Published online Nov 27, 2023. doi: 10.4254/wjh.v15.i11.1226
Peer-review started: July 8, 2023
First decision: August 15, 2023
Revised: September 5, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 27, 2023
Processing time: 138 Days and 13.3 Hours
Rifaximin is frequently administered to critically ill patients with liver disease and hepatic encephalopathy, but patients currently or recently treated with antibiotics were frequently excluded from studies of rifaximin efficacy. Due to overlapping spectrums of activity, combination therapy with broad-spectrum antibiotics and rifaximin may be unnecessary. A pharmacist-driven protocol was piloted to reduce potentially overlapping therapy in critically ill patients with liver disease. It was hypothesized that withholding rifaximin during broad-spectrum antibiotic therapy would be safe and reduce healthcare costs.
To determine the clinical, safety, and financial impact of discontinuing rifaximin during broad-spectrum antibiotic therapy in critically ill liver patients.
This was a single-center, quasi-experimental, pre-post study based on a pilot pharmacist-driven protocol. Patients in the protocol group were prospectively identified via the medical intensive care unit (ICU) (MICU) protocol to have rifaximin withheld during broad-spectrum antibiotic treatment. These were compared to a historical cohort who received combination therapy with broad-spectrum antibiotics and rifaximin. All data were collected retrospectively. The primary outcome was days alive and free of delirium and coma (DAFD) to 14 d. Safety outcomes included MICU length of stay, 48-h change in vasopressor dose, and ICU mortality. Secondary outcomes characterized rifaximin cost savings and protocol adherence. Multivariable analysis was utilized to evaluate the association between group assignment and the primary outcome while controlling for potential confounding factors.
Each group included 32 patients. The median number of delirium- and coma-free days was similar in the control and protocol groups [3 interquartile range (IQR 0, 8) vs 2 (IQR 0, 9.5), P = 0.93]. In multivariable analysis, group assignment was not associated with a reduced ratio of days alive and free of delirium or coma at 14 d. The protocol resulted in a reduced median duration of rifaximin use during broad-spectrum antibiotic therapy [6 d control (IQR 3, 9.5) vs 1 d protocol (IQR 0, 1); P < 0.001]. Rates of other secondary clinical and safety outcomes were similar including ICU mortality and 48-h change in vasopressor requirements. Overall adherence to the protocol was 91.4%. The median estimated total cost of rifaximin therapy per patient was reduced from $758.40 (IQR $379.20, $1200.80) to $126.40 (IQR $0, $126.40), P < 0.01.
The novel pharmacist-driven protocol for rifaximin discontinuation was associated with significant cost savings and no differences in safety outcomes including DAFD.
Core Tip: Critically ill patients with liver disease receiving broad-spectrum antibiotic therapy have been frequently excluded from clinical trials of rifaximin efficacy. Therefore, despite overlapping spectrums of antibacterial activity, it is not known if rifaximin provides additional clinical benefit in these patients. In this study, pharmacist-guided rifaximin discontinuation during broad-spectrum antibiotic therapy resulted in significant cost savings and was not associated with negative short-term cognitive effects or adverse events.
- Citation: Ward JA, Yerke J, Lumpkin M, Kapoor A, Lindenmeyer CC, Bass S. Evaluation of a protocol for rifaximin discontinuation in critically ill patients with liver disease receiving broad-spectrum antibiotic therapy. World J Hepatol 2023; 15(11): 1226-1236
- URL: https://www.wjgnet.com/1948-5182/full/v15/i11/1226.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i11.1226
Hepatic encephalopathy (HE) encompasses a spectrum of neurocognitive alterations in patients with liver dysfunction and/or porto-systemic shunting, and is associated with symptoms that range in severity from minimal neuropsychiatric manifestations to cerebral edema and coma in the most severe cases[1,2]. The pathogenesis of HE has not been fully elucidated and is likely multifactorial. Ammonia has been implicated as a contributing factor to the development of HE due to its association with direct neurotoxicity mediated by astrocyte swelling and modification of glutamine and gamma-amino-n-butyric acid systems[3]. Rifaximin is an oral, non-absorbable rifamycin derivative with antibiotic activity against ammonia-producing gram-positive, gram-negative, and anaerobic species[4,5]. Long-term rifaximin use is associated with clinically important reductions in infections, hospital re-admissions, durations of hospital stays, and overt HE recurrence[6-8]. The exact mechanism by which rifaximin exerts benefit remains unclear[9]. Previous hypotheses focused on the control of ammonia-producing enteric bacteria via antibiotic activity[10,11]. However, a growing body of evidence depicts increasingly understood mechanisms of rifaximin activity including decreased circulating endotoxin burden, decreased microbiota-derived systemic inflammation, and improvement in cirrhosis-related dysbiosis which suggests the presence of multi-factorial benefits of rifaximin in the pathobiology of cirrhosis[12]. Rifaximin is recommended by the American Association for the Study of Liver Diseases (AASLD) as adjunctive therapy for the prevention of overt HE recurrence (grade I, A, 1)[13]. Similarly, the European Association for the Study of the Liver (EASL) recommends rifaximin as an adjunct to lactulose as secondary prophylaxis following ≥ 1 additional episodes of overt HE within 6 mo of the first episode (LoE 2, strong)[14]. Use of rifaximin for the treatment of HE is not recom
Patients receiving or recently treated with antibiotics were frequently excluded from these trials[15-17]. These studies, which included patients with both acute and chronic HE, excluded cases with precipitants or recent medication exposures which could interfere with HE or therapeutic effect monitoring. Infection is a frequent precipitant of overt HE in critically ill patients, the treatment of which commonly relies on broad-spectrum antibiotics[19]. In many cases of infection, patients also receive rifaximin either as a continuation of home therapy or newly initiated treatment. Because these patients are infrequently studied, it remains unclear if rifaximin provides additional therapeutic benefit when combined with broad-spectrum antibiotics.
In 2018, Cleveland Clinic established the medical intensive liver unit (MILU), a specialty unit of the MICU supported by a multidisciplinary team including intensivists, hepatologists, and critical care clinical pharmacy specialists caring for patients with a variety of hepatic pathologies. Many patients admitted to the MILU are initiated on broad-spectrum antibiotics for empiric or targeted therapy for infections in addition to HE treatment with rifaximin. A pharmacist-driven pilot protocol was implemented to reduce potentially overlapping therapy through the discontinuation of rifaximin during broad-spectrum antibiotic treatment. Pharmacists were also responsible for the coordination of rifaximin re-initiation following antibiotic therapy narrowing or discontinuation. This study aimed to evaluate the impact of this protocol on clinical, safety, and financial outcomes.
This was an Institutional Review Board-approved, quasi-experimental, pre-post study conducted at a large quaternary academic medical and liver transplant center in the United States. The pharmacist-driven protocol for rifaximin discontinuation was implemented beginning August 1, 2020. Adult patients in the medical intensive care unit (ICU) (MICU) were prospectively screened by clinical pharmacy specialists and eligible for the protocol if they had orders for rifaximin and a qualifying antibiotic regimen (Table 1). Discontinuation of rifaximin was recommended and recorded by the pharmacist and research team. Duration of antibiotic therapy was tracked and reviewed daily by a small group of critical care clinical pharmacy specialists to ensure re-initiation of rifaximin upon antibiotic narrowing or discontinuation. Before implementation, education was provided to all pharmacists who would manage or verify orders for MICU patients to reduce time to rifaximin discontinuation for patients admitted to the MICU during evenings and weekends. Physician leadership and medical teams were also involved in education about the protocol and its implementation.
| Monotherapy | Gram-positive/negative | Gram-positive | Gram-negative | Anaerobic |
| Ampicillin-sulbactam | Cefazolin | Vancomycin | Aminoglycosides | Metronidazole |
| Amoxicillin-clavulanate | Cephalexin | Linezolid | Polymyxin B and colistin | Clindamycin |
| Piperacillin-tazobactam | Cefuroxime | Daptomycin | Aztreonam | |
| Cefoxitin | Cefdinir | Quinupristin-dalfopristin | Cefiderocol | |
| Meropenem +/- vaborbactam | Cefixime | Rifampin | ||
| Imipinem-cilastatin +/- relebactam | Ceftazidime +/- avibactam | Rifabutin | ||
| Ertapenem | Ceftriaxone | Penicillins | ||
| Tigecycline | Cefepime | |||
| Eravacycline | Ceftaroline | |||
| Ceftolozane-tazobactam | ||||
| Ciprofloxacin | ||||
| Levofloxacin | ||||
| Doxycycline | ||||
| Sulfamethoxazole-trimethoprim |
Patients[3] 18 years old were eligible for study inclusion if they received at least 3 d of broad-spectrum antibiotics and had an order for rifaximin during MICU admission. Additional inclusion criteria for the control group were: (1) Admission to the MICU between August 1 and October 31, 2019; and (2) rifaximin therapy for ≥ 3 d or 75% of the antibiotic treatment duration during MICU admission, whichever was longer. In the protocol group inclusion criteria were (1) admission to the MICU between August 1, 2020 and January 31, 2021; and (2) ≥ 3 d of broad-spectrum antibiotics without rifaximin and concomitant rifaximin for ≤ 25% of the antibiotic duration during MICU admission. Any patient with a positive test for severe acute respiratory syndrome coronavirus 2 during admission was excluded.
The primary outcome was days alive and free of delirium and coma (DAFD) to day 14. Secondary outcomes were days alive and free of delirium to day 14, days alive and free of coma to day 14, ICU length of stay, ICU mortality, time to first extubation, rate of reintubation, days of combination therapy during MICU admission, rate of protocol adherence, time to rifaximin discontinuation in the protocol group, and the per-patient cost of rifaximin therapy during the follow-up period. The cost of rifaximin therapy was calculated using the average wholesaler price as of January 2023 to reflect the increase in rifaximin cost since initial protocol implementation[20]. The minimum cost of rifaximin was calculated based on one tablet given (control) or saved (protocol) per day of therapy while the maximum cost assumed two tablets given or saved per day. Changes in vasopressor requirements and Glasgow Coma Score (GCS) during the first 48 h of MICU combination therapy or withholding rifaximin were evaluated as additional safety measures.
Day one for the 14-d study period was defined as the first day during MICU admission on which patients received broad-spectrum antibiotics and rifaximin (control group) or broad-spectrum antibiotics without rifaximin (protocol group). Broad-spectrum antibiotic regimens were defined as providing gram-positive, gram-negative, and anaerobic coverage (Table 1). A day of therapy was defined as a 24-h period from midnight to 11:59 pm during which at least one-half of the scheduled doses of rifaximin and/or broad-spectrum antibiotics were received. Days were considered delirium-free if patients were alive and without a positive confusion assessment method for the ICU (CAM-ICU) assessment during the 24-h period and coma-free if patients were alive and with zero hours spent with a Richmond Agitation Sedation Scale (RASS) score of -4 or -5 or with GCS of 3 during the 24-h period. Days of mechanical ventilation were defined as the use of positive pressure ventilation during any one hour of the 24-h period from midnight to 11:59 pm for use in the multivariable model. All admission days in non-ICUs during which the patient was alive were considered to be free of delirium as brief CAM (bCAM) and West-Haven grades (WHG) were not routinely recorded. Protocol adherence was defined as the discontinuation of rifaximin occurring within 72-h of protocol-defined broad-spectrum antibiotic therapy initiation. All vasopressor doses were converted to norepinephrine equivalents according to the following equation: [norepinephrine (mcg/min) + (epinephrine (mcg/min)] + [(dopamine (mcg/kg/min) ÷ 2] + [(phenylephrine (mcg/min) ÷ 10] + [vasopressin (units/hour) × 8.33][21,22]. Sedative agents included propofol, dexmedetomidine, ketamine, lorazepam, or midazolam when administered as a continuous infusion. Ileus was defined as > 48 h with zero bowel movements or fecal management system output recorded. Orders for octreotide continuous infusions were used as a surrogate to identify episodes of gastrointestinal bleeding according to routine institutional practice. Occurrences of Clostridioides difficile infection were recorded if the patient had either a positive polymerase chain reaction or enzyme-linked immunosorbent assay test.
Continuous data were assessed for normality by the Shapiro-Wilk test. Parametric continuous data are reported as mean ± SD and were analyzed using a two-sample t-test. Non-parametric continuous data are reported as median (IQR) and were analyzed using the Wilcoxon rank-sum test. Categorical data are presented as number (%) and were analyzed by chi-squared or Fisher’s exact tests, based on sample size. The primary outcome of DAFD was compared using a one-sided Wilcoxon rank-sum test, assuming greater median DAFD in the control group. It was calculated that the inclusion of 32 patients in each group would provide 80% power to detect a 0.65-d difference in DAFD, with a one-sided significance level of 0.025. Multivariable analysis of the primary outcome was planned to include covariates of biologic plausibility (duration of mechanical ventilation, use of deep sedation, MELD-Na score, gastrointestinal bleeding) and those with a P-value of < 0.05 in univariable analysis. A negative binomial model was selected for the multivariable analysis to account for the observed over-dispersion of the primary outcome which violated foundational assumptions of a Poisson distribution that was attempted after similar violations of linear regression despite log-transformation of the variables. All variables included in the model were selected based on prior literature and biological plausibility to contribute to or interfere with the assessment of delirium and coma or to indicate a clinically significant baseline difference in illness severity between the groups. All analyses were performed based on an overall significance level of 0.05 using either SAS software (version 9.4, Cary, NC) or Stata/IC software, v.14 (StataCorp LP, College Station, TX). Data extracted from the electronic medical record were collected and managed using REDCap electronic data capture tools hosted by Cleveland Clinic[23,24].
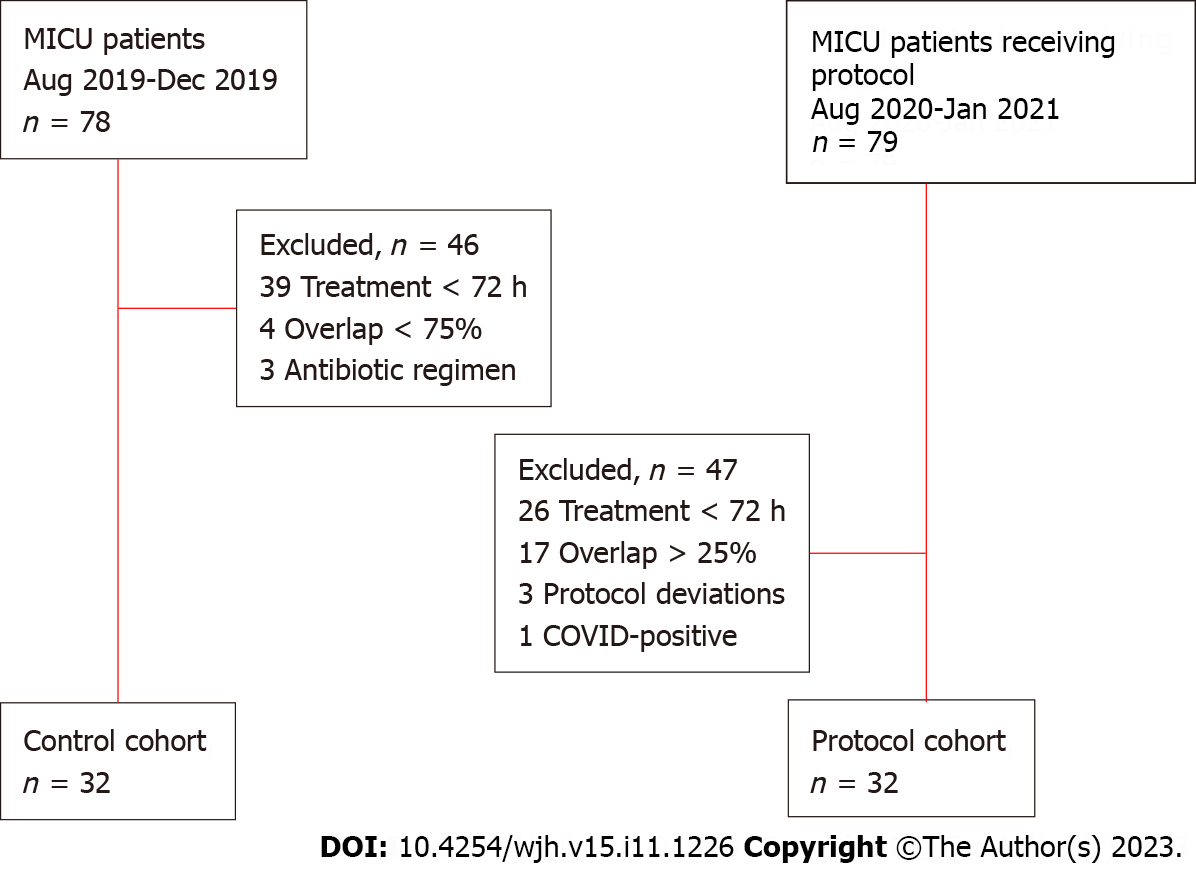
A total of 159 patients were screened for inclusion and 32 were included in both groups (Figure 1). The most common reason for exclusion in both groups was insufficient treatment duration. The two groups were well-balanced at baseline with the exception of norepinephrine requirements on the day of MICU admission, which were higher in the protocol group, and race (Table 2). There was no difference in high-grade HE at baseline; however, due to intubation and sedation on study day 1, many patients were unable to be assigned baseline WHG. Though none of these differences were statistically significant, deep sedation, paralysis, and gastrointestinal bleeding were more common among protocol patients while control patients more often received scheduled benzodiazepines and were admitted directly to the ICU.
| Variable | Control (n = 32) | Protocol (n = 32) | P value |
| Male | 19 (59.4) | 18 (56.2) | 0.21 |
| Age (yr) | 57.9 (± 12.9) | 53.0 (± 12.7) | 0.13 |
| Race | 0.01 | ||
| White | 26 (81.3) | 24 (75) | |
| Unavailable | 4 (12.5) | 2 (6.3) | |
| Black | 1 (3.1) | 1 (3.1) | |
| American Indian/Alaskan native | 1 (3.1) | 0 (0) | |
| Asian | 0 (0) | 0 (0) | |
| Multiracial-Multicultural | 0 (0) | 0 (0) | |
| Weight (kg) | 85.4 (± 23.1) | 91.3 (± 29.2) | 0.37 |
| Direct Intensive Care Unit Admission | 15 (46.9) | 12 (37.5) | 0.45 |
| SOFA score | 10.2 (± 3.0) | 11 (± 3.2) | 0.34 |
| NE requirements, mcg/kg/min | 0 (0, 0.136) | 0.023 (0, 0.309) | 0.02 |
| MELD-Na | 28.5 (± 8.5) | 30.0 (± 8.2) | 0.48 |
| West-Haven grade | 0.31 | ||
| Unavailable | 10 (31.3) | 6 (18.8) | |
| 0 | 6 (18.8) | 8 (25) | |
| 1 | 6 (18.8) | 6 (18.8) | |
| 2 | 4 (12.5) | 6 (18.8) | |
| 3 | 5 (15.6) | 5 (15.6) | |
| 4 | 1 (3.1) | 1 (3.1) | |
| Glasgow Coma Score | 9 (6, 14) | 11 (7, 15) | 0.69 |
| Cirrhosis Etiology | 0.21 | ||
| Ethanol | 17 (53.1) | 21 (65.6) | |
| Non-alcoholic steatohepatitis | 9 (28.1) | 5 (15.6) | |
| Primary biliary cholangitis | 2 (6.3) | 1 (3.1) | |
| Autoimmune hepatitis | 2 (6.3) | 1 (3.1) | |
| Primary sclerosing cholangitis | 0 (0) | 1 (3.1) | |
| Unknown | 2 (6.3) | 0 (0) | |
| Other | 0 (0) | 3 (9.4) | |
| Pre-ICU rifaximin treatment | 12 (37.5) | 17 (48.6) | 0.21 |
| Antibiotic Indication | 0.80 | ||
| Empiric; source unknown | 20 (62.5) | 23 (71.9) | |
| Pneumonia | 6 (18.8) | 3 (9.4) | |
| Intraabdominal | 4 (12.5) | 4 (12.5) | |
| Bloodstream infection | 1 (3.1) | 1 (3.1) | |
| Skin and soft tissue | 1 (3.1) | 0 (0) | |
| Bone and joint infection | 0 (0) | 1 (3.1) | |
| Rifaximin regimen | 0.37 | ||
| 550 mg BID | 31 (96.9) | 31 (96.9) | |
| 400 mg BID | 1 (3.1) | 0 (0) | |
| 200 mg BID | 0 (0) | 1 (3.1) | |
| Deep sedation use | 5 (15.6) | 9 (28.1) | 0.38 |
| Benzodiazepine use | 3 (9.4) | 0 (0) | 0.29 |
| Continuous neuromuscular blockade use | 2 (6.3) | 3 (9.4) | 1.00 |
| Polyethylene glycol use | 9 (28.1) | 13 (40.6) | 0.29 |
| Lactulose use | 29 (90.6) | 31 (96.9) | 0.30 |
| Gastrointestinal bleeding treatment | 8 (25) | 14 (43.8) | 0.11 |
| Alcohol withdrawal diagnosis | 1 (3.1) | 0 (0) | 1.00 |
| Ileus | 6 (18.8) | 9 (28.1) | 0.56 |
| C. difficile infection | 1 (3.1) | 2 (6.3) | 1.00 |
No significant differences were observed in the primary outcome (DAFD to day 14), between the control and protocol groups [3 (interquartile range (IQR 0, 8) vs 2 (IQR 0, 9.5); P = 0.93] (Table 3). After adjustment for deep-sedation, gastrointestinal bleeding treatment, MELD-Na score, and duration of mechanical ventilation in a negative binomial regression there remained no significant difference in the primary outcome between the control and protocol groups (ratio 0.78, 95% confidence interval 0.39-1.56, P = 0.48) (Table 4).
| Outcome | Control (n = 32) | Protocol (n = 32) | P value |
| Primary outcome | |||
| Days alive and free of delirium and coma to day 14 | 3 (0, 8) | 2 (0, 9.5) | 0.93 |
| Secondary outcomes | |||
| Days alive and free of delirium | 3 (0, 8.5) | 2 (0, 9) | 0.85 |
| Days alive and free of coma (RASS) | 6 (3.5, 12) | 8 (4, 11) | 0.81 |
| Days alive and free of coma (GCS) | 8 (5.5, 13) | 7.5 (5.5, 9) | 0.21 |
| Glasgow Coma Score 48-h change | 0 (-3, 1.5) | 0 (-1, 0.5) | 0.43 |
| ICU mortality (%) | 13 (40.6) | 15 (46.9) | 0.61 |
| ICU length of stay | 10 (4.5, 20.5) | 11 (7, 17) | 0.73 |
| Time to first extubation from day 1 of intubation | 6 (4, 14) (n = 23) | 5 (4, 9) (n = 26) | 0.49 |
| Rate of reintubation (%) | 4 (17.4) | 3 (11.5) | 0.56 |
| IVVP requirement 48-h change, NE mcg/kg/min equivalents | 0 (0, 0.12) | 0 (0, 0.01) | 0.45 |
| Days of MICU combination therapy | 6 (3, 9.5) | 1 (0, 1) | < 0.01 |
| Protocol adherence | - | 91.4% | - |
| Time to rifaximin discontinuation, days | - | 1 (0, 1) | - |
| Cost of rifaximin therapy per patient to day 14, USD | |||
| Minimum | 379.20 (189.60, 600.40) | 63.20 (0, 63.20) | < 0.01 |
| Maximum | 758.40 (379.20, 1200.80) | 126.40 (0, 126.40) | < 0.01 |
| (n = 60) | DAFD ratio (95%CI) |
| Group assignment (protocol) | 0.78 (0.39, 1.56) |
| Deep sedation (yes) | 0.89 (0.39, 2.02) |
| Gastrointestinal bleeding (yes) | 0.65 (0.32, 1.31) |
| MELD-Na (per unit increase) | 0.97 (0.94, 1.01) |
| Mechanical ventilation duration (per day) | 0.79 (0.72, 0.87) |
The observed ICU mortality rate was high in both groups [control 13 (40.6%) vs protocol 15 (46.9%); P = 0.61]. Days alive and free of either delirium or coma, ICU length of stay, and time to extubation were similar between the groups. Similarly, no significant differences in vasopressor requirements and GCSs in the 48 h following rifaximin discontinuation were observed between groups (Table 3). For patients included in the protocol group, the median time to rifaximin discontinuation was approximately 24 h from MICU admission. Protocol adherence was 91.4% with the most common reason for non-adherence being rifaximin discontinuation during antibiotic therapy not meeting the protocol definition of broad-spectrum. Days of rifaximin therapy during broad-spectrum antibiotics were significantly reduced in the protocol group [6 (IQR 3-9.5) vs 1 (IQR 0-1); P < 0.001]. The cost of rifaximin therapy was also significantly reduced in the protocol group with an estimated cost savings of $316.00 [United States dollar (USD)] to $632.00 (USD) per patient.
The addition of rifaximin to broad-spectrum antibiotic therapy may provide overlapping antibacterial activity without additional therapeutic benefit in critically ill patients with HE. Notably, patients on broad-spectrum antibiotics have been generally excluded from studies of rifaximin efficacy in HE. This gap in the literature represents a need to better understand the role of rifaximin in this unique patient population, as ICU hospitalizations for patients with HE are typically characterized by severe disease and increased morbidity and mortality. This study is the first to evaluate the feasibility and safety of rifaximin discontinuation during broad-spectrum antibiotic therapy.
In our pilot investigation, rifaximin discontinuation during broad-spectrum antibiotic therapy in critically ill patients with liver disease was not associated with higher rates of delirium or coma. This result was robust to adjustment in multivariable analysis. As demonstrated in Table 3, no negative associations were observed between rifaximin discontinuation and short-term cognitive outcomes. Neither was rifaximin discontinuation associated with increased adverse effects, which adds support to the hypothesis that withholding rifaximin during broad-spectrum antibiotic therapy is safe. The lack of observed differences in cognitive outcomes is an important contribution to the existing understanding of the interaction between treatment with rifaximin and other broad-spectrum antibiotics.
Much of the promising data for the benefit of rifaximin therapy in patients with cirrhosis, including reduced infections and hospitalizations, have been produced in the outpatient setting and reflect chronic use (> 30 d in most cases)[6-8]. In the present study, rifaximin was held for a limited time in hospitalized, critically ill patients during concomitant broad-spectrum antibiotic treatment. Few trials of rifaximin efficacy have included or focused on a critically ill population, however, the very limited data available suggest potential for harm with rifaximin discontinuation[19,25,26]. Sharma et al[18] published the largest single cohort evaluating rifaximin efficacy including critically ill patients. The authors reported a decrease in mortality among patients treated with rifaximin and lactulose compared with those treated with lactulose and placebo. Importantly, this finding is limited by the high rate of sepsis-related mortality in the placebo group. In contrast to the study by Sharma et al[18], all patients included in the present study had known or suspected infection but observed mortality rates were similar among patients who continued rifaximin during broad-spectrum antibiotic therapy and those who did not. In an open-label single-center study including 15 patients, Kalambokis et al[24] demonstrated that rifaximin therapy was associated with increased systemic vascular resistance after four weeks. This finding raised a question about the potential impact sudden discontinuation of therapy might have in critically ill patients predisposed to clinically significant vasodilation exacerbated in the setting of active infection. To address this question, vasopressor requirements in the first 48-h after rifaximin discontinuation (protocol) or antibiotic initiation (control) were compared. In the current larger cohort, rifaximin discontinuation was not associated with changes in vasopressor requirements despite severe and progressive illness in many included patients. Finally, in a single-center retrospective cohort analysis of mechanically ventilated critically ill patients with decompensated cirrhosis, rifaximin administration within the first 24-h post-intubation was associated with shorter time to extubation [hazard ratios 1.74 (1.21-2.50)], although pre-intubation rifaximin and lactulose administration was associated with a delayed time to extubation[26]. Rifaximin discontinuation was not associated with delays in extubation or rates of reintubation in the present study, though the sample size was smaller. In summary, although several studies in critically ill patients had demonstrated potential associations with mortality, vasopressor requirements, and duration of mechanical ventilation, the current study evaluated several potential safety concerns in a highly vulnerable patient population and did not reveal any negative signals associated with rifaximin discontinuation.
Notably, the present study demonstrates the feasibility and benefit of a pharmacist-driven, manually applied protocol with multidisciplinary support aimed at antimicrobial stewardship in a critically ill population. Though likely to be applicable to many centers in the United States, opportunities to optimize protocol execution exist including streamlining patient identification and enrollment, minimizing delays in rifaximin discontinuation, and ensuring rifaximin re-initiation after broad-spectrum antibiotic therapy completion or narrowing.
Several limitations exist within this evaluation. First, this was a single-center study with retrospective data collection. Second acute (overt) HE was not a requirement for inclusion, nor was the chronicity of HE episodes able to be quantified. Patients were stratified according to WHG and/or GCS in order to describe clinical status and align with available guideline recommendations for HE assessment. Similarly, it was not possible to definitively identify the specific indication for rifaximin for every patient nor to confirm the prescription of rifaximin prior to hospital admission with the limited available insurance claim records. Though based on available records, pre-hospital rifaximin therapy was prescribed at a similar rate in both the control and protocol groups, 37.5% vs 48.6%, respectively. Third, the high frequency of missing WHG due to retrospective clinical assessment in the setting of critical illness requiring mechanical ventilation and sedation necessitated the use of multiple surrogate endpoints. The primary outcome of DAFD was selected based on previously published studies evaluating critically ill patients’ level of awareness[27,28]. While CAM scoring is routine in ICUs at our institution, delirium assessments (i.e. bCAM) are not routine in non-ICU care areas. The decision to consider all patients in non-ICU care areas delirium-free was based on an understanding of the clinical improvements required to support ICU discharge and the lack of routinely available validated scores to collect for the endpoint. This may have led to an over-estimation of the days free of delirium, however, this is likely balanced by the high average proportion of time spent in the ICU compared to non-ICU care areas. The 48-h change in GCS was also collected as a sensitive marker for any negative cognitive effect rifaximin discontinuation may have exerted. GCS was selected as current AASLD guidelines recommend this score as an alternative measure to the WHG for the diagnosis of HE (grade II-2, B, 1)[13]. The EASL guidelines recommend the addition of GCS to West-Haven criteria in patients with impaired consciousness, including those treated in an ICU (LoE 5, strong)[14]. Several outcomes of interest were unable to be assessed due to the absence of baseline WHG including the achievement and time to resolution or improvement of HE. Despite a robust effort to characterize the patient population and describe the severity and extent of illness, there are illness-specific variables and outcomes that were unable to be feasibly assessed, including indication for MICU admission and infection resolution which may have contributed to cognitive and clinical outcomes. Additionally, this study was not designed to evaluate long-term outcomes or impact of withheld rifaximin therapy. Despite these limitations, no differences were found in the available and utilized markers of cognitive outcomes between patients who did or did not have rifaximin discontinued during broad-spectrum antimicrobial therapy. Finally, the few baseline differences observed in the two cohorts may have been smaller or eliminated in a larger sample size. However, the authors anticipate the strong left skew in the primary outcome with a predominance of zero or minimal days spent alive and free of delirium and coma to persist, even in a larger sample, given the tenuous nature of critically ill patients with liver disease. Similarly, rates of HE resolution in a comparable population would be expected to be very low. These data characteristics would likely render future non-inferiority trials difficult or impossible to complete.
In conclusion, no significant differences were noted between the control and protocol groups in key clinical or safety outcomes. The robustness of the primary outcome to multivariable analysis strengthens the conclusion that rifaximin discontinuation during broad-spectrum antibiotic therapy does not appear to negatively impact the cognitive status of critically ill liver patients. This study demonstrates the feasibility of a pharmacist-driven protocol to reduce combination therapy in critically ill patients with liver disease treated with rifaximin and broad-spectrum antibiotics. Given the significant cost savings achieved during ICU and hospital admission, a prospective, multi-center evaluation of a similar protocol in a larger sample is warranted, including investigation into longer-term outcomes. Investigation of the impact of this type of protocol in non-critically ill liver patients should likewise be considered.
Rifaximin is frequently administered to critically ill patients with liver disease and hepatic encephalopathy (HE). However, data supporting the use of rifaximin in this population, particularly in combination with broad-spectrum antibiotics, are extremely limited. Due to the overlapping spectrums of antibiotic activity, it was hypothesized that withholding rifaximin during broad-spectrum antibiotic therapy would be safe and reduce healthcare costs. The present study is the first to evaluate the feasibility and safety of rifaximin discontinuation during broad-spectrum antibiotic therapy and represents a highly vulnerable patient population.
The gap in available evidence demonstrates the need to better understand the role of rifaximin in this unique population, as intensive care unit (ICU) hospitalizations for patients with HE are typically characterized by severe disease and increased morbidity and mortality. Therefore, after protocol development the need to assess clinical and safety outcomes was clear. Additionally, given the opportunity to reduce healthcare expenditures with decreased use of rifaximin during ICU admission, costs of therapy were quantified. This proof-of-concept evaluation also provides a foundation for future, larger-scale, well-controlled studies to confirm and expand on the findings.
The present study aimed to evaluate the safety, efficacy, and financial impact of discontinuing rifaximin during broad-spectrum antibiotic use. The efficacy of withholding rifaximin was evaluated using a surrogate marker for short-term cognitive impact, days alive and free of delirium and coma. Multiple, robust safety outcomes were considered including mortality, ICU length of stay, 48-h change in vasopressor requirements, duration of mechanical ventilation, and successful extubation. Cost avoidance was evaluated by comparing rifaximin drug costs during the observation period pre- and post-protocol. The outcomes utilized provided an initial, comprehensive assessment of the pilot protocol that could be replicated in further investigations.
This was a single-center, quasi-experimental study evaluating outcomes pre- and post-implementation of a pharmacist-driven protocol for rifaximin discontinuation in critically ill liver patients being treated in a medical ICU. To address potential sources of bias, multivariable analysis of the primary outcome was performed with characteristics selected based on biological plausibility and univariate screening. Inferential statistics were performed in the usual fashion based on data type and distribution. The study achieved 80% power to detect a 0.65 d difference in the primary outcome.
In this pilot investigation, rifaximin discontinuation during broad-spectrum antibiotic therapy in critically ill patients with liver disease was not associated with more days of delirium or coma [3 (0, 8) vs 2 (0, 9.5); P = 0.93]. Protocol application was associated with a high rate of adherence (91.4%) and resulted in a significant reduction in days of combination therapy [6 (3-9.5), 1 (0-1); P < 0.001] and medication expenditures (estimated per patient cost avoidance $316.00 to $632.00 USD). No signals of harm were detected in any safety endpoint. The results of this study support the safety and feasibility of a protocolized discontinuation of rifaximin during broad-spectrum antibiotic therapy. Due to the limited sample size and retrospective nature of the present study, future evaluations should prioritize larger sample sizes and prospective designs to the greatest extent possible. Many questions remain regarding the optimal use of rifaximin among patients being treated with broad-spectrum antibiotics, including non-critically ill patients and those receiving long courses of therapy.
This was a novel evaluation that provides new insight about the potential safety of discontinuing rifaximin during short-term, broad-spectrum antibiotic therapy in critically ill patients with liver disease which has not yet been investigated in the literature. The safety, efficacy, and cost-saving results of this study warrant confirmation in an investigation with a larger sample size and prospective, well-controlled methods which could lead to broader application of a similar protocol. Finally, this study provides further support that pharmacists may be leveraged to assist with antimicrobial stewardship efforts in specific and dynamic patient populations.
Future research related to this question should focus on: Confirmation of the reported findings; longer-term outcomes of withholding rifaximin therapy, particularly during prolonged courses of broad-spectrum antibiotics; the impact of a similar protocol among non-critically ill patients; and opportunities to optimize protocol application.
The authors would like to thank the leadership of the medical ICU and medical intensive liver unit for their support in the implementation and review of this protocol. The authors would also like to thank Ms. Lu Wang for her support with statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Virovic-Jukic L, Croatia S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 867] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 2. | Nicoletti V, Gioia S, Lucatelli P, Nardelli S, Pasquale C, Nogas Sobrinho S, Pentassuglio I, Greco F, De Santis A, Merli M, Riggio O. Hepatic encephalopathy in patients with non-cirrhotic portal hypertension: Description, prevalence and risk factors. Dig Liver Dis. 2016;48:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity--a review. Chemotherapy. 2005;51 Suppl 1:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Volk ML, Burne R, Guérin A, Shi S, Joseph GJ, Heimanson Z, Ahmad M. Hospitalizations and healthcare costs associated with rifaximin versus lactulose treatment among commercially insured patients with hepatic encephalopathy in the United States. J Med Econ. 2021;24:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Salehi S, Tranah TH, Lim S, Heaton N, Heneghan M, Aluvihare V, Patel VC, Shawcross DL. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol Ther. 2019;50:435-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | De Graeve J, Vanderstraeten E, Delvaeye T, De Lepeleere S, De Somer T, Van Steenkiste C. The impact of rifaximin on the hospital burden and infections in patients with hepatic encephalopathy: a retrospective observational study. Acta Gastroenterol Belg. 2022;85:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Wright G, Jalan R. Management of hepatic encephalopathy in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:95-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Munoz SJ. Hepatic encephalopathy. Med Clin North Am. 2008;92:795-812, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Patel VC, Lee S, McPhail MJW, Da Silva K, Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons N, Galleron N, Huang X, Gencer S, Coen M, Tranah TH, Wendon JA, Bruce KD, Le Chatelier E, Ehrlich SD, Edwards LA, Shoaie S, Shawcross DL. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol. 2022;76:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1407] [Article Influence: 127.9] [Reference Citation Analysis (1)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 220] [Article Influence: 73.3] [Reference Citation Analysis (1)] |
| 15. | Loguercio C, Federico A, De Girolamo V, Ferrieri A, Del Vecchio Blanco C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Gastroenterol Dietol. 2003;49:53-62. [PubMed] |
| 16. | Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I, Pardo A; Spanish Association for the Study of the Liver Hepatic Encephalopathy Cooperative Group. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Paik YH, Lee KS, Han KH, Song KH, Kim MH, Moon BS, Ahn SH, Lee SJ, Park HJ, Lee DK, Chon CY, Lee SI, Moon YM. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Rifaximin. In: Lexi-Drugs Online. Up to Date, Inc. Jan 15, 2022. [cited 30 January, 2022]. Available from: https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7630?cesid=5MCoLonRAKR&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Drifaximin%26t%3Dname%26acs%3Dfalse%26acq%3Drifaximin#monograph-tab-content. |
| 21. | Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1182] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 22. | Ensor CR, Sabo RT, Voils SA. Impact of Early Postoperative Hydrocortisone Administration in Cardiac Surgical Patients After Cardiopulmonary Bypass. Ann Pharmacother. 2011;45:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36729] [Article Influence: 2295.6] [Reference Citation Analysis (0)] |
| 24. | Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 14627] [Article Influence: 2437.8] [Reference Citation Analysis (0)] |
| 25. | Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Gibbs JT, Louissaint J, Tapper EB. Rate of Successful Extubation in Mechanically Ventilated Patients with Cirrhosis and Hepatic Coma. Dig Dis Sci. 2022;67:5336-5344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ, Khan B, Pisani MA, Hyzy RC, Schmidt GA, Schweickert WD, Hite RD, Bowton DL, Masica AL, Thompson JL, Chandrasekhar R, Pun BT, Strength C, Boehm LM, Jackson JC, Pandharipande PP, Brummel NE, Hughes CG, Patel MB, Stollings JL, Bernard GR, Dittus RS, Ely EW; MIND-USA Investigators. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med. 2018;379:2506-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 28. | Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |