Published online Dec 27, 2023. doi: 10.4254/wjh.v15.i12.1284
Peer-review started: August 1, 2023
First decision: October 9, 2023
Revised: November 27, 2023
Accepted: December 5, 2023
Article in press: December 5, 2023
Published online: December 27, 2023
Processing time: 145 Days and 22.2 Hours
Intrahepatic cholangiocarcinoma (iCCA) is recognized as the second most frequently diagnosed liver malignancy, following closely after hepatocellular carcinoma. Its incidence has seen a global upsurge in the past several years. Unfortunately, due to the lack of well-defined risk factors and limited diagnostic tools, iCCA is often diagnosed at an advanced stage, resulting in a poor progno
Core Tip: Intrahepatic cholangiocarcinoma (iCCA), the second most common type of liver cancer, is frequently diagnosed at an advanced stage due to limited diagnostic tools and undefined risk factors. Surgery, the potential cure, is often infeasible. Ongoing investigations into unresectable iCCA treatment include chemotherapy, targeted therapy, immunotherapy, and locoregional treatments. Transarterial radioembolization (TARE) demonstrates safety and effectiveness, with a median response rates (34%-86%) and OS (12-16 mo) varying due to patient heterogeneity. Key prognostic factors include tumor burden, portal vein involvement, and patient performance status. The median overall survival reported after TARE is of 22 mo with 22% of tumor down-staging. TARE is a viable unresectable iCCA treatment, especially when combined with systemic chemotherapy. Nonetheless, further research is needed to optimize treatment combinations and identify predictive factors for favorable responses in iCCA patients.
- Citation: Elvevi A, Laffusa A, Elisei F, Morzenti S, Guerra L, Rovere A, Invernizzi P, Massironi S. Any role for transarterial radioembolization in unresectable intrahepatic cholangiocarcinoma in the era of advanced systemic therapies? World J Hepatol 2023; 15(12): 1284-1293
- URL: https://www.wjgnet.com/1948-5182/full/v15/i12/1284.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i12.1284
Cholangiocarcinoma (CCA) is a diverse group of bile duct tumors, divided into three subtypes: intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA)[1]. With increasing rates, CCA is the second most common liver cancer globally[2]. CCA rates are stable in regions with reduced alcohol-related live disease, but increasing in Europe and America due to higher alcohol intake, viral hepatitis infections, an increase in obesity rates, and the prevalence of non-alcoholic fatty liver disease (NAFLD)[1,3]. The prevalence and incidence of CCA result from a complex interplay of evolving risk factors, including chronic biliary tract inflammation and biliary stasis[1]. Risk factors include primary sclerosing cholangitis, congenital choledochal cysts, liver fluke infections, hepatolithiasis, cirrhosis, hepatitis B and C, NAFLD, obesity, asbestos exposure, diabetes, smoking, and alcohol. Some cases have no identifiable risk factors, making early diagnosis challen
Local-regional therapies are an option for iCCA patients to manage tumor growth and complications. Limited evidence supports their use in pCCA and dCCA[7]. Common loco-regional treatments include external beam radiotherapy, trans-arterial chemoembolization (TACE), image-guided thermal ablation, trans-arterial radioembolization (TARE), and hepatic chemosynthesis. TARE has gained attention, though with limited evidence[1,6,7].
TARE is a minimally invasive technique to treat liver cancer, used for both hepatocellular carcinoma (HCC) and CCA. TARE involves the delivery of small beads, called microspheres, which are coated with a radioactive material, to the tumor through the blood vessels.
The liver receives blood from two sources: The hepatic artery and the portal vein; moreover, the hepatic parenchyma has a sinusoidal cytoarchitecture. Both these aspects promote the use of intra-arterially delivered treatments, such as TACE and TARE[8]. TARE is a technique that allows the delivery of a radioactive drug directly to the tumor, minimizing systemic irradiation and preserving the health of the liver. This is achieved by introducing microspheres into the tumor’s blood vessels through a catheter inserted into the femoral artery and guided to the liver using imaging techniques like angiography. These microspheres lodge in the small blood vessels that supply the tumor, leading to a reduction in blood flow to the cancer cells and ultimately causing their death. Different types of microspheres, such as Lipiodol, glass, resin, or polymer, have been used, along with various radioisotopes like Phosphorus-32 (32P), Yttrium-90 (90Y), Iodine-131 (131I), Holmium-166 (166Ho), Lutetium-177 (177Lu), and Rhenium-186/188 (186/188Re), all of which are beta emitters[8,9]. Current TARE agents use β-particle-emitting radioisotopes, although some pioneering studies are now investigating the development and use of α-particle-emitting radioisotopes[10]. Targeted alpha therapy is a highly effective treatment due to the densely ionizing track and the short path length (40-90 μm) of the emitted α-particles, resulting in a high linear energy transfer (50-30 keV/μm). Actinium-225 is one of the α-particle emitting radionuclides currently being explored for clinical applications. A recent study utilizing a mouse model demonstrated that survival rates significantly improved when using [225Ac]Ac-DOTA-TDA-Lipiodol® in comparison to control groups[10].
90Y-loaded microspheres are commonly used in TARE and have proven to be a crucial tool in the treatment of primary and secondary liver tumors, with a positive safety profile[11].
90Y, a radionuclide that emits beta particles, has a physical half-life duration of 64.2 hours. It does not emit gamma photons but produces secondary “bremsstrahlung” photons. Although it has a low positron emission of 32 decays per million, the maximum and mean energies of its beta particles are 2.28 MeV and 0.94 MeV, respectively, and in soft tissue, it has maximum and mean penetration depths of 11 mm and 4 mm, respectively[12]. 90Y can be loaded onto either glass or ion-exchange resin microspheres, enabling the delivery of high radiation doses to tumors while sparing normal hepatic parenchyma[13,14].
Observations indicate that radioembolization is well-tolerated by patients with a good performance status, consistent with current literature[14,15].
Prior to TARE, angiography of the aorta, superior mesenteric artery, and celiac trunk is performed to assess the hepatic vascular architecture, liver contact with surrounding structures, portal vein patency, and the presence of arterio-portal shunting[16]. Non-target vessels with microspheres injection can lead to adverse events, and although coil embolization of non-target vessels was previously routine, it is no longer recommended as a standard procedure[17]. One common arterio-portal shunt is between the liver and the lung, which is accurately visualized using 99m-Technetium labeled macroaggregated albumin (99mTc-MAA). It acts as a surrogate marker for 90Y microspheres and is injected into the left and right hepatic arteries. After injection, any arteriovenous connections around the tumor are sharpened[17]. After 99mTc-MAA injection, to assess and quantify pulmonary shunts, known as Lung Shunt Fraction, hepatic planar scintigraphy and single-photon emission computed tomography/computed tomography (SPECT/CT) scans are subsequently conducted[18], as significant pulmonary shunts can cause late radiation lung toxicity. 99mTc-MAA SPECT/CT imaging also aids in identifying possible gastrointestinal shunts, which, if not correctable through catheter embolization, can be an absolute contraindication for treatment[19,20]. Furthermore, 99mTc-MAA SPECT/CT acquisition helps define the treatment field and calculate the required activity of 90Y microspheres. When planning whole liver or selective, non-ablative or ablative TARE, it is advised to adopt a personalized method that utilizes dosimetry, grounded either in partition models or voxel-based models[21].
After microspheres injection a 90Y PET/CT scan is performed to assess treatment success by visualizing tumor targeting, quantifying absorbed dose post-treatment, and predicting complications such as radioembolization-induced liver disease[22,23].
TARE is considered a safe and effective treatment option with minimal side effects, and it can be repeated as necessary. It is a valuable alternative for patients who are not suitable for surgery, and it can also be combined with other treatments such as systemic chemotherapy, immunotherapy, or other local therapies to improve outcomes in iCCA.
A comprehensive literature search was conducted using PubMed, Embase (from 2008 to 2021), and the Cochrane Library to identify relevant studies. In order to maximize the inclusion of pertinent articles, personal knowledge of the relevant literature was utilized, the reference lists of retrieved papers were reviewed, and a manual search was performed in key journals. The search strategy employed a combination of medical subject headings (MeSH) terms and free language words to capture a wide range of relevant publications.
Locoregional treatments, including radiofrequency ablation, hepatic artery infusion, TACE, drug-eluting bead TACE, and TARE, have been extensively studied for unresectable cholangiocarcinoma iCCA[24,25]. These treatments have shown increased median overall survival (OS) compared to traditional systemic chemotherapy, with acceptable toxicity profiles[24].
In general patients eligible for locoregional treatments should meet specific criteria, including: (1) Eastern Cooperation Oncology Group (ECOG) performance status ≤ 2; (2) Adequate laboratory tests (A neutrophil count exceeding 1.5 × 109/L, A platelet count over 50 × 109/L); (3) Adequate kidney function (creatinine level below 2.0 mg/dL); (4) Proper liver functionality (bilirubin level under 2.0 mg/dL); and (5) The ability to undergo hepatic angiography[7].
Locoregional treatments are not recommended in cases of pregnancy, breastfeeding, an expected lifespan of under three months, or in instances of clinical liver failure. TARE is typically recommended for scenarios with minimal or no spread of the tumor beyond the liver, although interpretations of this condition vary[12]. It’s important to note that in patients with iCCA, lymph node metastases haven’t demonstrated a detrimental effect on OS and thus shouldn’t be viewed as a disqualifying factor for TARE. Nevertheless, the presence of solid organ metastases necessitates individualized treatment decisions[12,25].
TARE as an option for locoregional treatment was first described in a study by Ibrahim et al[26], which included 24 patients with histologically diagnosed iCCA. The median OS was 14 months, with two patients achieving downstage with resectable disease and/or liver transplantation. At follow-up imaging, the tumor response indicated partial shrinkage in 27% of cases, stable disease in 68%, and only 5% showed disease progression. In total, an objective tumor response, defined as any reduction in size, was noted in 86% of the patients[26].
Moreover, for inoperable patients treated with TARE, a systematic review by Boehm et al[24] reported a cumulative median OS of 13.9 months (95%CI: 9.5-18.3) and a radiologic response according to RECIST criteria of 27.4% (95%CI: 17.4%-37.5%) for complete or partial response and 54.8% (95%CI: 45.2%-56.7%) for stable disease. Another retrospective study by Gangi et al[15] of a cohort of patients with unresectable iCCA who underwent TARE as first- or second-line treatment showed a median OS of 12.0 months (95%CI: 8.0-15.2), with a radiologic partial response in 6.2% of patients, stable disease in 64.2%, and progressive disease in 29.6% of patients at 3 mo.
Two other studies showed encouraging results for inoperable ICCA: Pellegrinelli et al[27] showed a median OS of 16 mo at three months, while Robinson et al[14], analyzing data from the Registry for Radiation-Emitting SIR-Spheres in non-resectable Liver Tumors, reported a median OS of 14.0 mo (95%CI: 12.1-22.3) and a median progression-free survival of 5.8 months (95%CI: 4.6-7.2) for the entire cohort of patients treated with TARE. Notably, this last study demonstrated an objective radiologic response, assessed by RECIST criteria, in 34% of patients (8% complete response, 26% partial response), while 67% of patients achieved disease control[14].
TARE has been explored in combination with systemic chemotherapy to further enhance treatment success.
A recent phase-2 trial evaluated the combination of systemic chemotherapy using cisplatin and gemcitabine with TARE for unresectable iCCA[28]. The study reported a median OS of 22 mo (95%CI: 14-52 mo), with a one-year OS rate of 75% (95%CI: 62%-89%) and a two-year OS rate of 45% (95%CI: 30%-61%). The objective response, based on RECIST criteria at three months, was 39% (90%CI: 26%-53%). Additionally, the disease control rate at three months was 98% (95%CI: 89%-99%). Additionally, 22% of patients (9 patients) were able to undergo downstaging for potential surgical intervention. Although these results are promising, they still need to be confirmed through a phase-3 trial. Unfortunately, a similar trial combining cisplatin plus gemcitabine chemotherapy and TARE was prematurely halted due to insufficient patient recruitment[6]. A recent study shared findings on the combined use of TARE and CT-guided high-dose-rate interstitial brachytherapy (CT-HDRBT)[29]. A further possible approach involves an ablative method where a radioactive source, specifically Iridium 192, is inserted directly into neoplastic lesions via catheters under the guidance of CT imaging[30,31]. This technique overcomes size limitations and restrictions due to tumor location[32]. Among patients with CCA treated with either TARE or CT-HDRBT, the median OS was 29 mo, overall, the available evidence suggests that TARE could play a role in treating unresectable iCCA, both as a standalone therapy and when combined with systemic chemotherapy (Table 1).
| Ref. | Study design | N patients | Inclusion criteria | Technique | Median OS (mo) | R-response (%) |
| Ibrahim et al[26], 2008 | Report | 24 | Histological diagnosis; Unresectable disease; ECOG ≤ 2; Neutrophil > 1.5 × 109/L; Platelet > 50 × 109/L; Creatinine < 2.0 mg/dL; Bilirubin < 2.0 mg/dL; Able to undergo angiography | 90Yttrium (90Y); Radioembolization | 14 | 86 |
| Boehm et al[24], 2015 | Systematic Review and Metanalysis | 127 | Unresectable disease; TARE treatment | 90Yttrium (90Y); Radioembolization | 13.9 | 27.4 (partial or complete; 54 (stable disease) |
| Gangi et al[15], 2018 | Single Center; Retrospective study | 85 | Histological diagnosis; Unresectable disease; ECOG ≤ 2; Platelet > 50 × 109/L; Creatinine ≤ 2.0 mg/dL; Bilirubin ≤ 2.0 mg/dL; INR ≤ 1.5 | 90Yttrium (90Y); Radioembolization | 12 | 6.2 (partial response); 64 (stable disease) |
| Pellegrinelli et al[27], 2021 | Single center; Retrospective study | 6 | Unresectable disease; ECOG ≤ 2; Platelet > 50 × 109/L; Bilirubin < 2.0 mg/dL; Prothrombin time > 50%; Able to undergo angiography | 90Yttrium (90Y); Radioembolization | 16 | NA |
| Robinson et al[14], 2022 | Registry data | 95 | NA | 14 | 34 | |
| Edeline et al[28], 2020 | Phase 2; Clinical Trial | 41 | Unresectable disease; Never received CT; Never received intra-arterial treatment | 90Yttrium (90Y); Radioembolization + Cisplatin and Gemcitabine | 22 | 39 |
| Fleckenstein et al[29], 2022 | Single center; Retrospective study | 9 | Unresectable disease; At least one TARE treatment; At least one CT-HDRBT treatment | 90Yttrium (90Y); Radioembolization + CT-HDRBT | 29 | NA |
Discrepancies in OS among the available studies are due to significant differences in study designs, sample size, and association with other treatment options. Therefore further studies are required to establish TARE effectiveness in randomized trial and to better investigate its potential in combination with other approaches[6].
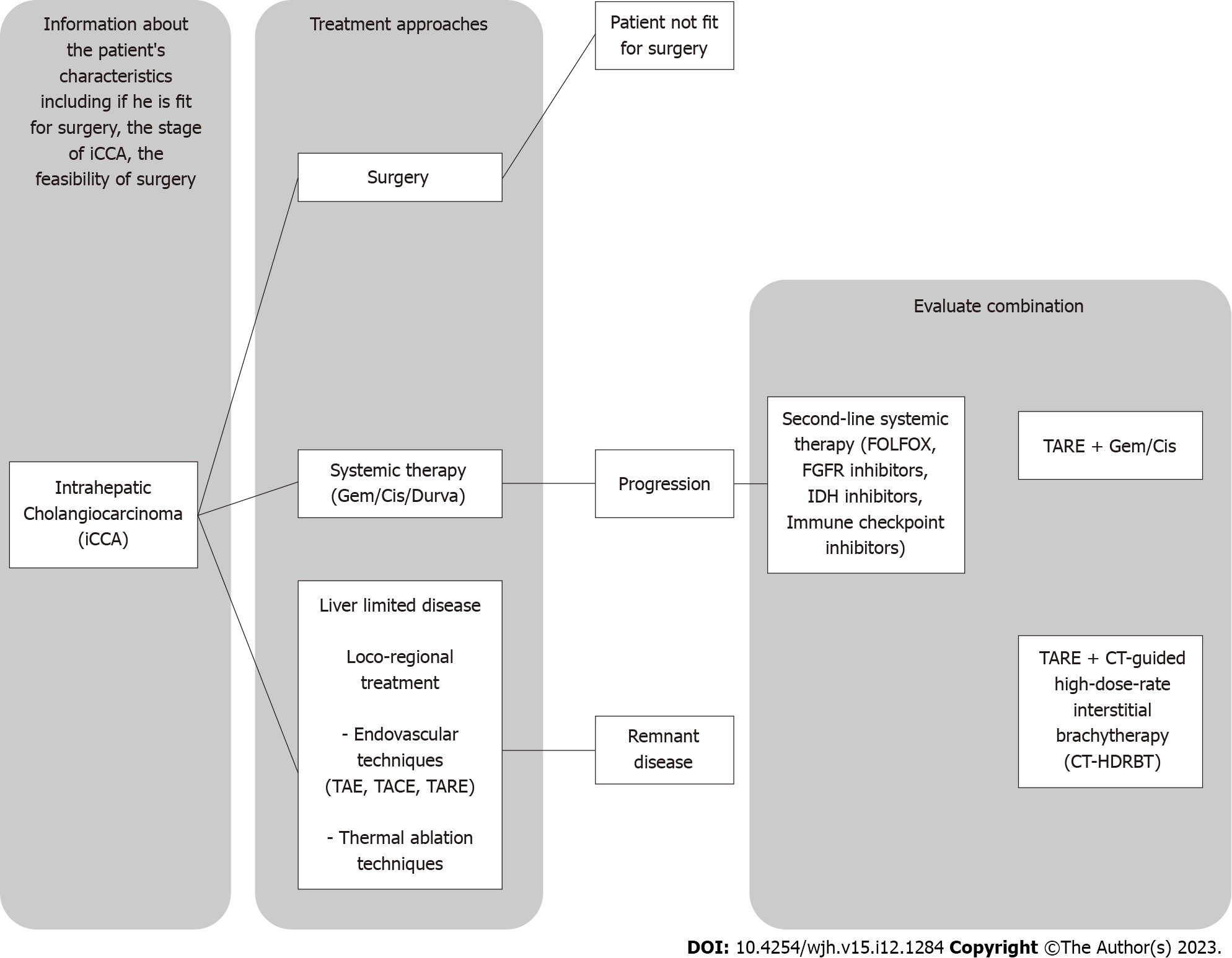
However, the treatment of advanced-stage iCCA remains a complex task that often requires the combination of different therapeutic strategies to develop the optimal treatment approach for patients. In this context, TARE may play a role, also as observed in in real life experience[9], as a combination treatment to further increase treatment success and improve patient care (Figure 1).
Patient selection is a critical aspect of TARE for iCCA. It involves assessing the extent of the disease, extrahepatic tumor spread, liver function, and overall health. Lobar or segmental perfusion involves the selective delivery of radioactive microspheres to specific regions of the liver, making precise patient selection even more crucial. High-quality imaging, such as angiography and CT or magnetic resonance imaging (MRI) scans, is essential to identify the arterial supply to the tumor and determine the optimal catheter placement for microsphere delivery. Advanced software tools are used for treatment planning to calculate the required dose and ensure minimal radiation to healthy liver tissue.
Moreover, the optimal radiation dose and the selection of embolic agents represent important issues.
Concerning the radiation dosage, with the use of resin microspheres, the activity level of 90Y to be administered is determined based on the assumption that a mean absorbed dose of 40 Gy or less to the non-tumoral liver is safe. Additionally, for iCCA, a minimum mean target-absorbed dose to the tumor of 100-120 Gy is recommended[21]. The cut-offs for calculating 90Y activity change when glass microspheres are used. In this case, the mean absorbed dose for the nontumorous liver, which is considered safe, is less than 75 Gy, and the minimum mean absorbed target dose for the tumor, which is recommended to significantly increase OS, is higher than 260 Gy[12,33]. A systematic review of dosimetry after iCCA treatment shows that the mean delivered tumor dose is approximately 200 to 250 Gy for glass-based treatments and 80 to 130 Gy for resin-based treatments[34]. A recent study using glass microspheres further increased the dose for treatment of iCCA, suggesting that segmental transarterial radioembolization at > 400 Gy is an ablative approach that is feasible in terms of safety and efficacy[35].
A more subtle point, but one of potential clinical importance, is the selection of embolic agents and their infusion methods tailored to TARE in the context of iCCA. The glass microspheres, which are insoluble and infused with Y90, measure between 20-30 μmol/L in diameter and possess an activity of 2500 Bq per sphere at calibration time. These microspheres are designated for use in cases of inoperable HCC and HCC with complications due to portal vein thrombosis. They have received approval from the United States Food and Drug Administration under a humanitarian exemption, which is based on their established safety and potential clinical benefits. A total count of 1.2 million microspheres generates an activity of 3 GBq (as stated in the TheraSphere® Yttrium-90 microspheres package insert, Kanata CMN. http://www.therasphere.com/physicians-package-insert/TS_PackageInsert_USA_v12.pdf). Resin microspheres are made of biocompatible resin and have a diameter ranging from 20-60 μmol/L, with an activity of 50 Bq per sphere. They contain a lower concentration of Y90 per sphere compared to glass microspheres, necessitating a larger number of spheres to administer a specific dose. This results in a higher embolic effect for the same dose delivery. To achieve 3 GBq of activity, between 40-80 million resin microspheres (each with 50 Bq) are required, in contrast to the 1.2 million needed for glass microspheres, as detailed in the SIRS-Spheres® Yttrium-90 microspheres package insert from Singapore Science Park SSM. http://www.sirtex.com/media/29845/ssl-us-10.pdf). Consequently, glass microspheres have the least embolic effect for the same prescribed activity because they are injected in much smaller numbers. The increased quantity of resin microspheres, despite the same prescribed activity level, could potentially lead to a more even distribution of the dose, resulting in a heightened biological effect, which includes both toxicity and efficacy. For resin 90Y-microspheres, given the higher embolic load, no blind infusions should be performed. The microspheres are delivered slowly at a rate of no more than 5 mL/min, as rapid delivery may cause reflux. During the procedure, the radiologist must repeatedly check the position of the catheter to ensure its position and continued forward flow. In the case of glass 90Y-microspheres, due to the small number of microspheres used, it’s not necessary to completely saturate the entire vascular bed or to use continuous fluoroscopic guidance during infusion. Typically, a full infusion can be completed in about 5 minutes through a slow, manual injection while the patient breathes normally[12].
Another recent option is the use of microspheres containing holmium-166 (166Ho), which are now available in Europe as an alternative to 90Y microspheres[12,36]. 166Ho offers advantages with its shorter half-life (26.8 h), high-energy beta and gamma radiation, and MRI-friendly properties[37]. Treatment planning uses the same microspheres as radioembolization, eliminating the need for 99mTc MAA. Clinical evidence supports the efficacy and safety of 166Ho in unresectable cancer[37]. Based on the latest usage guidelines, it’s permissible for the average dose absorbed by the treated volume to surpass 60 Gy, provided that the average dose absorbed by the entire liver remains below 60 Gy. This approach aims to strike a balance between effective treatment and the safety of the liver[38].
TARE for iCCA has demonstrated a favorable safety profile overall (Table 2). Ibrahim et al[26] reported a low incidence of severe adverse events (grade 3 or 4) in their study, with 17% of patients experiencing grade 3 albumin toxicities and 4% experiencing grade 3 bilirubin toxicities. Mild to moderate (grade 1 or 2) clinical toxicities were more common and included fatigue (75% of patients), transient abdominal pain (38%), vomiting (13%), anorexia (8%), and nausea (4%). Moderate to severe clinical toxicities were infrequent, with one patient (4%) developing a gastroduodenal ulcer, three patients (14%) developing ascites, and two patients (9%) developing pleural effusion. Two patients died within 30 d after the procedure. A subsequent review and meta-analysis by Boehm et al[24] found that TARE was associated with minor treatment-related complications such as minor pain, fatigue, nausea/vomiting, and fever, while major complications were rare, occurring in less than 1 event per patient. Liver-related complications varied widely, including increases in liver enzyme levels, the development of hepatic abscesses, and cases of liver failure. Overall, grade 3 or 4 toxicities were low following TARE.
| Paper | Mild to moderate (grade 1-2) | Severe (grade 3-4) | ||
| Biochemical (%) | Constitutional (%) | Biochemical (%) | Constitutional (%) | |
| Ibrahim et al[26], 2008 | - | Fatigue (75); Abdominal pain (38); vomiting (13); anorexia (8) | Albumin (17); Bilirubin (4) | Gastroduodenal Ulcer (4); Ascites (14); Pleural effusion (9) |
| Gangi et al[15], 2018 | (53) | Fatigue (42.3); Abdominal Pain (18.8); Weight loss (7.1); Ascites (5.9) | Bilirubin and Alkaline phosphatase elevation (9) | Liver abscess (2) |
| Pellegrinelli et al[27], 2021 | - | - | - | Cholecystitis and angiocholitis (2.85) |
| Robinson et al[14], 2022 | - | - | Bilirubin (10.5), Albumin (2.6), AST increase (7.8), ALT increase (5.2) | Abdominal pain, Cholecystitis (4.1) |
Pellegrinelli et al[27] reported a low incidence of complications and side effects in their study of TARE. Out of 70 patients, 12 developed grade 1 side effects such as nausea, abdominal pain, fever, and vascular-like pseudoaneurysm. Only two patients encountered severe (grade 3) side effects: one suffered from radiation-induced cholecystitis due to unintended accumulation of 90Y microsphere, while the other developed angiocholitis. No hepatic dysfunction or deaths related to TARE were observed[27]. Similarly, other studies, including those by Robinson et al[14] and Gangi et al[15] reported no mortality associated with TARE. Biochemical toxicities were generally mild to moderate, with 3%-10% of patients experiencing grade 3 biochemical toxicities. Constitutional toxicities were mostly grade 1 or 2, such as abdominal pain, fatigue, nausea, and vomiting, with rare occurrences of grade 3 or 4 constitutional toxicities. The toxicities observed in the study by Fleckenstein et al[29] were also mild to moderate, with few adverse events reported. When TARE was combined with systemic chemotherapy in a study by Fleckenstein et al[29], the toxic effects observed were primarily due to chemotherapy, with prevalent hematologic toxic effects of grade 3 or higher. The authors suggested a possible association between TARE and chemotherapy in the development of these hematologic toxic effects. However, in patients with cirrhosis, the number of hepatic toxicities was high. Liver toxic effects such as ascites, altered liver function tests, cholangitis, and acute cholecystitis were more common in cirrhotic patients. Among cirrhotic patients treated with TARE without chemotherapy, liver failure occurred in 75% of cases (9 out of 12 patients), including non-reversible cases, compared to 17% in patients without cirrhosis (all reversible cases). It was concluded that the combination of chemotherapy and TARE should be avoided in patients with cirrhosis, while liver toxicities in patients without cirrhosis were manageable, and no irreversible liver toxic effects were observed[28].
The researchers determined that combining chemotherapy with TARE is not advisable for patients who have cirrhosis. On the contrary, liver toxicities was passable in patients without cirrhosis, and no irreversible liver toxic effect was seen.
Several studies have investigated both tumor-independent and tumor-dependent factors that may influence the prognosis of patients undergoing TARE for iCCA. The initial study by Ibrahim et al[26] showed that ECOG performance status had an impact on OS, with grade 0 having a significantly higher median OS compared to grade 1 or 2. On the other hand, tumor-dependent factors such as previous systemic chemotherapy, portal vein invasion, and infiltrative tumor morphology were associated with a poorer prognosis. These factors indicate a more advanced or aggressive disease, suggesting that TARE may be a viable treatment option for unresectable iCCA without portal vein involvement and/or infiltrative behavior.
Similarly, Gangi et al[15] found a correlation between ECOG performance status score and median OS. Patients treated with TARE who had ECOG performance status scores of 0 and 1 had a median OS of 18.5 mo, while those with a score of 2 had a median OS of 5.5 mo (P = 0.0012). Additionally, patients with symptoms or signs of liver failure, indicated by low serum albumin levels, low international normalized ratio, and elevated aspartate aminotransferase, had lower OS. Poorly differentiated tumor histology was also associated with lower OS, as previously described by Ibrahim et al[26]. The relationship between metastatic disease and prognosis remains more controversial[14,15].
In contrast, Edeline et al[28] did not find any correlation between ECOG performance status and median OS in their study. Similarly, Pellegrinelli et al[27] did not identify any tumor-independent factors that correlated with patients’ median OS.
To summarize, the available literature suggests that TARE may have a role in the treatment of iCCA, either as a standalone treatment or in combination with systemic chemotherapy. However, further randomized trials and investigations of combination therapies are necessary to establish its efficacy and confirm its safety. TARE for iCCA is generally considered to have a favorable safety profile, with low rates of severe adverse events. However, caution should be exercised when combining TARE with systemic chemotherapy, particularly in patients with underlying cirrhosis, as they may be at a higher risk of liver toxic effects. Concomitant use of chemotherapy and TARE should be avoided in these patients. It is important to note that advancements in systemic therapies have resulted in improved prognosis for iCCA patients. Therefore, the role of TARE should be assessed within the context of these newer treatment options. Further randomized studies are needed to determine the optimal patient population, treatment regimen, and long-term outcomes associated with TARE in iCCA.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu K, China; Qi L, China; Zeng YY, China S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 967] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 2. | Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, Invernizzi P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 4. | Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27:100737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The Clinical Management of Cholangiocarcinoma in the United States and Europe: A Comprehensive and Evidence-Based Comparison of Guidelines. Ann Surg Oncol. 2021;28:2660-2674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Mosconi C, Calandri M, Javle M, Odisio BC. Interventional radiology approaches for intra-hepatic cholangiocarcinoma. Chin Clin Oncol. 2020;9:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Bouvry C, Palard X, Edeline J, Ardisson V, Loyer P, Garin E, Lepareur N. Transarterial Radioembolization (TARE) Agents beyond (90)Y-Microspheres. Biomed Res Int. 2018;2018:1435302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Schaarschmidt BM, Kloeckner R, Dertnig T, Demircioglu A, Müller L, Auer TA, Santos DPD, Steinle V, Miederer M, Gebauer B, Radunz S, Kasper S, Weber M, Theysohn J. Real-Life Experience in the Treatment of Intrahepatic Cholangiocarcinoma by (90)Y Radioembolization: A Multicenter Retrospective Study. J Nucl Med. 2023;64:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Josefsson A, Cortez AG, Rajkumar H, Latoche JD, Jaswal AP, Day KE, Zarisfi M, Rigatti LH, Huang Z, Nedrow JR. Evaluation of the pharmacokinetics, dosimetry, and therapeutic efficacy for the α-particle-emitting transarterial radioembolization (αTARE) agent [(225)Ac]Ac-DOTA-TDA-Lipiodol(®) against hepatic tumors. EJNMMI Radiopharm Chem. 2023;8:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17:1571-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, Gnesin S, Bodei L, Kracmerova T, Luster M, Garin E, Herrmann K. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49:1682-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 13. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 487] [Article Influence: 54.1] [Reference Citation Analysis (30)] |
| 14. | Robinson TJ, Du L, Matsuoka L, Sze DY, Kennedy AS, Gandhi RT, Kouri BE, Collins ZS, Kokabi N, Grilli CJ, Wang EA, Lee JS, Brown DB. Survival and Toxicities after Yttrium-90 Transarterial Radioembolization of Cholangiocarcinoma in the RESiN Registry. J Vasc Interv Radiol. 2023;34:694-701.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, El-Haddad G, Biebel B, Parikh N, Arslan B, Hoffe SE, Frakes JM, Springett GM, Anaya DA, Malafa M, Chen DT, Chen Y, Kim RD, Shridhar R, Kis B. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J Vasc Interv Radiol. 2018;29:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Liu DM, Salem R, Bui JT, Courtney A, Barakat O, Sergie Z, Atassi B, Barrett K, Gowland P, Oman B, Lewandowski RJ, Gates VL, Thurston KG, Wong CY. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol. 2005;16:911-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv Ther. 2016;33:699-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Georgiou MF, Kuker RA, Studenski MT, Ahlman PP, Witte M, Portelance L. Lung shunt fraction calculation using (99m)Tc-MAA SPECT/CT imaging for (90)Y microsphere selective internal radiation therapy of liver tumors. EJNMMI Res. 2021;11:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A 3rd, Espat J, Bilbao JI, Sharma RA, Thomas JP, Coldwell D. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Sancho L, Rodriguez-Fraile M, Bilbao JI, Beorlegui Arteta C, Iñarrairaegui M, Moran V, Sangro B. Is a Technetium-99m Macroaggregated Albumin Scan Essential in the Workup for Selective Internal Radiation Therapy with Yttrium-90? An Analysis of 532 Patients. J Vasc Interv Radiol. 2017;28:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Levillain H, Bagni O, Deroose CM, Dieudonné A, Gnesin S, Grosser OS, Kappadath SC, Kennedy A, Kokabi N, Liu DM, Madoff DC, Mahvash A, Martinez de la Cuesta A, Ng DCE, Paprottka PM, Pettinato C, Rodríguez-Fraile M, Salem R, Sangro B, Strigari L, Sze DY, de Wit van der Veen BJ, Flamen P. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48:1570-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 22. | Alsultan AA, Braat AJAT, Smits MLJ, Barentsz MW, Bastiaannet R, Bruijnen RCG, de Keizer B, de Jong HWAM, Lam MGEH, Maccauro M, Chiesa C. Current Status and Future Direction of Hepatic Radioembolisation. Clin Oncol (R Coll Radiol). 2021;33:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Stephan W, Michel H. PET/CT Specificities in (90)Y Imaging Post Radioembolization. PET Clin. 2019;14:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, Gamblin TC. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Köhler M, Harders F, Lohöfer F, Paprottka PM, Schaarschmidt BM, Theysohn J, Herrmann K, Heindel W, Schmidt HH, Pascher A, Stegger L, Rahbar K, Wildgruber M. Prognostic Factors for Overall Survival in Advanced Intrahepatic Cholangiocarcinoma Treated with Yttrium-90 Radioembolization. J Clin Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, Newman SB, Benson A 3rd, Omary RA, Salem R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Pellegrinelli J, Chevallier O, Manfredi S, Dygai-Cochet I, Tabouret-Viaud C, Nodari G, Ghiringhelli F, Riedinger JM, Popoff R, Vrigneaud JM, Cochet A, Aho S, Latournerie M, Loffroy R. Transarterial Radioembolization of Hepatocellular Carcinoma, Liver-Dominant Hepatic Colorectal Cancer Metastases, and Cholangiocarcinoma Using Yttrium90 Microspheres: Eight-Year Single-Center Real-Life Experience. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, Ayav A, Campillo-Gimenez B, Beuzit L, Pracht M, Lièvre A, Le Sourd S, Boudjema K, Rolland Y, Boucher E, Garin E. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 29. | Fleckenstein FN, Roesel MJ, Krajewska M, Auer TA, Collettini F, Maleitzke T, Böning G, Torsello GF, Fehrenbach U, Gebauer B. Combining Transarterial Radioembolization (TARE) and CT-Guided High-Dose-Rate Interstitial Brachytherapy (CT-HDRBT): A Retrospective Analysis of Advanced Primary and Secondary Liver Tumor Treatment. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Ricke J, Wust P, Stohlmann A, Beck A, Cho CH, Pech M, Wieners G, Spors B, Werk M, Rosner C, Hänninen EL, Felix R. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Bretschneider T, Mohnike K, Hass P, Seidensticker R, Göppner D, Dudeck O, Streitparth F, Ricke J. Efficacy and safety of image-guided interstitial single fraction high-dose-rate brachytherapy in the management of metastatic malignant melanoma. J Contemp Brachytherapy. 2015;7:154-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Jonczyk M, Collettini F, Schnapauff D, Geisel D, Böning G, Feldhaus F, Denecke T, Wieners G, Hamm B, Gebauer B. Cholangiocarcinoma: CT-guided High-Dose Rate Brachytherapy (CT-HDRBT) for Limited (<4 cm) and Large (>4 cm) Tumors. Anticancer Res. 2018;38:5843-5852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Bourien H, Palard X, Rolland Y, Le Du F, Beuzit L, Uguen T, Le Sourd S, Pracht M, Manceau V, Lièvre A, Boudjema K, Garin E, Edeline J. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: a large single-center experience. Eur J Nucl Med Mol Imaging. 2019;46:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Hosseini Shabanan S, Nezami N, Abdelsalam ME, Sheth RA, Odisio BC, Mahvash A, Habibollahi P. Selective Internal Radiation Therapy with Yttrium-90 for Intrahepatic Cholangiocarcinoma: A Systematic Review on Post-Treatment Dosimetry and Concomitant Chemotherapy. Curr Oncol. 2022;29:3825-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 35. | Yu Q, Patel M, Kwak D, Ungchusri E, Wang Y, Van Ha T, Zangan S, Marshall E, Little K, Baker T, Liao CY, Pillai A, Ahmed O. Segmental Yttrium-90 Radioembolization Using Glass Microspheres Greater than 400 Gray for the Treatment of Intrahepatic Cholangiocarcinoma: A Preliminary Experience. J Vasc Interv Radiol. 2023;34:1970-1976.e1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Klaassen NJM, Arntz MJ, Gil Arranja A, Roosen J, Nijsen JFW. The various therapeutic applications of the medical isotope holmium-166: a narrative review. EJNMMI Radiopharm Chem. 2019;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Reinders MTM, Smits MLJ, van Roekel C, Braat AJAT. Holmium-166 Microsphere Radioembolization of Hepatic Malignancies. Semin Nucl Med. 2019;49:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Drescher R, Seifert P, Gühne F, Aschenbach R, Kühnel C, Freesmeyer M. Radioembolization With Holmium-166 Polylactic Acid Microspheres: Distribution of Residual Activity in the Delivery Set and Outflow Dynamics During Planning and Treatment Procedures. J Endovasc Ther. 2021;28:452-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |