Published online Dec 27, 2023. doi: 10.4254/wjh.v15.i12.1258
Peer-review started: August 21, 2023
First decision: September 27, 2023
Revised: November 6, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 27, 2023
Processing time: 125 Days and 15.5 Hours
Primary liver cancer is a severe and complex disease, leading to 800000 global deaths annually. Emerging evidence suggests that inflammation is one of the critical factors in the development of hepatocellular carcinoma (HCC). Patients with viral hepatitis, alcoholic hepatitis, and steatohepatitis symptoms are at higher risk of developing HCC. However, not all inflammatory factors have a pathogenic function in HCC development. The current study describes the process and mechanism of hepatitis development and its progression to HCC, particularly focusing on viral hepatitis, alcoholic hepatitis, and steatohepatitis. Furthermore, the roles of some essential inflammatory cytokines in HCC progression are described in addition to a summary of future research directions.
Core Tip: Primary liver cancer is the second most common tumor in the world, and the number of deaths due to this disease is increasing every year. A large number of studies have shown that inflammation has a certain regulatory effect in the occurrence and exacerbation of liver cancer. However, the function of inflammation in liver cancer remains to be studied. This review introduces the classification of hepatitis, the correlation between various inflammatory factors and hepatocellular carcinoma (HCC), and some of the anti-inflammatory drugs used in the treatment of HCC.
- Citation: Chen HJ, Huang TX, Jiang YX, Chen X, Wang AF. Multifunctional roles of inflammation and its causative factors in primary liver cancer: A literature review. World J Hepatol 2023; 15(12): 1258-1271
- URL: https://www.wjgnet.com/1948-5182/full/v15/i12/1258.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i12.1258
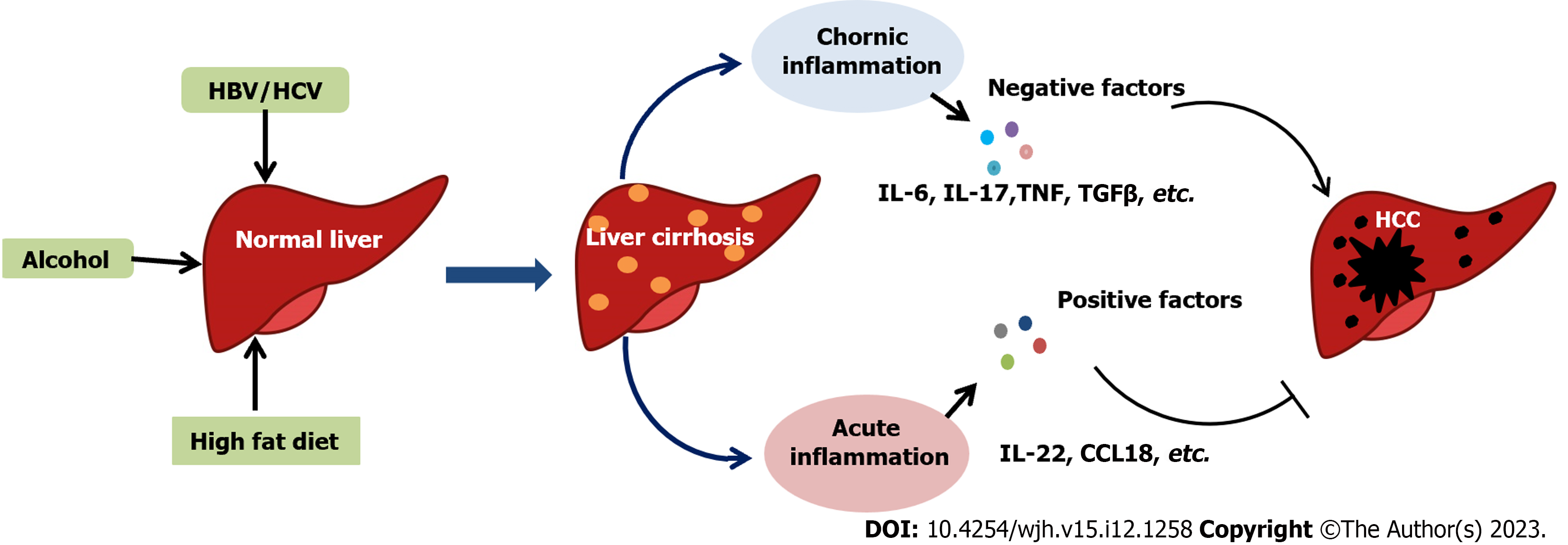
Liver cancer is categorized into primary and secondary liver cancer. Primary liver cancer involves hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, and other rare cancer types. In contrast, secondary liver cancer is due to cancer cell metastasis from different body parts to the liver via the bloodstream[1]. Notably, HCC accounts for 95% of primary liver cancer cases and is one of the leading types and fatal liver cancer forms. HCC development is closely associated with hepatitis C virus (HCV), hepatitis B virus (HBV), and nonalcoholic fatty liver disease (NAFLD)[2]. Alcoholic fatty liver may cause alcoholic steatohepatitis (ASH), leading to progressive fibrosis and cirrhosis, and can develop into HCC[3]. All these processes leading to HCC involve a series of reactions from inflammation to cirrhosis, resulting in HCC. Therefore, inflammation is clinically significant as the initiating factor in HCC.
Inflammation is a defensive response of the human body against stimulation and is divided into acute and chronic inflammation. Acute or short-term hepatic inflammation is a nonfibrotic condition caused by lipopolysaccharide, hepatitis virus, and other factors, and disappears within hours or days. Chronic or long-term inflammation, driven by chronic oxidative stress, is one of the critical processes in HCC development and progression[4]. More studies are investigating the presence of inflammation in the occurrence and development of liver cancer, but its exact role remains unclear. Immune cells in the tumor microenvironment either suppress or promote tumorigenesis, participating in adaptive and innate immunity and defense mechanisms to eliminate foreign agents. Persistent chronic inflammation accelerates the growth and proliferation of tumor cells[5]. Bioactive molecules released from immune cells in the tumor microenvironment stimulate carcinogenesis programming and enhance tumor development[6]. Several inflammatory cytokines, including interleukin (IL)-22, a member of the IL-10 family[7], play a positive role in liver regeneration and the anti-inflammatory response. Other cytokines, including IL-1β and IL-17A, serve as tumor-promoting cytokines, inducing liver disease progression and hepatocarcinogenesis[8,9]. This review summarizes the recent evidence on HCC mechanisms caused by various hepatitis viruses and discusses the role of inflammatory signaling pathways in HCC progression and development (Figure 1).
Viral hepatitis caused by infection with hepatitis viruses A, B, C, D, and E is a global epidemic leading to acute or chronic hepatitis, and even acute severe hepatitis related to a high mortality rate. Due to differences in the structure and features of viruses, they selectively infect the liver using various routes[10]. Approximately 80% of HCC cases are related to HBV or HCV infections, leading to cirrhosis and progressing to HCC.
Hepatitis B virus: Hepatitis B virus (HBV) can integrate its double-stranded DNA (dsDNA) into host cells to develop pregenomic RNA (pgRNA). Then, pgRNA is encapsulated into icosahedral capsids formed by the hepatitis B virus core antigen protein, meditated by polymerase action. Within the capsid, gpRNA is reverse-transcribed into single-stranded DNA (ssDNA), after which the DNA is enveloped to become infectious virions. HBV contains the gene fragments HBV X protein and HBV C protein in its genome. These gene fragments are critical regulatory proteins with crucial roles in HBV-induced HCC pathogenesis. They directly activate or inhibit the expression of hepatocyte growth-related genes, including CTbp2, HMBGA1, and CA10, affecting its transformation to HCC[11-13]. In addition to the direct effects on the host genome to attenuate stability and enhance gene mutations and chromosomal rearrangements with oncogenic or proto-oncogene expression, HBV accelerates HCC progression through multiple mechanisms. For instance, HBV promotes HCC by inducing inflammation and oxidative stress, and altering the immune cell interaction for immune evasion. Bing-Qing Zheng reported that HBsAg (surface antigen) suppressed STAT3 expression and activation in natural killer (NK) cells of chronic hepatitis B (CHB) patients by reducing the IL-21 stimulation response[14]. HBV also activates the phosphatase and tensin homolog (PTEN)/β-actin/c-Myc pathway to promote programmed cell death protein 1 expression, inhibiting T-cell activity and indirectly enhancing the immune evasion of HBV in CHB infection[15]. Furthermore, chronic HBV infection leads to CHB-induced inflammatory damage in hepatic cells due to the persistent activation of inflammatory cells and chemokines[16], causing chronic severe hepatitis or liver cancer. Overall, CHB linked with HBV infection has a weak direct stimulatory role in HCC progression. However, the infection depends more on regulating various immune-related active molecules within the hepatocyte microenvironment.
Hepatitis C virus: HCV belongs to the Flaviviridae family and is an enveloped ssRNA virus. Unlike HBV infection, HCV infection mainly presents as asymptomatic chronic hepatitis, of which 20%-30% of patients progress to liver cirrhosis, and 7% suffer liver cancer[10]. As the released immune cells form a complex HCV-induced HCC tumor microenvironment, Guo-He Song performed single-cell RNA sequencing on immune cells from nontumor and HCV-associated HCC liver tissues[17]. This discovery highlighted novel macrophage and T-cell subsets, of which M2 macrophages significantly expressing CCL18 were enriched in advanced HCC patients. CCL2, CCL20, CXCL8, or CXCL10 were highly induced by the synergistic activity of HCV core protein and chemokines such as interferon (IFN)-γ and IL-1β in fibroblasts or liver sinusoidal endothelial cells (LSECs). These chemokines result in HCV-induced hepatic injury of the LSECs by recruiting leukocytes and activating hepatic stellate cells (HSCs), enabling the development and progression of fibrosis and cirrhosis[18]. CCL2 and CXCL10 are upregulated in macrophages, promoted by the HCV core protein, by interacting with the gC1qR and nuclear factor-kappaB (NF-κB) signaling pathways[19]. Tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-10, IL-18, and transforming growth factor (TGF)-β are the most relevant inflammatory cytokines associated with HBV/HCV-induced HCC via multiple pathways[20]. The IL-6 GC and TGF-β1 TT genotypes promoted HCC development in the HCV-infected population by altering the transcription and stability of the protein structures. These could be potential markers for the early diagnosis of HCC[21].
Excessive alcohol consumption can cause alcoholic liver disease (ALD), such as steatosis, ASH, fibrosis, cirrhosis, and HCC. In the liver, alcohol is metabolized using three major oxidative pathways. First, alcohol is oxidized to acetaldehyde by alcohol dehydrogenase, with NAD+ as the cofactor[22], cytochrome P450 2E1 (CYP2E1) in the microsomal ethanol oxidizing system[23], and the heme-containing enzyme catalase[24]. Subsequently, acetaldehyde is oxidized to acetate by aldehyde dehydrogenase (ALDH). Acetaldehyde damages DNA and impairs the antioxidant defense system, decreasing antioxidant and detoxification enzymes. Adducts from acetaldehyde can disturb cellular function, promoting alcohol-induced liver injury. CYP2E1 induced by chronic alcohol intake enhances alcohol metabolism to acetaldehyde, leading to liver injury and producing reactive oxygen species (ROS)[25]. These ROS attack the hepatocyte mitochondria and reduce ALDH activity. Additionally, mutagenic etheno-DNA adducts, stimulated by CYP2E1, are essential in genetic damage and liver carcinogenesis[26]. Long-term alcohol use causes excessive CYP2E1 along with oxidative stress, producing ROS[27]. Such exposure results in structural damage, mitochondrial dysfunction, mitochondrial stress in hepatocytes, and apoptotic signal upregulation.
Long-term alcohol consumption and liver dysfunction induce alcoholic hepatitis (AH), which is linked with severe ASH and high mortality rates in the short term[28]. Excessive consumption of alcohol causes damage to the microtubule structure and dysfunction of liver cells in patients with AH, which affects the efficiency of nutrient transport. Protein adducts formed by acetaldehyde can block DNA repair and hepatocyte mitochondria, contributing to the dysfunction of oxygen utilization, collagen synthesis, and extracellular matrix accumulation, resulting in liver fibrosis, cirrhosis, and carcinogenesis[29].
Interestingly, innate immunity activation leads to carcinogenesis in two ways: it leads to alcohol-induced liver injury and results in hepatoprotection, regeneration, and anti-inflammatory reactions to decrease alcohol-induced liver damage[30]. Alcohol consumption elevates lipopolysaccharides and activates the MyD88-independent TRIF/IRF-3 pathway using Toll-like receptor 4 (TLR4), causing oxidative stress, TNF-α release, and liver damage[31]. However, TLR4 and complement factors also promote Kupffer cells to secrete protective cytokines such as IL-6 and anti-inflammatory cytokines such as IL-10. Inflammatory cytokines such as TNF-α, IL-1, and IL-6 are enhanced in the serum of ALD patients[32]. IL-10 plays a positive hepatoprotective role via the STAT3 signaling pathway[33]. In contrast, IL-6 and p-STAT3 are highly expressed in HCC patients[34]. TNF-α acts as a pro-tumorigenic cytokine and activates NF-κB and c-Jun N-terminal kinase (JNK) signaling pathways in liver carcinogenesis[35]. NK cells can develop IFN-γ to attenuate liver cell regeneration and kill hepatocytes[36]. However, the function of NK and NK T cells in hepatocytes remains unexplored. IL-1β plays an essential role in the progression of inflammation, alcohol-induced liver steatosis, and liver injury[37]. IL-22 has beneficial effects on hepatic inflammation and regeneration, while F-652, an IL-22 agonist, is a promising AH treatment candidate[38]. IL-17A functions as a tumor-promoting cytokine regulating inflammatory responses and cholesterol synthesis in developing hepatic steatosis, fibrosis, and HCC in an experimental alcohol-induced mouse model[9]. Some of the inflammatory factors have various roles in different stages. If their expression can be upregulated or downregulated during a specific period, these factors could exert their unique therapeutic effects on AH to HHC.
NAFLD is a global disease characterized by excessive fat accumulation in the liver and is not associated with excessive alcohol use. NAFLD progression occurs through several stages, such as simple steatosis, steatohepatitis, fibrosis, and cirrhosis, leading to HCC. NAFLD encompasses a group of liver diseases from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH)[39]. NAFL is a simple steatosis of liver cells without inflammation[40]. Furthermore, NAFL development is accompanied by an inflammatory response, causing NASH and liver cancer with cirrhosis[41]. NASH is characterized by the long-term accumulation of triglycerides or clearance disorders in liver cells, progressing to HCC[42]. The presence of steatosis, inflammation, and hepatocyte damage typically characterizes NASH. These are associated with a higher incidence of cirrhosis and liver cancer with NASH mortality than in NAFL[43-45]. TLR9-MyD88 signaling stimulates Kupffer cells to synthesize IL-1β, which contributes to hepatocyte damage and activates HSCs, promoting NASH development[46]. IL-33 is released during chronic hepatocellular stress to activate ILC-2 in the liver and produce IL-13, facilitating HSC activation and the onset of hepatic fibrosis[47]. Notably, the IL-33/ST2 axis has dual roles in diet-induced NASH, wherein an IL-33 supplement ameliorates hepatic steatosis but exacerbates hepatic fibrosis[48]. TNF-α promotes liver fibrosis while cooperating with TIMP-1 produced by HSCs[49]. In a recent study, IL-17A was tested at a high concentration in early-stage fibrosis with increased expression of profibrotic markers in the tissue slice culture, which revealed a significant role of IL-17A in promoting liver fibrosis in human liver tissue[50]. IL-22 treatment ameliorated CXCL1/high-fat diet-induced NASH and methionine choline-deficient diet-induced NASH via multiple targets, suppressing liver inflammation[51].
Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease with chronic and persistent bile stasis in the liver while causing cirrhosis and liver failure[52]. Some case reports show that cirrhosis is an HCC risk factor in PBC patients[53,54]. Diabetes is categorized into type 1 (T1DM) and type 2 (T2DM) diabetes. Diabetes liver fibrosis (DHF) is a chronic complication that progresses to liver disease. The main reason for DHF is to activate quiescent HSCs via high glucose stimulation[55]. T2DM possesses an elevated risk of advanced fibrosis in NAFL patients[56]. Clinical analysis revealed that 1 out of 20 T1DM patients and 1 out of 5 T2DM patients have elevated liver hardness (an indicator to evaluate liver fibrosis), suggesting severe or advanced liver fibrosis. Obese or T2DM patients have an increased risk of developing NASH, which can progress to cirrhosis and HCC if unchecked.
The IL family, with more than 40 members, was first investigated in 1976. According to the structural homology of cytokines, the IL family has seven subfamilies, including IL-1, IL-2, IL-6/IL-12, IL-10, IL-17, and chemokine α subfamilies.
IL-1 subfamily: The IL-1 subfamily includes IL-1α, IL-1β, IL-18, and IL-37[57]. Inhibition of IL-1 signaling using its agonist weakens hepatic inflammation and promotes liver regeneration, helping recovery from liver injury in AH[58,59]. In NAFLD, mice lacking IL-1α and IL-1β had inhibition of hypercholesterolemia steatosis to steatohepatitis and liver fibrosis[60]. Lack of IL-1α in Kupffer cells of mice with hypercholesterolemia weakens liver inflammation and inflammatory cytokine expression[61]. IL-1α release at different locations affects the development direction of HCC differently. Urinary excretion of IL-1α suggests an HCC-promoting effect, wherein the antitumor immune response is inhibited through myeloid-derived suppressor cells recruitment into the tumor microenvironment. Simultaneously, systemic IL-1α administration directly activates T cells to inhibit HCC development[62]. IL-1β secretion by macrophages was reduced in HBV and hepatitis D virus (HDV) infection, while IL-1β inhibited HBV and HDV replication[63]. IL-1β exerts antiviral effects by inhibiting ERK2 activation by elevating IFN-α, which inhibits HCV replication[64]. IL-1 receptor antagonists improve inflammasome-dependent ASH in mice[37]. Mice lacking the IL-1β activation gene can inhibit the development of obesity-induced NAFLD[65]. IL-1β receptor antagonists can inhibit liver fibrosis in mice, while IL-1β, a component of the NLRP3 inflammasome, can reduce liver fibrosis in NASH mice[66]. IL-1β is highly involved in hepatic lipogenesis by enhancing triglyceride accumulation and induces pathogenic liver steatosis in obesity-induced NAFLD[67]. M1 macrophages induce programmed cell death ligand 1 (PD-L1) expression in hepatoma cells via IL-1β signaling. This key checkpoint molecule mediates HCC immune escape[68]. IL-1β-mediated homologous box C10 overexpression enhances HCC metastasis by upregulating 3-phosphoinositol-dependent protein kinase 1 (PDPK1) and vasodilator-stimulated phosphoprotein (VASP) expressions[69].
IL-6/IL-12 subfamily: This subfamily consists of IL-6, IL-12, IL-23, IL-27, and IL-35A[70]. A case-control experimental study unraveled the potential susceptibility of IL-6 gene polymorphisms against HBV infection[71]. IL-6 regulates microRNA-125b expression in HCV infection using the STAT3 pathway, causing HCV infection onset and possibly progressing to HCC[72]. In AH, IL-6 promotes microRNA-223-rich exosome production, mitigating NAFLD-associated fibrosis[73]. Additionally, caffeine improves NAFLD with a tandem between muscle production of IL-6 and liver STAT3 activation[74]. The activation of IL-6/STAT3 signaling enhances LCSC production by hepatoma cells and resists sorafenib in hepatoma cells. This is an essential factor in inducing the occurrence, development, and metastasis of liver cancer[75]. Inhibiting IL-6/STAT3 signaling can lead to HCC cell apoptosis[76].
IL-10 subfamily: This subfamily consists of IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26[77]. In a clinical study, polymorphisms in IL-19 increased susceptibility to HBV infection in children[78]. IL-19 inhibits the progression from NAFLD to NASH in vitro, while its deficiency in mice leads to pro-inflammatory cytokine expression in the liver[79]. IL-22 positively affects liver inflammation and impaired hepatic regeneration in AH patients and reduces ethanol-induced liver steatohepatitis in mice[38,80]. IL-22 exerts hepatoprotective effects in NAFLD-related liver fibrosis and injury[51,81,82]. However, the role of IL-22 in viral hepatitis is controversial, wherein some studies have reported its positive effects[83], while others indicated that it promotes liver fibrosis and HCC[84,85]. IL-22 exerts pro-tumorigenic effects on hepatocytes in HCC, while IL-22 BP ameliorates liver carcinogenesis[86]. IL-22 overexpression promotes HCC progression, while metformin treatment suppresses IL-22-induced liver cell proliferation, migration, and invasion by reacting with the Hippo signaling pathway[87].
IL-17 subfamily: The IL-17 subfamily comprises IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F[88]. IL-17 expression and the methylation status of its gene promoter can enhance CHB progression[89]. Polymorphisms in the IL-17 gene are related to HCV infection in humans[90,91]. In NAFLD, IL-17 promotes M1 macrophage polarization and exacerbates the hepatic inflammatory response, accelerating NAFLD progression in mice[92]. High-fat diets lead to IL-17A expression, accelerate NAFLD progression by inhibiting fatty acid β oxidation, and promote triglyceride accumulation[93]. This prevents fibrosis in steatohepatitis in mice by inhibiting IL-17-mediated inflammation[94]. In an experimental model of alcohol-induced HCC, IL-17 promotes HCC by regulating the inflammatory response of macrophages and cholesterol synthesis in fatty hepatocytes[9]. IL-17 can also promote non-ASH and HCC[95]. In particular, IL-17A can enhance HCC invasion via the AKT pathway and restrict the autophagy of HCC cells by inhibiting Bcl2 degradation[96,97]. IL-17 can improve HepG2 cell proliferation in vitro and in vivo by activating the IL-2/STAT6 pathway[98].
Chemokine α subfamily: Endoplasmic reticulum stress induces IL-8 transcription and inhibits interferon reactivity in human hepatocytes to increase HBV proliferation[99]. Interferon induces IL-8 to inhibit the production of HBV surface antigen using human hepatocytes[100]. Blocking the recruitment effect of IL-8 on neutrophils can reverse ASH in mice[101,102]. In NAFLD, liver TLR2 expression is positively associated with circulating IL-8 levels. TLR2-mediated pathways are critical for NAFLD/NASH progression, and NASH progression is slower in TLR2 knockout mouse models than in wild-type mouse models[103]. HBV-induced IL-8 inhibits antitumor immunity and elevates HCC metastasis[104]. IL-8 promotes the upregulated signaling of integrin-β3 and HCC cell invasion by activating the PI3K/Akt pathway[105]. Thus, inhibiting IL-8 expression can suppress HCC growth[106,107].
TNF is a cytokine and an adipokine that plays significant roles in various cellular events, including cell proliferation, cell differentiation, and cell death. As a pro-inflammatory cytokine, TNF is actively involved in inflammation-related carcinogenesis. Gene variation in TNF is associated with increased susceptibility to HBV and HCV infection[108,109]. One study evaluated the inhibition of TNF/NF-κB signaling and macrophage M1-type polarization, suggesting a promising approach for attenuating NAFLD progression to NASH[110]. Anti-TNFR1 treatment significantly reduces liver injury and fibrosis without affecting protective TNFR2 signaling in high-fat diet-induced NAFLD[111]. Anti-TNF-α compromises HCC progression and prolongs survival time in mice by decreasing tumor cell viability[112]. TNF-α induces mesenchymal stem cells mobilization to the injured liver site to participate in the inflammatory microenvironment formation and promotes liver cancer development[113]. TNF-α-mediated extracellular Ca2+ influx in HCC accelerates cell apoptosis, suggesting the function of TNF-α as a tumor-killing (pro-apoptotic) cytokine[114]. In addition, TNF-α polymorphism is associated with an elevated risk of HCC[115-117]. The role of TNF-α in the development and progression of HCC requires further exploration.
Hepatic stellate cell–induced CXCL1 enhances the malignant development of HCC through the MIR4435-2HG/miR-506-3p/TGFβ axis, which could be a potential target in HCC therapy[118]. Inhibiting the CXCL1-CXCR2 loop improves doxorubicin efficacy in HCC, reducing macrophage recruitment in the tumor microenvironment and restricting tumor progression[119]. CXCL2 is a tumor suppressor, and its high expression significantly enhances the overall survival rate in HCC. Exogenous expression of CXCL2 inhibits cell proliferation in HCC by causing cell cycle arrest and apoptosis[120]. CXCL3 expression is upregulated in HCC and is highly associated with poor prognosis. This promotes CD133 + CSC proliferation through Erk1/2 phosphorylation[121]. CXCL5 knockdown inhibits cell proliferation and invasion through the miR-577/NF-κB axis, while CXCL5 overexpression is a potential indicator of poor prognosis in HCC patients[122]. Circ-HOMER1 causes cell growth and HCC aggressiveness by suppressing the miR-1322 function on CXCL6[123]. The expression level of CXCL6 in HCC tissues is significantly lower than in the adjacent normal tissues[124]. Tumor-associated macrophages caused by the CXCL8/miR-17 cluster enhance tumor cell growth and metastasis in HCC[125]. CXCL10 accelerates epithelial-mesothelial transition of HCC cells through MMP-2 activation[126]. CXCL10 remodels the intrahepatic tumor microenvironment of fibrosis-related HCC, while CXCL10 depletion promotes the invasion and infiltration of immune cells in the invasive tumor margin, resulting in an antitumorigenic microenvironment[127]. CXCL11/CXCR3 can positively regulate the stemness of α2δ1+ HCC tumor-initiating cells by improving self-renewal and tumorigenic properties via the ERK1/2 pathway[128]. SOX4-induced CXCL12 in HCC leads to tumor-distant metastasis by regulating CXCR4 in endothelial cells and reticular fibers while shaping the tumor microenvironment and neovascularization[129]. Compared with CHB patients or healthy control subjects, serum CXCL13 is significantly higher in HCC patients, and a positive result is associated with tumor size and metastasis[130]. In a clinical study, CXCL14 mRNA expression and serum CXCL14 levels were decreased in HBV-related HCC tissues. This indicates an advanced disease stage with severe hepatitis and impaired liver function[131]. CXCL14 represses cell proliferation in HCC and expedites apoptosis by inhibiting the Akt/mTOR signaling pathway[132]. Exogenous administration of CXCL14 prohibits angiogenesis in HCC and decelerates cell proliferation, invasion, and migration[133]. Allograft inflammatory factor 1 (AIF1)-induced M2 polarization macrophages secrete CXCL16, facilitating microvascular invasion and tumor progression[134]. Upregulated expression of CXCL17 in HCC promotes tumor cell proliferation and inhibits autophagy by controlling the LKB1-AMPK pathway[135]. MiR-325-3p overexpression attenuates angiogenesis, cell proliferation, migration, and invasion in HCC by restricting the CXCL17/CXCR8 axis[136]. Thus, CXCL2, CXCL6, and CXCL14 are negatively associated with HCC development and progression, while CXCL1, CXCL3, CXCL5, CXCL8, CXCL10, CXCL11, CXCL12, CXCL13, and CXCL17 play an inverse role.
TGF-β is a multifunctional regulator of various processes, including angiogenesis, immunity, and cancer[137,138]. TGF-β exists as three isoforms: TGF-β1, TGF-β2, and TGF-β3. All these can interrupt different stages of HCV propagation via the TGF-β/SMAD signaling pathway[139]. ECM1-mediated TGF-β activation promotes liver fibrosis by initiating HSCs[140]. TGF-β1 promotes HBV/HCV-induced fibrogenesis in hepatocytes and HSCs by interacting with the OCT4/Nanog pathway[141]. TGF-β inhibition significantly suppresses high-fat diet-induced inflammation and hepatic fibrosis, ameliorating obesity-related NAFLD and NASH[142,143]. Breviscapine and corosolic acid, TGF-β inhibitors, can alleviate NASH via multiple pathways by decreasing hepatic lipid accumulation, inflammation, and fibrogenesis[144,145]. In HCC, high TGF-β1 expression predicted shorter survival and poor disease prognosis in HCC patients[146]. In clinical studies, treating advanced HCC patients with the TGF-βR1/ALK5 inhibitor galunisertib can reduce AFP (alpha fetoprotein) and TGF-β1 in the body and prolong survival time[147,148]. In addition, galunisertib can improve sorafenib effectiveness in HCC patients[149]. In summary, TGF-β promotes the occurrence and development of HCC via inflammation-mediated cancer development (Table 1).
| Disease | Promotion genes | Inhibition genes |
| Virus hepatitis | IL-6[71]; IL-8[101,102]; IL-17[90,91]; IL-22[84,85]; TNF[108,109]; TGF-β[139]; | IL-1β[63,64]; IL-22[83] |
| Alcoholic hepatitis | IL-1β[37]; IL-8[101,102] | IL-6[73]; IL-22[38,80] |
| NAFLD | IL-1α[60,61]; IL-1β[60,67-69]; IL-8[103]; IL-17[92-94]; TNF[110-112]; TGF-β[144,145] | IL-19[79]; IL-22[51,81,82] |
| HCC | IL-1β[68,69]; IL-6[75,76]; IL-8[105-107]; IL-17[96-98]; IL-22[86,87]; CXCL1[118,119]; CXCL3[121]; CXCL5[122]; CXCL8[125]; CXCL10[126,127]; CXCL11[128]; CXCL12[129]; CXCL13[130]; CXCL16[134]; CXCL17[135]; CXCR3[128]; CXCR4[129]; TGF-β[146-148] | CXCL2[120]; CXCL6[123,124]; CXCL14[131,132] |
There is a significant correlation between inflammation and tumors, and regulating inflammation to treat the tumor could be an effective approach. The efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) in treating tumors is evident. They can exert their anticancer effect regardless of whether administered alone or combined[150]. The therapeutic effect of NSAIDs on HCC has been demonstrated, and aspirin can decrease the risk of death from liver cancer induced by chronic liver disease[151]. Celecoxib also promotes the apoptosis of HCC cells by inhibiting Akt expression[152]. In addition, inhibiting certain inflammatory factors can inhibit HCC development. Inhibition of the NLRP3 inflammasome can hinder the growth of HCC cells and promote autophagy[153,154]. 17β-Estradiol (E2) can induce NLRP3 inflammasome activation, trigger pyroptosis, and inhibit HCC progression[155]. Furthermore, IL-6 inhibition can cause HCC cell senescence[156]. The IL-6/STAT3 pathway can enable the metastasis and proliferation of HCC. Thus, inhibiting this pathway can enhance malignant HCC progression[157,158]. Trilobolide-6-O-isobutyrate inhibited IL-6/STAT3 pathway activation to decrease HCC progression[159]. Ursodeoxycholic acid inhibited IL-8 induced ERK phosphorylation, suppressing IL-8 induced angiogenesis[160]. Neurotensin controls IL-8 expression and interferes with EMT (epithelial-mesenchymal transition)-mediated HCC invasion and migration[161]. Dicer collaborates with lenvatinib to downregulate the expression of IL-8 and inhibit HCC growth[106]. In an alcoholic hepatitis mouse model, IL-22 can improve non-ASH through multiple targets while inhibiting inflammation and anti-fibrosis. Moreover, metformin inhibits IL-22 expression, attenuating HCC cell proliferation, migration, and invasion, and promotes apoptosis[87]. Targeting IL-22 has performed well in early HCC clinical experiments, with a good safety and efficacy profile[38,162].
Notably, anti-inflammatory drugs are combined to treat HCC with beneficial therapeutic effects. Pre-clinical studies have indicated that aspirin, a nonsteroidal anti-inflammatory drug, can elevate the sensitivity to various anti-cancer drugs. These include sorafenib and doxorubicin while overcoming sorafenib resistance in vitro and in vivo[163]. Additionally, aspirin limits NF-κB activation of SLC7A11 transcription by B inhibits the growth of HCC, leading to ferroptosis[164]. However, aspirin is negatively related to the early reported incidence rate of HCC in the general population, which should be considered in the future, particularly in gastrointestinal ulcer patients[165,166]. Another cohort study discovered that using NSAIDs could decrease the risk of early HCC recurrence two years after radical hepatectomy, irrespective of the patient's age, hepatectomy range, viral hepatitis status, basic diabetes, and cirrhosis[167]. Curcumin, a traditional Chinese medicine extract, has excellent anti-inflammatory effects. Curcumin overcame lenvatinib resistance, a first-line treatment drug for unresectable advanced liver cancer, by inhibiting epidermal growth factor receptor[168]. Combining steroid anti-inflammatory drugs dexamethasone and N-acetylcysteine can be employed for post-thrombotic syndrome and post-conventional transcatheter arterial chemoembolization, which is the standard treatment for mid-term HCC. Only two out of 50 participants experienced mild allergic dermatitis[169,170]. Currently, only a few anti-inflammatory drugs have undergone clinical trials. More effective anti-inflammatory drugs can be applied in clinical trials of HCC by continuously enhancing fundamental experiments.
Emerging studies demonstrated that inflammation, particularly chronic inflammation, is crucial in liver deterioration. Moreover, uncontrolled inflammation is a critical factor in liver cancer development. However, at this stage, some acute inflammatory factors have the opposite effect on HCC, indicating that the role of inflammation in HCC requires more exploration regarding new regulatory factors. These factors have great development prospects for the mechanism underlying malignant HCC progression and future clinical treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Massimi M, Italy; Tsoulfas G, Greece; Yang SS, Taiwan S-Editor: Liu JH L-Editor: Webster JR P-Editor: Cai YX
| 1. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 838] [Article Influence: 104.8] [Reference Citation Analysis (2)] |
| 2. | Yamashita T, Kaneko S. [Liver Cancer]. Rinsho Byori. 2016;64:787-796. [PubMed] |
| 3. | Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 807] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 4. | Refolo MG, Messa C, Guerra V, Carr BI, D'Alessandro R. Inflammatory Mechanisms of HCC Development. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 5. | Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921-R925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1524] [Article Influence: 381.0] [Reference Citation Analysis (0)] |
| 6. | Wen Y, Zhu Y, Zhang C, Yang X, Gao Y, Li M, Yang H, Liu T, Tang H. Chronic inflammation, cancer development and immunotherapy. Front Pharmacol. 2022;13:1040163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 96] [Reference Citation Analysis (0)] |
| 7. | Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y, Zhou D. Interleukin 22 in Liver Injury, Inflammation and Cancer. Int J Biol Sci. 2020;16:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Fischer J, Long S, Koukoulioti E, Müller T, Fueloep B, Heyne R, Eslam M, George J, Finkelmeier F, Waidmann O, Berg T, van Bömmel F. Association of Common Polymorphisms in the Interleukin-1 Beta Gene with Hepatocellular Carcinoma in Caucasian Patients with Chronic Hepatitis B. Pathogens. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Ma HY, Yamamoto G, Xu J, Liu X, Karin D, Kim JY, Alexandrov LB, Koyama Y, Nishio T, Benner C, Heinz S, Rosenthal SB, Liang S, Sun M, Karin G, Zhao P, Brodt P, Mckillop IH, Quehenberger O, Dennis E, Saltiel A, Tsukamoto H, Gao B, Karin M, Brenner DA, Kisseleva T. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J Hepatol. 2020;72:946-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (2)] |
| 10. | Pisano MB, Giadans CG, Flichman DM, Ré VE, Preciado MV, Valva P. Viral hepatitis update: Progress and perspectives. World J Gastroenterol. 2021;27:4018-4044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (11)] |
| 11. | Shen Z, Wu J, Gao Z, Zhang S, Chen J, He J, Guo Y, Deng Q, Xie Y, Liu J, Zhang J. High mobility group AT-hook 1 (HMGA1) is an important positive regulator of hepatitis B virus (HBV) that is reciprocally upregulated by HBV X protein. Nucleic Acids Res. 2022;50:2157-2171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Liu X, Zhu C, Li J, Xu F, Huang G, Xu L, Zhang B. HBV Upregulates CtBP2 Expression via the X Gene. Biomed Res Int. 2018;2018:6960573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Chung KM, Chen YT, Hong CC, Chang IC, Lin SY, Liang LY, Chen YR, Yeh CT, Huang SF. CA10 is associated with HBV-related hepatocarcinogenesis. Biochem Biophys Rep. 2022;31:101303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Zheng B, Yang Y, Han Q, Yin C, Pan Z, Zhang J. STAT3 directly regulates NKp46 transcription in NK cells of HBeAg-negative CHB patients. J Leukoc Biol. 2019;106:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Sun Y, Yu M, Qu M, Ma Y, Zheng D, Yue Y, Guo S, Tang L, Li G, Zheng W, Wang M, Guo D, Li C. Hepatitis B virus-triggered PTEN/β-catenin/c-Myc signaling enhances PD-L1 expression to promote immune evasion. Am J Physiol Gastrointest Liver Physiol. 2020;318:G162-G173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Li JZ, Ye LH, Wang DH, Zhang HC, Li TY, Liu ZQ, Dai EH, Li MR. The identify role and molecular mechanism of the MALAT1/hsa-mir-20b-5p/TXNIP axis in liver inflammation caused by CHB in patients with chronic HBV infection complicated with NAFLD. Virus Res. 2021;298:198405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Song G, Shi Y, Zhang M, Goswami S, Afridi S, Meng L, Ma J, Chen Y, Lin Y, Zhang J, Liu Y, Jin Z, Yang S, Rao D, Zhang S, Ke A, Wang X, Cao Y, Zhou J, Fan J, Zhang X, Xi R, Gao Q. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. 2020;6:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Abouelasrar Salama S, Gouwy M, De Zutter A, Pörtner N, Vanbrabant L, Berghmans N, De Buck M, Struyf S, Van Damme J. Induction of Chemokines by Hepatitis C Virus Proteins: Synergy of the Core Protein with Interleukin-1β and Interferon-γ in Liver Bystander Cells. J Interferon Cytokine Res. 2020;40:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Song X, Gao X, Wang Y, Raja R, Zhang Y, Yang S, Li M, Yao Z, Wei L. HCV Core Protein Induces Chemokine CCL2 and CXCL10 Expression Through NF-κB Signaling Pathway in Macrophages. Front Immunol. 2021;12:654998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Timperi E, Barnaba V. Viral Hepatitides, Inflammation and Tumour Microenvironment. Adv Exp Med Biol. 2020;1263:25-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Badshah Y, Shabbir M, Khan K, Fatima M, Majoka I, Aslam L, Munawar H. Manipulation of Interleukin-6 (IL-6) and Transforming Growth Factor Beta-1(TGFβ-1) towards viral induced liver cancer pathogenesis. PLoS One. 2022;17:e0275834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Meroni M, Longo M, Rametta R, Dongiovanni P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Jiang Y, Zhang T, Kusumanchi P, Han S, Yang Z, Liangpunsakul S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 24. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (5)] |
| 25. | Teschke R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 26. | Jain D, Murti Y, Khan WU, Hossain R, Hossain MN, Agrawal KK, Ashraf RA, Islam MT, Janmeda P, Taheri Y, Alshehri MM, Daştan SD, Yeskaliyeva B, Kipchakbayeva A, Sharifi-Rad J, Cho WC. Roles of Therapeutic Bioactive Compounds in Hepatocellular Carcinoma. Oxid Med Cell Longev. 2021;2021:9068850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Tan HK, Yates E, Lilly K, Dhanda AD. Oxidative stress in alcohol-related liver disease. World J Hepatol. 2020;12:332-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (2)] |
| 28. | Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G; NIAAA Alcoholic Hepatitis Consortia. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 29. | Kong LZ, Chandimali N, Han YH, Lee DH, Kim JS, Kim SU, Kim TD, Jeong DK, Sun HN, Lee DS, Kwon T. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 30. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 31. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30 Suppl 1:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 34. | Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 35. | Sethi JK, Hotamisligil GS. Metabolic Messengers: tumour necrosis factor. Nat Metab. 2021;3:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 239] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 36. | Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 298] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 37. | Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 587] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 38. | Arab JP, Sehrawat TS, Simonetto DA, Verma VK, Feng D, Tang T, Dreyer K, Yan X, Daley WL, Sanyal A, Chalasani N, Radaeva S, Yang L, Vargas H, Ibacache M, Gao B, Gores GJ, Malhi H, Kamath PS, Shah VH. An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology. 2020;72:441-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 39. | Hochreuter MY, Dall M, Treebak JT, Barrès R. MicroRNAs in non-alcoholic fatty liver disease: Progress and perspectives. Mol Metab. 2022;65:101581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 40. | Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. 2022;292:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 41. | Murphy WA, Adiwidjaja J, Sjöstedt N, Yang K, Beaudoin JJ, Spires J, Siler SQ, Neuhoff S, Brouwer KLR. Considerations for Physiologically Based Modeling in Liver Disease: From Nonalcoholic Fatty Liver (NAFL) to Nonalcoholic Steatohepatitis (NASH). Clin Pharmacol Ther. 2023;113:275-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 43. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 44. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 799] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 45. | Vonghia L, Michielsen P, Francque S. Immunological mechanisms in the pathophysiology of non-alcoholic steatohepatitis. Int J Mol Sci. 2013;14:19867-19890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-34.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 622] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 47. | McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 421] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 48. | Gao Y, Liu Y, Yang M, Guo X, Zhang M, Li H, Li J, Zhao J. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget. 2016;7:33649-33661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, Kozawa O. Tumor necrosis factor-α promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013;8:e65251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Kartasheva-Ebertz D, Gaston J, Lair-Mehiri L, Mottez E, Buivan TP, Massault PP, Scatton O, Gaujoux S, Vaillant JC, Pol S, Lagaye S. IL-17A in Human Liver: Significant Source of Inflammation and Trigger of Liver Fibrosis Initiation. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 51. | Hwang S, He Y, Xiang X, Seo W, Kim SJ, Ma J, Ren T, Park SH, Zhou Z, Feng D, Kunos G, Gao B. Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets. Hepatology. 2020;72:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 52. | Tanaka A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol. 2021;27:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 53. | Natarajan Y, Tansel A, Patel P, Emologu K, Shukla R, Qureshi Z, El-Serag HB, Thrift AP, Kanwal F. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;66:2439-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Sy AM, Ferreira RD, John BV. Hepatocellular Carcinoma in Primary Biliary Cholangitis. Clin Liver Dis. 2022;26:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 55. | Zhao B, Li S, Guo Z, Chen Z, Zhang X, Xu C, Chen J, Wei C. Dopamine receptor D2 inhibition alleviates diabetic hepatic stellate cells fibrosis by regulating the TGF-β1/Smads and NFκB pathways. Clin Exp Pharmacol Physiol. 2021;48:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Singh A, Garg R, Lopez R, Alkhouri N. Diabetes Liver Fibrosis Score to Detect Advanced Fibrosis in Diabetics with Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2022;20:e624-e626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Chan AH, Schroder K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 58. | Iracheta-Vellve A, Petrasek J, Gyogyosi B, Bala S, Csak T, Kodys K, Szabo G. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017;37:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Szabo G, Mitchell M, McClain CJ, Dasarathy S, Barton B, McCullough AJ, Nagy LE, Kroll-Desrosiers A, Tornai D, Min HA, Radaeva S, Holbein MEB, Casey L, Cuthbert J. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. 2022;76:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 60. | Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, Olteanu S, Barshack I, Dotan S, Voronov E, Dinarello CA, Apte RN, Harats D. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 61. | Olteanu S, Kandel-Kfir M, Shaish A, Almog T, Shemesh S, Barshack I, Apte RN, Harats D, Kamari Y. Lack of interleukin-1α in Kupffer cells attenuates liver inflammation and expression of inflammatory cytokines in hypercholesterolaemic mice. Dig Liver Dis. 2014;46:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Lin D, Mei Y, Lei L, Binte Hanafi Z, Jin Z, Liu Y, Song Y, Zhang Y, Hu B, Liu C, Lu J, Liu H. Immune suppressive function of IL-1α release in the tumor microenvironment regulated by calpain 1. Oncoimmunology. 2022;11:2088467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Delphin M, Faure-Dupuy S, Isorce N, Rivoire M, Salvetti A, Durantel D, Lucifora J. Inhibitory Effect of IL-1β on HBV and HDV Replication and HBs Antigen-Dependent Modulation of Its Secretion by Macrophages. Viruses. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 64. | Guo M, Ye L, Yu T, Han L, Li Q, Lou P, Gan T, Jin X, Xiao H, Meng G, Zhong J, Xu Y. IL-1β Enhances the Antiviral Effect of IFN-α on HCV Replication by Negatively Modulating ERK2 Activation. ACS Infect Dis. 2020;6:1708-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Mirea AM, Stienstra R, Kanneganti TD, Tack CJ, Chavakis T, Toonen EJM, Joosten LAB. Mice Deficient in the IL-1β Activation Genes Prtn3, Elane, and Casp1 Are Protected Against the Development of Obesity-Induced NAFLD. Inflammation. 2020;43:1054-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 827] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 67. | Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, Kim JK, Czech MP. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9:e107265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 68. | Zong Z, Zou J, Mao R, Ma C, Li N, Wang J, Wang X, Zhou H, Zhang L, Shi Y. M1 Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells Through IL-1β Signaling. Front Immunol. 2019;10:1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 69. | Dang Y, Chen J, Feng W, Qiao C, Han W, Nie Y, Wu K, Fan D, Xia L. Interleukin 1β-mediated HOXC10 Overexpression Promotes Hepatocellular Carcinoma Metastasis by Upregulating PDPK1 and VASP. Theranostics. 2020;10:3833-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 70. | Scheller J, Berg A, Moll JM, Floss DM, Jungesblut C. Current status and relevance of single nucleotide polymorphisms in IL-6-/IL-12-type cytokine receptors. Cytokine. 2021;148:155550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | El-Maadawy EA, Talaat RM, Ahmed MM, El-Shenawy SZ. Interleukin-6 promotor gene polymorphisms and susceptibility to chronic hepatitis B virus in Egyptians. Hum Immunol. 2019;80:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Dai CY, Tsai YS, Chou WW, Liu T, Huang CF, Wang SC, Tsai PC, Yeh ML, Hsieh MY, Huang CI, Vanson Liu SY, Huang JF, Chuang WL, Yu ML. The IL-6/STAT3 pathway upregulates microRNA-125b expression in hepatitis C virus infection. Oncotarget. 2018;9:11291-11302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, Li Y, Zheng MH, Kunos G, Gao B, Wang H. Myeloid-Cell-Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology. 2021;74:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 74. | Fang C, Cai X, Hayashi S, Hao S, Sakiyama H, Wang X, Yang Q, Akira S, Nishiguchi S, Fujiwara N, Tsutsui H, Sheng J. Caffeine-stimulated muscle IL-6 mediates alleviation of non-alcoholic fatty liver disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Wang X, Sun W, Shen W, Xia M, Chen C, Xiang D, Ning B, Cui X, Li H, Li X, Ding J, Wang H. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol. 2016;64:1283-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 76. | Wang ST, Huang SW, Liu KT, Lee TY, Shieh JJ, Wu CY. Atorvastatin-induced senescence of hepatocellular carcinoma is mediated by downregulation of hTERT through the suppression of the IL-6/STAT3 pathway. Cell Death Discov. 2020;6:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Wei H, Li B, Sun A, Guo F. Interleukin-10 Family Cytokines Immunobiology and Structure. Adv Exp Med Biol. 2019;1172:79-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Fan J, Mu LH, Zhou L, Huang X, Huang YF. [Association between IL-19 gene polymorphisms and hepatitis B virus susceptibility in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 79. | Azuma YT, Fujita T, Izawa T, Hirota K, Nishiyama K, Ikegami A, Aoyama T, Ike M, Ushikai Y, Kuwamura M, Fujii H, Tsuneyama K. IL-19 Contributes to the Development of Nonalcoholic Steatohepatitis by Altering Lipid Metabolism. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 80. | Hendrikx T, Duan Y, Wang Y, Oh JH, Alexander LM, Huang W, Stärkel P, Ho SB, Gao B, Fiehn O, Emond P, Sokol H, van Pijkeren JP, Schnabl B. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019;68:1504-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 81. | Zai W, Chen W, Wu Z, Jin X, Fan J, Zhang X, Luan J, Tang S, Mei X, Hao Q, Liu H, Ju D. Targeted Interleukin-22 Gene Delivery in the Liver by Polymetformin and Penetratin-Based Hybrid Nanoparticles to Treat Nonalcoholic Fatty Liver Disease. ACS Appl Mater Interfaces. 2019;11:4842-4857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Abdelnabi MN, Flores Molina M, Soucy G, Quoc-Huy Trinh V, Bédard N, Mazouz S, Jouvet N, Dion J, Tran S, Bilodeau M, Estall JL, Shoukry NH. Sex-Dependent Hepatoprotective Role of IL-22 Receptor Signaling in Non-Alcoholic Fatty Liver Disease-Related Fibrosis. Cell Mol Gastroenterol Hepatol. 2022;14:1269-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Sertorio M, Hou X, Carmo RF, Dessein H, Cabantous S, Abdelwahed M, Romano A, Albuquerque F, Vasconcelos L, Carmo T, Li J, Varoquaux A, Arnaud V, Oliveira P, Hamdoun A, He H, Adbelmaboud S, Mergani A, Zhou J, Monis A, Pereira LB, Halfon P, Bourlière M, Parana R, Dos Reis M, Gonnelli D, Moura P, Elwali NE, Argiro L, Li Y, Dessein A. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Zhang J, Liu Z, Liu L, Huang M, Huang Y. Th22/IL-22 mediates the progression of HBV-related hepatocellular carcinoma via STAT3. Cytotechnology. 2022;74:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Wu LY, Liu S, Liu Y, Guo C, Li H, Li W, Jin X, Zhang K, Zhao P, Wei L, Zhao J. Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol. 2015;158:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Giannou AD, Lücke J, Kleinschmidt D, Shiri AM, Steglich B, Nawrocki M, Zhang T, Zazara DE, Kempski J, Zhao L, Giannou O, Agalioti T, Brockmann L, Bertram F, Sabihi M, Böttcher M, Ewald F, Schulze K, von Felden J, Machicote A, Maroulis IC, Arck PC, Graß JK, Mercanoglu B, Reeh M, Wolter S, Tachezy M, Seese H, Theodorakopoulou M, Lykoudis PM, Heumann A, Uzunoglu FG, Ghadban T, Mann O, Izbicki JR, Li J, Duprée A, Melling N, Gagliani N, Huber S. A Critical Role of the IL-22-IL-22 Binding Protein Axis in Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 87. | Zhao D, Xia L, Geng W, Xu D, Zhong C, Zhang J, Xia Q. Metformin suppresses interleukin-22 induced hepatocellular carcinoma by upregulating Hippo signaling pathway. J Gastroenterol Hepatol. 2021;36:3469-3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Meehan EV, Wang K. Interleukin-17 Family Cytokines in Metabolic Disorders and Cancer. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 89. | Tian CH, Dai J, Zhang W, Liu Y, Yang Y. Expression of IL-17 and its gene promoter methylation status are associated with the progression of chronic hepatitis B virus infection. Medicine (Baltimore). 2019;98:e15924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Massabayeva MR, Aukenov NE, Mussazhanova ZB, Saenko VA, Rogounovitch TI, Shaimardanov NK, Kurmanova BR, Barkibaeva NR, Rakhypbekov TK. IL17A gene polymorphisms: relationship to predisposition for chronic viral hepatitis and progression to liver cirrhosis in kazakh population. Vopr Virusol. 2016;61:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 91. | Ren W, Wu Z, Ma R, Liu Z, Wang Y, Wu L, Liu S, Wang Z. Polymorphisms in the IL-17 Gene (rs2275913 and rs763780) Are Associated with Hepatitis B Virus Infection in the Han Chinese Population. Genet Test Mol Biomarkers. 2017;21:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Yang Y, Han CY, Guan QB, Ruan SL. [Interleukin-17-mediated inflammation promotes nonalcoholic fatty liver disease in mice with regulation of M1-type macrophage polarization]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 93. | Shen T, Chen X, Li Y, Tang X, Jiang X, Yu C, Zheng Y, Guo H, Ling W. Interleukin-17A exacerbates high-fat diet-induced hepatic steatosis by inhibiting fatty acid β-oxidation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Yamato M, Sakai Y, Mochida H, Kawaguchi K, Takamura M, Usui S, Seki A, Mizukoshi E, Yamashita T, Ishida K, Nasti A, Tuyen HTB, Komura T, Yoshida K, Wada T, Honda M, Kaneko S. Adipose tissue-derived stem cells prevent fibrosis in murine steatohepatitis by suppressing IL-17-mediated inflammation. J Gastroenterol Hepatol. 2019;34:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Gomes AL, Teijeiro A, Burén S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, Djouder N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2016;30:161-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 96. | Li S, Lin Z, Zheng W, Zheng L, Chen X, Yan Z, Cheng Z, Yan H, Zheng C, Guo P. IL-17A inhibits autophagic activity of HCC cells by inhibiting the degradation of Bcl2. Biochem Biophys Res Commun. 2019;509:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Xu QG, Yu J, Guo XG, Hou GJ, Yuan SX, Yang Y, Liu H, Pan ZY, Yang F, Gu FM, Zhou WP. IL-17A promotes the invasion-metastasis cascade via the AKT pathway in hepatocellular carcinoma. Mol Oncol. 2018;12:936-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Hu Z, Luo D, Wang D, Ma L, Zhao Y, Li L. IL-17 Activates the IL-6/STAT3 Signal Pathway in the Proliferation of Hepatitis B Virus-Related Hepatocellular Carcinoma. Cell Physiol Biochem. 2017;43:2379-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 99. | Tsuge M, Hiraga N, Zhang Y, Yamashita M, Sato O, Oka N, Shiraishi K, Izaki Y, Makokha GN, Uchida T, Kurihara M, Nomura M, Tsushima K, Nakahara T, Murakami E, Abe-Chayama H, Kawaoka T, Miki D, Imamura M, Kawakami Y, Aikata H, Ochi H, Hayes CN, Fujita T, Chayama K. Endoplasmic reticulum-mediated induction of interleukin-8 occurs by hepatitis B virus infection and contributes to suppression of interferon responsiveness in human hepatocytes. Virology. 2018;525:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Haga Y, Kanda T, Nakamoto S, Nakamura M, Sasaki R, Wu S, Yokosuka O. Interferon induces interleukin 8 and bone marrow stromal cell antigen 2 expression, inhibiting the production of hepatitis B virus surface antigen from human hepatocytes. Biochem Biophys Res Commun. 2017;486:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Wieser V, Adolph TE, Enrich B, Kuliopulos A, Kaser A, Tilg H, Kaneider NC. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors. Gut. 2017;66:930-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | French SW, Mendoza AS, Afifiyan N, Tillman B, Vitocruz E, French BA. The role of the IL-8 signaling pathway in the infiltration of granulocytes into the livers of patients with alcoholic hepatitis. Exp Mol Pathol. 2017;103:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Auguet T, Bertran L, Binetti J, Aguilar C, Martínez S, Sabench F, Lopez-Dupla JM, Porras JA, Riesco D, Del Castillo D, Richart C. Relationship between IL-8 Circulating Levels and TLR2 Hepatic Expression in Women with Morbid Obesity and Nonalcoholic Steatohepatitis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Zhang C, Gao Y, Du C, Markowitz GJ, Fu J, Zhang Z, Liu C, Qin W, Wang H, Wang F, Yang P. Hepatitis B-Induced IL8 Promotes Hepatocellular Carcinoma Venous Metastasis and Intrahepatic Treg Accumulation. Cancer Res. 2021;81:2386-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 105. | Sun F, Wang J, Sun Q, Li F, Gao H, Xu L, Zhang J, Sun X, Tian Y, Zhao Q, Shen H, Zhang K, Liu J. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 106. | Han X, Wu J, Sha Z, Lai R, Shi J, Mi L, Yin F, Guo Z. Dicer Suppresses Hepatocellular Carcinoma via Interleukin-8 Pathway. Clin Med Insights Oncol. 2023;17:11795549231161212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 107. | Krause GC, Lima KG, Haute GV, Schuster AD, Dias HB, Mesquita FC, Pedrazza L, Marczak ES, Basso BS, Velasque AC, Martha BA, Nunes FB, Donadio MV, de Oliveira JR. Fructose-1,6-bisphosphate decreases IL-8 levels and increases the activity of pro-apoptotic proteins in HepG2 cells. Biomed Pharmacother. 2017;89:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Woziwodzka A, Rybicka M, Sznarkowska A, Romanowski T, Dręczewski M, Stalke P, Bielawski KP. TNF-α polymorphisms affect persistence and progression of HBV infection. Mol Genet Genomic Med. 2019;7:e00935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Yue M, Huang P, Wang C, Fan H, Tian T, Wu J, Luo F, Fu Z, Xia X, Zhu P, Li J, Han Y, Zhang Y, Hou W. Genetic Variation on TNF/LTA and TNFRSF1A Genes is Associated with Outcomes of Hepatitis C Virus Infection. Immunol Invest. 2021;50:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Zhou W, Zhu Z, Xiao X, Li C, Zhang L, Dang Y, Ge G, Ji G, Zhu M, Xu H. Jiangzhi Granule attenuates non-alcoholic steatohepatitis by suppressing TNF/NFκB signaling pathway-a study based on network pharmacology. Biomed Pharmacother. 2021;143:112181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Wandrer F, Liebig S, Marhenke S, Vogel A, John K, Manns MP, Teufel A, Itzel T, Longerich T, Maier O, Fischer R, Kontermann RE, Pfizenmaier K, Schulze-Osthoff K, Bantel H. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020;11:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 112. | Li W, Jian YB. Antitumor necrosis factor-α antibodies as a noveltherapy for hepatocellular carcinoma. Exp Ther Med. 2018;16:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Zong C, Meng Y, Ye F, Yang X, Li R, Jiang J, Zhao Q, Gao L, Han Z, Wei L. AIF1 + CSF1R + MSCs, induced by TNF-α, act to generate an inflammatory microenvironment and promote hepatocarcinogenesis. Hepatology. 2023;78:434-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 114. | Zhu J, Jin M, Wang J, Zhang H, Wu Y, Li D, Ji X, Yang H, Yin C, Ren T, Xing J. TNFα induces Ca(2+) influx to accelerate extrinsic apoptosis in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Heidari Horestani M, Atri Roozbahani G, Sheidai M. The Potential Role of TNF-α (rs361525 and rs1800629) in Hepatocellular Carcinoma: Multivariate Analysis (Meta-Analysis). J Gastrointest Cancer. 2019;50:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 116. | Verma HK, Merchant N, Bhaskar LVKS. Tumor Necrosis Factor-Alpha Gene Promoter (TNF-α G-308A) Polymorphisms Increase the Risk of Hepatocellular Carcinoma in Asians: A Meta-Analysis. Crit Rev Oncog. 2020;25:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Wungu CDK, Ariyanto FC, Prabowo GI, Soetjipto, Handajani R. Association between five types of Tumor Necrosis Factor-α gene polymorphism and hepatocellular carcinoma risk: a meta-analysis. BMC Cancer. 2020;20:1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Li S, Hu X, Yu S, Yi P, Chen R, Huang Z, Huang Y, Zhou R, Fan X. Hepatic stellate cell-released CXCL1 aggravates HCC malignant behaviors through the MIR4435-2HG/miR-506-3p/TGFB1 axis. Cancer Sci. 2023;114:504-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 119. | Zhao H, Wei S, Zhou D, Liu Y, Guo Z, Fang C, Pang X, Li F, Hou H, Cui X. Blocking the CXCL1-CXCR2 axis enhances the effects of doxorubicin in HCC by remodelling the tumour microenvironment via the NF-κB/IL-1β/CXCL1 signalling pathway. Cell Death Discov. 2023;9:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Ding J, Xu K, Zhang J, Lin B, Wang Y, Yin S, Xie H, Zhou L, Zheng S. Overexpression of CXCL2 inhibits cell proliferation and promotes apoptosis in hepatocellular carcinoma. BMB Rep. 2018;51:630-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Zhang L, Zhang L, Li H, Ge C, Zhao F, Tian H, Chen T, Jiang G, Xie H, Cui Y, Yao M, Li J. CXCL3 contributes to CD133(+) CSCs maintenance and forms a positive feedback regulation loop with CD133 in HCC via Erk1/2 phosphorylation. Sci Rep. 2016;6:27426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 122. | Jia X, Wei S, Xiong W. CXCL5/NF-κB Pathway as a Therapeutic Target in Hepatocellular Carcinoma Treatment. J Oncol. 2021;2021:9919494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Zhao M, Dong G, Meng Q, Lin S, Li X. Circ-HOMER1 enhances the inhibition of miR-1322 on CXCL6 to regulate the growth and aggressiveness of hepatocellular carcinoma cells. J Cell Biochem. 2020;121:4440-4449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Wang J, Zhang C, Chen X, Li Y, Li A, Liu D, Li F, Luo T. Functions of CXC chemokines as biomarkers and potential therapeutic targets in the hepatocellular carcinoma microenvironment. Transl Cancer Res. 2021;10:2169-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Yin Z, Huang J, Ma T, Li D, Wu Z, Hou B, Jian Z. Macrophages activating chemokine (C-X-C motif) ligand 8/miR-17 cluster modulate hepatocellular carcinoma cell growth and metastasis. Am J Transl Res. 2017;9:2403-2411. [PubMed] |
| 126. | Ren T, Zhu L, Cheng M. CXCL10 accelerates EMT and metastasis by MMP-2 in hepatocellular carcinoma. Am J Transl Res. 2017;9:2824-2837. [PubMed] |
| 127. | Brandt EF, Baues M, Wirtz TH, May JN, Fischer P, Beckers A, Schüre BC, Sahin H, Trautwein C, Lammers T, Berres ML. Chemokine CXCL10 Modulates the Tumor Microenvironment of Fibrosis-Associated Hepatocellular Carcinoma. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 128. | Zhang Y, Zhao W, Li S, Lv M, Yang X, Li M, Zhang Z. CXCL11 promotes self-renewal and tumorigenicity of α2δ1(+) liver tumor-initiating cells through CXCR3/ERK1/2 signaling. Cancer Lett. 2019;449:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 129. | Tsai CN, Yu SC, Lee CW, Pang JS, Wu CH, Lin SE, Chung YH, Tsai CL, Hsieh SY, Yu MC. SOX4 activates CXCL12 in hepatocellular carcinoma cells to modulate endothelial cell migration and angiogenesis in vivo. Oncogene. 2020;39:4695-4710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 130. | Li B, Su H, Cao J, Zhang L. CXCL13 rather than IL-31 is a potential indicator in patients with hepatocellular carcinoma. Cytokine. 2017;89:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 131. | Lin Y, Chen BM, Yu XL, Yi HC, Niu JJ, Li SL. Suppressed Expression of CXCL14 in Hepatocellular Carcinoma Tissues and Its Reduction in the Advanced Stage of Chronic HBV Infection. Cancer Manag Res. 2019;11:10435-10443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Bi J, Liu Q, Sun Y, Hu X, He X, Xu C. CXCL14 inhibits the growth and promotes apoptosis of hepatocellular carcinoma cells via suppressing Akt/mTOR pathway. J Recept Signal Transduct Res. 2021;41:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Liu Y, Chang Q, Wu X, Yu Y, Zhang H. Effect of chemokine CXCL14 on in vitro angiogenesis of human hepatocellular carcinoma cells. Arch Physiol Biochem. 2022;128:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Cai H, Zhu XD, Ao JY, Ye BG, Zhang YY, Chai ZT, Wang CH, Shi WK, Cao MQ, Li XL, Sun HC. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. Oncoimmunology. 2017;6:e1333213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 135. | Wang L, Li H, Zhen Z, Ma X, Yu W, Zeng H, Li L. CXCL17 promotes cell metastasis and inhibits autophagy via the LKB1-AMPK pathway in hepatocellular carcinoma. Gene. 2019;690:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 136. | Li L, Ji Y, Chen YC, Zhen ZJ. MiR-325-3p mediate the CXCL17/CXCR8 axis to regulate angiogenesis in hepatocellular carcinoma. Cytokine. 2021;141:155436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Larson C, Oronsky B, Carter CA, Oronsky A, Knox SJ, Sher D, Reid TR. TGF-beta: a master immune regulator. Expert Opin Ther Targets. 2020;24:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 138. | Syed V. TGF-β Signaling in Cancer. J Cell Biochem. 2016;117:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 139. | Zou LL, Li JR, Li H, Tan JL, Wang MX, Liu NN, Gao RM, Yan HY, Wang XK, Dong B, Li YH, Peng ZG. TGF-β isoforms inhibit hepatitis C virus propagation in transforming growth factor beta/SMAD protein signalling pathway dependent and independent manners. J Cell Mol Med. 2021;25:3498-3510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 140. | Fan W, Liu T, Chen W, Hammad S, Longerich T, Hausser I, Fu Y, Li N, He Y, Liu C, Zhang Y, Lian Q, Zhao X, Yan C, Li L, Yi C, Ling Z, Ma L, Xu H, Wang P, Cong M, You H, Liu Z, Wang Y, Chen J, Li D, Hui L, Dooley S, Hou J, Jia J, Sun B. ECM1 Prevents Activation of Transforming Growth Factor β, Hepatic Stellate Cells, and Fibrogenesis in Mice. Gastroenterology. 2019;157:1352-1367.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 141. | Li W, Duan X, Zhu C, Liu X, Jeyarajan AJ, Xu M, Tu Z, Sheng Q, Chen D, Shao T, Cheng Z, Salloum S, Schaefer EA, Kruger AJ, Holmes JA, Chung RT, Lin W. Hepatitis B and Hepatitis C Virus Infection Promote Liver Fibrogenesis through a TGF-β1-Induced OCT4/Nanog Pathway. J Immunol. 2022;208:672-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 142. | Pahk K, Lee SG, Joung C, Kim EO, Kwon HW, Kim DH, Hwang JI, Kim S, Kim WK. SP-1154, a novel synthetic TGF-β inhibitor, alleviates obesity and hepatic steatosis in high-fat diet-induced mice. Biomed Pharmacother. 2022;145:112441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 143. | Hui ST, Wang F, Stappenbeck F, French SW, Magyar CE, Parhami F, Lusis AJ. Oxy210, a novel inhibitor of hedgehog and TGF-β signalling, ameliorates hepatic fibrosis and hypercholesterolemia in mice. Endocrinol Diabetes Metab. 2021;4:e00296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 144. | Lan T, Jiang S, Zhang J, Weng Q, Yu Y, Li H, Tian S, Ding X, Hu S, Yang Y, Wang W, Wang L, Luo D, Xiao X, Piao S, Zhu Q, Rong X, Guo J. Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology. 2022;76:155-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 145. | Liu G, Cui Z, Gao X, Liu H, Wang L, Gong J, Wang A, Zhang J, Ma Q, Huang Y, Piao G, Yuan H. Corosolic acid ameliorates non-alcoholic steatohepatitis induced by high-fat diet and carbon tetrachloride by regulating TGF-β1/Smad2, NF-κB, and AMPK signaling pathways. Phytother Res. 2021;35:5214-5226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 146. | Peng L, Yuan XQ, Zhang CY, Ye F, Zhou HF, Li WL, Liu ZY, Zhang YQ, Pan X, Li GC. High TGF-β1 expression predicts poor disease prognosis in hepatocellular carcinoma patients. Oncotarget. 2017;8:34387-34397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 147. | Giannelli G, Santoro A, Kelley RK, Gane E, Paradis V, Cleverly A, Smith C, Estrem ST, Man M, Wang S, Lahn MM, Raymond E, Benhadji KA, Faivre S. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-βRI inhibitor galunisertib. PLoS One. 2020;15:e0222259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 148. | Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond E, Benhadji KA, Giannelli G. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 149. | Kelley RK, Gane E, Assenat E, Siebler J, Galle PR, Merle P, Hourmand IO, Cleverly A, Zhao Y, Gueorguieva I, Lahn M, Faivre S, Benhadji KA, Giannelli G. A Phase 2 Study of Galunisertib (TGF-β1 Receptor Type I Inhibitor) and Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Clin Transl Gastroenterol. 2019;10:e00056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 150. | Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, Filosa R, Caraglia M. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 151. | Yan LJ, Yao SY, Li HC, Meng GX, Liu KX, Ding ZN, Hong JG, Chen ZQ, Dong ZR, Li T. Efficacy and Safety of Aspirin for Prevention of Hepatocellular Carcinoma: An Updated Meta-analysis. J Clin Transl Hepatol. 2022;10:835-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 152. | Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 153. | Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol Res. 2019;27:827-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 154. | Chen W, Wei T, Chen Y, Yang L, Wu X. Downregulation of IRAK1 Prevents the Malignant Behavior of Hepatocellular Carcinoma Cells by Blocking Activation of the MAPKs/NLRP3/IL-1β Pathway. Onco Targets Ther. 2020;13:12787-12796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 155. | Awwad SF, Assaf RH, Emam AA, Fouad AA, Arafa LF, El-Hanafy AA. NLRP3 inflammasome activation By 17β-estradiol is a potential therapeutic target in hepatocellular carcinoma treatment. Med Oncol. 2023;40:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 156. | Wang L, Zhu L, Liang C, Huang X, Liu Z, Huo J, Zhang Y, Chen L, Xu H, Li X, Xu L, Kuang M, Wong CC, Yu J. Targeting N6-methyladenosine reader YTHDF1 with siRNA boosts antitumor immunity in NASH-HCC by inhibiting EZH2-IL-6 axis. J Hepatol. 2023;79:1185-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 157. | Liu D, Luo X, Xie M, Zhang T, Chen X, Zhang B, Sun M, Wang Y, Feng Y, Ji X, Li Y, Liu B, Huang W, Xia L. HNRNPC downregulation inhibits IL-6/STAT3-mediated HCC metastasis by decreasing HIF1A expression. Cancer Sci. 2022;113:3347-3361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 158. | Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front Oncol. 2021;11:760971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 159. | Zhou XQ, Mao XM, Fan R, Li SY, Shang J, Zhang T, Li RH, Li HQ, Hui Y, Chen WH, Wang ZX, Shen DY. Trilobolide-6-O-isobutyrate suppresses hepatocellular carcinoma tumorigenesis through inhibition of IL-6/STAT3 signaling pathway. Phytother Res. 2021;35:5741-5753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 160. | Lin W, Li S, Meng Y, Huang G, Liang S, Du J, Liu Q, Cheng B. UDCA Inhibits Hypoxic Hepatocellular Carcinoma Cell-Induced Angiogenesis Through Suppressing HIF-1α/VEGF/IL-8 Intercellular Signaling. Front Pharmacol. 2021;12:755394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 161. | Xiao P, Long X, Zhang L, Ye Y, Guo J, Liu P, Zhang R, Ning J, Yu W, Wei F, Yu J. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology. 2018;7:e1440166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 162. | Tang KY, Lickliter J, Huang ZH, Xian ZS, Chen HY, Huang C, Xiao C, Wang YP, Tan Y, Xu LF, Huang YL, Yan XQ. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol. 2019;16:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 163. | Wang J, Hu F, Yu P, Wang J, Liu Z, Bao Q, Zhang W, Wen J. Sorafenib inhibits doxorubicin-induced PD-L1 upregulation to improve immunosuppressive microenvironment in Osteosarcoma. J Cancer Res Clin Oncol. 2023;149:5127-5138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 164. | Wang YF, Feng JY, Zhao LN, Zhao M, Wei XF, Geng Y, Yuan HF, Hou CY, Zhang HH, Wang GW, Yang G, Zhang XD. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-κB p65-activated SLC7A11 transcription. Acta Pharmacol Sin. 2023;44:1712-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 165. | Ricciotti E, Wangensteen KJ, FitzGerald GA. Aspirin in Hepatocellular Carcinoma. Cancer Res. 2021;81:3751-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 166. | Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 167. | Yeh CC, Lin JT, Jeng LB, Ho HJ, Yang HR, Wu MS, Kuo KN, Wu CY. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 168. | Miyazaki K, Morine Y, Xu C, Nakasu C, Wada Y, Teraoku H, Yamada S, Saito Y, Ikemoto T, Shimada M, Goel A. Curcumin-Mediated Resistance to Lenvatinib via EGFR Signaling Pathway in Hepatocellular Carcinoma. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 169. | Simasingha N, Tanasoontrarat W, Claimon T, Sethasine S. Efficacy of dexamethasone and N-acetylcysteine combination in preventing post-embolization syndrome after transarterial chemoembolization in hepatocellular carcinoma. World J Gastroenterol. 2023;29:890-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 170. | Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, Chun HJ, Jang JW, Bae SH, Choi JY, Yoon SK. Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Study. J Vasc Interv Radiol. 2017;28:1503-1511.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |