Published online Nov 27, 2023. doi: 10.4254/wjh.v15.i11.1188
Peer-review started: July 23, 2023
First decision: September 15, 2023
Revised: October 23, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 27, 2023
Processing time: 123 Days and 20.5 Hours
Classical Philadelphia-negative myeloproliferative neoplasms (MPNs), i.e., polyc
Core Tip: The JAK inhibitor ruxolitinib has been approved for the treatment of classical Philadelphia-negative myeloproliferative neoplasms (MPNs), i.e., polycythemia vera, essential thrombocythemia and primary/secondary myelofibrosis. However, its use has been associated with a potential risk of opportunistic infections, including hepatitis B reactivation. Herein, we briefly overview the association between ruxolitinib treatment in MPNs and hepatitis B reactivation.
- Citation: Adesola AA, Cozma MA, Chen YF, Srichawla BS, Găman MA. Risk of hepatitis B reactivation in patients with myeloproliferative neoplasms treated with ruxolitinib. World J Hepatol 2023; 15(11): 1188-1195
- URL: https://www.wjgnet.com/1948-5182/full/v15/i11/1188.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i11.1188
Hepatitis B virus (HBV) infection is the most common chronic viral infection in the world. It affects more than 350 million people worldwide as chronic carriers, and more than 2 billion (30% of the world’s population) people show evidence of past exposure. Additionally, HBV infection has accounted for roughly half of total liver cancer mortality in 2010[1,2]. Once contacted, the virus cannot be eliminated, even with proper and rapid antiviral treatment, but the infection is self-limiting in more than 95% of immunocompetent adults. These patients are now known as carriers 'anti-HBc positive'. They do not require specific management or monitoring unless immunosuppression is suspected[3].
If HBV persists for more than 6 mo in the body, the affected individual is considered to have chronic hepatitis B. Its incidence depends on the time of exposure: 95% of newborns, 20%-30% of children aged 1 to 5 years, and less than 5% of adults[3]. The reason for this dormant state of HBV is the presence of covalently closed circular viral DNA (cccDNA) that penetrates and persists indefinitely in hepatocyte DNA[2-4]. This cccDNA acts as a template for future viral components in the case of HBV reactivation (HBVr). Viral transmission has been greatly slowed recently by the advent of a safe and effective vaccine, available since 1981 and introduced in 2011 in routine vaccination schedules in more than 180 countries[1,5].
The number of cases of HBVr after treatment with immunosuppressive agents is increasing worldwide, mostly attributed to an increase in the prevalence of positive HBV serology and, at the same time, an increase in the number of clinical indications for potent immunosuppression, including solid malignancies, inflammatory bowel disease, autoimmune disorders, blood cancers, e.g. myeloproliferative neoplasms (MPNs), and rheumatic diseases[3].
There are, although very similar, several definitions of HBVr, proposed by several medical associations from around the globe. All of them take into account both virological and serological criteria and describe HBVr as either an exacerbation of chronic hepatitis B or a reactivation of past hepatitis B infection. The most used definition is the one proposed by the American Association for the Study of Liver Diseases, last updated in 2020, which defines HVBr according to the virological status of the patient[4,6-8].
For HBsAg-positive patients with or without detectable HBV DNA: (1) At least 2 Log (or 100-fold) increase in HBV DNA compared to the baseline level; (2) HBV DNA at least 3 Log (or 1000) IU/mL in patients with previously undetectable HBV DNA; or (3) HBV DNA at least 4 Log (or 10000) IU/mL if the baseline level is unavailable[4,6-8].
For patients with HBsAg negative and HBV DNA negative: (1) HBV DNA becomes detectable; or (2) reverse HBsAg seroconversion (reappearance of HBsAg)[4,6-8].
The natural history of HBVr depends, among others, on the underlying disease requiring immunosuppressives, host immunity and the immunosuppressive agents used. Evolution can be classified into multiple stages[4,6-8].
After the initiation of immunosuppressive therapy, viral replication resumes, leading to a gradual increase in serum HBV DNA levels. The patient is still asymptomatic and, in general, HBVr-related hepatitis, described as an increase in alanine transaminase (ALT) or aspartate transaminase (AST) to 3 times upper limit of normal (ULN), does not develop[4,6-8].
ALT or AST increases to ≥ 3 times ULN (in some cases between 5-10 times ULN). Although most patients may remain asymptomatic, a small number might experience constitutional symptoms, such as pain in the right upper quadrant and jaundice. In rare cases, hepatic injury could further progress and cause liver failure, fulminant hepatitis or even death[4,6-8].
Normalization of serum ALT and AST levels, due to completion of immunosuppressive therapy, due to antiviral therapy, or due to host immunological mechanisms[4,6-8].
Found in a small number of individuals who continue to have a progressive decline in liver function, it is characterized by increased levels of bilirubin, prolonged prothrombin time, and, in very rare cases, even signs and symptoms of acute liver failure and hepatic decompensation (ascites and encephalopathy)[4,6-8].
As previously mentioned, after entering the hepatocytes, the viral genome is converted into plasmid-like cccDNA which can persist in liver cells in a latent state, serving as a reservoir for HBVr, in spite of active anti-HBV immune response. Compared to the hepatitis C virus (HCV) infection, complete eradication of both HBV cccDNA and integrated DNA is impossible with current antiviral treatment with nucleos(t)ide analogs. Thus, these cells constitute a reservoir of persistent HBV. Although HBVr can occur in a variety of settings, immunosuppressive therapies are the most commonly reported. A detailed description of the HBVr induction mechanisms of immunosuppressive therapies is provided in Table 1[3,4,6-12].
| Immunosuppressive therapies with high risk of HBVr | ||||||||
| B-cell depleting therapies (rituximab and ofatumumab) | Anthracycline derivatives (doxorubicin and epirubicin) | Corticosteroids | TNF-α inhibitors (infliximab, adalimumab, certolizumab) | Anti-CD52 monoclonal antibody (alemtuzumab) | ||||
| Increased HBVr risk in positive HBsAg and negative HBsAg and anti-HBc subjects by acting against the B-lymphocyte antigen CD20; The Food and Drug Administration has placed a black box warning for rituximab regarding HBVr in rituximab-treated individuals; used to treat CD20+ blood cancers (lymphomas, CLL) and IRD; B cells play a previously underestimated role in HBV immune control by producing neutralizing antibodies; rituximab associated with > 5× increase in HBVr risk (incidence 3%–55%, overall mortality rate 30%–38%) | High-risk for patients with hepatocellular carcinoma and hepatitis B undergoing TACE; used to treat lymphomas and acute leukemias, breast and ovarian cancer, and sarcoma; HBVr rate = 41% in patients with HBsAg positive | Prednisone use > 20 mg p.o. daily > 4 wk | TNF-α can activate the APOBEC antiviral pathway which causes the degradation of cccDNA in HBV-infected cells. HBVr pooled incidence in patients with resolved HBV infection = 3.0% vs 15.4% in HBsAg positive patients | Used for refractory CLL; causes reverse HBsAg seroconversion and reactivation-related hepatitis | ||||
| Immunosuppressive agents with moderate risk of HBVr | ||||||||
| Less potent TNF-α inhibitors (etanercept) | Cytokine or integrin inhibitors (abatacept, ustekinumab, natalizumab, vedolizumab) | Tyrosine kinase inhibitors (imatinib, nilotinib, dasatinib) | Proteasome inhibitors: (Bortezomib) | Histone deacetylase inhibitors (HDIs) (romidepsin) | Prednisone 10-20 mg p.o. daily > 4 wk | Calcineurin inhibitors (cyclosporine or tacrolimus) | ||
| Moderate risk of HBVr in patients with HBsAg positive (1%-5%) and even lower in patients with HBsAg negative | Commonly utilized in the treatment of IBD, IRD and dermatologic conditions; inhibit local inflammatory response associated with immune-mediated diseases by blocking the localization and traffic of activated lymphocytes | Standard of treatment for all phases of CML; also used in the treatment of GIST; inhibit various kinase signaling pathways, essential for immune activation and proliferation of lymphocytes, with an important role in immune control of HBV replication; prophylactic antiviral therapy and regular monitoring of HBV DNA and liver enzymes are essential; reported HBVr rates of 26%–34.8% | Used for the treatment of MM and induction therapy for transplant- eligible patients prior to stem cell harvest; target cellular pathways that interfere with the functions of healthy B cells, which are important in HBV immune control | Used in the treatment of T-cell lymphomas; inhibit histone deacetylase, a histone-modifying enzyme that is important for epigenetic regulation of gene expression with possible deacetylation status of silent cccDNA, resulting in active HBV transcription and then HBVr | The mechanism is two-fold: The HBV genome contains a transcription regulatory element responsive to glucocorticoid that is up-regulated by corticosteroids, resulting in increased viral replication; a directly suppressive effect on cytotoxic T cells that are involved in HBV control; risk of HBVr of 10%-15.8% in HBsAg positive individuals | Suppress T cell function by inhibiting calcineurin required for signal transduction of T cell activation and inhibiting transcription of interleukin required for T cell proliferation | ||
| Immunosuppressive agents with low risk of HBVr | ||||||||
| Methotrexate, azathioprine or 6-mercaptopurine | Intra-articular steroid injections or prednisone < 10 mg p.o. daily | |||||||
| Documented cases of HBVr are rather rare | ||||||||
| Novel therapies | ||||||||
| Immune checkpoint inhibitors such as anti-PD-L1 (nivolumab) and anti-CTLA4 (ipilimumab) | BTK inhibitor ibrutinib and PI3K delta inhibitor idelalisib | Ruxolitinib | Mogamulizumab | Brentuximab | Obinutuzumab | Hypomethylating agents: Decitabine, azacitidine | Daratumumab | |
| HBVr rarely reporter; anti-HBV prophylaxis is recommended | B-cell receptor signaling modulators; approved by the FDA for the treatment of CLL and certain low-grade NHL; HBVr has been rarely been reported; anti-HBV prophylaxis is recommended | A novel inhibitor of JAK1 and JAK2 that has been approved for the treatment of patients with MPNs; There are reported cases of HBVr | Humanized monoclonal antibody targeting the C-C chemokine receptor 4; Used for ATLL; HBVr cases have been reported | Anti-CD30 drug conjugated antibody; used in the treatment of relapsed or refractory HL and CD30 positive T-cell lymphoma; There are reported cases of HBVr | Newer generation anti-CD20 monoclonal antibody, similar to rituximab but with greater efficacy; FDA has mandated a warning of the risk of HBVr with obinutuzumab and HBVr has been reported | Used in the treatment of AML; anti-HBV prophylaxis is recommended | Monoclonal antibody against CD38; used in the treatment of hematologic malignancies of B cells; HBVr cases have been reported | |
Host-related risk factors for HBVr include male sex, younger age and older age (the elderly are more likely to have HBsAg seroclearance but persistent levels of total HBV DNA and cccDNA in the liver) and have been associated with increased risk of HBVr. Preexisting conditions, for example, cirrhosis or MPNs, also play a role in HBVr. HBVr has been reported in patients with MPNs, lymphomas, myeloma, and acute myeloid leukemia. However, it is not yet clear whether this association is attributed to the underlying disease or to the potent immunosuppressants used in the management of these blood cancers[7-9].
Virological factors include HBsAg and HBeAg positivity (adding a 5- to 8-fold risk for HBVr), non-A HBV genotypes, elevated HBV DNA levels before starting immunosuppressive therapy, and co-infection of HBV with other viruses such as HIV and HCV[4,7,8].
Type of immunosuppression: the greatest risk of HBVr is represented by the use of B-cell depleting therapies, used in the therapeutic armamentarium of blood and solid cancers and in the setting of bone marrow or solid organ trans
Identifying infected individuals is the first and most important step for HBVr prophylaxis. According to the latest specialty guidelines, HBV infection screening must be performed in all patients who are receiving immunosuppressive treatment. Furthermore, all patients who are HBcAg positive, regardless of the status of HBsAg or the HBV DNA values, must receive prophylactic antiviral treatment. In numerous studies, prophylactic antiviral treatment has been shown to reduce the rate of HBVr, liver failure, and death in these categories of patients. Even if lamivudine was the first and for many years the most used oral antiviral agent for HBVr prophylaxis, YMDD gene mutations cause a high incidence of viral resistance if used for > 6 mo. This is why entecavir or tenofovir are recommended as therapies for HBVr prevention if intended for longer periods of time[4,6-8].
In general, the duration of antiviral therapy varies depending on the type of immunosuppressives used. General recommendations include the use of antiviral therapy for at least 6 mo after the last dose of immunosuppressive agents is administered. However, in the case of B cell-depleting therapies (such as rituximab or obinutuzumab), it is recommended that antiviral prophylaxis be continued up to 12 mo after the last dose. Another important step is routine testing for HBV DNA and serum ALT and AST 3-6 mo after discontinuation of immunosuppressives[3,7].
Moreover, particular attention should be given to preventive measures, such as instructing patients to withdraw from alcohol consumption, as well as close monitorization of liver function tests in subjects who are prescribed pharmacological agents with a potentially hepatotoxic effect[13,14]. According to the findings of the Dionysos Study, individuals diagnosed with HBV who consume alcohol experience elevated rates of hepatic fibrosis and death[13].
Ruxolitinib is a commonly used medication to treat MPNs, a group of blood disorders characterized by excessive blood cell production in the bone marrow. One of the common manifestations of MPNs is splenomegaly. Ruxolitinib acts by inhibiting Janus kinases (JAK1 and JAK2), which are enzymes involved in signaling pathways associated with cytokine receptors. By inhibiting these enzymes, ruxolitinib effectively helps control MPNs, particularly intermediate and high-risk myelofibrosis (MF) and high-risk polycythemia vera (PV). Importantly, its effect is not specific to any particular mutation. Ruxolitinib shows good oral bioavailability and reaches its maximum plasma concentration within 1-2 h after administration. Plasma half-life of this drug is approximately 3 h when administered at a maximum tolerated dose of 100 mg once a day. It is mainly metabolized through the CYP3A4 pathway, an important liver enzyme system involved in drug metabolism. Consequently, ruxolitinib has the potential for interactions with medications that induce or inhibit the CYP3A4 pathway. Ruxolitinib is primarily eliminated from the body through metabolism in urine and feces. Therefore, dosage adjustments are necessary for patients with renal or liver impairments, as these conditions can affect the clearance of the drug from the body[15].
It is important to note that this pharmacological agent exhibits immunomodulatory effects, meaning that it can modify the functioning of the immune system. As a result, ruxolitinib treatment may increase susceptibility to opportunistic infections in patients prescribed this drug. Thus, regular monitoring for signs of infection is important when subjects diagnosed with MPNs start taking this medicine[16]. In particular, this pharmacological agent exhibits immunomodulatory and anti-inflammatory actions and can interfere with or impair the innate/adaptive immune response due to its interplay with dendritic cells, regulatory/T-helper lymphocytes or natural killer cells[17,18].
In a case report by Sjoblom et al[19], a patient with a history of PV received initial treatment with hydroxyurea. However, due to progressive splenomegaly and fatigue, his treatment was changed to pegylated interferon. Furthermore, to more effectively manage his symptoms, ruxolitinib was introduced. The patient experienced HBVr while on ruxolitinib, which was confirmed by abnormal liver function test results, positive viremia, and newly positive surface antigen for hepatitis B (HbsAg). With the initiation of tenofovir disoproxil, the patient's liver function gradually normalized, indicating successful management of HBVr[19]. In another report by Shen et al[20], a patient with MF and a history of HBV infection experienced HBVr during ruxolitinib treatment. The initial elevation in transaminase levels was mistakenly attributed to drug toxicity. Subsequent detection of high plasma levels of HBV DNA confirmed the reactivation. Ruxolitinib was discontinued and antiviral therapy was started, resulting in a gradual decrease in transaminase levels[19,20]. Additionally, in another report by Passucci et al[21], a patient with PMF and previous HBV infection achieved resolution of splenomegaly with ruxolitinib therapy. However, HBVr occurred after the patient discontinued prophylactic lamivudine. De-escalation of ruxolitinib and the initiation of anti-HBV therapy led to a gradual decline in HBV DNA levels without signs of active hepatitis[21]. Kirito et al[22] highlight the importance of considering prophylactic antiviral therapy in patients with chronic HBV infection before starting treatment with ruxolitinib, as such a proactive measure can help prevent HBVr, as observed in their patient[22].
Ruxolitinib has an immunosuppressive effect, leading to an increased risk of serious infections. The immunosuppressive effect of ruxolitinib is due to its interaction with multiple pathways of the immune system, affecting both adaptive and innate immune responses. This can result in the reactivation of silent infections such as tuberculosis, HBV, and varicella-zoster virus. Therefore, proactive infection surveillance, baseline screening for latent infections, and considering prophylactic or preventive interventions for specific infections such as varicella-zoster virus and HBV virus are crucial[23]. A pilot study conducted by Crodel et al[24] investigated the frequency of infections in patients with MPNs. The study included multiple centers and relied on patient-reported data. The findings revealed that over 50% of MPN patients experienced one or more episodes of infection within a 12-mo period. The most frequently reported infections were upper respiratory tract infections, herpes virus infections, and gastrointestinal infections. Among the different subtypes of MPNs, subjects with MF had the highest percentage of infectious events, followed by PV and essential thrombocythemia[24]. Furthermore, Lussana et al[25] conducted a systematic review and meta-analysis examining the safety and efficacy of ruxolitinib in the treatment of MF and PV. The study specifically focused on the incidence of infections in patients receiving ruxolitinib. It was found that ruxolitinib, with its immunosuppressive effects, can affect immune functions and increase the risk of infections. Herpes zoster, pneumonia, bronchitis, and urinary tract infections were among the most frequently reported infectious complications. The aforementioned quantitative assessment emphasized the importance of carefully evaluating infection risk before initiating ruxolitinib therapy and highlighted the need to monitor and address infections in patients receiving ruxolitinib for MF and PV[25].
In a paper by Perricone et al[26], two case reports of HBVr in MF patients treated with ruxolitinib are discussed. The immunosuppressive effects of ruxolitinib, particularly in dendritic cells and T cells, may contribute to an increased risk of infections, including HBVr. The article emphasizes the need for vigilance among physicians when considering infectious causes when using immunosuppressive agents such as ruxolitinib[26]. In a prospective study by Gill et al[27], 40 patients with MPNs were included. Among the 37 subjects who were negative for HBsAg, 15 tested positive for anti-HBc antibodies, indicating occult HBV infection. Prophylactic treatment for HBV was administered to the three HBsAg positive patients. During a median follow-up of 19.2 mo, four patients (26.7%) experienced HBVr, occurring at a median of 10.5 mo after starting ruxolitinib therapy. The estimated cumulative incidence rates of HBVr at 6 and 12 mo were 7.7% and 30.8%, respectively. This investigation emphasizes the need to monitor HBVr in patients with occult HBV infection who receive ruxolitinib therapy[27]. Garcia-Horton et al[28] conducted a retrospective cohort study involving 1171 individuals with MPNs to evaluate the risk of HBVr in subjects treated with ruxolitinib. Among the 58 patients with prior HBV infection, 20 received ruxolitinib. Only one patient experienced HBVr during ruxolitinib therapy, and their HBV DNA levels peaked, but subsequently returned to undetectable levels without interrupting or reducing the ruxolitinib dose[28]. Duan et al[29] conducted a retrospective analysis to evaluate the incidence of HBVr in MPN patients treated with ruxolitinib. The study included 62 patients with a history of HBV infection, 56 with resolved infection and 6 with chronic HBV infection. Among patients with chronic HBV infection, two experienced HBVr and hepatitis flare-up after ruxolitinib therapy. None of the patients with resolved HBV infection experienced reactivation. In particular, the two patients with chronic HBV infection did not receive antiviral prophylaxis[29]. Caocci et al[30] presented a case report of a patient with MF who experienced HBVr during treatment with ruxolitinib. The patient had a history of HBV infection and initially received ruxolitinib for symptoms related to MF. Although there was improvement in MF symptoms, HBVr was observed through increased levels of HBV-DNA. Adjusting the dose of ruxolitinib resulted in an improvement in symptoms, but HBV-DNA levels remained fluctuating. This case report raises concerns about the management of MF patients with HBV infection receiving ruxolitinib and emphasizes the importance of careful monitoring and potential prophylactic treatment[30].
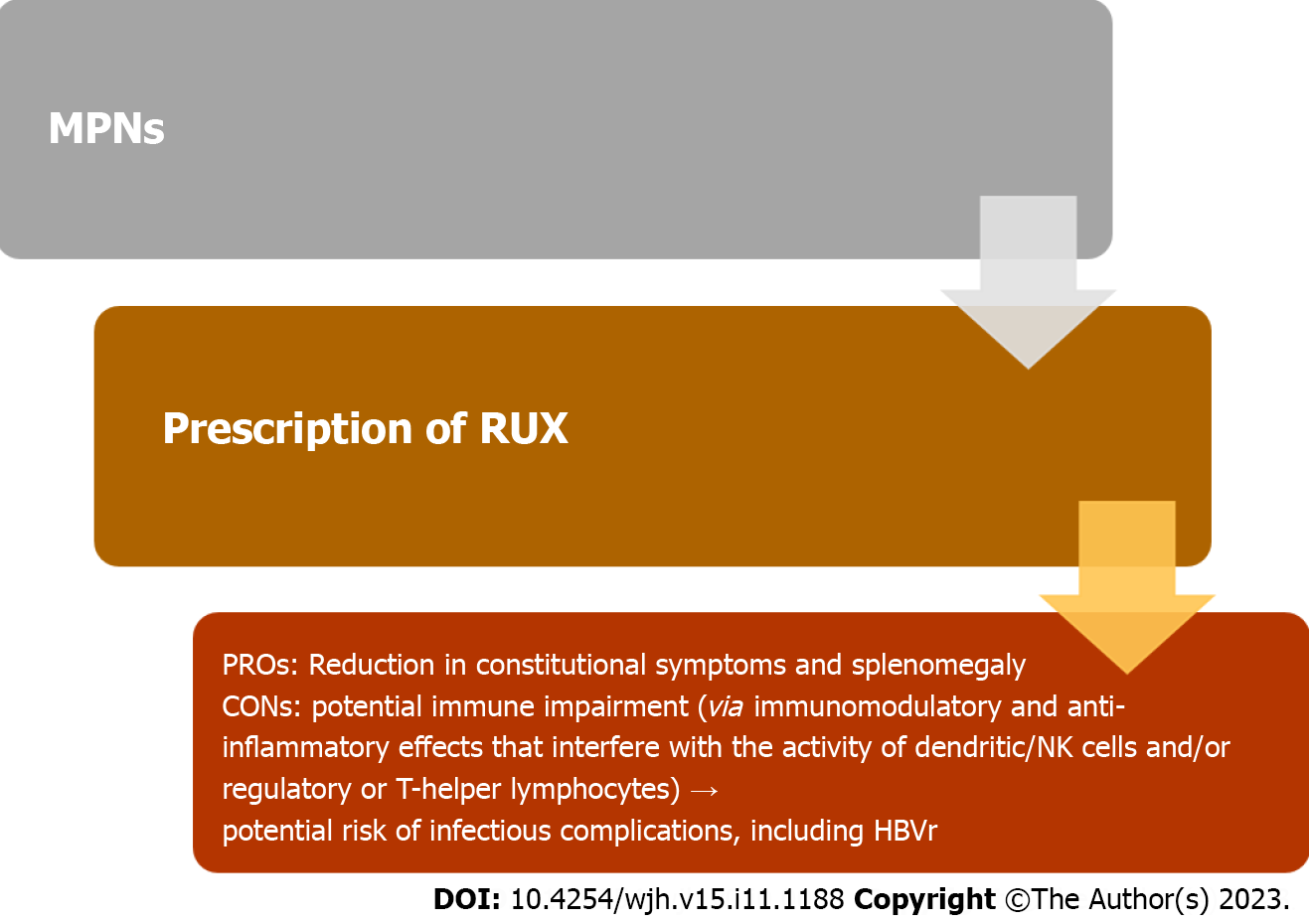
A schematic representation between the benefits and risks of ruxolitinib use in terms of opportunistic infections in MPNs is depicted in Figure 1.
In conclusion, ruxolitinib is an effective medication to manage MPNs such as MF and PV, particularly in intermediate and high-risk cases. By inhibiting JAK1 and JAK2, ruxolitinib helps control excessive blood cell production and reduce splenomegaly. However, its use carries certain risks and considerations. The interaction of ruxolitinib with the immune system can increase the susceptibility to opportunistic infections, highlighting the need for vigilant monitoring and timely intervention. Furthermore, there is a potential for HBVr, especially in patients with a history of HBV infection. Close monitoring of liver function and proactive measures, such as prophylactic antiviral therapy, are crucial to managing these risks. In general, ruxolitinib offers therapeutic benefits for MPNs, but careful evaluation of infection risk, regular monitoring, and appropriate interventions are essential to ensure patient safety.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beenet L, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 2. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 3. | Koffas A, Dolman GE, Kennedy PT. Hepatitis B virus reactivation in patients treated with immunosuppressive drugs: a practical guide for clinicians. Clin Med (Lond). 2018;18:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 5. | Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, Locarnini S, Zoulim F, Chang KM, Lok AS. Present and future therapies of hepatitis B: From discovery to cure. Hepatology. 2015;62:1893-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 6. | Chang Y, Jeong SW, Jang JY. Hepatitis B Virus Reactivation Associated With Therapeutic Interventions. Front Med (Lausanne). 2021;8:770124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Shih CA, Chen WC. Prevention of hepatitis B reactivation in patients requiring chemotherapy and immunosuppressive therapy. World J Clin Cases. 2021;9:5769-5781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 8. | Law MF, Ho R, Cheung CK, Tam LH, Ma K, So KC, Ip B, So J, Lai J, Ng J, Tam TH. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol. 2016;22:6484-6500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Wang B, Mufti G, Agarwal K. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica. 2019;104:435-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Shen J, Wang X, Wang N, Wen S, Yang G, Li L, Fu J, Pan X. HBV reactivation and its effect on survival in HBV-related hepatocarcinoma patients undergoing transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors. Front Cell Infect Microbiol. 2023;13:1179689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Chiu CY, Ahmed S, Thomas SK, Wang LS, Mustafayev K, Fayad LE, Wierda WG, Khawaja F, Torres HA. Hepatitis B Virus Reactivation in Patients Receiving Bruton Tyrosine Kinase Inhibitors. Clin Lymphoma Myeloma Leuk. 2023;23:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, Peters MG, Tanaka Y, Zelenetz AD, Kuriki H, Fingerle-Rowson G, Nielsen T, Ueda E, Piper-Lepoutre H, Sellam G, Tobinai K. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. 2019;133:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Moore DC, Elmes JB, Arnall JR, Strassels SA, Patel JN. Hepatitis B reactivation in patients with multiple myeloma treated with anti-CD38 monoclonal antibody-based therapies: a pharmacovigilance analysis. Int J Clin Pharm. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bedogni G, Miglioli L, Masutti F, Ferri S, Castiglione A, Lenzi M, Crocè LS, Granito A, Tiribelli C, Bellentani S. Natural course of chronic HCV and HBV infection and role of alcohol in the general population: the Dionysos Study. Am J Gastroenterol. 2008;103:2248-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Purwar S, Fatima A, Bhattacharyya H, Simhachalam Kutikuppala LV, Cozma MA, Srichawla BS, Komer L, Nurani KM, Găman MA. Toxicity of targeted anticancer treatments on the liver in myeloproliferative neoplasms. World J Hepatol. 2023;15:1021-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 16. | Curto-Garcia N, Harrison CN. An updated review of the JAK1/2 inhibitor (ruxolitinib) in the Philadelphia-negative myeloproliferative neoplasms. Future Oncol. 2018;14:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dioverti MV, Abu Saleh OM, Tande AJ. Infectious complications in patients on treatment with Ruxolitinib: case report and review of the literature. Infect Dis (Lond). 2018;50:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Front Oncol. 2019;9:1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Sjoblom M, Chtioui H, Fraga M, Stalder G, Grandoni F, Blum S. Hepatitis B reactivation during ruxolitinib treatment. Ann Hematol. 2022;101:2081-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Shen CH, Hwang CE, Chen YY, Chen CC. Hepatitis B virus reactivation associated with ruxolitinib. Ann Hematol. 2014;93:1075-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Passucci M, Masucci C, Paoletti F, Ielo C, Costa A, Carmosino I, Scalzulli E, Martelli M, Gentile G, Breccia M. Case Report: Infectious prophylaxis in hematological malignancies. Front Oncol. 2023;13:1163175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Kirito K, Sakamoto M, Enomoto N. Elevation of the Hepatitis B Virus DNA during the Treatment of Polycythemia Vera with the JAK Kinase Inhibitor Ruxolitinib. Intern Med. 2016;55:1341-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Sant'Antonio E, Bonifacio M, Breccia M, Rumi E. A journey through infectious risk associated with ruxolitinib. Br J Haematol. 2019;187:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Crodel CC, Jentsch-Ullrich K, Koschmieder S, Kämpfe D, Griesshammer M, Döhner K, Jost PJ, Wolleschak D, Isfort S, Stegelmann F, Jilg S, Hofmann V, Auteri G, Rachow T, Ernst P, Brioli A, von Lilienfeld-Toal M, Hochhaus A, Palandri F, Heidel FH. Frequency of infections in 948 MPN patients: a prospective multicenter patient-reported pilot study. Leukemia. 2020;34:1949-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: A systematic review and meta-analysis. Am J Hematol. 2018;93:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 26. | Perricone G, Vinci M, Pungolino E. Occult hepatitis B infection reactivation after ruxolitinib therapy. Dig Liver Dis. 2017;49:719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Gill H, Leung GMK, Seto WK, Kwong YL. Risk of viral reactivation in patients with occult hepatitis B virus infection during ruxolitinib treatment. Ann Hematol. 2019;98:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Garcia-Horton A, Smith E, Maze D, McNamara C, Sibai H, Gupta V. Risk of hepatitis B virus reactivation in HBsAg-negative, anti-HBc-positive patients with myeloproliferative neoplasms treated with ruxolitinib. Leuk Lymphoma. 2021;62:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Duan MH, Cao XX, Chang L, Zhou DB. Risk of hepatitis B virus reactivation following ruxolitinib treatment in patients with myeloproliferative neoplasms. Hematology. 2021;26:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Caocci G, Murgia F, Podda L, Solinas A, Atzeni S, La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28:225-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |