treatment, but assessing relapse is hindered for non-hepatological reasons. Lastly, type 5 encompasses individuals with DILI and positive autoimmune antibodies. The significance of these antibodies remains uncertain.
This case outlines a rare presentation of DILI from Skullcap supplements. Hepatologists must be aware of this association as the popularity of herbal supplements continues to rise.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American College of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esakkimuthu DS, India; Skrypnyk I, Ukraine S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | US Food and Drug Administration. Dietary supplement health and education act of 1994. December. 1995; 1. |
| 2. | Commission on Dietary Supplement Labels. Report of the Commission on Dietary Supplement Labels. Published online 1997. |
| 3. | Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 4. | Geller AI, Shehab N, Weidle NJ, Lovegrove MC, Wolpert BJ, Timbo BB, Mozersky RP, Budnitz DS. Emergency Department Visits for Adverse Events Related to Dietary Supplements. N Engl J Med. 2015;373:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 5. | Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmerón J, Martin-Vivaldi R; Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 690] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 7. | Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 8. | Hillman L, Gottfried M, Whitsett M, Rakela J, Schilsky M, Lee WM, Ganger D. Clinical Features and Outcomes of Complementary and Alternative Medicine Induced Acute Liver Failure and Injury. Am J Gastroenterol. 2016;111:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Strader DB, Navarro VJ, Seeff LB. Chapter 26 - Hepatotoxicity of Herbal Preparations. In: Boyer TD, Manns MP, Sanyal AJ, eds. Zakim and Boyer's Hepatology (Sixth Edition). W.B. Saunders; 2012: 462-475. [DOI] [Full Text] |
| 10. | Scutellaria - an overview | ScienceDirect Topics. Accessed July 10, 2023. Available from: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/scutellaria. |
| 11. | Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy. 2010;30:750, 258e-262e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |