Published online Oct 27, 2023. doi: 10.4254/wjh.v15.i10.1153
Peer-review started: August 25, 2023
First decision: September 15, 2023
Revised: September 21, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 27, 2023
Processing time: 59 Days and 13.3 Hours
The existing literature suggests that exercise for cirrhotic patients is safe and favours significant improvement to their physical capacity. However, exercise training for this population and how to deliver activities, especially in severe stages of the disease and while waiting for a liver transplant (LT), remain undefined.
To review the existing exercise prescriptions for cirrhotic patients on the waiting list for LT, their results for frailty evolution and their effect on clinical outcomes.
A systematic review was performed following the Preferred Reporting Review and Meta-Analysis guidelines and searching the PubMed, MEDLINE, and Scopus databases. The keyword “liver transplant” was used in combination with the free terms “frailty” and “exercise” for the literature review. Clinical studies that evaluated the effect of a regular training program, independent of supervision or the duration or intensity of physical exercise, in cirrhotic patients on the waiting list for LT were reviewed. The data on safe physical activity prescriptions following Frequency, Intensity, Time, and Type recommendations were extracted and summarised.
Nine articles met the inclusion criteria for this review. Various instruments for frailty assessment were used, frequently in combination. Five studies prescribed physical activity for patients, one in-person and four to be performed remotely and unsupervised. The remaining four studies only used a self-report instrument to assess the level of physical activity. None reported adverse events related to exercise training. The exercise frequency mainly varied from daily to a minimum of twice per week. The intensity depended on frailty and included increasing levels of activity. The type of exercise was predominantly a combination of aerobic and resistance training. The duration of exercise varied from 4 to 12 wk. Three articles evaluated the effect of the exercise program on clinical outcomes, reporting a reduction in 90-d readmission rates post-transplant and improved frailty scores, as well as improved survival of cirrhotic patients waiting for LT.
Routine frailty assessment is essential for this population. Although more robust evidence is required, the prescription of exercise is safe and can improve patients’ functional capacity, improving pre- and post-LT outcomes.
Core Tip: Frailty negatively affects the outcomes of patients waiting for liver transplants (LTs). However, the tools used to assess frailty and functional performance vary amongst the existing studies, limiting the estimation of the real and accurate prevalence of the condition and the effectiveness of proposed treatments. So far, existing studies suggest that exercise may improve cirrhotic patients’ functional capacity and frailty while they are on the waiting list for LTs. In addition, although evidence is scarce, studies affirm that exercise training improves pre- and post-LT outcomes.
- Citation: Loschi TM, Baccan MDTA, Della Guardia B, Martins PN, Boteon APCS, Boteon YL. Exercise training as an intervention for frailty in cirrhotic patients on the liver transplant waiting list: A systematic review. World J Hepatol 2023; 15(10): 1153-1163
- URL: https://www.wjgnet.com/1948-5182/full/v15/i10/1153.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i10.1153
Frailty is a multidimensional clinical syndrome that encompasses physical, psychological, social, and environmental components[1,2]. The physical component relates to sarcopenia, reduced physical function, reduced aerobic function, and physical disability[2]. Frailty is defined as a clinical state of decreased physiological reserves and increased vulnerability to health stressors, which predisposes individuals to adverse clinical outcomes[1]. It develops in elderly patients and earlier in patients with debilitating chronic diseases. In patients with end-stage liver disease (ESLD) who are candidates for liver transplant (LT), frailty is associated with decreased strength, physical deconditioning, and worse clinical outcomes with a higher probability of death and dependence after LT[2-4].
Approximately 18%-43% of cirrhotic patients are expected to be considered frail[5,6]. These figures vary largely according to the definition used and the population studied. Although there are some similarities, the mechanisms of frailty in ESLD patients are distinct from those in the geriatric population. The latter entails the original concept of frailty and the multidimensional derangement of physiological systems and is also called global frailty. In ESLD patients, the aetiology of the condition is complex but predominantly driven by liver impairment, such as synthetic protein dysfu
Sarcopenia assessment involves tests to quantify muscle mass; for example, cross-sectional imaging via computed tomography or magnetic resonance imaging, bioelectrical impedance analysis, dual-energy X-ray absorptiometry, or ultrasound, and a deep discussion of these is beyond the scope of this review[2,10]. To assess physical frailty in ESLD patients, three tests have been used: The Fried Frailty Index[1], the Clinical Frailty Scale (CFS)[12], and the Liver Frailty Index (LFI)[13]. Other tests that are also typically used in the care of these patients to assess their physical function and the relationship between muscle strength and function are the Short Physical Performance Battery[14], the Six-Minute Walk Test (6MWT)[15], Gait Speed[16], Hand Grip Strength[17] tests. In an inpatient setting, generic instruments can be used to measure global frailty, including the Frailty Index, the Hospital Frailty Risk Score[18], Activities of Daily Living[19,20], and Karnofsky Performance Status[21]. Regardless of the instrument used for the assessment, frailty in cirrhotic patients is associated with worse pre- and post-transplant outcomes[22-25].
The identification of interventions that could modify or reverse this condition is, therefore, crucial. Although exercise training is widely recommended for people with other chronic diseases[26], evidence for this treatment lags well behind for the population of cirrhotic patients on a waiting list for LT. Perhaps this is due to concerns about exercise causing increased portal pressure[27] or the lack of a standardised exercise protocol. Exercise programs for this population remain uncommon, and debate about the optimal method to deliver exercise (in-person supervised exercise or home-based programs) continues. Regular physical activity and exercise may favour better outcomes by adding muscle mass, as sarcopenia is associated with poor prognostic outcomes[11], and improving these individuals’ functional capacity - i.e., reducing their frailty. Therefore, this review aims to investigate the exercise programmes and prescriptions currently used for cirrhotic patients on the waiting list for LT, their results for frailty pre- and post-intervention and their effect on clinical outcomes.
This systematic review was performed according to the Preferred Reporting Systematic Review and Meta-Analysis protocol[28]. No review protocol was registered before this review began.
Articles were identified with the keyword “liver transplant” in combination with the free terms “frailty” and “exercise”. The PubMed, MEDLINE and Scopus databases were searched for this review. There was no limitation according to publication date, and the search ended in January 2023.
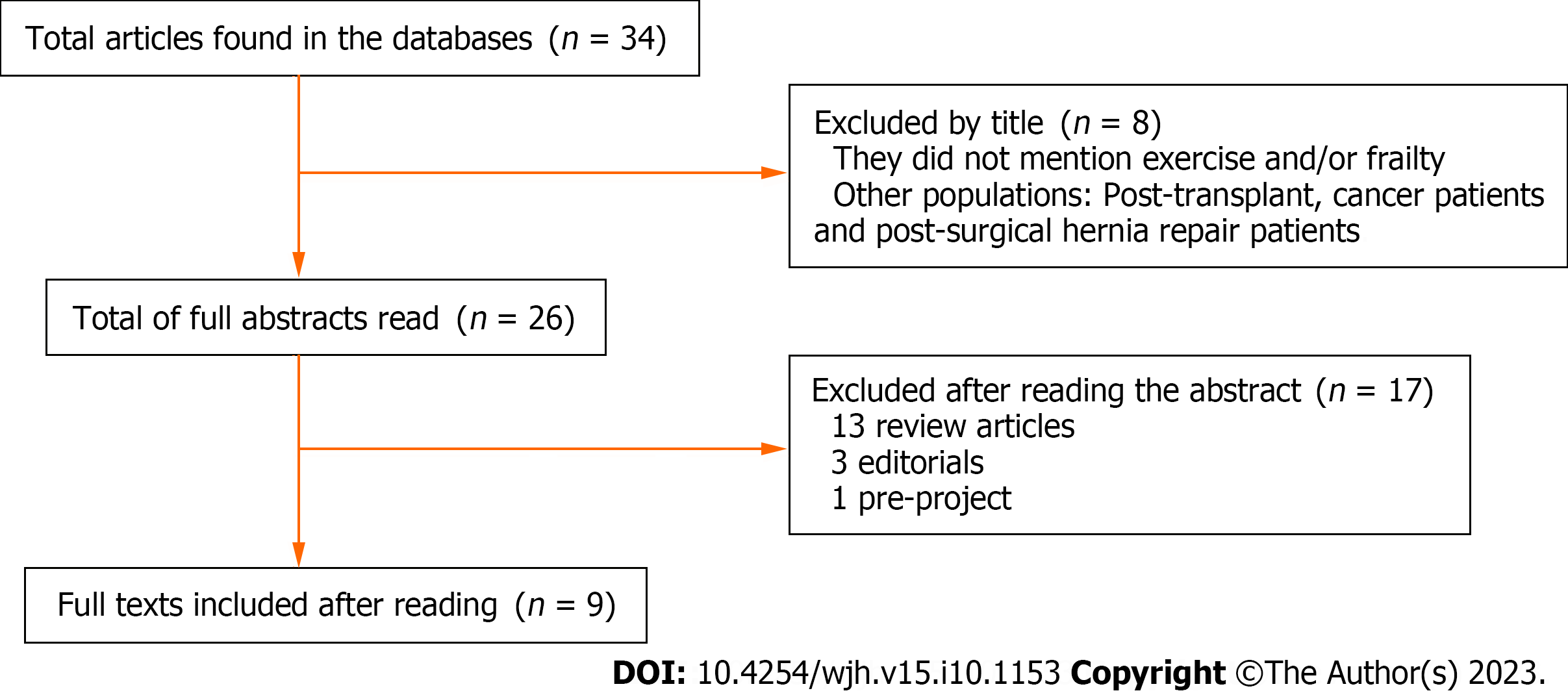
Two authors (Loschi TM and Baccan MDTA) screened the articles independently, and there were no disagreements about the studies selected. In the first step, studies with titles that were not related to the theme were excluded. The second step involved reading the full abstracts and excluding articles that did not address important topics for this review. Finally, the full text of the remaining articles was evaluated for eligibility and articles that met the criteria were included in this review. The flow chart for the literature selection process is shown in Figure 1.
The inclusion criteria were: (1) Articles that assessed physical frailty in cirrhotic patients on waiting lists for LT and the effect of physical exercise in this setting using any measurement instrument; and (2) Articles written in English and published. Physical exercise was considered a regular training program regardless of supervision, duration, or intensity. The exclusion criteria were: (1) A study population that was not candidates for LT; (2) Review articles; (3) Editorials and pre-projects; or (4) Articles not written in English.
Details about the exercise program, method of exercise delivery (supervised or unsupervised), and results regarding frailty pre- and post-intervention and the evaluated clinical outcomes were retrieved from each manuscript and analysed. The main outcomes evaluated were the assessment instrument for physical frailty, whether any pre-established criteria for inclusion in the transplant list related to frailty assessment were included; the frequency, intensity, type, and duration of exercise performed; and the primary and secondary outcomes evaluated after the physical exercise program.
The quality of the included studies was assessed based on the National Institutes of Health Study Quality Assessment Tools. Due to the variable study designs, any identified risk of bias was discussed in the study. No simplifications or assumptions were made.
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the studies included in this review[29]. The tool applies a “star system” to evaluate three broad perspectives: The selection of the study groups, the comparability of the groups, and the ascertainment of exposure or assessment of the outcome of interest for case-control and cohort studies, respectively. The high-quality features of the numbered items evaluating each of the three domains are identified with a star. A study can be awarded a maximum of one star for each item across a total of four for the selection category, three for the outcome/exposure category, and one for comparability. Therefore, each article can score up to 9 points. Scores of 7 to 9, 4 to 6, and < 4 points indicate high, moderate, and low methodological quality, respectively[29].
The search strategy yielded nine articles that met the eligibility criteria and were, therefore, included in this systematic review.
The included studies used various instruments to assess frailty; most of them used a combination of instruments. The tools used were LFI (frailty for chronic liver disease), dynamometry (objective measure of handgrip strength), the Duke Activity Status Index (self-reported estimate of functional capacity), 6MWT (functional capacity), Gait Speed (functional capacity), the Rosow-Breslau scale (self-reported assessment of independence for activities), the Incremental Shuttle Walking Test (functional capacity), step count, the Fried Frailty Scale (objective score for assessing frailty), and Cardiopulmonary Exercise Testing (exercise capacity).
Of the nine studies included in this review, only one used in-person supervised exercise[30], four prescribed physical activity to be performed remotely and unsupervised[31-34], and the remaining did not specify exercise but recorded participants’ self-reported levels of physical activity[35-38].
From the articles that prescribed exercise, we extracted data on the prescription of safe physical activity for this population. The acronym Frequency, Intensity, Time, and Type (FITT) was used to guide us in achieving this aim. We evaluated whether the exercise was supervised or not and whether the activity was performed in person or remotely.
The exercise frequency was 1-5 times per week for the study that prescribed in-person exercise. The studies that adopted remote exercises prescribed daily to a minimum of twice per week exercise, usually for 30 min per session. The intensity of training depended on the existence of frailty and included increasing levels of activity. The intensity progression was mainly based on professional evaluation. The type of exercise was predominantly a combination of aerobic and resistance training, although one study opted for aerobic exercise alone. The duration of exercise varied from 4 to 12 wk, and most studies included reassessment during the program. A detailed description of the exercise prescriptions is provided in Table 1.
| Ref. | Frequency | Intensity | Type | Time | Supervised? | In-person? |
| Al-Judaibi et al[30], 2019 | In-person group: 1 to 5 times/wk. Remote group: 2 to 3 times/wk | - | In-person group: Aerobic and resistance training | - | Yes, remote group was supervised by videoconference | Yes |
| Lin et al[31], 2021 | 30 min a day, 5 times/wk | Frail and pre-frail: Aerobic: Encouraged to walk; purchasing a stationary bicycle or a pedal boat was suggested if the patient was at risk for walking. Resistance: Initial prescription at the time of evaluation with the physiotherapist. Weights or elastic bands can be used. Initially, 1 series of 10 repetitions, increase by 5 to 10 repetitions until reaching 30. Only then progress the load | Aerobic and resistance training | 150 min of exercise per week | No | No, only if complications prevented the performance of the unsupervised exercise |
| Williams et al[32], 2019 | Resistance: 20 min 2 times/wk. Aerobic: 10 min of brisk walking 3 times/d | Resisted: BORG between 12 and 14. Three levels: Low, moderate, and high. Aerobic: Increasing the number of steps | Aerobic and resistance training | Total of 12 wk, with reassessment at 6 and 12 wk and telephone contact 1 time/wk until 6 wk | No | No |
| Chen et al[33], 2020 | Daily | Increase of 500 steps/d at each evaluation | Walking | Total: 8 wk, with a baseline assessment and every 2 wk | No | No |
| Lin et al[34], 2022 | - | The group that used the app: The app’s algorithm established the intensity after an initial assessment by a professional | - | Baseline assessment and 4 wk after | No | No |
Only one article of the nine used frailty to indicate the decision to include a cirrhotic patient on the LT waiting list[30]. In that study, patients with acceptable functional mobility were considered fit for inclusion on the list. The parameters were: a 6MWT of > 250 m, handgrip strength of > 30 kg for men and > 14 kg for women, and a Duke Activity Status Index of > 4 METS. Patients who were not listed were closely monitored, and goals related to the 6MWT were established to optimise functional mobility.
Post-transplant (length of hospital stay; hospital readmission rate) and pre-transplant (survival rate; quality of life assessment) endpoints were investigated as study outcomes. Physical activity and frailty improvement were consistently associated with favourable outcomes. Detailed information on the results is presented in Table 2. In addition, we used the NOS to assess article quality. The NOS scores were between 4 and 9, indicating moderate to high quality for the included articles (Table 2).
| Ref. | Outcomes analysed | Adverse events | Adherence | NOS quality assessment |
| Al-Judaibi et al[30], 2019 | Patients who participated in the rehabilitation program showed a tendency towards a decrease in length of stay and a reduction in readmission rates in patients undergoing liver transplantation. Although not statistically significant, the observed trend towards early discharge after liver transplantation was observed in patients in the intervention group | Selection: 4*. Comparability: 0*. Outcome: 3* | ||
| Lin et al[31], 2021 | LFI: Patients more adherent to the prehabilitation program have better results. Frailty is associated with mortality. An improvement of 0.3 in LFI is potentially associated with improved survival. 6MWT: Tendency of improvement with the pre-rehabilitation program, especially in the most adherent patients, after visit 4. Frailty is associated with mortality. Gait speed: There was no change with pre-rehabilitation. Frailty is associated with mortality. Frailty is more prevalent in females, higher BMI (as assessed by the 6MWT and GST), cirrhosis from alcohol or NASH, HB values, albumin, and bulky appearance, do not correlate with MELD. Patients with COPD and CVD are frailer by 3 frailty assessment metrics | Reposted by the patient at follow-up assessment: non-adherent < 20%, partially adherent: 20%-79%, adherent: ≥ 80%. Members: 38%. Partially adherent: 51% and non-adherent: 11%. Adherence to physical therapy was independently associated with increased survival | Selection: 2*. Comparability: 0*. Outcome: 2* | |
| Dunn et al[35], 2016 | Karnofsky scale and Rossow-Breslau indicated habitual physical activity performance close to normal. Comparing with objective data of counting daily steps, it showed that 75.9% of waking time was in sedentary activity. There was a significant association between the percentage of sedentary behaviour and deaths on the waiting list | Selection: 4*. Comparability: 0*. Outcome: 3* | ||
| Williams et al[32], 2019 | SPPB, ISWT: Improved at 6 wk and no improvement at 12 wk. Step count: Improvement in the index at 12 wk. Quality of life improves in the 12th wk, mainly regarding mobility | No events | Adherence up to 6 wk: 82% step target and 90% resistance exercises. Already at 12 wk, they dropped to 53% and 78%, respectively | Selection: 4*. Comparability: 0*. Outcome: 3* |
| Ney et al[36], 2017 | IPAQ: 47%, 38% and 15% of patients had low, moderate and high activity levels, respectively. The main barrier perceived for not performing physical exercise was fatigue | Selection: 2*. Comparability: 0*. Outcome: 2* | ||
| Lai et al[37], 2016 | Fried Frailty Index > 3 frail. 6MWT: Frail with less walking distance. Frail: Less sit and stand up and lower isometric knee extension strength-tendency of critically ill patients to be more fragile | Selection: 2*. Comparability: 0*. Outcome: 2* | ||
| Chen et al[33], 2020 | The control group had more patients who walked less than 2500 steps/d. 6MWT: Greater distance walked in the group with home exercises. CPET: No difference between groups. Computed tomography: Increased psoas muscle mass in the group with home exercises. Quality of life: No differences between groups | No events | Selection: 4*. Comparability: 2*. Outcome: 3* | |
| Oikonomou et al[38], 2022 | Significant correlation between LFI and physical activity level. LFI: Best in active (active: 3.75, sedentary: 4.42). No frail in the active group. Six frails in the sedentary group. Greater distance in active compared to sedentary (458.2 × 324.7). Peak VO2 and the highest AT in the active | Selection: 3*. Comparability: 0*. Outcome: 2* | ||
| Lin et al[34], 2022 | They were considered fragile if LFI was 4.5 or greater, 6MWT was less than 250 m, or GST was less than 0.8 m/s. Patients who walk less than 1200 steps/d have a higher LFI. Every additional 500 steps taken per day reduces the risk of hospitalisation by 5% and the risk of death by 12%. Patients in the group using an exercise app took more daily steps. Daily step count was moderately correlated with frailty metrics, and frail patients walked less than their less frail peers by any metric |
Three articles evaluated the effects of exercise programs on clinical outcomes[30,31,34]. Al-Judaibi et al[30] in a retrospective single-centre study that analysed 458 LT patients, showed a trend towards shorter hospital stays (14 vs 17 d) and a reduction in 90-d readmission rates (17.9% vs 20%) in patients who underwent comprehensive exercise training programs before LT. Lin et al[31] analysed the effects of a prehabilitation strategy that involved home-based exercise in a study that included 517 patients and reported that a median improvement of 0.3 points in frail patients’ LFI scores was associated with improved survival. Adherence to a physical therapy program was independently associated with increased survival. One year later, the same group reported more hospital admissions and high mortality rates among waiting list patients in the lowest quartile of the daily step count (i.e., < 1200 steps per day). When adjusted by the Model for End-stage Liver Disease (MELD)-Na and the use of a physical training-dedicated smartphone application (Exercise and Liver FITness, or EL-FIT), both hospital admissions and death were significantly associated with the lowest quartile [hazard ratio (HR) = 1.9, confidence interval (CI): 1.09-3.30 and HR = 3.42, CI: 1.23-9.68], 6MWT (HR = 0.63, CI: 0.47-0.83 and HR = 0.66, CI: 0.44-0.99 per 100 m), and Gait Speed Test (HR = 0.29, CI: 0.11-0.72 and HR = 0.21, CI: 0.05-0.84). Notably, there was a 5% reduction in the risk of admission and a 12% reduction in the risk of death for every additional 500 steps taken per day regardless of MELD-Na score and exercise program use[34]. None of the articles reported adverse events related to exercise training in this population.
Frailty is a clinical condition that negatively affects the outcomes of cirrhotic patients on waiting lists for LT[22-24]. Although the benefits of exercise training are well-established for other chronic conditions, it has not been deeply investigated in this population, mainly due to concerns for their safety[27] and the lack of a standard recommendation. In this review, we assessed the existing literature investigating the effect of exercise training on patients on the waiting list for LT, particularly the prescription of exercise. The current, extremely limited literature suggests benefits from exercise training programmes for clinical outcomes; however, frailty assessment tools lack standardisation, and the evidence for exercise therapy prescription is currently in its early stages.
In a large multicentric retrospective study in the United States that encompassed 1044 patients, frailty (defined as LFI ≥ 4.5) was associated with a 1.82-fold increase in the adjusted risk of mortality on the waiting list compared to non-frail patients[39]. Another North American study found that a 0.1 unit increase in the LFI at three months was associated with a 2-fold increased risk of death or delisting (95%CI: 1.35-3.09) regardless of baseline LFI score or MELD-Na value[22]. When applied to patients listed for LT, the 6-minute walk distance showed a moderate inverse correlation with the MELD score (r = -0.61). In addition, a 6-min walk distance of < 250 m was significantly associated with an increased risk of death on the waiting list (P = 0.0001), and every 100-m decrease in performance predicted a 2-fold increase in mortality[24]. Studies that applied other instruments to measure frailty, such as the Fried Frailty Index[23], the CFS score[5], the Gait Speed test[16], and the 6MWT[24], also found associations between high frailty and increased mortality.
Nowadays, LT specialists accept the benefits of regular physical activity and exercise to improve the functional capacity of cirrhotic patients on waiting lists for LT. The studies summarised in review articles in the literature suggested that exercise is safe for cirrhotic patients and favours a significant improvement in peak exercise capacity (VO2), a significant increase in the distance walked in the 6MWT, improved muscle strength and function, decreased fatigue, a reduced hepatic portal venous gradient, increased lean mass, and improved quality of life[40-42]. Nevertheless, exercise prescriptions for this population remain poorly defined, likely because frailty assessment is predominantly based on subjective clinician assessment. These professionals focus mainly on managing the underlying disease aetiology and liver-related complications. Moreover, practical tools for clinicians to translate their knowledge into a safe and effective exercise prescription are limited. In a review article, experts suggest using the simple and time-efficient CFS and LFI scores alongside routine clinical patient evaluation to identify the patients at the highest risk for physical frailty and recommend them for prehabilitation with specialists, dieticians, and physiotherapists[2].
Therefore, one of the factors affecting the use of physical activity in this population is the physician-centred care model, which frequently delays or prevents access to professionals who prescribe exercise, such as physiotherapists and physical educators. In Brazil, few transplant centres have specialist teams to assess frailty and decreased physical performance, which can reduce the perception of the condition and, consequently, the prescription of exercise and the use of a monitoring program for these patients. In developed countries, the situation is similar. For example, a Canadian study investigating barriers to lifestyle modification in cirrhotic patients reported that only 1 in 7 centres offer their patients regular exercise programming[36]. A professional, detailed functional assessment and patient-specific exercise program that is adapted at regular follow-up sessions are desirable for optimal results. Other barriers to the adoption of prehabilitation exercise training programs are logistical - related to the distance from the transplant centre or caregiver availability due to the need to maintain a constant and continuous exercise regimen for approximately 8-12 wk - or related to the care team’s fear of exacerbating or promoting an ESLD complication[42]. In addition, cost is an essential variable as that not all insurance covers more than a few sessions of physiotherapy.
While studies with cirrhotic patients generally focused on physical frailty, the tools currently used to measure frailty and functional performance varied. In addition, the method of exercise delivery encompassed in-person or remote and supervised or unsupervised approaches. Independent of the method employed, the existing studies show favourable results from exercise training in terms of improved functional capacity and frailty[30,31,34]. This finding suggests the need to encourage healthy and active lifestyles for cirrhotic patients; physicians should advise them to stay active and adhere to exercise training. This need is even more crucial in transplant centres with a predominantly physician-centred care model because adherence to these programs depends strongly on physicians raising patients’ awareness of the importance of this strategy. Instruments that take objective measures, such as the LFI[13], the Short Physical Performance Battery[43], the Gait Speed test and the 6MWT[44], evaluate the strength and resistance of the lower limbs, the strength of the upper limbs, and balance. According to the literature, these are relevant predictive tools in this setting and appropriate drivers for prescribing exercise therapy.
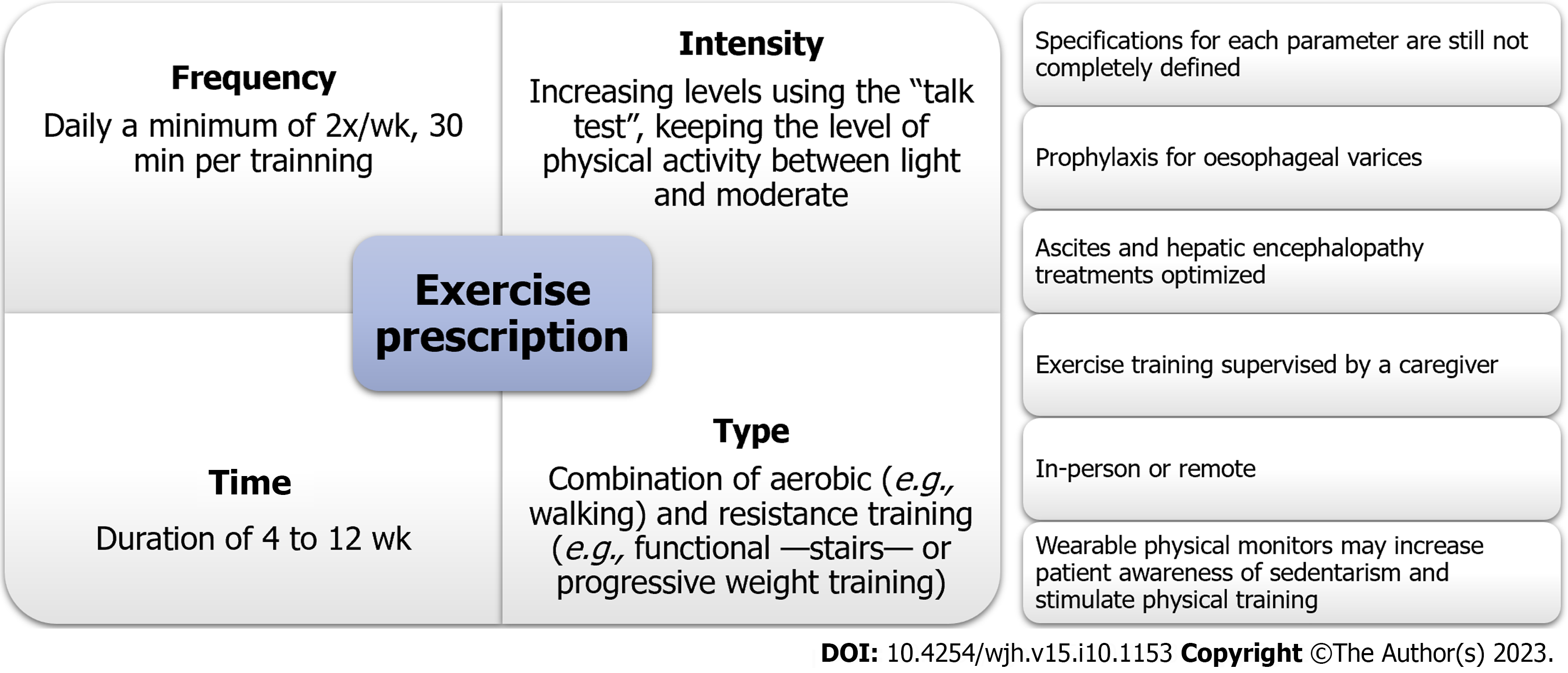
Thus far, we know that exercise prescriptions should adhere to the FITT recommendations. However, specifications for each parameter have not been completely defined for this population. In recent review articles, the authors proposed relevant, useful orientations based mostly on expert opinions[7,45]. An exercise prescription must contain aerobic training (e.g., walking), resistance exercises for the lower and upper extremities (e.g., functional - stairs - or progressive weight training) and flexibility or balance training (e.g., stretching and balance exercises) and use the talk test to ensure adequate intensity throughout the training[7,42].
Cirrhotic patients are more prone to remaining sedentary more frequently. Dunn et al[35] showed, by analysing the level of physical activity exhibited by cirrhotic patients on the transplant waiting list, that they engaged in some of the lowest reported activity of all chronic disease patients, similar to that of patients with advanced chronic lung disease or renal failure. On average, they spent 75.9% of their waking hours in sedentary activities and only 18.9% in moderate-vigorous activity[35]. In addition, Dunn et al[35] stated that sedentariness was associated with patient death while on the list.
Technology may contribute to monitoring these critical patients’ physical activity, increasing their compliance and response to interventions. Wearable physical monitors may provide a more realistic estimation of sedentary cirrhotic patients’ activity levels as patient self-assessments and provider physical activity assessments do not reliably indicate the actual level of physical exercise[35]. In addition, the use of these gadgets may increase patients’ awareness of sedentary physical activities and even stimulate their engagement in physical training, as Lin et al[34] showed.
None of the reviewed articles reported adverse events related to exercise training in this population, suggesting that this approach is safe. Pre-exercise safety may be assessed through disease-related safety issues, screening for cardiopulmonary diseases, and evaluating the effects of other comorbidities[40]. Uniformly, the reviewed studies excluded patients who lacked adequate primary or secondary prophylaxis for oesophageal varices. Other worrying conditions include prohibitive cardiopulmonary diseases, recent alcohol consumption, haemoglobin levels of < 11 g/dL, and incapacitating mental or physical disabilities[40]. Ascites and hepatic encephalopathy treatments should be optimised, and the exercise training should be supervised by a caregiver[7].
Regarding the format for delivering exercise, only one of the articles in this review applied an in-person approach for patients who lived near the rehabilitation centre[30]. Others adopted remote exercise training to facilitate patient engagement and showed benefits of frailty improvement[31,34]. Thus, the evidence suggests that both approaches are safe and effective, allowing the transplant centre and/or the patient to choose the modality that most fits their agenda. In a non-cirrhotic context, a randomised clinical trial of sarcopenic elders suggested that early exercise (supervised exercise followed by home-based exercise) and nutritional intervention aided in restoring lower extremity muscle mass but not physical function[46].
Although the results that these studies reported were not consistent for each criterion of the FITT principles individually, keeping the level of physical activity light-to-moderate, i.e., using exercise that is not so strenuous that the patient cannot talk while performing it (“talk test”), offers a good starting point regardless of the chosen delivery modality. A summary of the exercise recommendations for cirrhotic patients waiting for LT based on the evidence in the literature is presented in Figure 2. A literature review from North America on the use of expert opinion to guide professionals in the prescription of exercise for patients with chronic liver disease recommends moderate-intensity aerobic exercise daily to achieve the goal of walking 150 min a week, which corroborates the findings of this systematic review. Experts also suggest resistance exercises with stretching focused on large muscle groups on alternate days at least twice a week with progressive intensity[40].
This review has some limitations to define. Primarily, due to the limited number of existing studies on this subject, the risk of bias and heterogeneity assessments were not strict, and we opted not to follow with a meta-analysis. Therefore, whilst an acceptable level of design was set, design-based quality checklists were not used. Given the weaknesses of the current evidence and the heterogeneity of the reviewed studies, the above recommendations should be considered carefully. In addition, this review focused on transplant candidates; therefore, extrapolation to other stages of chronic liver diseases or in-hospital patients due to decompensation must be done carefully. Exercise training in this population is intended to maintain physical function during a period of illness and prepare patients for a major surgery that requires hospitalisation and gradual rehabilitation. In addition, no studies thus far have stratified their exercise recommendations based on the severity of liver disease. Future studies should apply well-established frail assessment tools and the existing exercise recommendations, seek to achieve a clinically significant endpoint, and employ a randomly assigned control group.
Although further studies are still needed to guide the prescription of exercise and validate the use of regular physical activity to prevent and treat physical frailty in cirrhotic patients who are on the waiting list for LT, the routine assessment of frailty and practice of regular physical exercise, either in-person or remote, of low to moderate intensity is safe and can improve patients’ functional capacity and pre- and post-LT outcomes.
In patients with end-stage liver disease candidates for liver transplant (LT), frailty is associated with worse clinical outcomes and a higher probability of death. Therefore, the identification of interventions which could modify or reverse this condition is of paramount importance. Although exercise training is widely recommended for individuals with other chronic diseases, evidence still lags well behind for this population.
Exercise programs for cirrhotic patients are still not so frequent, and there is still debate about the optimal method to deliver exercises to them (in-person supervised exercise or home-based programs).
To investigate the existing exercise programmes and prescriptions for cirrhotic patients on the waiting list for liver transplantation, their results concerning frailty pre- and post-intervention and their impact on clinical outcomes.
We searched the PubMed, MEDLINE and Scopus databases using the keyword and free terms “liver transplant”, “frailty”, and “exercise”. The research findings, their contributions to the research in this field, and the problems that remain to be solved should be described in detail. The results were subsequently analysed for the instrument for physical frailty assessment, whether there were any pre-established criteria for inclusion in the transplant list related to frailty assessment, frequency, intensity, type and time of exercise performed, and primary and secondary outcomes evaluated after the physical exercise program.
We identified nine research articles that were included in this review. The instruments for frailty assessment varied amongst them, and five studies prescribed physical activity to patients, one in-person and four to be performed remotely and unsupervised. None reported adverse events related to exercise training. Three articles evaluated the impact of the exercise program on clinical outcomes, reporting a reduction in 90-d readmission rates post-transplant and improvement of frailty scores followed by improved survival of cirrhotic patients waiting for a transplant.
We found that the routine assessment of frailty and practice of regular physical exercise, either in-person or remote, of low to moderate intensity is safe and capable of improving the patient’s functional capacity and favour positive pre- and post-LT outcomes.
Although further studies are still required to guide exercise prescription and validate the practice of regular physical activity for cirrhotic patients on the waiting list for LT, it may improve their outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: International Liver Transplantation Society; The Transplantation Society; Associação Brasileira de Transplante de Órgãos; Academia Nacional de Medicina.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang KV, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 15805] [Article Influence: 658.5] [Reference Citation Analysis (1)] |
| 2. | Williams FR, Milliken D, Lai JC, Armstrong MJ. Assessment of the Frail Patient With End-Stage Liver Disease: A Practical Overview of Sarcopenia, Physical Function, and Disability. Hepatol Commun. 2021;5:923-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Gordon AL, Masud T, Gladman JR. Now that we have a definition for physical frailty, what shape should frailty medicine take? Age Ageing. 2014;43:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E; FOD-CC group (Appendix 1). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 5. | Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, Ma M, Bailey RJ, Jayakumar S, Burak KW, Abraldes JG, Brisebois A, Ferguson T, Majumdar SR. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients With Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am J Gastroenterol. 2016;111:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Cron DC, Friedman JF, Winder GS, Thelen AE, Derck JE, Fakhoury JW, Gerebics AD, Englesbe MJ, Sonnenday CJ. Depression and Frailty in Patients With End-Stage Liver Disease Referred for Transplant Evaluation. Am J Transplant. 2016;16:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (1)] |
| 8. | Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 9. | Chen HW, Dunn MA. Arresting frailty and sarcopenia in cirrhosis: Future prospects. Clin Liver Dis (Hoboken). 2018;11:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J Gastroenterol. 2015;21:7637-7647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 11. | Chang KV, Chen JD, Wu WT, Huang KC, Lin HY, Han DS. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J Formos Med Assoc. 2019;118:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Kremer WM, Nagel M, Reuter M, Hilscher M, Michel M, Kaps L, Labenz J, Galle PR, Sprinzl MF, Wörns MA, Labenz C. Validation of the Clinical Frailty Scale for the Prediction of Mortality in Patients With Liver Cirrhosis. Clin Transl Gastroenterol. 2020;11:e00211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 370] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 14. | Treacy D, Hassett L. The Short Physical Performance Battery. J Physiother. 2018;64:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six Minute Walk Test to assess functional capacity in chronic liver disease patients. World J Gastroenterol. 2007;13:3996-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, Kallenborn EA, Behari J, Landsittel DP, DiMartini AF, Delitto A. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol. 2016;111:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 18. | Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, Bardsley M, Conroy S. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1008] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 19. | Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB; Women's Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 773] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 20. | Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Charette RS, Sarpong NO, Weiner TR, Shah RP, Cooper HJ. Registration of Bony Landmarks and Soft Tissue Laxity during Robotic Total Knee Arthroplasty is Highly Reproducible. Surg Technol Int. 2022;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, Ganger DR, Rahimi RS, McCulloch CE, Haugen CE, McAdams-DeMarco M, Ladner DP, Segev DL, Verna EC; Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020;73:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 23. | Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 24. | Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, Vargas HE, Douglas DD. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Mathur S, Janaudis-Ferreira T, Wickerson L, Singer LG, Patcai J, Rozenberg D, Blydt-Hansen T, Hartmann EL, Haykowsky M, Helm D, High K, Howes N, Kamath BM, Lands L, Marzolini S, Sonnenday C. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Webborn ADJ. ACSM's exercise management for persons with chronic diseases and disabilities. Br J Sports Med. 1997;31:35427. |
| 27. | García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, Rodés J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8858] [Article Influence: 553.6] [Reference Citation Analysis (0)] |
| 29. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12532] [Article Influence: 835.5] [Reference Citation Analysis (0)] |
| 30. | Al-Judaibi B, Alqalami I, Sey M, Qumosani K, Howes N, Sinclair L, Chandok N, Eddin AH, Hernandez-Alejandro R, Marotta P, Teriaky A. Exercise Training for Liver Transplant Candidates. Transplant Proc. 2019;51:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Lin FP, Visina JM, Bloomer PM, Dunn MA, Josbeno DA, Zhang X, Clemente-Sanchez A, Tevar AD, Hughes CB, Jakicic JM, Duarte-Rojo A. Prehabilitation-Driven Changes in Frailty Metrics Predict Mortality in Patients With Advanced Liver Disease. Am J Gastroenterol. 2021;116:2105-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Williams FR, Vallance A, Faulkner T, Towey J, Durman S, Kyte D, Elsharkawy AM, Perera T, Holt A, Ferguson J, Lord JM, Armstrong MJ. Home-Based Exercise in Patients Awaiting Liver Transplantation: A Feasibility Study. Liver Transpl. 2019;25:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, Park S, Bartter T, Dunn MA, Ruiz-Margain A, Kim WR, Duarte-Rojo A. Home-Based Physical Activity and Diet Intervention to Improve Physical Function in Advanced Liver Disease: A Randomized Pilot Trial. Dig Dis Sci. 2020;65:3350-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Lin FP, Bloomer PM, Grubbs RK, Rockette-Wagner B, Tevar AD, Dunn MA, Duarte-Rojo A. Low Daily Step Count Is Associated With a High Risk of Hospital Admission and Death in Community-Dwelling Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1813-1820.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl. 2016;22:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Ney M, Gramlich L, Mathiesen V, Bailey RJ, Haykowsky M, Ma M, Abraldes JG, Tandon P. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol. 2017;23:97-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Lai JC, Volk ML, Strasburg D, Alexander N. Performance-Based Measures Associate With Frailty in Patients With End-Stage Liver Disease. Transplantation. 2016;100:2656-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Oikonomou IM, Sinakos E, Antoniadis N, Goulis I, Giouleme O, Anifanti M, Katsanos G, Karakasi KE, Tsoulfas G, Kouidi E. Effects of an active lifestyle on the physical frailty of liver transplant candidates. World J Transplant. 2022;12:365-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 39. | Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 40. | Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, Holman J, Howes N, Haykowsky MJF, Josbeno DA, McNeely M. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol. 2018;69:1164-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 42. | Brustia R, Savier E, Scatton O. Physical exercise in cirrhotic patients: Towards prehabilitation on waiting list for liver transplantation. A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 6713] [Article Influence: 216.5] [Reference Citation Analysis (0)] |
| 44. | ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6981] [Cited by in RCA: 8256] [Article Influence: 359.0] [Reference Citation Analysis (0)] |
| 45. | Tandon P, Zanetto A, Piano S, Heimbach JK, Dasarathy S. Liver transplantation in the patient with physical frailty. J Hepatol. 2023;78:1105-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | Chang KV, Wu WT, Huang KC, Han DS. Effectiveness of early versus delayed exercise and nutritional intervention on segmental body composition of sarcopenic elders - A randomized controlled trial. Clin Nutr. 2021;40:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |