Published online Oct 27, 2023. doi: 10.4254/wjh.v15.i10.1127
Peer-review started: May 22, 2023
First decision: July 8, 2023
Revised: August 11, 2023
Accepted: September 18, 2023
Article in press: September 18, 2023
Published online: October 27, 2023
Processing time: 154 Days and 18.2 Hours
Alpha-1 antitrypsin deficiency is a rare genetic disease and a leading cause of inherited alterations in plasma protein metabolism (APPM).
To understand the prevalence, burden and progression of liver disease in patients with APPM including alpha-1 antitrypsin deficiency.
We conducted a retrospective analysis of anonymized patient-level claims data from a German health insurance provider (AOK PLUS). The APPM cohort comprised patients with APPM (identified using the German Modification of the International Classification of Diseases-10th Revision [ICD-10-GM] code E88.0 between 01/01/2010-30/09/2020) and incident liver disease (ICD-10-GM codes K74, K70.2-3 and K71.7 between 01/01/2012-30/09/2020). The control cohort comprised patients without APPM but with incident liver disease. Outcomes were inci
Overall, 2680 and 26299 patients were included in the APPM (fibrosis, 96; cirrhosis, 2584) and control (fibrosis, 1444; cirrhosis, 24855) cohorts, respectively. Per 100000 individuals, annual incidence and prevalence of APPM and liver disease was 10-15 and 36-51, respectively. In the APPM cohort, median survival was 4.7 years [95% confidence interval (CI): 3.5-7.0] and 2.5 years (95%CI: 2.3-2.8) in patients with fibrosis and cirrhosis, respectively. A higher proportion of patients in the APPM cohort experienced disease progression (92.0%) compared with the control cohort (67.2%). Median PFS was shorter in the APPM cohort (0.9 years, 95%CI: 0.7-1.1) compared with the control cohort (3.7 years, 95%CI: 3.6-3.8; P < 0.001). Patients with cirrhosis in the control cohort had longer event-free survival for ascites, hepatic encephalopathy, hepatic failure and esophageal/gastric varices than patients with cirrhosis in the APPM cohort (P < 0.001). Patients with fibrosis in the control cohort had longer event-free survival for ascites, cirrhosis, hepatic failure and esophageal/gastric varices than patients with fibrosis in the APPM cohort (P < 0.001). In the APPM cohort, the most common diagnostic procedures within 12 mo after the first diagnosis of liver disease were imaging procedures (66.3%) and laboratory tests (51.0%).
Among patients with liver disease, those with APPM experience substantial burden and earlier liver disease progression than patients without APPM.
Core Tip: This was a retrospective analysis of anonymized, patient-level, insurance claims data from a German health insurance provider (AOK PLUS), which demonstrated that a diagnosis of alterations in plasma protein metabolism (APPM) (E88.0) in patients with liver disease was associated with a substantial burden and higher rate of liver disease progression compared with patients with liver disease but without APPM. To enable accurate diagnosis and inform disease management, it is important to have specific diagnostic codes that differentiate between genetic liver disease and liver manifestations from other causes.
- Citation: Picker N, Hagiwara M, Baumann S, Marins EG, Wilke T, Ren K, Maywald U, Karki C, Strnad P. Liver disease epidemiology and burden in patients with alterations in plasma protein metabolism: German retrospective insurance claims analysis. World J Hepatol 2023; 15(10): 1127-1139
- URL: https://www.wjgnet.com/1948-5182/full/v15/i10/1127.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i10.1127
Alterations in plasma protein metabolism (APPM) can either be inherited or acquired[1]. As hepatocytes are responsible for the majority of protein production (approximately 10-20 g/d)[2], liver disease is frequently observed among patients with APPM[1].
Alpha-1 antitrypsin (AAT) deficiency (AATD) is the most common form of inherited APPM and is caused by mutations in serpin family A member 1 (SERPINA1), which encodes AAT, a serum protein produced primarily by hepatocytes that protects the lungs from protease-mediated degradation[3]. A homozygous mutation (Glu342Lys) in SERPINA1, named protease inhibitor (Pi) ZZ, is estimated to affect approximately 1 in 2000-5000 newborn infants in Europe and North America[4]. Mutations in SERPINA1 cause a reduction in serum AAT levels and promote the development of respiratory diseases, such as emphysema or chronic obstructive pulmonary disease[5]. In addition, AATD can result in liver diseases such as liver cirrhosis or hepatocellular carcinoma (HCC) due to the accumulation of hepatic AAT, which can trigger proteotoxic stress and lead to hepatocyte death and liver injury[6,7]. Approximately 20%-36% of patients with a PiZZ genotype develop significant fibrosis and approximately 10%-15% develop advanced fibrosis[8]. The only available curative treatment for end-stage liver disease in patients with AATD is liver transplantation[9]. Liver transplants in Germany have been allocated based on urgency according to the model for end-stage liver disease scoring system[10].
Given the lack of information on the natural history and epidemiology of AATD, we conducted a retrospective analysis of insurance claims data from Germany to better understand overall prevalence, burden and progression of liver disease in patients with APPM, including AATD. To improve our understanding of the natural history of APPM and liver disease, we compared patients with APPM and liver disease with a control cohort of patients with liver disease but without APPM.
This retrospective study used anonymized, patient-level, insurance claims data from 01 January 2010 to 30 September 2020, provided by the German regional health insurance provider, AOK PLUS. This data set covers approximately 3.3 million individuals from the federal states of Saxony and Thuringia, accounting for approximately 4.5% of the German statutory health insured (SHI) population in 2020. The age and comorbidity characteristics of patients insured by AOK PLUS are similar to those in the general German population who are insured by sickness funds[11-14].
The cohort of patients with APPM and incident liver disease was identified using the German Modification of the International Classification of Diseases-10th Revision (ICD-10-GM) code E88.0 for disorders of plasma protein metabolism (which includes AATD and other metabolic disorders such as plasminogen deficiency and bisalbuminaemia), and codes K74, K70.2-3 and K71.7 for liver disease.
Patients were included in the APPM cohort if they had APPM (diagnosed between 01 January 2010 and the end of the study) and incident liver disease (diagnosed between 01 January 2012 and the end of the study; Supplementary Figure 1). Patients were excluded if their liver disease was diagnosed in 2010 or 2011, to guarantee a liver disease-free period of 2 years. Continuous insurance coverage (no interruption of insurance for > 30 d) in 2010 and/or 2011 was required. Patients were observed from the date of the first diagnosis of liver disease (index date) until death, loss to follow-up due to end of insurance or end of the study. In patients with incident fibrosis who developed cirrhosis after 01 January 2012, the index date was defined as the date of the first diagnosis of cirrhosis for analyses of the subgroup of patients with cirrhosis. Patients were included in the control cohort if they did not have a diagnosis of APPM, but had incident liver disease. All patients with incident liver disease in both cohorts were further divided into two sub-cohorts: (1) Those with fibrosis at index date (ICD-10-GM codes: K74.0-2 and K70.2); and (2) those with cirrhosis at index date or those with fibrosis who developed cirrhosis during the study period (ICD-10-GM codes: K74.3-7, K70.3 and K71.7).
The study evaluated point prevalence and cumulative incidence of patients with APPM and liver disease, stratified by sex. In addition, demographics and baseline disease characteristics, including the Charlson Comorbidity Index, were measured at index date, with comorbidities identified during the previous 12 mo. Comorbidities were identified based on three-digit ICD-10-GM codes and evidence of confirmatory diagnosis/diagnoses (at least one inpatient or at least two outpatient diagnoses). Diagnostic procedures (liver biopsy, imaging, laboratory tests, AAT phenotyping and liver function tests) in the 12 mo after the index date were identified based on German procedure codes (Operationen-und Prozedurenschlüssel and einheitlicher Bewertungsmaßstab). A composite endpoint of progression-free survival (PFS) was defined as the time from index date until the first date with selected liver disease-related clinical events (acute peritonitis, ascites, cirrhosis, only among patients with fibrosis), esophageal/gastric varices, HCC, hepatic encephalopathy, hepatic failure, liver transplantation or all-cause death (used to assess mortality). Disease progression events were also analyzed separately, and comprised the following (one inpatient or one confirmed outpatient diagnosis): Ascites, esophageal/gastric varices, acute peritonitis, hepatic encephalopathies, gastrointestinal bleeding (e.g., melaena or hematemesis), hepatic failure, malignant neoplasm of the liver and intrahepatic bile ducts and HCC. Procedure-related events were analyzed, which comprised liver incision, liver resection, other operations on the liver, failure or rejection of transplant, and infection due to prosthesis, implant or graft.
To calculate the point prevalence of liver disease in patients with APPM, the denominator was the number of individuals insured by AOK PLUS on 01 January of the respective calendar year (2011-2020) and during the preceding 12 mo. The numerator was the number of patients alive on the 1st January of each year, with evidence of confirmatory diagnosis/diagnoses of APPM (made in two different quarters within the same year) and a diagnosis of liver disease during the previous year, and with continuous insurance coverage during that year.
The cumulative incidence of liver disease in patients with APPM was estimated for 2012-2019 by dividing the number of new cases in a calendar year by the total number of insured patients at risk of liver disease (i.e., those with no current evidence of liver disease) at the beginning of the same year. The numerator was the number of patients with APPM diagnosed at any point and with liver disease diagnosed during the year of the index date, but without any liver disease diagnosis within the previous 2 years and with continuous insurance coverage during this period. The denominator was the number of patients alive at the beginning of the respective calendar year for whom no liver disease diagnosis was documented in the 24 mo before index date, and with continuous insurance over this period.
Point prevalence and cumulative incidence were adjusted for age and sex differences compared with the German SHI population.
Patient demographics and baseline characteristics were analyzed using summary statistics (mean, standard deviation, median and interquartile range) for continuous variables and frequency statistics for categorical variables.
Time to disease progression was estimated using the Kaplan-Meier method. Patients were censored if they were lost to follow-up or had reached the end of the study. In addition to the number of patients with a progression event, the following Kaplan-Meier estimates were reported: median follow-up time in patients without an event, the 25th, 50th and 75th percentiles of time without an event, and event rates at years 1, 3 and 5 post-baseline. All time to event analyses were compared via a log-rank test.
Time from index date to all-cause death was estimated for patients with APPM and incident fibrosis or cirrhosis, and separately for patients with APPM and cirrhosis with and without a previous documented diagnosis of fibrosis.
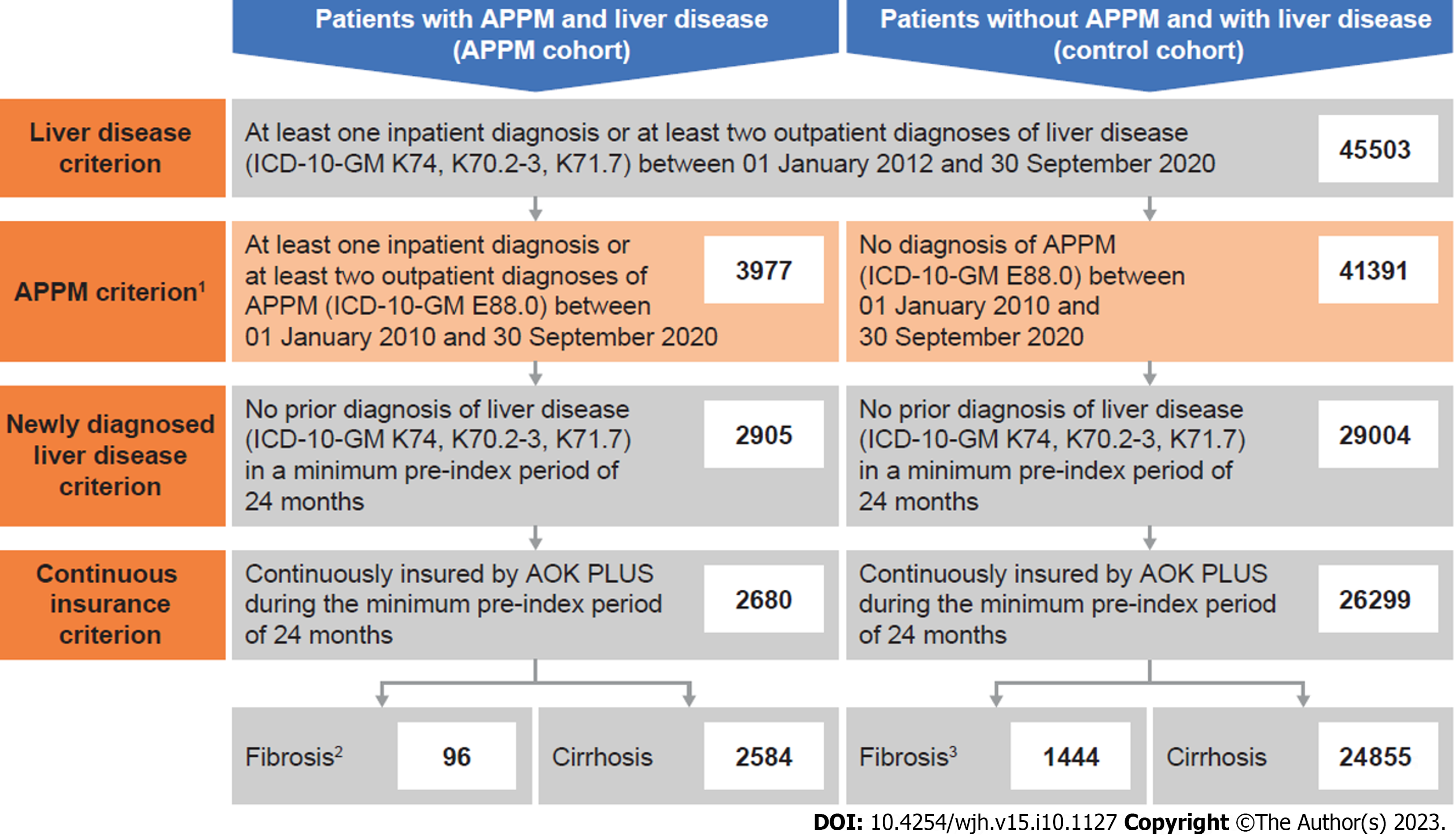
In total, 45503 patients had confirmatory diagnosis(es) of liver disease between 01 January 2012 and the end of the study (Figure 1). Of these, 2680 fulfilled the criteria to be included in the APPM cohort. In total, 96 of these patients had fibrosis and 2584 had cirrhosis at their index date (between 01 January 2012 and the end of the study).
In total, 26299 patients were included in the corresponding control cohort with no diagnosis of APPM. Of these, 1444 had fibrosis and 24855 had cirrhosis at their index date (Figure 1).
Between 2012 and 2019, the annual cumulative incidence of liver disease per 100000 individuals with APPM was 10-15, and was higher in males (15-22) than females (5-9; Supplementary Figure 2). When adjusted for age and sex differences vs the SHI population, the cumulative incidence was 8-13 per 100000 individuals. Between 2011 and 2020, the point prevalence of liver disease per 100000 individuals with APPM was 36-51 and was again higher in males (52-74) than females (22-32; Supplementary Figure 3). When adjusted for age and sex differences compared with the SHI population, the point prevalence was 33-47 per 100000 individuals.
Demographics and baseline characteristics were similar between cohorts (Table 1). In the APPM and control cohorts, respectively, 840 patients (31.3%) and 8595 patients (32.7%) were female (P = 0.159 between cohorts). Patients in the APPM cohort were significantly younger than in the control cohort, with a median age (interquartile range) of 63 years (54-73) and 65 years (56-76; P < 0.001), respectively. The most common liver-related comorbidities in the APPM and control cohorts, respectively, were “other liver disease” [679 (25.3%) and 6298 (23.9%)], non-alcoholic steatohepatitis [17 (0.6%) and 150 (0.6%)] and chronic hepatitis [12 (0.5%) and 163 (0.6%)]. The proportion of patients with respiratory system-related comorbidities was similar between the APPM [827 (30.9%)] and control [8333 (31.7%)] cohorts.
| Patients with APPM and liver disease: APPM cohort, n = 2680 | Patients without APPM and with liver disease: Control cohort, n = 26299 | |||
| All patients, n = 2680 | Patients with fibrosis, n = 96 | Patients with cirrhosis, n = 26261 | ||
| Total observed patient-years | 6118 | 283 | 5944 | 70261 |
| Median follow-up, yr (IQR) | 1.5 (0.4-3.7) | 2.3 (0.8-4.7) | 1.5 (0.4-3.7) | 2.0 (0.5-4.4) |
| Female sex2 | 840 (31.3) | 41 (42.7) | 815 (31.0) | 8595 (32.7) |
| Median age, yr (IQR)3 | 63.0 (54.0-73.0) | 64.5 (53.0-76.5) | 63.0 (54.0-73.0) | 65.0 (56.0-76.0) |
| 0-14 yr | 14 (0.5) | 6 (6.3) | 10 (0.4) | 49 (0.2) |
| 15-29 yr | 10 (0.4) | 0 | 10 (0.4) | 156 (0.6) |
| 30-44 yr | 183 (6.8) | 10 (10.4) | 177 (6.7) | 1420 (5.4) |
| 45-59 yr | 818 (30.5) | 21 (21.9) | 814 (31.0) | 7516 (28.6) |
| 60-74 yr | 1046 (39.0) | 31 (32.3) | 1029 (39.2) | 9564 (36.4) |
| 75-89 yr | 577 (21.5) | 25 (26.0) | 557 (21.2) | 7090 (27.0) |
| ≥ 90 yr | 32 (1.2) | 3 (3.1) | 29 (1.1) | 504 (1.9) |
| With care needs4 | 547 (20.4) | 33 (34.3) | 528 (20.1) | 4934 (18.8) |
| Median CCI5 (IQR) | 3.0 (1.0-5.0) | 4.0 (1.5-6.0) | 3.0 (1.0-5.0) | 3.0 (1.0-5.0) |
| Most common liver-related comorbidities | ||||
| “Other” liver disease6 | 679 (25.3) | 26 (27.1) | 669 (25.5) | 6298 (23.9) |
| Non-alcoholic steatohepatitis | 17 (0.6) | 1 (1.0) | 16 (0.6) | 150 (0.6) |
| Chronic hepatitis | 12 (0.5) | 0 | 12 (0.5) | 163 (0.6) |
| Respiratory system-related comorbidities | 827 (30.9) | 41 (42.7) | 806 (30.7) | 8333 (31.7) |
| Most common comorbidities | ||||
| Hypertension | 1751 (65.3) | 64 (66.7) | 1710 (65.1) | 17278 (65.7) |
| Type 2 diabetes mellitus | 1103 (41.2) | 40 (41.7) | 1082 (41.2) | 10766 (40.9) |
| Dyslipidaemia | 832 (31.0) | 35 (36.5) | 813 (31.0) | 8932 (34.0) |
| Alcohol-related disorders7 | 650 (24.3) | 17 (17.7) | 649 (24.7) | 6127 (23.3) |
| Heart failure | 605 (22.6) | 21 (21.9) | 591 (22.5) | 6071 (23.1) |
Minor differences in demographics and baseline characteristics were observed when the APPM cohort was stratified by the presence of fibrosis or cirrhosis. There was a higher proportion of females with fibrosis [41 (42.7%)] compared with cirrhosis [815 (31.0%)] and alcohol-related disorders were more common in patients with cirrhosis [649 (24.7%)] compared with fibrosis [17 (17.7%)].
The most common diagnostic procedures within 12 mo after the index date in the APPM cohort were imaging procedures [1778 (66.3%)] and laboratory tests [1366 (51.0%); Table 2]. Only 55 patients (2.1%) underwent AAT phenotyping.
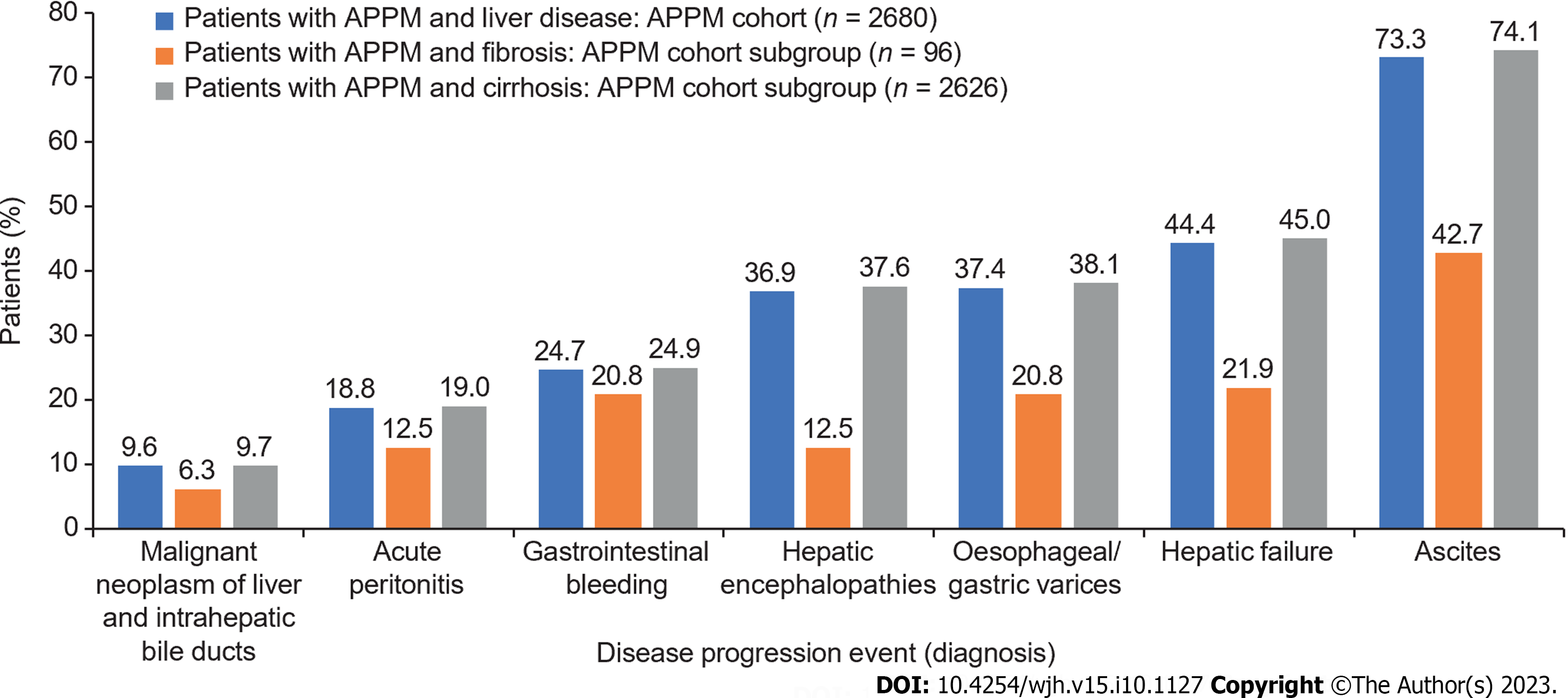
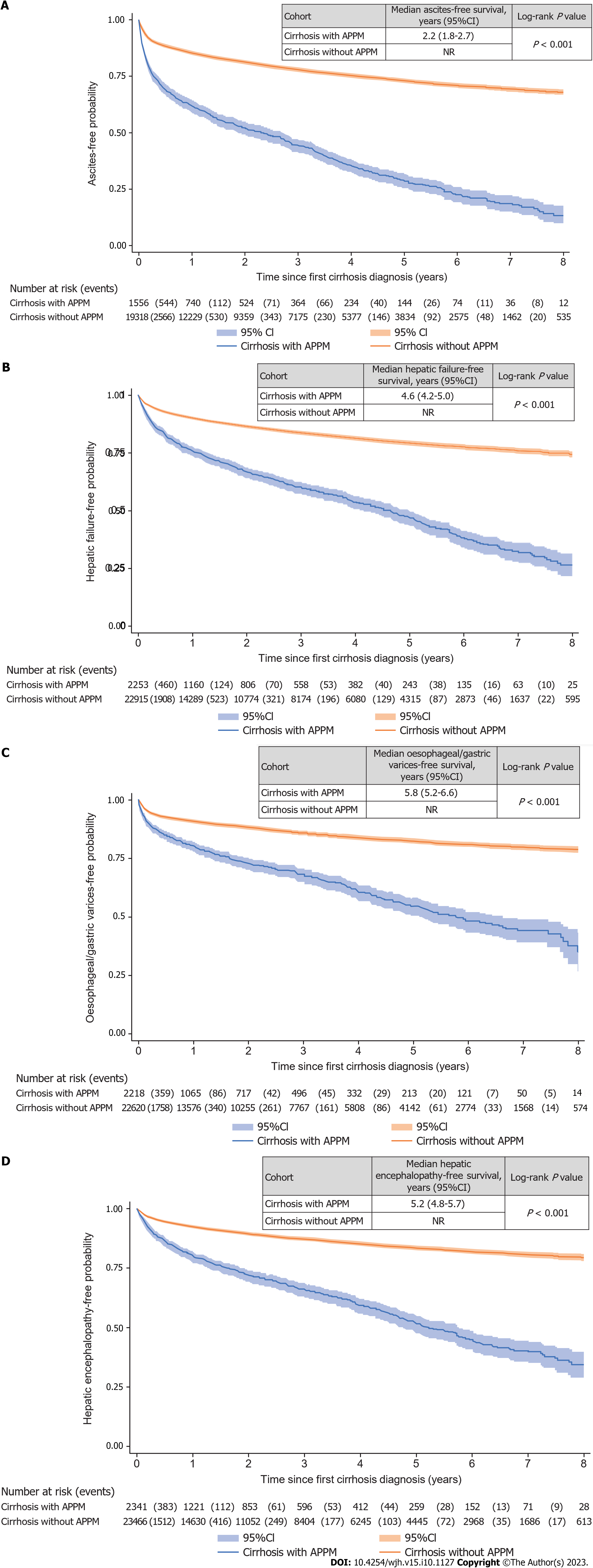
A higher proportion of patients in the APPM cohort experienced disease progression [2465 (92.0%)] compared with the control cohort [17682 (67.2%)]. Median PFS (composite endpoint) was significantly shorter in the APPM cohort [0.9 years (95%CI: 0.7-1.1)] compared with the control cohort [3.7 years (95%CI: 3.6-3.8); P < 0.001; Supplementary Figure 4]. The most common disease progression events were ascites, hepatic failure, esophageal/gastric varices, and hepatic encephalopathies (Figure 2). Patients with cirrhosis but without APPM had significantly longer event-free survival for ascites, hepatic failure, esophageal/gastric varices, and hepatic encephalopathy compared with those with APPM and cirrhosis (all P < 0.001; Figure 3). Similarly, patients with fibrosis but without APPM had significantly longer event-free survival for ascites, cirrhosis, hepatic failure and esophageal/gastric varices compared with those with APPM and fibrosis (all P < 0.001; Supplementary Figure 5). In the subgroup of 96 patients in the APPM cohort with fibrosis, median time from fibrosis to cirrhosis was 2.9 years (95%CI: 1.2-not reached; Supplementary Figure 5B). In the control cohort, median time from fibrosis to cirrhosis was not reached.
The most common procedures and procedure-related events indicating disease progression were liver resection, liver transplantation, failure and rejection of liver transplant and other operations on the liver (Supplementary Figure 6). A significantly higher proportion of patients with APPM and fibrosis had a liver resection [15 (15.6%) vs 93 (6.4%); P = 0.025] and a liver transplantation [6 (6.3%) vs 3 (0.2%); P < 0.001] compared with patients without APPM but with fibrosis (Table 3). Similarly, a significantly higher proportion of patients with APPM and cirrhosis had a liver resection [87 (3.3%) vs 488 (1.9%); P < 0.001] and a liver transplantation [78 (3.0%) vs 31 (0.1%); P < 0.001] indicating greater disease progression compared with patients without APPM but with cirrhosis (Table 3; Supplementary Figure 6).
| Parameter | Patients with fibrosis | Patients with cirrhosis | ||||||
| Liver resection | Liver transplantation | Liver resection | Liver transplantation | |||||
| Patients with APPM, n = 96 | Patients without APPM, n = 1445 | Patients with APPM, n = 96 | Patients without APPM, n = 1445 | Patients with APPM, n = 2626 | Patients without APPM, n = 25134 | Patients with APPM, n = 2626 | Patients without APPM, n = 25134 | |
| Patients with event during follow-up | 15 (15.6) | 93 (6.4) | 6 (6.3) | 3 (0.2) | 87 (3.3) | 488 (1.9) | 78 (3.0) | 31 (0.1) |
| Patients included in the KM analysis1 | 85 (88.5) | 1373 (95.0) | 94 (97.9) | 1442 (99.8) | 2606 (99.2) | 24849 (98.9) | 2621 (99.8) | 25034 (99.6) |
| Median follow-up in patients without an event, yr (IQR)2 | 2.3 (0.7-5.0) | 3.0 (1.2-5.2) | 2.3 (0.7-4.7) | 2.8 (1.2-5.1) | 1.5 (0.4-3.7) | 1.9 (0.5-4.4) | 1.5 (0.4-3.7) | 1.9 (0.5-4.4) |
| Failure rate | ||||||||
| After 1 yr | 3 (4.2) | 15 (1.2) | 1 (1.2) | 1 (0.1) | 40 (2.0) | 198 (1.0) | 26 (1.4) | 8 (< 0.1) |
| After 3 yr | 3 (4.2) | 18 (1.5) | 3 (4.8) | 1 (0.1) | 56 (3.4) | 258 (1.5) | 53 (3.6) | 23 (0.2) |
| After 5 yr | 3 (4.2) | 20 (1.9) | 4 (7.3) | 1 (0.1) | 64 (4.7) | 289 (1.9) | 66 (5.6) | 25 (0.2) |
| P value (log-rank test) | 0.025 | < 0.001 | < 0.001 | < 0.001 | ||||
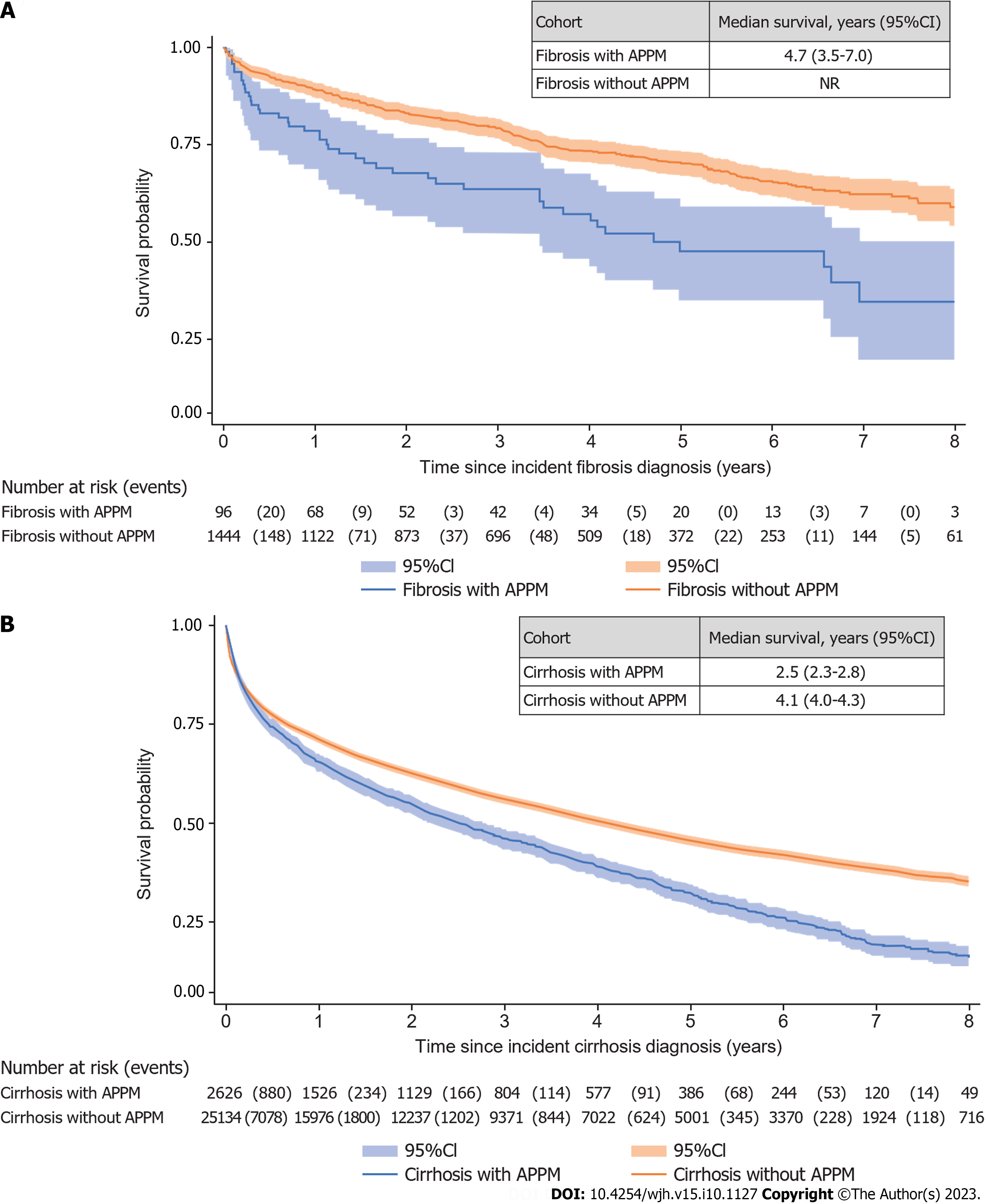
Median survival was shorter in the APPM cohort [2.6 years (95%CI: 2.3-2.8)] than in the control cohort [4.3 years (95%CI: 4.2-4.5)]. Median survival was 4.7 years (95%CI: 3.5-7.0) in patients with APPM and fibrosis and 2.5 years (95%CI: 2.3-2.8) in patients with APPM and cirrhosis (Figure 4). In the 42 patients in the APPM cohort with fibrosis who developed cirrhosis during the follow-up period, median survival was 4.1 years (95%CI: 2.2-7.1). In the 2584 patients in the APPM cohort with cirrhosis and without previous fibrosis, the median survival was 2.5 years (95%CI: 2.2-2.7).
This retrospective insurance claims-based study demonstrated that, per 100000 individuals, the annual incidence (2012-2019) and point prevalence (2011-2020) of APPM was 10-15 and 36-51, respectively, with higher rates in males than in females. Patients in the APPM cohort experienced shorter PFS, higher mortality and a higher rate of liver decompensation events compared with patients in the control cohort. In addition, patients with fibrosis in the APPM cohort had significantly shorter cirrhosis-free survival compared with patients with fibrosis in the control cohort (P < 0.001). This may have been because APPM is indicative of a more advanced stage of fibrosis that is more likely to decompensate, or alternatively may reflect how the E88.0 code is used in clinical practice. Liver cirrhosis is often associated with decreased plasma levels of hepatocyte-derived proteins[15]. Some of these proteins, such as albumin and transferrin, are well-established indicators of poor prognosis[1]. Previous studies have demonstrated that patients with cirrhosis and a heterozygous Pi mutation, PiMZ, in SERPINA1 decompensate faster than patients with cirrhosis but without AATD[16,17]. However, only 2.1% of patients in the APPM cohort underwent AAT phenotyping in our study, and therefore we were unable to further evaluate the association between decompensation and AATD genotype. The median age of patients in the APPM cohort was 2 years younger than in the control cohort, yet the APPM cohort had a higher risk of liver disease-related clinical events. This supports that patients with APPM are at a higher risk of liver disease-related clinical events than patients without APPM irrespective of age.
As AATD is also associated with the development of respiratory system comorbidities such as chronic obstructive pulmonary disease[18], it was anticipated that the APPM cohort would have a higher incidence of such comorbidities compared with the control cohort. However, we observed a similar incidence in the APPM and control cohorts (30.9% and 31.7%), which might indicate that the APPM cohort included a substantial number of patients without AATD. The E88.0 code we used to identify patients with APPM cannot be equated with AATD as the code includes a broad range of acquired and inherited APPM disorders, such as plasminogen deficiency. The European Commission Expert Group on Rare Diseases currently recommends the Orphanet nomenclature of rare diseases (ORPHA) codes to identify rare disorders[19]. The adoption of ORPHA codes is expected to facilitate the transition to ICD-11 codes, which include an expanded set of rare disorder codes compared with ICD-10[19]. In addition, developments of the ICD coding system, such as the addition of the E88.0A code for AATD, could improve the identification of patients with AATD in future administrative insurance claims analyses. In a recent registry-based cohort study of the prevalence, incidence and mortality associated with AATD in Denmark using the E88.0A code, a sensitivity analysis demonstrated a predominance of AATD in the E88.0 category for APPM and a near complete shift to the more specific E88.0A code for AATD between 2000 and 2018[20]. The adoption of diagnosis codes specific to patients with AATD may facilitate earlier diagnosis and improved patient management, which may, in turn, contribute to slowing disease progression and decreasing the burden of disease in these patients with a rare, chronic disease.
The limitations of this study are typical of those seen in other insurance claims-based analyses. As noted previously, we were unable to determine the proportion of patients included in the study who had AATD due to the limitations of the ICD-10-GM coding system. In addition, as AATD is a highly underdiagnosed disease[21], we cannot exclude the possibility that some cases may have been included in the control cohort. The general lack of laboratory test results, direct clinical measures and biomarkers in the database confounded our ability to analyze the AATD genotype distribution. Only 96 patients in the APPM cohort and 1444 in the control cohort were recorded as having fibrosis, which was lower than anticipated, likely owing to underdiagnosis (patients are often asymptomatic in the early stages of fibrosis) and/or underreporting[22]. Furthermore, a substantial number of patients with fibrosis developed cirrhosis and liver decom
Among patients with liver disease in Germany, those with APPM experience substantial burden and a higher rate of liver disease progression than patients without APPM. To enable accurate diagnosis and inform disease management, it is important to have specific diagnostic codes that differentiate between genetic liver disease and liver manifestations from other causes.
Alpha-1 antitrypsin deficiency (AATD) is a rare genetic disease that can result in the development of liver and/or lung disease, and is a leading cause of inherited alterations in plasma protein metabolism (APPM).
Currently, there is a lack of information on the natural history and epidemiology of AATD.
To understand the prevalence, burden and progression of liver disease in patients with APPM, which includes patients diagnosed with AATD, in Germany.
A retrospective analysis of anonymized, patient-level, insurance claims data from a German health insurance provider (AOK PLUS) was conducted. The APPM cohort comprised patients with APPM (01/01/2010-30/09/2020) and incident liver disease (01/01/2012-30/09/2020) and the control cohort comprised patients without APPM but with incident liver disease. Outcomes were incidence/prevalence of liver disease in patients with APPM, demographics/baseline characteristics, disease progression, progression-free survival, mortality, and diagnostic procedures.
Overall, 2680 and 26299 patients were included in the APPM [fibrosis (96); cirrhosis (2584)] and control [fibrosis (1444); cirrhosis (24855)] cohorts, respectively. The annual incidence and prevalence of APPM and liver disease was 10-15/100000 and 36-51/100000, respectively. Median survival was shorter in the APPM cohort (2.6 years) than in the control cohort (4.3 years). In patients in the APPM cohort with fibrosis and cirrhosis, respectively, median survival was 4.7 years and 2.5 years. More patients in the APPM cohort (92.0%) experienced liver disease progression than in the control cohort (67.2%). Median progression-free survival was shorter in the APPM cohort [0.9 years (95%CI: 0.7-1.1)] compared with the control cohort [3.7 years (95%CI: 3.6-3.8); P < 0.001]. In patients with cirrhosis, event-free survival for ascites, hepatic encephalopathy, hepatic failure, and esophageal/gastric varices was longer in the control cohort than in the APPM cohort (P < 0.001). In patients with fibrosis, event-free survival for ascites, cirrhosis, hepatic failure, and esophageal/gastric varices was longer in the control cohort than in the APPM cohort (P < 0.001). The most common diagnostic procedures within 12 mo after the first diagnosis of liver disease in the APPM cohort were imaging procedures (66.3%) and laboratory tests (51.0%).
In Germany, patients with APPM and liver disease experience substantial burden and a higher rate of and earlier liver disease progression than patients without APPM.
The adoption of diagnosis codes specific to AATD should enable differentiation of this disease from other APPM disorders and facilitate earlier diagnosis and patient management. This should contribute to slowing disease progression and decreasing the burden of disease in patients with this rare, chronic disease.
Medical writing and submission assistance was provided by Matthew Reynolds of Oxford PharmaGenesis, Oxford, United Kingdom and was supported by Takeda Development Center Americas, Inc.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gaspar R, Portugal; Ren WR, China; Zhang Y, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Cai YX
| 1. | Kuscuoglu D, Janciauskiene S, Hamesch K, Haybaeck J, Trautwein C, Strnad P. Liver - master and servant of serum proteome. J Hepatol. 2018;69:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 639] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Ferrarotti I, Carroll TP, Ottaviani S, Fra AM, O'Brien G, Molloy K, Corda L, Medicina D, Curran DR, McElvaney NG, Luisetti M. Identification and characterisation of eight novel SERPINA1 Null mutations. Orphanet J Rare Dis. 2014;9:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | O'Brien ML, Buist NR, Murphey WH. Neonatal screening for alpha1-antitrypsin deficiency. J Pediatr. 1978;92:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Strnad P, McElvaney NG, Lomas DA. Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2020;382:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 6. | Townsend SA, Edgar RG, Ellis PR, Kantas D, Newsome PN, Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Fromme M, Schneider CV, Trautwein C, Brunetti-Pierri N, Strnad P. Alpha-1 antitrypsin deficiency: A re-surfacing adult liver disorder. J Hepatol. 2022;76:946-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Hamesch K, Mandorfer M, Pereira VM, Moeller LS, Pons M, Dolman GE, Reichert MC, Schneider CV, Woditsch V, Voss J, Lindhauer C, Fromme M, Spivak I, Guldiken N, Zhou B, Arslanow A, Schaefer B, Zoller H, Aigner E, Reiberger T, Wetzel M, Siegmund B, Simões C, Gaspar R, Maia L, Costa D, Bento-Miranda M, van Helden J, Yagmur E, Bzdok D, Stolk J, Gleiber W, Knipel V, Windisch W, Mahadeva R, Bals R, Koczulla R, Barrecheguren M, Miravitlles M, Janciauskiene S, Stickel F, Lammert F, Liberal R, Genesca J, Griffiths WJ, Trauner M, Krag A, Trautwein C, Strnad P; European Alpha1-Liver Study Group. Liver Fibrosis and Metabolic Alterations in Adults With alpha-1-antitrypsin Deficiency Caused by the Pi*ZZ Mutation. Gastroenterology. 2019;157:705-719.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Karatas E, Di-Tommaso S, Dugot-Senant N, Lachaux A, Bouchecareilh M. Overview of alpha-1 antitrypsin deficiency-mediated liver disease. EMJ Hepatol. 2019;7:65-79. [DOI] [Full Text] |
| 10. | Schlitt HJ, Loss M, Scherer MN, Becker T, Jauch KW, Nashan B, Schmidt H, Settmacher U, Rogiers X, Neuhaus P, Strassburg C. [Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres]. Z Gastroenterol. 2011;49:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Flemming R. Patterns of pregabalin prescribing in four German federal states: analysis of routine data to investigate potential misuse of pregabalin. BMJ Open. 2022;12:e060104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Datzmann T, Schmitt J, Fuhrmann S, Roessler M, Meier F, Schoffer O. Implementation and Effectiveness of Novel Therapeutic Substances for Advanced Malignant Melanoma in Saxony, Germany, 2010-2020-Cohort Study Based on Administrative Data. Cancers (Basel). 2021;13:6150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Markevych I, Tesch F, Datzmann T, Romanos M, Schmitt J, Heinrich J. Outdoor air pollution, greenspace, and incidence of ADHD: A semi-individual study. Sci Total Environ. 2018;642:1362-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Trautmann F, Schuler M, Schmitt J. Burden of soft-tissue and bone sarcoma in routine care: Estimation of incidence, prevalence and survival for health services research. Cancer Epidemiol. 2015;39:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Gurbuz B, Guldiken N, Reuken P, Fu L, Remih K, Preisinger C, Brůha R, Leníček M, Petrtýl J, Reissing J, Aly M, Fromme M, Zhou B, Karkossa I, Schubert K, von Bergen M, Stallmach A, Bruns T, Strnad P. Biomarkers of hepatocellular synthesis in patients with decompensated cirrhosis. Hepatol Int. 2023;17:698-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Chen VL, Burkholder DA, Moran IJ, DiBattista JV, Miller MJ, Chen Y, Du X, Oliveri A, Cushing KC, Lok AS, Speliotes EK. Hepatic decompensation is accelerated in patients with cirrhosis and alpha-1 antitrypsin Pi*MZ genotype. JHEP Rep. 2022;4:100483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Balcar L, Tonon M, Semmler G, Calvino V, Hartl L, Incicco S, Jachs M, Bauer D, Hofer BS, Gambino CG, Accetta A, Brocca A, Trauner M, Mandorfer M, Piano S, Reiberger T; Baveno Cooperation: an EASL consortium. Risk of further decompensation/mortality in patients with cirrhosis and ascites as the first single decompensation event. JHEP Rep. 2022;4:100513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Sandhaus R, Strange C, Stone G, Runken MC, Blanchette CM, Howden R. Comorbidity Associations with AATD Among Commercially Insured and Medicare Beneficiaries with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2020;15:2389-2397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Aymé S, Bellet B, Rath A. Rare diseases in ICD11: making rare diseases visible in health information systems through appropriate coding. Orphanet J Rare Dis. 2015;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Acquavella J, Vágó E, Sorensen HT, Horváth-Puhó E, Hess GP. Registry-based cohort study of alpha-1 antitrypsin deficiency prevalence, incidence and mortality in Denmark 2000-2018. BMJ Open Respir Res. 2022;9:e001281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Barrecheguren M, Monteagudo M, Simonet P, Llor C, Rodriguez E, Ferrer J, Esquinas C, Miravitlles M. Diagnosis of alpha-1 antitrypsin deficiency: a population-based study. Int J Chron Obstruct Pulmon Dis. 2016;11:999-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, Pryke R, Hutchinson SJ, Sangro B, Martin NK, Cecchini M, Dirac MA, Belloni A, Serra-Burriel M, Ponsioen CY, Sheena B, Lerouge A, Devaux M, Scott N, Hellard M, Verkade HJ, Sturm E, Marchesini G, Yki-Järvinen H, Byrne CD, Targher G, Tur-Sinai A, Barrett D, Ninburg M, Reic T, Taylor A, Rhodes T, Treloar C, Petersen C, Schramm C, Flisiak R, Simonova MY, Pares A, Johnson P, Cucchetti A, Graupera I, Lionis C, Pose E, Fabrellas N, Ma AT, Mendive JM, Mazzaferro V, Rutter H, Cortez-Pinto H, Kelly D, Burton R, Lazarus JV, Ginès P, Buti M, Newsome PN, Burra P, Manns MP. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 372] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 23. | Labenz C, Arslanow A, Nguyen-Tat M, Nagel M, Wörns MA, Reichert MC, Heil FJ, Mainz D, Zimper G, Römer B, Binder H, Farin-Glattacker E, Fichtner U, Graf E, Stelzer D, Van Ewijk R, Ortner J, Velthuis L, Lammert F, Galle PR. Structured Early detection of Asymptomatic Liver Cirrhosis: Results of the population-based liver screening program SEAL. J Hepatol. 2022;77:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1424] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 25. | Dornquast C, Kroll LE, Neuhauser HK, Willich SN, Reinhold T, Busch MA. Regionale Unterschiede in der Prävalenz kardiovaskulärer Erkrankungen. Deutsches Ärzteblatt. 2016;42:704-11. |
| 26. | Bowlus CL, Willner I, Zern MA, Reuben A, Chen P, Holladay B, Xie L, Woolson RF, Strange C. Factors associated with advanced liver disease in adults with alpha1-antitrypsin deficiency. Clin Gastroenterol Hepatol. 2005;3:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |