Published online Oct 27, 2023. doi: 10.4254/wjh.v15.i10.1140
Peer-review started: August 17, 2023
First decision: September 5, 2023
Revised: September 14, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 27, 2023
Processing time: 67 Days and 14.4 Hours
The lymphatic system is crucial in maintaining the body fluid homeostasis. A dysfunctional lymphatic system may contribute to the refractoriness of ascites and edema in cirrhosis patients. Therefore, assessment of lymphatic dysfunction in cirrhosis patients with refractory ascites (RA) can be crucial as it would call for using different strategies for fluid mobilization.
To assessing the magnitude, spectrum, and clinical associations of lymphatic dysfunction in liver cirrhosis patients with RA.
This observational study included 155 consecutive cirrhosis patients with RA. The presence of clinical signs of lymphedema, such as peau d’orange appearance and positive Stemmer sign, intestinal lymphangiectasia (IL) on duodenal biopsy seen as dilated vessels in the lamina propria with strong D2-40 immunohistochemistry, and chylous ascites were used to diagnose the overt lymphatic dysfunctions.
A total of 69 (44.5%) patients out of 155 had evidence of lymphatic dysfunction. Peripheral lymphedema, found in 52 (33.5%) patients, was the most common manifestation, followed by IL in 42 (27.0%) patients, and chylous ascites in 2 (1.9%) patients. Compared to patients without lymphedema, those with lymphedema had higher mean age, median model for end-stage liver disease scores, mean body mass index, mean ascitic fluid triglyceride levels, and proportion of patients with hypoproteinemia (serum total protein < 5 g/dL) and lymphocytopenia (< 15% of total leukocyte count). Patients with IL also had a higher prevalence of lymphocytopenia and hypoproteinemia (28.6% vs. 9.1%, P = 0.004). Seven (13%) patients with lymphedema had lower limb cellulitis compared to none in those without it. On multivariate regression analysis, factors independently associated with lymphatic dysfunction included obesity [odds ratio (OR): 4.2, 95% confidence intervals (95%CI): 1.1–15.2, P = 0.027], lymphocytopenia [OR: 6.2, 95%CI: 2.9–13.2, P < 0.001], and hypoproteinemia [OR: 3.7, 95%CI: 1.5–8.82, P = 0.003].
Lymphatic dysfunction is common in cirrhosis patients with RA. Significant indicators of its presence include hypoproteinemia and lymphocytopenia, which are likely due to the loss of lymphatic fluid from the circulation. Future efforts to mobilize fluid in these patients should focus on methods to improve lymphatic drainage.
Core Tip: Lymphatic dysfunction is often underappreciated in advanced cirrhosis patients. Our study evaluated the magnitude, spectrum, and associations of lymphatic dysfunction in cirrhosis patients with refractory ascites (RA). Nearly half (44.5%) of the studied population (n = 155) revealed evidence of overt lymphatic dysfunction in the forms of peripheral lymphedema (33.5%), intestinal lymphangiectasia (27.0%), and chylous ascites (1.9%). Obesity, hypoproteinemia, and lymphocytopenia were independently associated with lymphatic dysfunction in said patients. From a therapeutic standpoint, it can be extremely important to evaluate lymphatic dysfunction in cirrhosis patients with RA since it would call for using different strategies for fluid mobilization.
- Citation: Arya R, Kumar R, Kumar T, Kumar S, Anand U, Priyadarshi RN, Maji T. Prevalence and risk factors of lymphatic dysfunction in cirrhosis patients with refractory ascites: An often unconsidered mechanism. World J Hepatol 2023; 15(10): 1140-1152
- URL: https://www.wjgnet.com/1948-5182/full/v15/i10/1140.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i10.1140
The lymphatic system is crucial in maintaining the body fluid homeostasis[1]. By recirculating surplus tissue fluid back into the bloodstream, the lymphatic system prevents tissues from becoming edematous. In patients with cirrhosis and portal hypertension (PHT), the production of lymph from the liver and intestines is significantly increased[2,3]. Increased production of lymph promotes lymphangiogenesis, which in turn tends to improve the functional capacity of the lymphatics. However, as cirrhosis progresses, these compensatory mechanisms become overwhelmed, leading to the development of ascites and edema[2-4]. Subsequently, functional impairment of the lymphatic system also sets in, resulting in further worsening of the fluid accumulation[4,5]. The consequent lymphatic flow stagnation and leakage lay the ground for lymphedema development, which is the deposition of protein-rich lymph fluid within the tissues[6]. The gut lymphatics are important for maintaining the abdominal fluid balance. Studies have shown that patients with cirrhosis have much higher abdominal lymph production (up to 30-fold) and lymph flow in the thoracic duct (8-9 L/day)[7,8]. Moreover, a persistently high lymphatic pressure associated with PHT has the potential to cause intestinal lymphangiectasia (IL) and chylous ascites (CA) in cirrhosis patients[9-11]. Rupture of IL with subsequent loss of lymph can result in hypoproteinemia, lymphocytopenia, and malabsorption of fat[4,12].
Lymphatic dysfunction occurs in patients with cirrhosis, although the amount of published data on this subject is extremely limited[2-5]. There have been only a few reports of CA and IL in these patients[10,11]. Lymphedema has not even been considered in cirrhosis patients with persistent peripheral edema. The structural and functional changes in gut lymphatics, which are vital in splanchnic lymph drainage, remain an unexplored area in cirrhosis patients. Despite being a significant factor in maintaining fluid homeostasis, the lymphatic system is commonly ignored when assessing the pathophysiology of refractory ascites (RA) in cirrhosis patients. RA, which represents an extreme form of fluid accumulation, eventually develops in about 10% of cirrhosis patients with advanced decompensation[13].
Over the past two decades, a better understanding of the lymphatic vascular system has emerged; however, little is known about how lymphatic dysfunction contributes to the pathophysiology of advanced cirrhosis. Given the significant role the lymphatic system plays in maintaining the balance of body fluids, it is reasonable to assume that a dysfunctional lymphatic system may contribute to the refractoriness of ascites and edema in cirrhosis patients. Therefore, assessing lymphatic dysfunction in such patients can provide a novel approach to tissue decongestion. Case studies have shown dietary changes to be effective in controlling ascites and improving liver stiffness in cirrhosis patients with IL[10,14].
In a recent study, treatment with recombinant vascular endothelial growth factor (VEGF) C, a lymphatic-specific growth factor, resulted in decreased portal pressure, enhanced lymphatic drainage, and decreased ascites in cirrhotic rats[15]. However, techniques for assessing lymphatic structure and function in cirrhosis are limited. Lymphography and lymphoscintigraphy lack sufficient accuracy and are not readily available. Lymphedema, IL, and CA are significant surrogate markers of lymphatic dysfunction that need to be investigated in patients with cirrhosis. Hence, this study assessed the magnitude, spectrum, and clinical associations of lymphatic dysfunction in patients with liver cirrhosis and RA using surrogate markers.
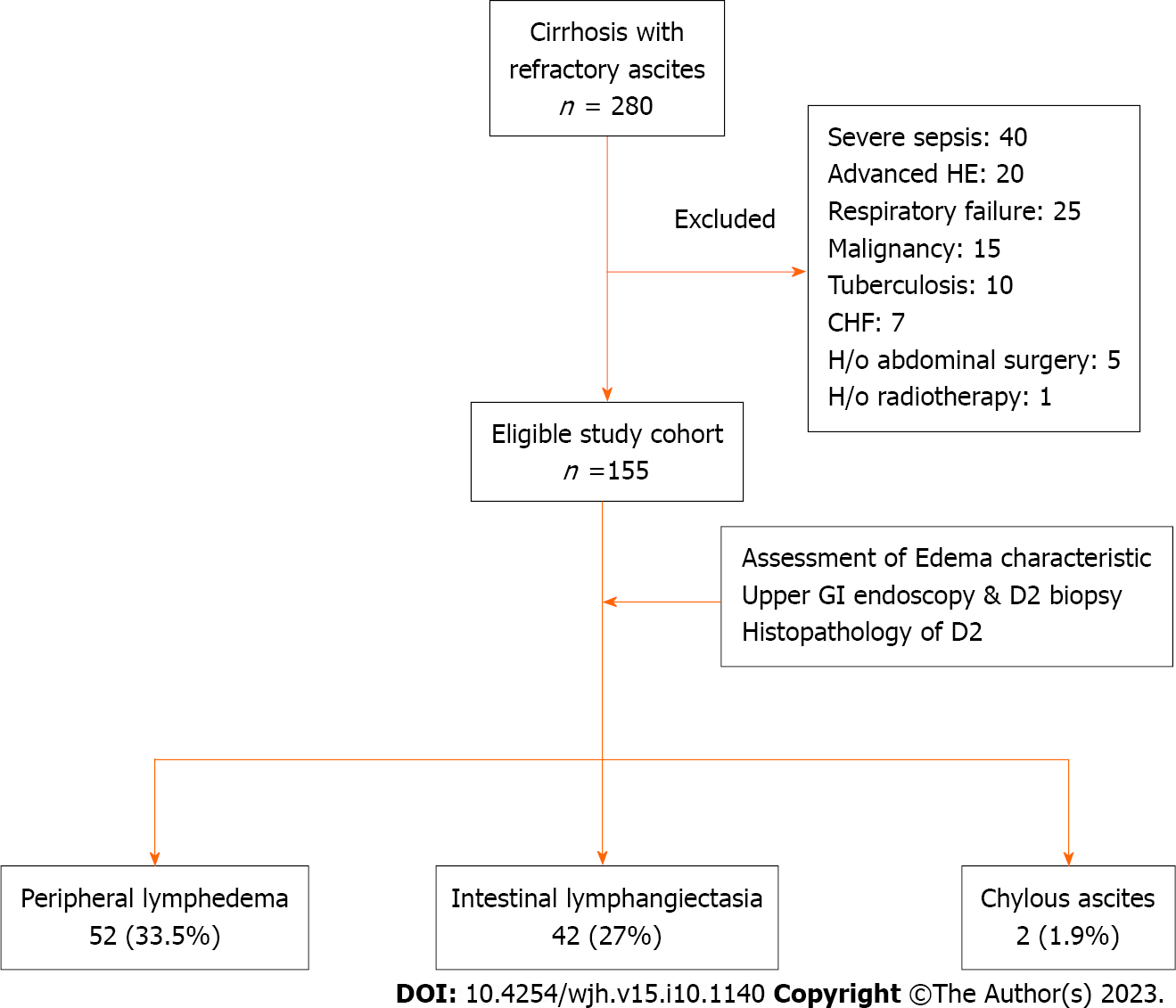
This observational study was conducted in the Department of Gastroenterology, All India Institute of Medical Sciences, Patna, a tertiary care medical center in India. The protocol was approved by the institute’s research board, and investigations were conducted according to the Declaration of Helsinki principles. Consecutive adult liver cirrhosis patients between 18 years and 75 years admitted with RA from December 2021 to March 2023 were screened according to the inclusion criteria. RA was diagnosed as ascites that could not be mobilized or the early recurrence of which could not be prevented because of a lack of response to a maximum dose of diuretic treatment or because patients developed complications that precluded the use of an effective dose of diuretics[16]. Liver cirrhosis was diagnosed by clinical features, imaging characteristics, and endoscopic findings. Patients with low serum ascites albumin gradient (< 1.1) ascites, congestive heart failure, primary or metastatic abdominal malignancy, history of radiation therapy, concomitant tuberculosis, history of abdominal surgery, and filariasis were excluded from the study. Cirrhosis patients with severe sepsis, advanced hepatic encephalopathy, and respiratory failure were also excluded from the study.
Demographic and clinical data, including the degree of ascites and duration of RA, were noted at baseline. Estimates of dry weight were made for the corrected body mass index calculation by deducting 15% of the actual weight due to grade-3 ascites and an additional 5% because of peripheral edema. Per the Asian standard, body mass index > 25 kg/m2 was considered obese. Routine investigations, including hemograms, liver function tests, kidney function tests, international normalized ratio, fasting blood sugar, and ascitic fluid analysis, including ascitic fluid triglyceride estimation, were performed in all patients, who also underwent a complete etiological workup. The severity of cirrhosis was assessed by Child-Pugh classification and model for end-stage liver disease (MELD) scores. Standard medical therapy, including etiology-specific treatment, was given to all patients.
The lymphatic dysfunction was ascertained by the presence of one or more of the following surrogate markers: (1) Features of peripheral lymphedema, as evident by physical characteristics such as painless leg edema, pitting or non-pitting, showing orange peel (peau d’orange) appearance, and positive Stemmer sign; (2) Presence of IL on endoscopy and/or histopathological examination of duodenal biopsy specimens; and (3) Presence of CA, as indicated by milky white ascites with elevated triglyceride level > 110 mg/dL.
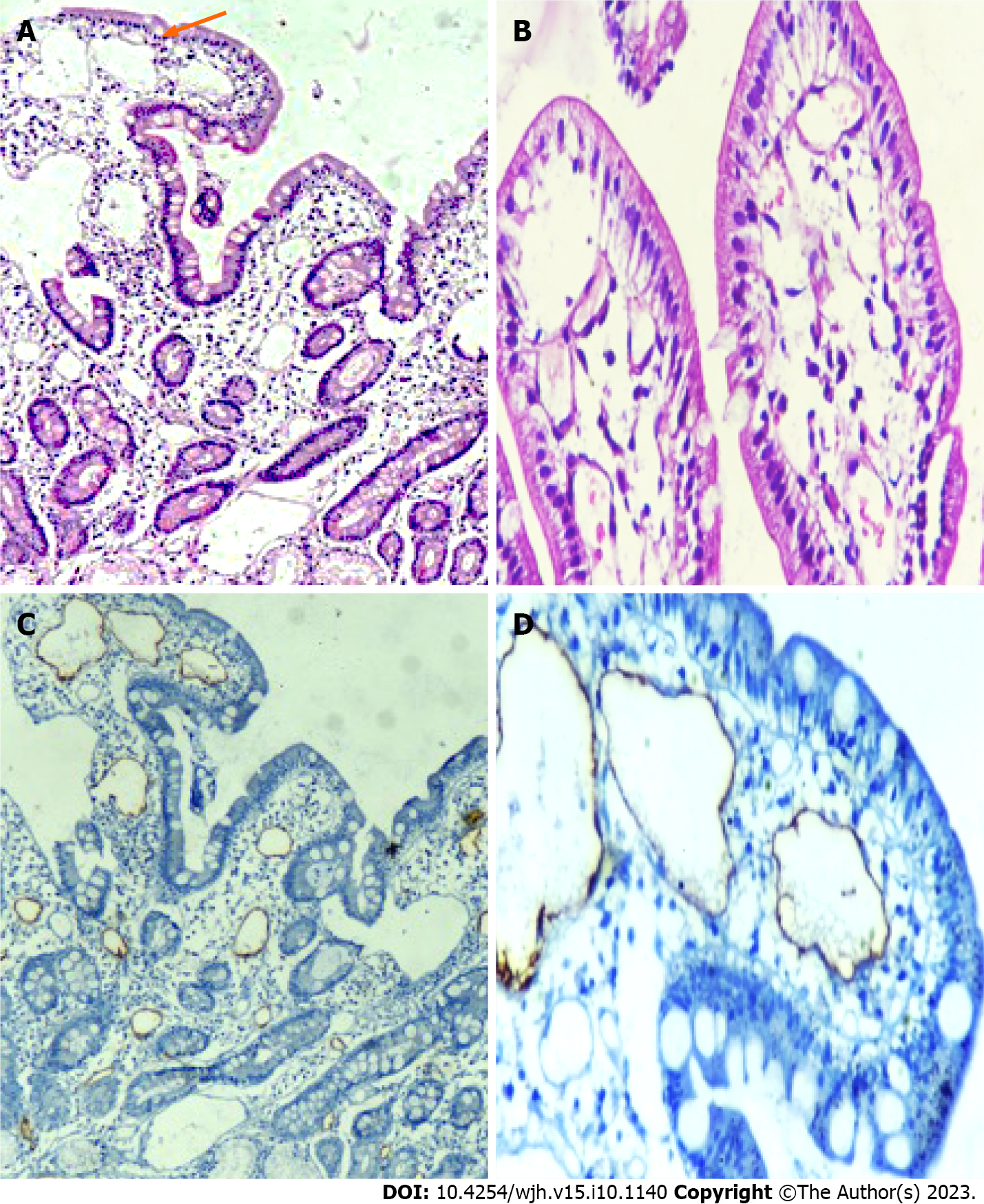
The diagnosis of IL on endoscopy was based on the presence of swollen mucosa with scattered white spots suggestive of dilated lacteals. Portal hypertensive duodenopathy (PHD) was considered in the presence of swollen duodenal mucosa with varying degrees of erythema, erosions, friability, and telangiectasia. Irrespective of endoscopic evidence of PHD, biopsies were obtained from the second part of the duodenum (D2), distal to the ampulla of Vater, using standard endoscopic biopsy forceps in all patients. The biopsies obtained were submitted in a vial containing diluted formalin for fixation. Histological examination (hematoxylin and eosin) was performed by an expert pathologist. A markedly dilated vessel in the lamina propria, which on immunohistochemistry showed strong D2-40 positivity, confirmed the presence of IL. Hypoproteinemia was considered when total serum protein was < 5 g/dL, with a decrease in both albumin and globulin. Lymphocytopenia was considered when the proportion of blood lymphocytes was < 15% of the total leukocyte count.
Because the magnitude and impact of lymphatic dysfunction in cirrhosis are not clearly defined, we conducted this observational study as an exploratory research project with a cross-sectional design intended to include a minimum of 100 eligible patients. Continuous variables, depending on the normalcy of distribution, were expressed as mean ± standard deviation or median (range). Categorical data were represented as proportion. To compare the normal covariates between patients with and without lymphatic dysfunction, independent sample t-test or Mann-Whitney U test was used when applicable. Comparisons in categorical variables were performed using χ2 or Fisher’s test. Multivariate regression analysis (MVA) was used to determine independent associates of lymphatic dysfunction. The relevant variables in the univariate analysis with P < 0.10 were considered for MVA. However, highly correlated variables were excluded to avoid multicollinearity in regression analysis. The odds ratio (OR) and 95% confidence interval (95%CI) for all significant variables in MVA were reported. Data were analyzed using SPSS software version 23.0 (SPSS, Chicago, IL, United States), wherein P < 0.05 was taken as significant.
A total of 280 cirrhosis patients with RA were screened during the study, but 125 of them were found to be ineligible based on the inclusion and exclusion criteria (Figure 1). The study cohort ultimately consisted of 155 cirrhosis patients with RA.
The mean age of the patients was 49.0 ± 13.1 years with a predominance of male subjects (77.0%). The predominant etiology of cirrhosis involved alcohol (37.4%), followed by non-alcoholic steatohepatitis (35.4%). The median Child-Pugh and MELD scores were 10.3 and 18.1, respectively. Twenty-seven (17.4%) patients were diabetic, 16 (10.3%) patients were obese, and 9 (5.8%) patients had functional chronic kidney disease. All patients had grade-3 ascites, and the median duration of RA was 5 (3-48) mo. On endoscopy, large esophageal varices were found in 60 (38.0%) patients, and severe portal hypertensive gastropathy (PHG) was noted in 35 (22.6%) patients. Additionally, 31 (20.0%) patients had evidence of PHD. The presence of PHD was independent of the severity of PHG as 70.0% of patients with PHD had mild PHG. Other baseline characteristics are specified in Table 1.
| Parameters | n = 155 |
| Age in yr, mean ± SD | 49.0 ± 13.1 |
| Male | 119 (77.0) |
| Etiology of cirrhosis | |
| Alcohol | 58 (37.4) |
| Hepatitis B | 23 (14.8) |
| Hepatitis C | 7 (4.5) |
| NASH | 55 (35.4) |
| Others | 14 (9.0) |
| Duration of RA in mo, median (range) | 5 (-48) |
| MELD scores, median (range) | 18.1 ± 8.6, 17(6-42) |
| Child-Pugh score, mean ± SD | 10.3 ± 1.8 |
| Diabetes mellitus | 27 (17.4) |
| Hypertension | 12 (7.7) |
| Corrected body mass index in kg/m2, mean ± SD | 20.25 ± 3.40 |
| Obesity | 16 (10.3) |
| Chronic kidney disease | 9 (5.8) |
| Serum total bilirubin in mg/dL, median (range) | 2.7 (0.3-28.5) |
| Serum AST, median (range) U/L | 60 (18-672) |
| Serum ALT, median (range) U/L | 32 (4-250) |
| Serum total protein, mean ± SD mg/dL | 5.8 ± 0.7 |
| Serum albumin, mean ± SD mg/dL | 2.5 ± 0.5 |
| Hypoproteinemia | 42 (27.0%) |
| INR, median (range) | 1.6 (0.4-6.7) |
| Serum sodium, mean ± SD meq/L | 130.0 ± 6.5 |
| Serum potassium, mean ± SD meq/L | 4.07 ± 2.40 |
| Serum urea in mg/dL, median (range) | 34 (12-191) |
| Creatinine in mg/dL, median (range) | 0.9 (0.3-3.7) |
| Hemoglobin in g/dL, mean ± SD | 8.8 (4.2-65.0) |
| Total leukocyte count as /cmm, median (range) | 6130 (1750-34300) |
| Lymphocytopenia | 72 (46.5) |
| Esophageal varices | |
| None | 3 (1.9) |
| Small grade | 92 (59.4) |
| Large grade | 60 (38.0) |
| PHG | |
| Mild | 118 (76.0) |
| Severe | 35 (22.6) |
| PHD | 31 (20.0) |
| Ascitic fluid analysis | |
| Cell count as /µL, median (range) | 100 (0-11200) |
| Protein median in gm/dL, median (range) | 1.1 (0.1-3.5) |
| ADA in U/L, median (range) | 10 (1-33) |
| Triglycerides in mg/dL, median (range) | 19.5 (0-224.0) |
| SAAG, median (range) | 2.0 (1.2-4.0) |
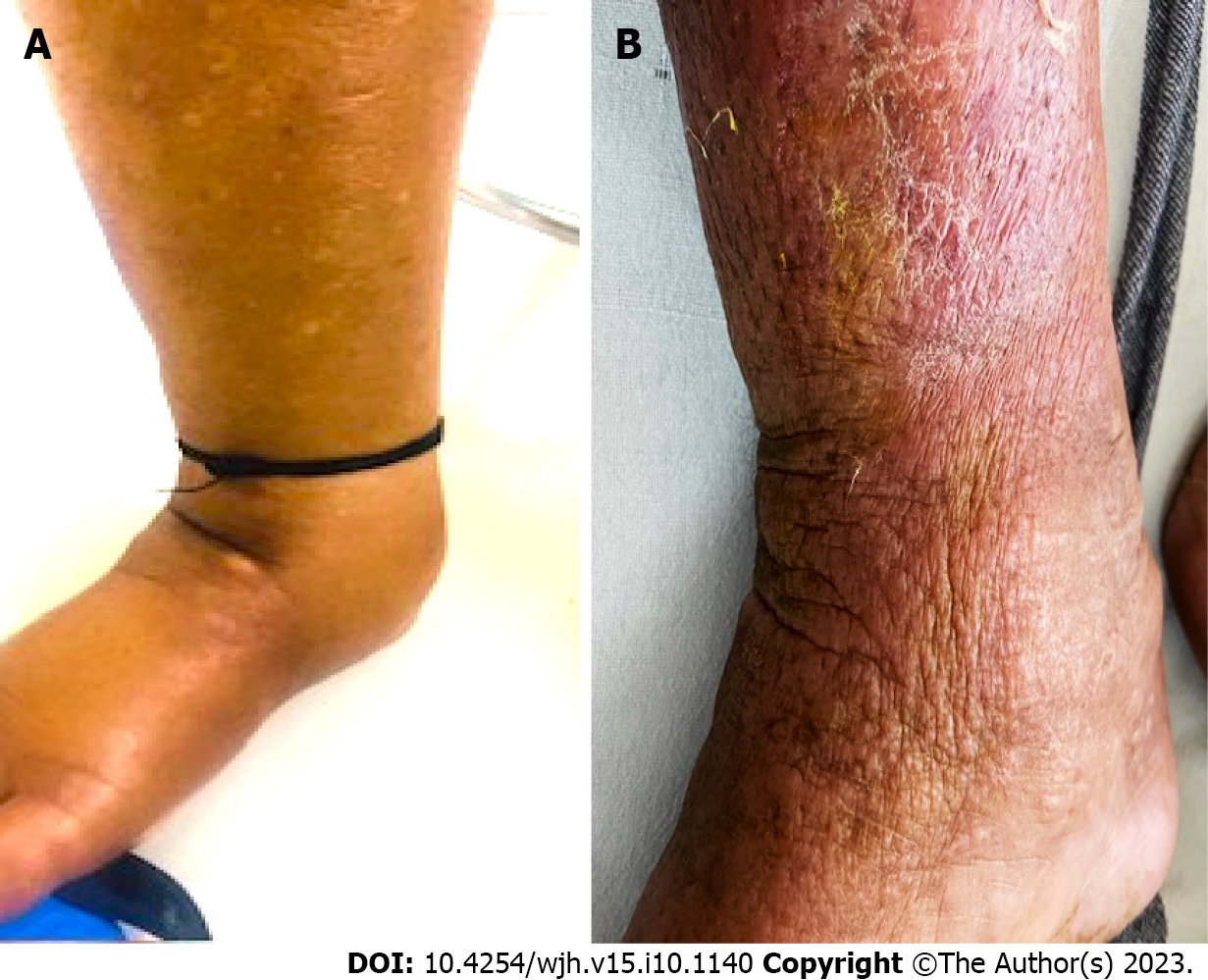
Edema characteristics: The physical appearance of peripheral edema revealed two distinct patterns (Figure 2). In 52 (33.5%) patients, edema was severe, pitting or non-pitting, with skin texture showing exaggerated dorsal skin creases, hyperkeratosis, and peau d’orange appearance. Moreover, these patients had a positive Stemmer sign, suggesting the presence of lymphedema. In the remaining 103 (66.5%) patients, edema was of the pitting type, skin texture was smooth, and the Stemmer sign was negative.
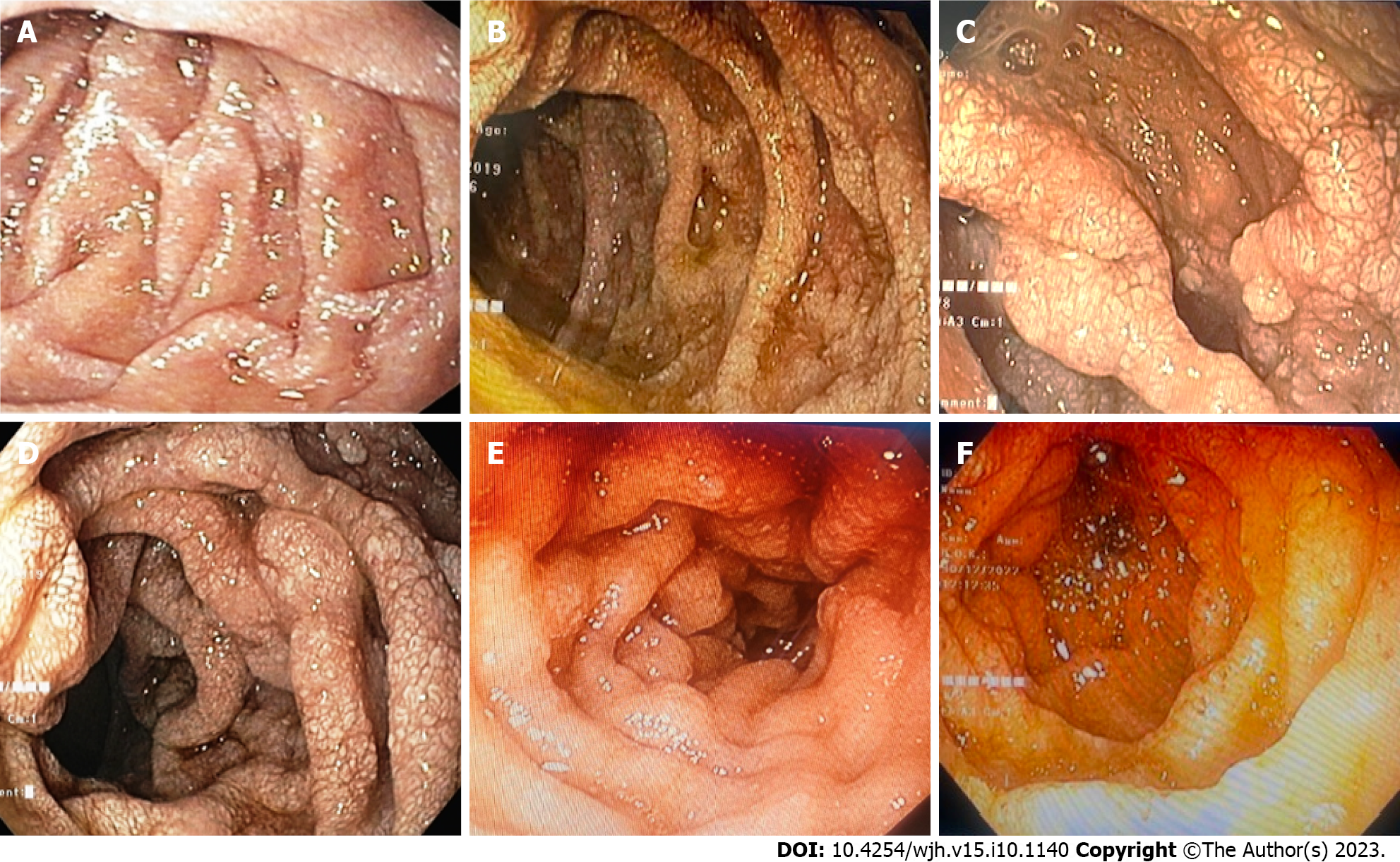
Endoscopic and histopathological evidence of IL: All patients tolerated the endoscopic procedure with D2 biopsy irrespective of coagulopathy and thrombocytopenia. Eight (5.1%) patients had endoscopic evidence of IL as a whitish enlarged villi on swollen mucosa (Figure 3). On histopathological examination of D2 biopsy specimens, 42 (27.0%) patients revealed markedly dilated vessels in the lamina propria, which on immunohistochemistry with D2-40 confirmed the presence of IL (Figure 4). Thus, 34 (22%) patients revealed IL on histopathological examination without endoscopic evidence of the same.
CA: The median level of triglyceride in ascitic fluid was 19.5 (0–224.0) mg/dL. Based on a physical examination of ascetic fluid and triglyceride levels, 2 patients (1.29%) were found to have CA.
Thus, a total of 69 (44.5%) cirrhosis patients with RA had evidence of lymphatic dysfunction in the forms of lymphedema, IL, and CA either alone or in combination. Lymphedema, present in 52 (33.5%) patients, was the most common manifestation of lymphatic dysfunction. Twenty-six patients had only lymphedema, 18 patients had only IL, 24 patients had both lymphedema and IL, and 2 patients had lymphedema as well as IL and CA.
Clinically, lymphedema was the most relevant marker of lymphatic dysfunction in the study population. Compared to patients without lymphedema, those with lymphedema were older (mean age of 53.3 years vs 47.7 years, P = 0.007), had more severe liver disease (median MELD scores of 21 vs 14, P < 0.001), and had a greater proportion of obese cirrhosis (21.0% vs 5.8%, P = 0.012). Patients with lymphedema had a higher prevalence of PHD (32.7% vs 13.6%, P = 0.001) and histopathological evidence of IL (46.2% vs 17.5%, P < 0.001) than those without it. Furthermore, the mean ascitic fluid triglyceride levels (P = 0.006), the proportion of lymphocytopenia (73.0% vs 33.0%, P < 0.001), and hypoproteinemia (50.0% vs 15.5%, P < 0.001) were significantly higher in patients with lymphedema than those without it (Supplementary Table 1). Lower limb cellulitis was noted in 7 (13.0%) patients with lymphedema vs none in patients where lymphedema was absent.
Patients with IL had a higher prevalence of lymphedema than those without IL (57.1% vs 23.6%, P < 0.001). The frequency of lymphocytopenia and hypoproteinemia was higher in patients with IL than those without it (28.6% vs 9.1%, P = 0.004). However, IL was not associated with age, severity of liver cirrhosis, or metabolic comorbidities. With regard to the only 2 patients with CA, the levels of triglyceride in their ascitic fluid were observed at 137 mg/dL and 224 mg/dL. Both patients were male, diabetic, and had high Child-Pugh scores (13 and 12). Both had evidence of IL on endoscopy and D2 biopsy.
The mean age (52.0 ± 13.8 vs 47.8 ± 12.3, P < 0.04) and proportion of obese cirrhosis (16.0% vs 5.8%) were higher in patients with lymphatic dysfunction than those without it. The median duration of RA (6 mo vs 4 mo, P = 0.02), median MELD score (18 vs 14, P = 0.003), and mean Child-Pugh score (10.0 ± 1.9 vs 9.9±1.6) were similarly higher in patients with lymphatic dysfunction (Table 2). Among the patients with lymphatic dysfunction, lymphopenia was noted in 49 (79.0%) patients, hypoproteinemia in 29 (42.0%) patients, and a combined hypoproteinemia plus lymphopenia in 21 (30.0%) patients. The values of serum bilirubin, serum aspartate aminotransferase, serum alanine aminotransferase, and international normalized ratio were higher, while serum sodium was lower in patients with lymphatic dysfunction compared to those without it.
| Parameters | LD present, n = 69 | LD absent, n = 86 | P value |
| Age in yr, mean ± SD | 52.0 ± 13.8 | 47.8 ± 12.3 | 0.040 |
| Male, n (%) | 56 (81.2%) | 63 (73.3%) | 0.320 |
| Etiology of cirrhosis, n (%) | 0.050 | ||
| Alcohol | 22 (31.8%) | 36 (41.8%) | |
| Hepatitis | 14 (20.2%) | 9 (10.4%) | |
| Hepatitis C | 01 (1.4%) | 6 (7.0%) | |
| NASH | 28 (40.5%) | 27 (31.3%) | |
| Others | 04 (5.7%) | 10 (11.6%) | |
| Duration of RA in mo, median (range) | 06 (5-48) | 4 (3-36) | 0.020 |
| MELD scores, median (range) | 18 (7-42) | 14 (6-38) | 0.003 |
| Child-Pugh score, mean ± SD | 10 ± 1.9 | 9.9 ± 1.6 | 0.001 |
| Diabetes mellitus, n (%) | 15 (21.7%) | 12 (14.0%) | 0.280 |
| Hypertension, n (%) | 06 (8.7%) | 6 (7.0%) | 0.760 |
| Corrected BMI in kg/m2, mean ± SD | 22.2 ± 2.3 | 20.8 ± 4.6 | 0.060 |
| Obesity, n (%) | 11 (16.0%) | 5 (5.8%) | 0.030 |
| Chronic kidney disease, n (%) | 4 (5.8%) | 5 (5.8%) | 1.000 |
| Serum total bilirubin in mg/dL, median (range) | 3.6 (0.3-28.5) | 2.1 (0.4-26.4) | 0.040 |
| Serum AST in U/L, median (range) | 67 (18-672) | 56 (22-238) | 0.010 |
| Serum ALT in U/L, median (range) | 34 (4-250) | 30 (7-202) | 0.020 |
| Serum total protein in mg/dL, mean ± SD | 5.6 ± 0.6 | 6.0 ± 0.8 | 0.001 |
| Severe hypoproteinemia, n (%) | 29 (42.0%) | 13 (15.1%) | < 0.001 |
| Albumin in mg/dL, mean ± SD | 2.4 ± 0.5 | 2.6 ± 0.5 | 0.005 |
| INR, median (range) | 1.8 (0.8-3.8) | 1.5 (0.4-6.7) | 0.007 |
| Serum sodium in meq/L, mean ± SD | 128.0 ± 7.2 | 131.0 ± 5.5 | 0.001 |
| Serum potassium in meq/L, mean ± SD | 4.2 ± 1.2 | 3.9 ± 2.4 | 0.130 |
| Serum urea, median (range) mg/dL | 42 (12-191) | 31 (12-188) | 0.060 |
| Creatinine in mg/dL, median (range) | 1.0 (0.4-2.9) | 0.9 (0.3-3.7) | 0.160 |
| Hemoglobin in g/dL, mean ± SD | 8.6 (5.3-12.3) | 8.8 (4.2-15) | 0.360 |
| Total leukocyte count as /cmm, median (range) | 7000 (1750-34300) | 5550 (2050-12680) | 0.960 |
| Lymphocytopenia, n (%) | 49 (79.0%) | 23 (26.7%) | < 0.001 |
| Combined lymphocytopenia and hypoproteinemia, n (%) | 21 (30.4%) | 2 (2.3%) | < 0.001 |
| Esophageal varices, n (%) | 0.610 | ||
| None | 02 (2.9%) | 1 (1.2%) | |
| Small grade | 40 (58.0%) | 52 (60.5%) | |
| Large grade | 27 (39.1%) | 33 (38.4%) | |
| PHG, n (%) | 0.025 | ||
| Mild | 46 (66.7%) | 72 (84.0%) | |
| Severe | 21 (30.4%) | 14 (16.0%) | |
| PHD, n (%) | 22 (31.8%) | 9 (10.4%) | |
| Ascitic fluid analysis | |||
| Cell count as /µL, median (range) | 150 (0-3500) | 100 (0-1120) | 0.970 |
| Protein in gm/dL, median (range) | 0.9 (0.1-2.5) | 1.1 (0.6-3.5) | 0.370 |
| ADA in U/L, median (range) | 8.5 (1.0-33.0) | 11.0 (2.0-30.0) | 0.200 |
| Triglycerides in mg/dL, median (range) | 23 (2-224) | 18 (0-64) | 0.070 |
| SAAG, median (range) | 1.8 (1.2-4.0) | 2.1 (1.2-3.5) | 0.390 |
| Lower limb cellulitis | 7 (10.0%) | 0 | 0.003 |
On MVA (Table 3), factors independently associated with lymphatic dysfunction included obesity (OR: 4.2, 95%CI: 1.1–15.2, P = 0.027), lymphocytopenia (OR: 6.2, 95%CI: 2.9–13.2, P < 0.001), and hypoproteinemia (OR: 3.7, 95%CI: 1.5–8.8, P = 0.003). When independent predictors of only lymphedema were assessed by MVA, age (OR: 1.06, P = 0.002) and Child-Pugh scores (OR: 1.82, P = 0.005) were found to be significant, apart from obesity (OR: 6.3, P = 0.012), lymphocytopenia (OR: 3.5, P = 0.01), and hypoproteinemia (OR: 7.1, P = 0.001) (Table 4).
| Parameters | P value | OR | 95%CI |
| Obesity | 0.027 | 4.2 | 1.1-15.2 |
| Lymphocytopenia | < 0.001 | 6.2 | 2.9-13.2 |
| Hypoproteinemia | 0.003 | 3.7 | 1.5-8.8 |
| Parameters | P value | OR | 95%CI |
| Age | 0.002 | 1.06 | 1.02-1.10 |
| Child-Pugh score | 0.005 | 1.82 | 1.19-2.79 |
| Obesity | 0.012 | 6.33 | 1.49-26.90 |
| Hypoproteinemia | 0.001 | 7.10 | 2.44-20.70 |
| Lymphocytopenia | 0.011 | 3.55 | 1.34-9.38 |
Our study was the first to assess the characteristics of lymphatic dysfunction in liver cirrhosis patients who have RA, an extreme form of fluid accumulation. We found evidence of lymphatic dysfunction in nearly half (44.5%) of the patients. The spectrum of dysfunctions included peripheral lymphedema (33.5%), IL (27.0%), and CA (1.3%). Obesity, lymphocytopenia, and hypoproteinemia independently predicted the presence of lymphatic dysfunction in such patients. Additionally, higher mean ages and Child-Pugh scores were independently associated with peripheral lymphedema, the most common manifestation of lymphatic dysfunctions.
Portal pressure in cirrhosis patients positively correlated with lymphatic flow[17,18]. As cirrhosis progresses, functional deficiencies in the lymphatic system emerge, causing flow stagnation and leakage of lymph from the ectatic lymphatic system[4,5]. These changes lay the ground for the lymphedema development. Our study detected evidence of lymphedema in one-third of cirrhosis patients with RA. Lymphedema should be common in patients with advanced cirrhosis, given the lymphatic failure that often follows cirrhosis, yet there is a dearth of research on it in the existing literature. It is difficult to distinguish early lymphedema from edema due to the change in plasma hydrostatic-oncotic pressure balance. The presence of physical signs, such as a peau d’orange appearance and the positive Stemmer sign, may reflect the advanced stage of lymphedema. Patients with lymphedema are more susceptible to cellulitis due to their hyperkeratotic surface, deep fissures, stagnant lymph, and weakened immunity. In our study, 13% of lymphedema patients had lower limb cellulitis, compared to none in those who did not have lymphedema. In fact, lymphedema is considered the most important risk factor for cellulitis[19].
In our study, 31 (20.0%) patients revealed evidence of PHD on endoscopy, though only 8 (5.1%) patients had macroscopic evidence of IL. PHD in cirrhosis has been reported at 8.4% by Menchén et al[20], 14% by Misra et al[21], and 51% by Barakat et al[22] in earlier studies. No study has so far reported the endoscopic prevalence of IL in cirrhosis patients. Notably, 27.0% of the study subjects had evidence of IL only on histological examination of D2 biopsy specimens. Very few studies have examined histopathological changes in the duodenum of cirrhosis patients[15,21,22]. Barakat et al[22] reported marked capillary congestion and capillary angiogenesis in duodenal mucosa of cirrhosis patients. The changes were mostly marked in the subepithelial location, which on the immunohistochemical stain was CD34 positive. Notably, CD34 is a pan-endothelial marker that can also be positive in the lymphatic endothelium[23]. As a selective marker of lymphatic endothelium (D2-40) was not used in that study, it is possible that IL could have been misinterpreted as capillary congestion and angiogenesis. In a recent prospective study, IL was found to be significantly higher in patients with decompensated cirrhosis than in compensated cirrhosis, and the density of IL on duodenal biopsy was associated with systemic inflammation and 3-mo mortality[24]. It is believed that IL in cirrhosis results from a persistent rise in lymphatic pressure secondary to PHT. However, it is unclear why IL does not manifest in all cirrhotic patients despite sustained PHT.
Because intestinal lymph contains proteins, chylomicrons, and lymphocytes, rupture of IL with subsequent leakage of lymph into the intestine can lead to hypoproteinemia and lymphocytopenia[4,5]. Regardless of the presence of IL, study participants with lymphatic dysfunction displayed lymphocytopenia in 79.0% of cases and hypoproteinemia in 42.0% of the cases, indicating that lymphatic dysfunction at non-enteric sites can also result in loss of lymphocytes and protein from circulation. CA, which was observed in 2 patients, is a rare complication caused by rupture of subserosal lymphatic vessels secondary to a sustained high portal pressure[25]. As intestinal lymph contains triglyceride-rich fat droplets (chylomicrons), CA appear milky in color. Notably, the rupture of hepatic lymphatics does not produce CA as it is devoid of fat droplets. Although < 1% of cirrhosis patients develop CA, cirrhosis has been attributed to 11% of atraumatic CA cases[11,26,27].
Our study revealed several risk factors and indicators of lymphatic dysfunction, including older age, higher MELD and Child-Pugh scores, obesity, lymphocytopenia, and hypoproteinemia, which may point to lymphatic dysfunction in a given patient. It is well recognized that aging alters the structure and function of the lymphatic system. Important aging-related lymphatic alterations include impaired contractile function, decreased nitric oxide lymphatic collectors, and loss of endothelial glycocalyx[28]. Similarly, recent evidence suggests that obesity can significantly impair lymphatic function and increase risk of lymphedema, whereas losing weight can enhance lymphatic functioning[29]. Moreover, lymphatic dysfunction is also involved in the pathogenesis of obesity and obesity-related chronic inflammation[30].
The pathophysiological process underlying lymphatic dysfunction in patients with cirrhosis needs to be explored at the molecular level. In addition to old age and obesity, other variables affecting lymphatic function in patients with cirrhosis include diabetes, dyslipidemia, neurohormonal alterations, and chronic inflammation[4,31,32]. Intestinal lymphatic function may be impacted by intestinal dysmotility and intestinal dysbiosis typically found in advanced cirrhosis[33,34]. Ribera et al[5] discovered that excess nitric oxide production by lymphatic endothelial cells was responsible for poor lymphatic drainage in cirrhotic rats with ascites. Interestingly, when these rats were given a nitric oxide synthase inhibitor, lymphatic drainage was improved and the ascitic volume was much reduced, suggesting an influential role for nitric oxide in the dysfunction of the lymphatic system.
From a therapeutic standpoint, the lymphatic dysfunction in cirrhosis patients with RA are worth evaluating because it would call for using different strategies for fluid mobilization. Some clinical and experimental studies have found improved ascites with dietary changes, VEGF C, and nitric oxide synthase inhibitors in cirrhosis subjects with evident lymphatic dysfunction[8,14,15]. Thus, future efforts to mobilize fluid in these patients might focus on methods to improve lymphatic drainage. Unfortunately, there is no recommendation on how to diagnose and evaluate lymphatic functions in patients with cirrhosis. There are many imaging techniques, such as lymphoscintigraphy and magnetic resonance lymphography, but they are frequently constrained by poor resolution, a lack of standardization, the need for invasive procedures, the danger of radiation exposure, and a lack of accessibility[4]. Low attenuation rims surrounding the portal veins and the intrahepatic vena cava on the computed tomography scan correspond to the lymph congestion secondary to impaired lymphatic drainage[35]. However, the clinical implications of these findings in cirrhotic patients need to be determined. In light of these limitations, surrogate markers of lymphatic dysfunction based on physical examination, routine blood investigations, and endoscopy can be an important leap forward.
Our study data was novel, relevant, and generalizable. It sheds light on a well-known but understudied area that calls for further research to determine the role of lymphatics in the complication of liver cirrhosis. It would be interesting to further investigate whether use of prolymphangiogenic substances like VEGF C and D can enhance lymphatic functioning and fluid mobilization in patients with advanced cirrhosis. It would also be worthwhile investigating whether the placement of a transjugular intrahepatic portosystemic shunt aids in improving lymphatic functions in cirrhosis patients, given the involvement of PHT in lymphatic stasis and leakage.
There were some limitations in our study. First, our study was an association study, so causal inference cannot be drawn. We also did not use any lymphangiographic methods to demonstrate lymphatic stasis or leakage. Further, the diagnosis of lymphedema based on physical characteristics has inherent limitations and is at risk of subjective bias and misclassification error in borderline cases. Hence, further studies are recommended to address such gaps.
In conclusion, patients with advanced liver cirrhosis frequently exhibit signs of overt lymphatic dysfunction. Given the crucial role of the lymphatic system in volume management, its failure may be at the root of many complications of cirrhosis, including RA. Therefore, addressing the lymphatic system in patients with liver cirrhosis may offer a novel strategy in decongesting tissue and improving outcomes.
Lymphatic dysfunction occurs in patients with liver cirrhosis, although the published data on this subject is extremely limited. Given the significant role the lymphatic system plays in maintaining the balance of body fluids, it is reasonable to assume that a dysfunctional lymphatic system may contribute to the refractoriness of ascites and edema in cirrhosis patients.
From a therapeutic standpoint, it can be extremely important to evaluate lymphatic dysfunction in cirrhosis patients with refractory ascites (RA) since it would call for using different strategies for fluid mobilization.
The objectives of this study were to assess the magnitude, spectrum, and clinical associations of lymphatic dysfunction in cirrhosis patients with RA using surrogate markers such as lymphedema, intestinal lymphangiectasia (IL), and chylous ascites (CA).
This observational study was conducted as an exploratory project with a cross-sectional design and included 155 consecutive cirrhosis patients with RA. The presence of clinical signs of lymphedema, IL on duodenal biopsy, and CA were used as surrogate markers of lymphatic dysfunction.
The study found evidence of lymphatic dysfunction in nearly half of the cirrhosis patients with RA. The spectrum of dysfunction included peripheral lymphedema in 33.5%, intestinal IL in 27.0%, and CA in 1.3%. Obesity, lymphocytopenia, and hypoproteinemia were independently associated with the presence of lymphatic dysfunction in such patients.
Lymphatic dysfunction is common in cirrhosis patients with RA. Hypoproteinemia and lymphocytopenia are significant indicators of its presence.
Evaluation of lymphatic dysfunction in cirrhosis patients with RA can serve as a guide for future research into novel approaches for tissue decongestion.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Ullah K, Pakistan S-Editor: Lin C L-Editor: Filipodia P-Editor: Lin C
| 1. | Olszewski WL. The lymphatic system in body homeostasis: physiological conditions. Lymphat Res Biol. 2003;1:11-21; discussion 21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Vollmar B, Wolf B, Siegmund S, Katsen AD, Menger MD. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol. 1997;151:169-175. [PubMed] |
| 3. | Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 4. | Kumar R, Anand U, Priyadarshi RN. Lymphatic dysfunction in advanced cirrhosis: Contextual perspective and clinical implications. World J Hepatol. 2021;13:300-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernández-Varo G, Held KF, Soria G, Tudela R, Planas AM, Fernández-Hernando C, Arroyo V, Jiménez W, Morales-Ruiz M. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Browse NL, Stewart G. Lymphoedema: pathophysiology and classification. J Cardiovasc Surg (Torino). 1985;26:91-106. [PubMed] |
| 7. | Aller MA, Prieto I, Argudo S, de Vicente F, Santamaría L, de Miguel MP, Arias JL, Arias J. The interstitial lymphatic peritoneal mesothelium axis in portal hypertensive ascites: when in danger, go back to the sea. Int J Inflam. 2010;2010:148689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Tanaka M, Iwakiri Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol. 2016;2:733-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Chindaratana K, Tanpowpong P, Lertudomphonwanit C, Treepongkaruna S. Gastrointestinal protein loss in children with portal hypertension. Indian J Gastroenterol. 2021;40:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kumar R, Kumar T, Anand U, Priyadarshi RN. Intestinal Lymphangiectasia Associated With Refractory Ascites in a Cirrhosis Patient. Cureus. 2021;13:e12567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 11. | Cheng WS, Gough IR, Ward M, Croese J, Powell LW. Chylous ascites in cirrhosis: a case report and review of the literature. J Gastroenterol Hepatol. 1989;4:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (39)] |
| 13. | Wong F. Management of refractory ascites. Clin Mol Hepatol. 2023;29:16-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Milazzo L, Peri AM, Lodi L, Gubertini G, Ridolfo AL, Antinori S. Intestinal lymphangiectasia and reversible high liver stiffness. Hepatology. 2014;60:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Juneja P, Ruhina Rahman SN, Jakhar D, Mourya AK, Tripathi DM, Kaur I, Tiwari V, Rohilla S, Gupta A, Rawal P, Baweja S, Rastogi A, Naidu VGM, Sarin SK, Banerjee S, Kaur S. Recombinant VEGF-C (Cys156Ser) improves mesenteric lymphatic drainage and gut immune surveillance in experimental cirrhosis. JHEP Rep. 2023;5:100816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 16. | Fukui H, Kawaratani H, Kaji K, Takaya H, Yoshiji H. Management of refractory cirrhotic ascites: challenges and solutions. Hepat Med. 2018;10:55-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Barrowman JA, Granger DN. Effects of experimental cirrhosis on splanchnic microvascular fluid and solute exchange in the rat. Gastroenterology. 1984;87:165-172. [PubMed] |
| 18. | Yokomori H, Oda M, Kaneko F, Kawachi S, Tanabe M, Yoshimura K, Kitagawa Y, Hibi T. Lymphatic marker podoplanin/D2-40 in human advanced cirrhotic liver--re-evaluations of microlymphatic abnormalities. BMC Gastroenterol. 2010;10:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, Sassolas B, Guillaume JC, Grob JJ, Bastuji-Garin S. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ. 1999;318:1591-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 307] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Menchén L, Ripoll C, Marín-Jiménez I, Colón A, Gómez-Camarero J, González-Asanza C, Menchén P, Cos E, Bañares R. Prevalence of portal hypertensive duodenopathy in cirrhosis: clinical and haemodynamic features. Eur J Gastroenterol Hepatol. 2006;18:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorphometric study of portal hypertensive enteropathy. Am J Clin Pathol. 1997;108:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Barakat M, Mostafa M, Mahran Z, Soliman AG. Portal hypertensive duodenopathy: clinical, endoscopic, and histopathologic profiles. Am J Gastroenterol. 2007;102:2793-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Basha BM, Hsi ED. Immunohistochemical Analysis of Endothelial Cells in Vascular Transformation of Lymph Node Sinuses: Vascular or Lymphatic Differentiation? Appl Immunohistochem Mol Morphol. 2019;27:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Juneja P, Sharma A, Shasthry SM, Kumar G, Tripathi DM, Rajan V, Rastogi A, Sarin SK, Kaur S. Podoplanin-positive dilated lymphatic vessels in duodenum associates with three-month mortality in patients with cirrhosis. Front Physiol. 2023;14:1045983. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther. 2017;46:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Tsauo J, Shin JH, Han K, Yoon HK, Ko GY, Ko HK, Gwon DI. Transjugular Intrahepatic Portosystemic Shunt for the Treatment of Chylothorax and Chylous Ascites in Cirrhosis: A Case Report and Systematic Review of the Literature. J Vasc Interv Radiol. 2016;27:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Steinemann DC, Dindo D, Clavien PA, Nocito A. Atraumatic chylous ascites: systematic review on symptoms and causes. J Am Coll Surg. 2011;212:899-905.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Shang T, Liang J, Kapron CM, Liu J. Pathophysiology of aged lymphatic vessels. Aging (Albany NY). 2019;11:6602-6613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Kataru RP, Park HJ, Baik JE, Li C, Shin J, Mehrara BJ. Regulation of Lymphatic Function in Obesity. Front Physiol. 2020;11:459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Jiang X, Tian W, Nicolls MR, Rockson SG. The Lymphatic System in Obesity, Insulin Resistance, and Cardiovascular Diseases. Front Physiol. 2019;10:1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol. 2008;295:H587-H597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Rehal S, Blanckaert P, Roizes S, von der Weid PY. Characterization of biosynthesis and modes of action of prostaglandin E2 and prostacyclin in guinea pig mesenteric lymphatic vessels. Br J Pharmacol. 2009;158:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol. 1988;254:G389-G398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Suh SH, Choe K, Hong SP, Jeong SH, Mäkinen T, Kim KS, Alitalo K, Surh CD, Koh GY, Song JH. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Aspestrand F, Schrumpf E, Jacobsen M, Hanssen L, Endresen K. Increased lymphatic flow from the liver in different intra- and extrahepatic diseases demonstrated by CT. J Comput Assist Tomogr. 1991;15:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |