Published online Jan 27, 2023. doi: 10.4254/wjh.v15.i1.89
Peer-review started: August 29, 2022
First decision: October 17, 2022
Revised: November 17, 2022
Accepted: December 31, 2022
Article in press: December 31, 2022
Published online: January 27, 2023
Processing time: 143 Days and 16.2 Hours
Liver disease incidence and hence demand on hepatology services is increasing.
To describe trends in incidence and natural history of liver diseases in Wales to inform effective provision of hepatology services.
The registry is populated by International Classification of Diseases-10 (ICD-10) code diagnoses for residents derived from mortality data and inpatient/day case activity between 1999-2019. Pseudo-anonymised linkage of: (1) Causative diag
The population of Wales in 2019 was 3.1 million. Between 1999 and 2019 73054 individuals were diagnosed with a hepatic disorder, including 18633 diagnosed with cirrhosis, 10965 with liver decompensation and 2316 with hepatocellular carcinoma (HCC). Over 21 years the incidence of liver diseases increased 3.6 fold, predominantly driven by a 10 fold increase in non-alcoholic fatty liver disease (NAFLD); the leading cause of liver disease from 2014. The incidence of cirrhosis, decompensation, HCC, and all-cause mortality tripled. Liver-related mortality doubled. Alcohol-related liver disease (ArLD), autoimmune liver disease and congestive hepatopathy were associated with the highest rates of decompensation and all-cause mortality.
A 10 fold increase in NAFLD incidence is driving a 3.6 fold increase in liver disease in Wales over 21 years. Liver-related morbidity and mortality rose more slowly reflecting the lower progression rate in NAFLD. Incidence of ArLD remained stable but was associated with the highest rates of liver-related and all-cause mortality.
Core Tip: In this paper we describe the following: (1) Novel methodology for developing a national liver registry; (2) The incidence of liver disease has increased 3.6-fold in Wales between 1999-2019 driven by a 10-fold increase in non-alcoholic fatty liver disease (NAFLD); (3) 3-fold increase in cirrhosis, portal hypertension, decompensation and hepatocellular carcinoma, 2-fold increase in liver disease related mortality between 1999-2019; and (4) Actuarial tables of 10-year liver disease progression: Alcohol-related liver disease, autoimmune liver disease and congestive hepatopathy are associated with increased rates of decompensation and death compared to viral hepatitis and NAFLD. Description of the proportion of patients dying from liver disease as directly, as a contributory cause or where liver disease has not been recording on the death certificate.
- Citation: Pembroke TPI, John G, Puyk B, Howkins K, Clarke R, Yousuf F, Czajkowski M, Godkin A, Salmon J, Yeoman A. Rising incidence, progression and changing patterns of liver disease in Wales 1999-2019. World J Hepatol 2023; 15(1): 89-106
- URL: https://www.wjgnet.com/1948-5182/full/v15/i1/89.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i1.89
Liver disease is the third most common cause of premature death in the United Kingdom[1]. Since 1970 mortality from liver disease has increased fourfold in the United Kingdom whilst all other major causes of death have declined[2]. The major causes of liver disease are alcohol-related liver disease (ArLD), non-alcoholic fatty liver disease (NAFLD) and viral hepatitis.
Liver disease typically progresses through several stages with increasing mortality regardless of the underlying aetiology[3]. Persistent inflammation may drive the accumulation of fibrosis resulting in cirrhosis and the development of portal hypertension over months to years[4]. Decompensation of chronic liver disease is defined by a deterioration in hepatic function and is represented by the sequalae of portal hypertension on a background of cirrhosis: Jaundice, ascites, encephalopathy, hepatorenal syndrome and variceal bleeding[5]. Up to a third of individuals with cirrhosis will develop liver cancer over their life time[6], which can precipitate liver decompensation. Decompensation may be the initial symptoms and first presentation to medical services and is associated with a high mortality[2,7]. Treatment of the underlying liver disease is key to improving prognosis, and can result in improvement in liver function, re-compensation of liver failure and even regression of cirrhosis[8,9]. Access to specialist care, surveillance for liver cancers and variceal bleeding and early discharge specialist follow up are all associated with reduced all-cause mortality for patients with cirrhosis[10,11].
Over the last decade there have been significant clinical advances in the management of liver disease, a striking example is the impact of direct acting antiviral drugs to eradicate hepatitis C virus (HCV) infection[12]. It is reasonable to assume that public health initiatives addressing lifestyle risk factors for liver disease may impact on the liver disease incidence and morbidity. Minimum unit pricing for alcohol in Scotland has resulted in an initial reduction in alcohol consumption, and taxation on sugary drinks has been introduced in an attempt to counter the obesity epidemic[13,14]. The long term impact of such interventions on liver disease incidence and mortality has yet to be established. Accurate epidemiological data may inform the effective provision of public health, primary and secondary care initiatives for liver disease and assess the future effectiveness of these interventions. Previous epidemiological studies have been limited by use of either mortality data alone[15,16], focusing of cohorts with advanced disease severity[3,10,17,18], or by specific disease aetiology such as autoimmune hepatitis, HCV or ArLD[4,19,20].
We set out to define the incidence of inpatient presentation, progression through significant stages of disease over time, and mortality of all liver diseases in Wales. We have developed a national liver disease registry populated by routinely coded diagnoses related to hospital admissions and death certificates. These diagnoses are recorded using the International Classification of Diseases (ICD)-10 classification[21]. Incorporating routinely coded data into a registry remains problematic for the following reasons: (1) ICD-10 codes for liver disease may be specific to individual aetiologies; descriptive of a disease process; or reflect the stage of disease i.e., ‘chronic viral hepatitis C’ (B18.2), ‘inflammatory liver disease, unspecified’ (K76.9) and portal hypertension (K76.6) respectively; (2) Diagnoses are recorded in the order of presentation rather than reflecting the natural history of disease described above. For example, variceal bleeding (I85.01) may be the initial presentation of autoimmune hepatitis (K75.4), however, a period of investigation including liver biopsy is commonly required before the aetiological diagnosis is later made. A code of ‘inflammatory liver disease, unspecified’ (K76.9) may be recorded during the diagnostic work up for this patient which becomes redundant once autoimmune hepatitis is confirmed; and (3) The progression of liver disease requires different levels of surveillance and monitoring and defining the stage of liver disease can be challenging. As a consequence, disease registries do not typically reflect multiple stages of disease.
In this paper we have applied a novel methodology to define: (1) The point of entry into a national liver registry; (2) Aetiological diagnoses from routinely coded data whilst removing redundant diagnoses; and (3) A decision tree to define the ordering of diagnostic codes for stages of liver disease. The aim was to develop novel insights into the changing aetiology and progression of liver disease, hence providing an analytic pipeline that will allow assessment of public health interventions, define clinical service requirements and aid redesign.
Between 1999 and 2019 the population of Wales increased from 2.9 to 3.1 million people; of whom 2.5 million are adults. Health care is devolved to the Welsh Government from the United Kingdom Government in Westminster. Primary and secondary health care services are delivered by 7 health boards each covering a population of approximately 400000 people. All medical diagnoses documented in medical notes from hospital admissions and day case procedures are recorded by dedicated teams of disease coders within health boards using the ICD-10 classification[21]. Mortality data including diagnoses recorded on death certificates are derived from the Office for National Statistics (ONS). The ONS record the ICD-10 code of the underlying cause of death, defined as “the disease or injury which initiated the train of morbid events leading directly to death” detailed in Part Ia-c of the United Kingdom death certificate[22]. Contributory diseases not part of the direct sequence resulting in death are recorded and are typically reported in part II of the death certificate. For all Welsh residents these data from hospitals in Wales or admissions to English hospitals are uploaded into the NHS Wales Informatics Services (NWIS) data warehouse. It is not possible to access primary care records to link risk factors for liver disease for all patients in Wales at present. Individuals with liver disease diagnosis recorded between 1991 and 1998 were excluded to reduce the risk of secular trend analysis bias through over-estimation of incidence in the early period of the study. All coded diagnoses between 1st January 1999 and 31st December 2019 were captured. Individuals were anonymised and given a unique identifier to link all demographic characteristics, 4-digit ICD-10 codes, and mortality data. The European Age Standardised Rate (EASR) of liver diseases was calculated using ONS census data for Wales from 2001-2019; the years for which census data has been used to estimate the European Age Standardised population for Wales[22].
ICD-10 classifications include codes for aetiology of liver disease, cirrhosis, portal hypertension, liver cancers and decompensation. We have sought to categorise ICD-10 codes into these stages of liver diseases as laid out below.
Aetiology: Building upon the recoding mapping in the Hepahealth project[15] and 2021 expert panel consensus[23], we grouped ICD-10 codes to represent the aetiology of liver diseases. In order to manage overlapping and compound liver diseases we designated a hierarchy of 3 tiers groups based upon perceived clinical importance and relevance to public health, primary and secondary care intervention (Table 1). Tier 1 diagnoses were defined as the 6 major categories of liver disease hepatitis B virus (HBV), HCV, autoimmune liver disease, ArLD, NAFLD, and metabolic liver diseases (haemochromatosis, alpha 1 antitrypsin deficiency and Wilson’s disease). Individuals with more than one of these Tier 1 diagnoses are recorded as an overlap aetiology. If alcohol was one of the overlapping Tier 1 aetiologies individuals were defined as ‘alcohol-overlap’, if alcohol was not one of multiple Tier 1 diagnoses they were defined as ‘not alcohol-overlap’. All codes recorded by clinical coders were included in this registry methodology and the relative position of codes complied by the clinical coders did not impact on the aetiology definition.
| Tier 1 | Tier 2 | Tier 3 | ||||||
| Descriptor | ICD-10 code | Descriptor | ICD-10 code | Descriptor | ICD-10 code | |||
| Autoimmune liver disease | Autoimmune hepatitis | K75.4 | Hepatitis not specified | Inflammatory liver disease unspecified | K76.9 | Miscellaneous | Peliosis hepatis | K76.4 |
| Primary biliary cholangitis | K74.3 | Other specified inflammatory liver disease | K75.8 | Other specified diseases of the liver | K76.8 | |||
| Granulomatous hepatitis not elsewhere specified | K75.3 | Chronic hepatitis not elsewhere classified | K73.0-K73.9 | Liver disorders in diseases classified elsewhere | K77 | |||
| Metabolic liver disease | Haemochromatosis | E83.11 | Congestive hepatopathy | Chronic passive congestion of the liver | K76.1 | |||
| Alpha 1 anti-trypsin deficiency | E88.01 | Central haemorrhagic necrosis of the liver | K76.2 | |||||
| Wilson’s disease | E83.01 | Hepatic veno-occlusive disease | K76.5 | |||||
| HBV | Hepatitis B without D | B18.1 | Toxic liver disease | Toxic liver disease | K71 | |||
| Hepatitis B with D | B18.0 | |||||||
| HCV | Hepatitis C | B18.2 | ||||||
| Alcohol-related liver disease | Alcoholic liver disease | K70-K70.9 | ||||||
| Non-alcoholic fatty liver disease | Non-alcoholic fatty liver disease | K76.0 | ||||||
Tier 2 diagnoses were toxic liver injury (K71), congestive hepatopathy, indicating passive venous congestion of the liver including right heart failure (K76.1, K76.2, K76.5 and I82.0), and hepatitis not specified (K76.9, K75.8 and K73.0-K73.9 capturing undefined liver inflammation). We propose that whilst these diagnoses may result in significant chronic liver disease, either they are not specific or they do not constitute the majority of hepatology workload within either primary or secondary care. Additionally, in the presence of Tier 1 diagnoses they are of secondary importance to understanding the epidemiology of liver disease. Tier 3 was designated as miscellaneous diagnoses (K76.4 peliosis hepatis, K76.8 ‘other specified diseases of the liver’ and K77 ‘liver disorders in diseases classified elsewhere’). Tier 3 diagnoses were only recorded in the absence of Tier 1 or 2 diagnoses. Finally, individuals without one of these aetiological codes but with a diagnosis of cirrhosis were grouped as ‘cirrhosis without defined aetiology’. Individuals were pseudo-anonymised with linkage of diagnoses allowing repeat codes to be removed.
We propose that liver disease is typically considered in 5 discrete stages (Supplementary Figure 1). The first stage is the underlying aetiological disease process in the absence of cirrhosis. The management of this stage focuses upon reversing causes of inflammation and, in specific subsets of patients with HBV, hepatocellular carcinoma (HCC) surveillance. The ICD-10 case definitions for stages 2-5 are detailed in Table 2 and described below. Stage 2 is cirrhosis without portal hypertension or synthetic failure. At this point HCC should be considered and signs of potentially significant portal hypertension sought to screen for varices by gastroscopy. Stage 3 is defined as portal hypertension without decompensation and represents an important group which should be screened for varices and medium/large varices treated to prevent bleeding. The presence of clinically significant portal hypertension is associated with increased mortality[5]. Portal vein thrombosis is included within this group as a significant complication of portal hypertension. Stage 4 denotes liver decompensation associated with variceal bleeding, hepatic synthetic failure, ascites and hepatorenal syndrome. Stage 5 represents development of HCC. Whilst this may develop spontaneously or in HBV in the absence of cirrhosis, HCC most commonly arises in cirrhotic livers. It is well recognised that liver injury may regress and decompensation may improve. There are no recorded codes for this process however, liver transplantation may be considered as a separate code applied to a small proportion of individuals with liver disease. As described above, patients may progress through these stages in turn but can present either in a stepwise fashion or at a later stage depending on symptoms, screening or incidental diagnosis. Each of these stages requires varying levels of surveillance and specialist input to ameliorate the risk of liver-related mortality.
| Aetiological diagnoses (stage 1) | Cirrhosis (stage 2) | Portal hypertension (stage 3) | Hepatic decompensation (stage 4) | Hepatocellular carcinoma (stage 5) |
| As defined in Table 1 | Alcoholic cirrhosis, K70.3 | Portal hypertension, K76.6 | Chronic hepatic failure, K72.1 | Primary liver cancer C22.0 |
| Hepatic fibrosis or sclerosis, K74.0-K74.2 | Portal vein thrombosis, I81 | Hepatorenal syndrome, K76.7 | Hepatocellular carcinoma C22.1 | |
| Secondary biliary cirrhosis, K74.4 | Oesophageal varices without bleeding I85.9 | Oesophageal varices with bleeding, I85.0 | ||
| Biliary cirrhosis unspecified, K74.5 | Oesophageal varices without bleeding in diseases specified elsewhere, I98.2 | Hepatic failure unspecified K72 | ||
| Cirrhosis, other, K74.6 |
The acute liver diseases are considered separately to these acquired chronic liver diseases (Supplementary Table 1) as they carry a different challenge to public health, primary, and secondary care. Whilst these codes are captured, they are not included within the aetiology of chronic liver diseases described above. Alcohol-related codes that did not indicate liver disease (for example F10; alcohol use disorder) were not recorded to maintain a focus on individuals with evidence of liver disease requiring hepatology services rather than broader substance misuse services.
We proposed that the time of the first liver-related diagnosis listed in either Table 1 or Table 2 is used as the point of entry into the registry and was defined as the index diagnosis. In order to assess the proportion of individuals presenting late the diagnoses were ordered in keeping with the natural history of disease. Thus, the highest tier aetiological diagnosis is always the first entry into the liver registry, followed by cirrhosis, portal hypertension, decompensation and liver cancer. The application of the Wales Liver Registry methodology to a hypothetical patient is described in Supplementary Figure 2. The proportion of patients at each stage of liver disease was recorded. Mortality was recorded and deaths were divided into liver disease underlying cause, liver disease contributing, and non-liver-related.
We also set out to assess the quality of routinely coded inpatient, day case and mortality diagnoses in Wales in comparison to outpatient liver disease data to identify if significant liver diagnoses are under-recorded. Consultant hepatologists within the Gwent region (Aneurin Bevan Health Board), have routinely entered ICD-10 liver disease codes at the end of each outpatient appointment since 2012. These diagnoses were made with the full benefit of clinical investigations and could be updated at subsequent appointments to reflect evolving clinical manifestations of liver disease and progression of liver disease. We defined this as the gold standard of aetiological diagnosis to compare the diagnoses recorded within hospitals and on death certificates. To assess the impact of our proposed methodology we interrogated the NWIS data warehouse and report the impact of this approach. Scripts were written in SQL to perform the data linkage and reordering. Data was then collated and reported as fold change in incidence and time to event by Kaplan Meier analysis in Power BI (Microsoft, United Kingdom) and Prism (GraphPad, United States). This study was conducted in keeping with the Helsinki declaration. The design of this study was discussed with the South Wales research ethics committee and was not classed as research requiring regulatory approvals by Health Research Authority.
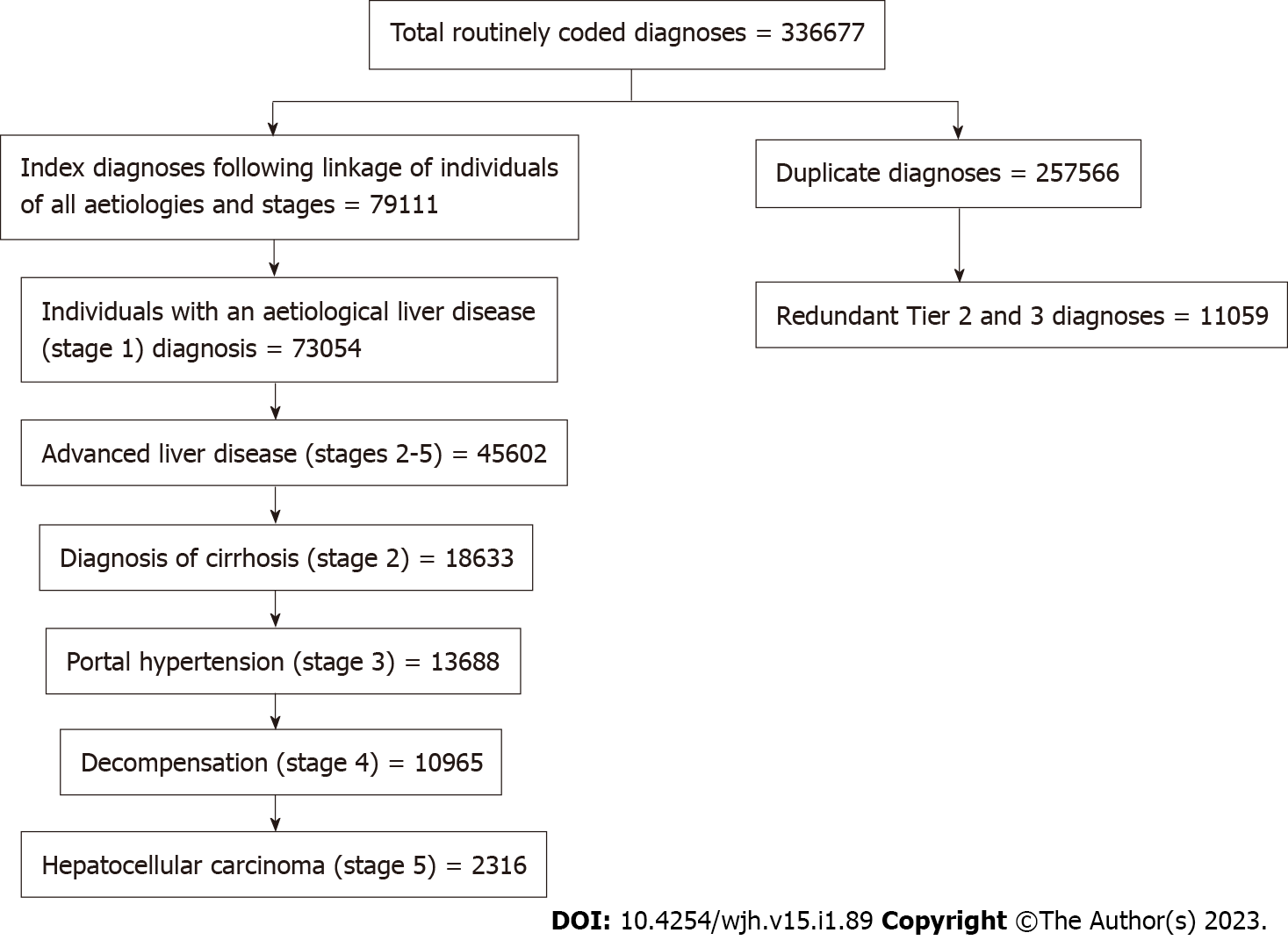
Between January 1, 1999 and December 31, 2019 there were 336677 routinely coded diagnoses of liver disease in Wales. There were 79111 index ICD-10 diagnosis of liver disease of any aetiology or stage in 73054 individuals (Figure 1). This indicates that there were approximately 3 times as many repeat diagnoses as indexed diagnoses during subsequent admissions. Fifty two percent of individuals (n = 37877) with a liver disease diagnosis between 1999 and 2019 died from all causes. A third of the individuals (n = 13266) who died, died from an underlying liver disease cause; equating to 18% of all individuals in the registry. In 2019 there were 35177 individuals alive with a secondary care diagnosis of chronic liver disease equating to a prevalence of 1.1% of the 3.1 million Welsh population.
The proposed hierarchy of aetiological diagnoses was applied to the liver registry cohort; 11059 Tier 2 and 3 diagnoses (including miscellaneous and hepatitis not specified) recorded in the presence of a Tier 1 diagnosis and therefore considered redundant. Following application of the staging criteria methodology there were 16992 individuals with cirrhosis, including 12858 diagnosed with portal hypertension and 10399 with an index diagnoses of decompensation, and 2316 with HCC (Figure 1). The aetiologies of liver disease in Wales are listed in Table 3; across the study period, the most frequent causes of liver disease in Wales were ArLD (26.1%), NAFLD (21.6%) metabolic disease (11.5%) and HCV (4.7%). The mean age of the entire cohort at diagnosis was 59.7 years and there was a slight male preponderance (54.5%, n = 39875). The mean age of diagnosis varied by aetiologies, ranging from 43 years in HCV to 64.7 years in congestive hepatology. Similarly, the proportion of males ranged from 20.5% to 67.8% in autoimmune liver diseases and ArLD respectively (Table 3).
| Aetiology | n | % of total cohort | Mean/median age (yr) | % male |
| Tier 1 | ||||
| ArLD | 16143 | 26.1% | 54.9/55 | 67.8% |
| NAFLD | 13390 | 21.6% | 57/58 | 47% |
| Metabolic | 7131 | 11.5% | 63/67 | 52.5% |
| HCV | 2889 | 4.7% | 43/41 | 66.8% |
| ArLD overlap | 2979 | 4.8% | 51.2/51 | 65.3% |
| Autoimmune liver diseases | 2312 | 3.7% | 61.8/64 | 20.5% |
| HBV | 904 | 1.5% | 43.5/39 | 53.3% |
| Non ArLD overlap | 955 | 1.5% | 51.2/51 | 55.8% |
| Tier 2 | ||||
| Hepatitis not specified | 6069 | 9.8% | 63/66 | 50.9% |
| Congestive hepatopathy | 894 | 1.4% | 64.7/70 | 49.6% |
| Toxic liver disease | 820 | 1.3% | 46.3/44 | 38.4% |
| Tier 3 | ||||
| Miscellaneous | 7430 | 12% | 66.7/70 | 42% |
Inpatient and day case coding may fail to capture the incidence of disease diagnosed in the outpatient clinic. We wished to assess the variation in incidence of aetiological diagnoses and staging diagnoses in the outpatient setting. When including outpatient coded diagnoses there was a substantial increase in the number of HBV (additional 112%), HCV (77%), autoimmune liver diseases (66%), NAFLD (40%), hepatitis not specified (34%), metabolic (23%), and ArLD (23%) cases captured (Supplement
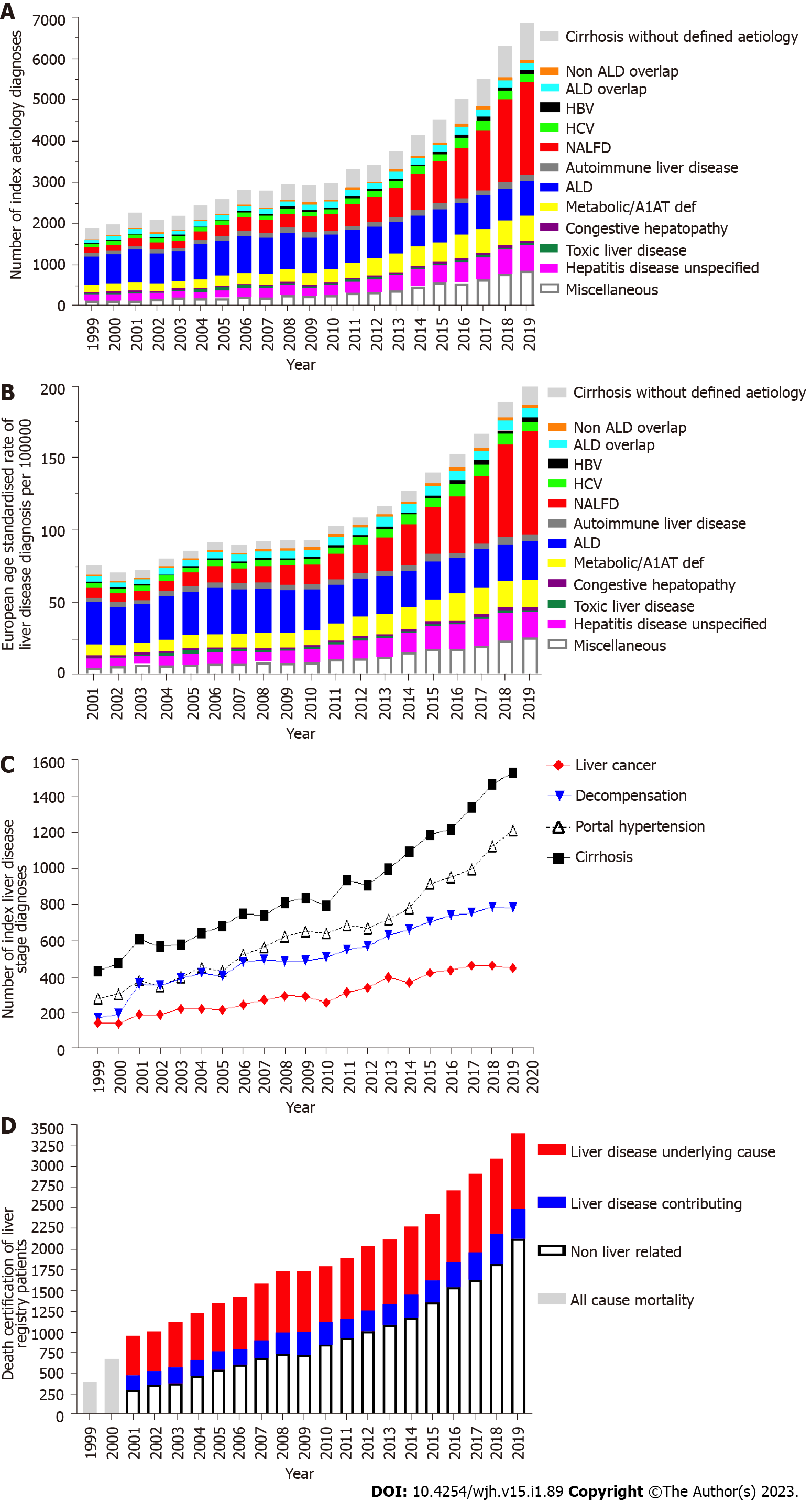
Between 1999 and 2019 the total number of new liver disease diagnosis rose 3.6-fold from 1916 to 6932 individuals per annum (Figure 2A). The total EASR of liver diseases increased from 75.9 to 199 per 100000 (Figure 2B) between 2001 and 2019. There was a marked increase in all liver disease diagnoses over this period apart from ArLD and toxic liver disease. NAFLD demonstrated a 10-fold increase over the 20-year period and in 2014 became the most common aetiological diagnosis of liver disease in Wales. Importantly, between 1999 and 2019 there was at least a 3-fold increase in the first recorded diagnosis of the clinically significant sequelae of chronic liver disease; cirrhosis, (435 to 1533) portal hypertension (282 to 1214), decompensation (173 to 785), and liver cancers (146 to 449, Figure 2B). The absolute number of deaths per year in a longitudinal cohort may be influence by lead time bias; the increasing age of the cohort, progressive and accumulation of comorbidities. However, between 2001 and 2019 the number of deaths in Wales with an underlying liver disease cause or in which liver disease contributed doubled (451 to 890 and 199 to 381 respectively, Figure 2C). In 2019 the number of all-cause deaths in the Wales Liver Registry was 3398.
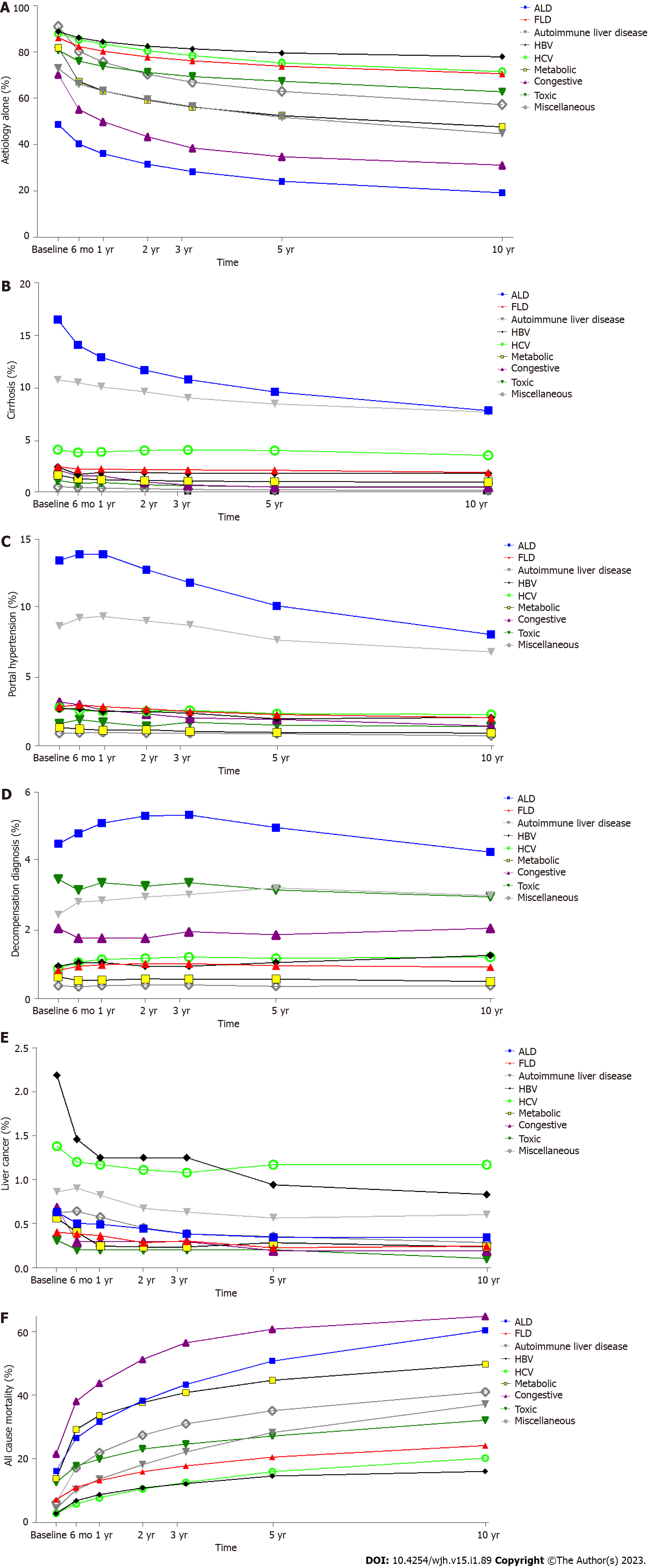
Different aetiologies of liver disease will have different rates of progression to advanced liver disease and risk of all-cause mortality thus requiring varying levels of specialist care and surveillance. For each aetiological group we analysed the proportion of patients by their most advanced stage of liver disease (aetiology only, cirrhosis, portal hypertension, decompensation, and liver cancer) and all-cause mortality from entry into the liver registry up until death or censure at the end of the study period. The proportion who had further diagnoses indicating progression to each stage of liver disease was recorded at baseline, and by 6 mo, 1, 2, 3, 5 and 10 years (Table 4, Supplementary Figures 4A-F). The aetiology of liver disease resulted in significantly different proportions of each stage of liver disease at each time point (Figures 3A-F; P < 0.001 one way ANOVA). In this respect, advanced liver disease and decompensation were more frequent in ArLD, autoimmune liver disease, HCV, and congestive hepatopathy than NAFLD, HBV, and metabolic liver disease. This ranking of more progressive liver diseases did not change significantly at different time points (Figure 3). A notable exception was the proportion of patients living with HCC diagnosis was greatest in those with HBV up to year 3, then it was greatest in those with HCV (Figure 3E). The highest rates of cirrhosis were in ArLD (35%-20%) and autoimmune liver diseases (23.5%-18.5%) over the 10 years of follow up. The rate of cirrhosis in NAFLD (2.5%-1.98%) was over 10-fold lower than for ArLD (Table 4). In the final year of the study the number of individuals with index ArLD aetiology, cirrhosis and decompensation diagnoses were 638, 537 and 238 respectively. In the same year index NAFLD aetiology, cirrhosis and decompensation diagnoses were 2242, 164 and 63 respectively (data not shown). The proportion of ArLD and autoimmune liver patients with cirrhosis decreased over 10 years whilst the proportion with portal hypertension and decompensation increased to a peak at 1 year and 3 years respectively before falling below baseline (Figures 3C and 3D). This suggests progression of liver disease drives the increasing morbidity for these aetiologies. Congestive hepatopathy had the greatest mortality increasing for 21% at entry into the registry, 42% at 1 year and 64% at 10 years but relatively low rates of cirrhosis. Alcohol and metabolic liver disease had similar high levels of all-cause mortality whilst HBV and HCV had the lowest levels of mortality over 10 years (Figure 3F). Progression to advanced stages of liver disease appeared to most frequently occurred in the first 3 years following entry into the registry (Figures 3A-F).
| 0 | 6 mo | 1 yr | 2 yr | 3 yr | 5 yr | 10 yr | |
| ArLD | |||||||
| Stage 1 | 48.75 | 40.27 | 36.14 | 31.54 | 28.36 | 24.21 | 19.18 |
| Stage 2 | 16.52 | 14.12 | 12.93 | 11.75 | 10.82 | 9.65 | 7.87 |
| Stage 3 | 13.47 | 13.93 | 13.92 | 12.8 | 11.86 | 10.18 | 8.1 |
| Stage 4 | 4.49 | 4.8 | 5.09 | 5.3 | 5.33 | 4.97 | 4.25 |
| Stage 5 | 0.63 | 0.5 | 0.49 | 0.44 | 0.38 | 0.34 | 0.34 |
| Stage 6 | 16.14 | 26.38 | 31.43 | 38.17 | 43.25 | 50.64 | 60.25 |
| NAFLD | |||||||
| Stage 1 | 86.3 | 82.52 | 80.42 | 77.98 | 76.25 | 73.93 | 70.7 |
| Stage 2 | 2.54 | 2.28 | 2.31 | 2.21 | 2.22 | 2.18 | 1.98 |
| Stage 3 | 2.87 | 2.99 | 2.87 | 2.7 | 2.52 | 2.29 | 2.07 |
| Stage 4 | 0.82 | 0.93 | 0.98 | 1.01 | 1.01 | 0.95 | 0.91 |
| Stage 5 | 0.4 | 0.38 | 0.36 | 0.28 | 0.3 | 0.22 | 0.24 |
| Stage 6 | 7.07 | 10.89 | 13.07 | 15.82 | 17.71 | 20.43 | 24.1 |
| Autoimmune liver disease | |||||||
| Stage 1 | 72.94 | 66.33 | 63.31 | 59.57 | 56.44 | 51.89 | 44.83 |
| Stage 2 | 10.79 | 10.53 | 10.12 | 9.67 | 9.07 | 8.51 | 7.73 |
| Stage 3 | 8.7 | 9.29 | 9.41 | 9.07 | 8.77 | 7.69 | 6.83 |
| Stage 4 | 2.43 | 2.8 | 2.84 | 2.95 | 3.02 | 3.21 | 2.99 |
| Stage 5 | 0.86 | 0.9 | 0.82 | 0.67 | 0.63 | 0.56 | 0.6 |
| Stage 6 | 4.29 | 10.15 | 13.51 | 18.07 | 22.06 | 28.14 | 37.03 |
| HBV | |||||||
| Stage 1 | 88.96 | 86.25 | 84.58 | 82.6 | 81.46 | 79.69 | 78.02 |
| Stage 2 | 2.5 | 1.77 | 1.98 | 1.98 | 1.88 | 1.88 | 1.88 |
| Stage 3 | 2.71 | 2.71 | 2.5 | 2.5 | 2.4 | 1.98 | 2.08 |
| Stage 4 | 0.94 | 1.04 | 1.04 | 0.94 | 0.94 | 1.04 | 1.25 |
| Stage 5 | 2.19 | 1.46 | 1.25 | 1.25 | 1.25 | 0.94 | 0.83 |
| Stage 6 | 2.71 | 6.77 | 8.65 | 10.73 | 12.08 | 14.48 | 15.94 |
| HCV | |||||||
| Stage 1 | 88.02 | 85.5 | 83.54 | 80.68 | 78.51 | 75.38 | 71.59 |
| Stage 2 | 4.15 | 3.88 | 3.94 | 4.06 | 4.12 | 4.06 | 3.61 |
| Stage 3 | 2.8 | 2.59 | 2.53 | 2.53 | 2.59 | 2.35 | 2.29 |
| Stage 4 | 0.87 | 1.05 | 1.14 | 1.17 | 1.2 | 1.17 | 1.2 |
| Stage 5 | 1.38 | 1.2 | 1.17 | 1.11 | 1.08 | 1.17 | 1.17 |
| Stage 6 | 2.77 | 5.78 | 7.67 | 10.44 | 12.49 | 15.86 | 20.13 |
| Metabolic | |||||||
| Stage 1 | 82.03 | 67.35 | 63.3 | 59.25 | 56.3 | 52.51 | 47.7 |
| Stage 2 | 1.73 | 1.37 | 1.29 | 1.21 | 1.16 | 1.11 | 1.05 |
| Stage 3 | 1.36 | 1.24 | 1.16 | 1.2 | 1.07 | 0.99 | 0.96 |
| Stage 4 | 0.63 | 0.52 | 0.53 | 0.57 | 0.56 | 0.56 | 0.49 |
| Stage 5 | 0.56 | 0.4 | 0.24 | 0.23 | 0.23 | 0.28 | 0.23 |
| Stage 6 | 13.69 | 29.12 | 33.48 | 37.54 | 40.69 | 44.55 | 49.57 |
| Congestive hepatopathy | |||||||
| Stage 1 | 70.36 | 55.3 | 50.05 | 43.44 | 38.58 | 34.79 | 31.1 |
| Stage 2 | 2.24 | 1.65 | 1.65 | 1.07 | 0.78 | 0.58 | 0.58 |
| Stage 3 | 3.21 | 3.01 | 2.62 | 2.33 | 2.04 | 1.94 | 1.46 |
| Stage 4 | 2.04 | 1.75 | 1.75 | 1.75 | 1.94 | 1.85 | 2.04 |
| Stage 5 | 0.68 | 0.29 | 0.29 | 0.29 | 0.29 | 0.19 | 0.19 |
| Stage 6 | 21.48 | 38 | 43.63 | 51.12 | 56.37 | 60.64 | 64.63 |
| Toxic | |||||||
| Stage 1 | 80.87 | 76.09 | 73.96 | 71.31 | 69.48 | 67.45 | 62.87 |
| Stage 2 | 1.22 | 0.92 | 1.02 | 0.81 | 0.71 | 0.61 | 0.61 |
| Stage 3 | 1.63 | 1.93 | 1.73 | 1.42 | 1.73 | 1.53 | 1.42 |
| Stage 4 | 3.46 | 3.15 | 3.36 | 3.26 | 3.36 | 3.15 | 2.95 |
| Stage 5 | 0.31 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 |
| Stage 6 | 12.51 | 17.7 | 19.74 | 22.99 | 24.52 | 27.06 | 32.04 |
| Miscellaneous | |||||||
| Stage 1 | 91.24 | 80.46 | 75.76 | 70.45 | 67.05 | 63.04 | 57.4 |
| Stage 2 | 0.62 | 0.57 | 0.49 | 0.42 | 0.35 | 0.31 | 0.29 |
| Stage 3 | 0.94 | 0.97 | 0.99 | 0.94 | 0.94 | 0.9 | 0.75 |
| Stage 4 | 0.37 | 0.34 | 0.37 | 0.39 | 0.39 | 0.35 | 0.37 |
| Stage 5 | 0.62 | 0.64 | 0.57 | 0.45 | 0.38 | 0.35 | 0.28 |
| Stage 6 | 6.21 | 17.02 | 21.82 | 27.35 | 30.89 | 35.04 | 40.91 |
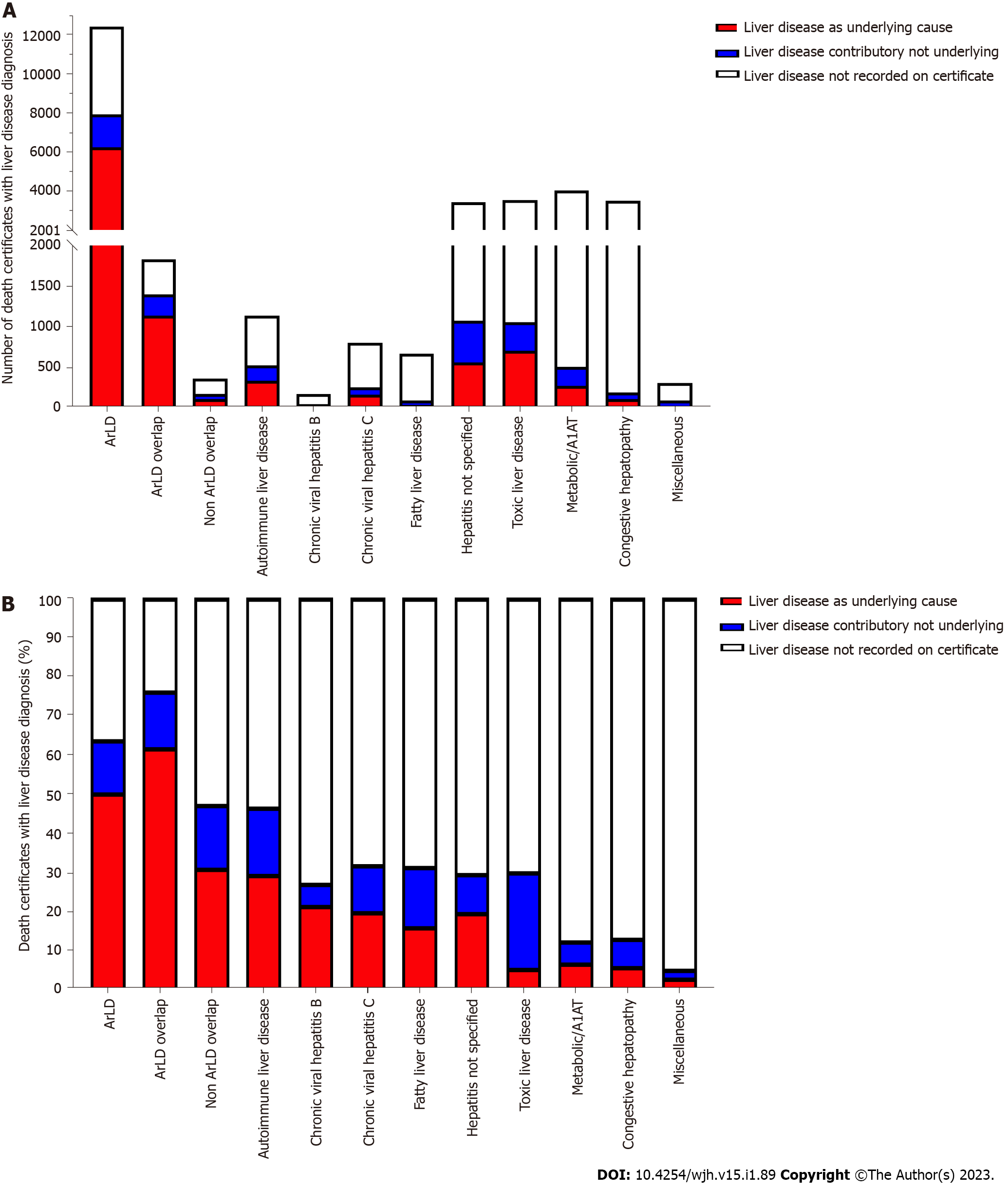
The variation in progression to advanced stages of liver disease suggests that liver-related mortality will vary considerable between aetiologies of liver disease. The ONS records diagnoses that are the underlying cause of death and contributary causes. We compared the number of individuals who had a liver disease diagnosis listed as the underlying cause of death, other recording of liver disease or no mention of liver disease on the death certificate. Absolute number of deaths caused directly by liver disease was highest in the cohort ArLD (n = 6238) and this was approximately 10-fold higher than deaths due to liver disease in hepatitis not specified (n = 704), NAFLD (n = 559) or autoimmune liver diseases (n = 334, Figure 4A). ArLD, overlap and autoimmune liver diseases were associated with the greatest proportion of liver-related deaths. A third of individuals with NAFLD who died had a liver disease diagnosis recorded on their death certificate. The lowest rates of liver-related death were associated with metabolic liver disease, congestive hepatopathy and miscellaneous liver disease diagnoses (Figure 4B). These data are in keeping with diagnoses associated with highest rates of progression to cirrhosis and decompensation (Figure 3).
Accurate epidemiological data covering the broad arc of liver disease diagnoses and stages has the potential to improve the planning of hepatology services. In this paper we have proposed a novel epidemiological approach to analysing routinely coded data with 3 key features: (1) Defined groups of ICD-10 codes with the application of a hierarchy order of the most clinically relevant codes; (2) Linkage of diagnoses on an individual level to assess the clinical stage of liver disease; and (3) Monitor progression and mortality. To knowledge this approach to capture all diagnoses and stages of liver disease has not been applied previously in the United Kingdom or internationally. We have applied this methodology to the Welsh national data warehouse to establish the first dedicated national liver disease registry providing novel insight into the changing incidence and progression associated with different liver diseases.
The Wales Liver Registry methodology builds on the Hepahealth project grouping of ICD-10 code which was predominantly applied to mortality data[15]. To increase the relevance to longitudinally collected routine hospital coding data these groupings have been modified with the addition of a hierarchical ordering of aetiological diagnoses removing less specific overlapping codes. We have prioritised diagnoses that predominantly drive the long-term liver disease outcomes to better inform appropriate service level interventions. Analysis of outpatient data suggests that routine inpatient coding will underestimate the incidence of most aetiologies, in particular viral hepatitis (Supple
The incidence of liver disease has increased 3.6-fold over the last 21 years in Wales, predominantly driven by a 10-fold increase in NAFLD diagnoses (Figure 2). This is in keeping with trends in NAFLD around the world[25]. Between 2004/5 and 2018/19 the proportion of overweight and obese individuals in Wales increased from 55% to 60% and obesity from 18% to 25%[26,27]. The rapid increase in incidence of NAFLD in the registry may reflect the evolution of establishing NAFLD in individuals over several years. Other co-morbidities that contribute to the incidence of NAFLD include diabetes and insulin resistance. The incidence of diabetes in Wales has doubled in the last 21 years and Wales has the highest prevalence of diabetes in the United Kingdom (7.4%)[28]. The precise proportion of the overweight and obesity population that will develop NAFLD remains to be defined, nonetheless, at present, the trajectory of NAFLD diagnoses continues to climb. The proportion of individuals with NALFD who had cirrhosis increased by 70% over the 21 year period (Figure 4A). This increase is likely to reflect more severe and earlier onset of obesity in Wales in combination with earlier detection using novel diagnostic approaches including fibroscan. The rising incidence of NAFLD may also be related to increased recognition and diagnosis of NAFLD through national guidelines and primary care abnormal liver function test pathways and an increasing number of hepatologists in Wales altering clinical practice.
ArLD has a high rate of cirrhosis and advanced liver disease at presentation (35%) which will have a significant demand on healthcare resources. Interestingly, the incidence of ArLD peaked between 2006 and 2008 (approximately 925 diagnoses per year), before reducing towards baseline levels seen in 1999 (630 diagnoses). The fall in ArLD incidence coincides with a trend to a reduction in the consumption of alcohol within the United Kingdom[29], and the introduction of the alcohol duty escalator between 2008 and 2013. This increased duty on alcohol by 2% above the rate of inflation reduced alcohol affordability[14,30]. Such measures demonstrate how targeted policy intervention in the community may further reduce the proportion of individuals with ArLD presenting with decompensation. Minimum unit pricing, introduced in Wales in March 2020, may further impact trends in ArLD incidence and disease behavior.
The annual all-cause mortality of individuals with a liver disease diagnosis has increased 30% over the last 21 years. This increase is a tenth of the increment of liver disease incidence. All cause and liver related mortality was greatest in individuals with an ArLD diagnosis and considerably lower for individuals with NAFLD. Advances in the management of chronic liver diseases are likely to have impacted upon mortality rates. In particular hepatitis C viral eradication with the advent of diatom diazotroph associations. Other factors that are likely to have had a significant impact are variceal screening programmes and alcohol and lifestyle interventions. Our data suggests that the early period following initial diagnosis is associated with the greatest morbidity and mortality (Figure 3). This supports previous data suggesting that early specialist follow up and presumably optimal management is associated improved outcomes following decompensation[11].
Taken together these data confirm that the major causes of liver disease in Wales are driven by population health behaviors and lifestyle, which are, crucially, modifiable. Given the scale of the obesity and diabetes epidemics in Wales, hepatology services will need to adapt and expand to manage the 5%-6% of individuals who develop cirrhosis and advanced liver disease. To improve population health and the impact on clinical services, further targeted interventions on alcohol excess and body weight are required to reverse the rising trends observed in this study and prevent the adverse health consequences also identified. Identification of patients who will most benefit from long term follow up requires further investigation.
We recognise the limitations of this study in regard to the lack of primary care and complete outpatient data. Firstly, there is a reduction in the capture of aetiology diagnoses compared to the outpatient department particularly for viral hepatitis (Supplementary Figure 3B). This may in part reflect the efficacy of nucleos(t)ide inhibitors in suppressing HBV[31,32] and direct acting antivirals to eradicate HCV[33], with a reduction in these patients developing HCC or decompensation requiring inpatient hepatology services[34,35]. Secondly whilst up to 50% of index liver disease diagnoses in the United Kingdom occur within the inpatient setting this is associated with a worse outcome[36], this is likely to reflect more advanced underlying liver disease. The described natural history of liver disease is likely to be less severe if primary care data is included. However, acute hospital admission marks a milestone in the prognosis of liver disease defining outcomes from this time point is useful when counselling the need for major lifestyle interventions such as alcohol cessation. In future, primary care diagnoses of liver disease could be included in the Liver Registry by mapping SNOMED or read codes onto ICD-10 codes and applying the same analytic approach. This would provide further insight into the time from first presentation to medical services to disease progression. The major practical challenge to accessing this data in Wales, as elsewhere, is the lack of primary care coding data in the national secondary care data warehouse. Thirdly routinely coded diagnoses do not define non-cirrhotic disease is mild or more advanced. There are inherent assumptions associated with applying a framework to a complex disease natural history and the current approach lacks a certain level of granularity, for example, HBV serological markers of disease activity or levels of fibrosis and steatohepatitis in NAFLD. This level of detail would require significant specialised data input and would probably be best served by separate or combined disease specific registries with additional clinical details and prospective data entry. Similarly cirrhosis could be stratified by inclusion of clinical scoring systems such as Childs Pugh or model for end-stage liver disease, however, it is not possible to access these results to incorporate into the Liver Registry.
We propose that the Wales Liver Registry methodology to group aetiological diagnoses and monitor time to progression is robust and has provided novel insights into liver disease in Wales prior to the coronavirus disease 2019 (COVID-19) pandemic. Development of an analytic pipeline will allow rapid assessment of COVID-19 which presents significant challenges in terms of a worsening picture in terms of liver disease lifestyle risk factors as well as impacting healthcare capacity. This data can be used to identify potential targets for the provision of hepatology services, including public health interventions, and assess their impact.
The incidence, morbidity, and mortality related to liver disease is not well understood.
To develop a national liver disease registry to inform the provision of hepatology services.
Develop and apply a novel methodology for a national liver registry incorporating aetiology, stage and mortality related to liver disease.
Novel methodology for developing a national liver registry using routinely coded secondary care and death certificate datasets.
The incidence of liver disease has increased 3.6-fold in Wales between 1999-2019 driven by a 10-fold increase in non-alcoholic fatty liver disease (NAFLD) 3-fold increase in cirrhosis, portal hypertension, decompensation and hepatocellular carcinoma, 2-fold increase in liver disease related mortality between 1999-2019. Actuarial tables of 10-year liver disease progression: Alcohol-related liver disease, autoimmune liver disease and congestive hepatopathy are associated with increased rates of decompensation and death compared to viral hepatitis and NAFLD.
The Wales Liver Registry methodology provides a novel approach to understand the progression of liver disease in the setting of a rapidly altering incidence of liver disease in Wales.
We have developed an analytic pipeline and will using this methodology to assess the impact of improvements in service provision.
We would like to thank Professor Richard Edwards, University of Otago, Wellington, New Zealand for his expert critical appraisal of this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: British Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang T, China; Welter J, Switzerland S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Office for National Statistics. Mortality statistics: deaths registered in England and Wales (series DR): 2013. [cited 15 July 2022]. Available from: https://www.gov.uk/government/statistics/mortality-statistics-deaths-registered-in-england-and-wales-series-dr-2013--4. |
| 2. | Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, Ferguson J, Forton D, Foster G, Gilmore I, Hickman M, Hudson M, Kelly D, Langford A, Lombard M, Longworth L, Martin N, Moriarty K, Newsome P, O'Grady J, Pryke R, Rutter H, Ryder S, Sheron N, Smith T. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 3. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 4. | Fontana RJ, Sanyal AJ, Ghany MG, Bonkovsky HL, Morgan TR, Litman HJ, Reid AE, Lee WM, Naishadham D; HALT-C Trial Study Group. Development and progression of portal hypertensive gastropathy in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among United States hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol. 2015;13:577-84; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, Agarwal K, Angus P, Yoshida EM, Colombo M, Rizzetto M, Dvory-Sobol H, Denning J, Arterburn S, Pang PS, Brainard D, McHutchison JG, Dufour JF, Van Vlierberghe H, van Hoek B, Forns X; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 9. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 971] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 10. | Serper M, Kaplan DE, Shults J, Reese PP, Beste LA, Taddei TH, Werner RM. Quality Measures, All-Cause Mortality, and Health Care Use in a National Cohort of Veterans With Cirrhosis. Hepatology. 2019;70:2062-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Roberts SE, John A, Brown J, Napier DJ, Lyons RA, Williams JG. Early and late mortality following unscheduled admissions for severe liver disease across England and Wales. Aliment Pharmacol Ther. 2019;49:1334-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Janjua NZ, Wong S, Abdia Y, Jeong D, Buller-Taylor T, Adu PA, Samji H, Wilton J, Pearce M, Butt ZA, Yu A, Binka M, Bartlett S, Alvarez M, Krajden M. Impact of direct-acting antivirals for HCV on mortality in a large population-based cohort study. J Hepatol. 2021;75:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | O'Donnell A, Anderson P, Jané-Llopis E, Manthey J, Kaner E, Rehm J. Immediate impact of minimum unit pricing on alcohol purchases in Scotland: controlled interrupted time series analysis for 2015-18. BMJ. 2019;366:l5274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Williams R, Alexander G, Aspinall R, Batterham R, Bhala N, Bosanquet N, Severi K, Burton A, Burton R, Cramp ME, Day N, Dhawan A, Dillon J, Drummond C, Dyson J, Ferguson J, Foster GR, Gilmore I, Greenberg J, Henn C, Hudson M, Jarvis H, Kelly D, Mann J, McDougall N, McKee M, Moriarty K, Morling J, Newsome P, O'Grady J, Rolfe L, Rice P, Rutter H, Sheron N, Thorburn D, Verne J, Vohra J, Wass J, Yeoman A. Gathering momentum for the way ahead: fifth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;392:2398-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 16. | Ly KN, Miniño AM, Liu SJ, Roberts H, Hughes EM, Ward JW, Jiles RB. Deaths Associated With Hepatitis C Virus Infection Among Residents in 50 States and the District of Columbia, 2016-2017. Clin Infect Dis. 2020;71:1149-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Ratib S, West J, Fleming KM. Liver cirrhosis in England-an observational study: are we measuring its burden occurrence correctly? BMJ Open. 2017;7:e013752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Philip G, Djerboua M, Carlone D, Flemming JA. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLoS One. 2020;15:e0229218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | Wong T, Dang K, Ladhani S, Singal AK, Wong RJ. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001-2016. JAMA. 2019;321:1723-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. ICD-10 Version: 2016. [cited 15 July 2022]. Available from: https://icd.who.int/browse10/2016/en. |
| 22. | Office for National Statistics. User guide to mortality statistics. [cited 27 July 2022]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/userguidetomortalitystatisticsjuly2017. |
| 23. | Hagström H, Adams LA, Allen AM, Byrne CD, Chang Y, Grønbaek H, Ismail M, Jepsen P, Kanwal F, Kramer J, Lazarus JV, Long MT, Loomba R, Newsome PN, Rowe IA, Ryu S, Schattenberg JM, Serper M, Sheron N, Simon TG, Tapper EB, Wild S, Wong VW, Yilmaz Y, Zelber-Sagi S, Åberg F. Administrative Coding in Electronic Health Care Record-Based Research of NAFLD: An Expert Panel Consensus Statement. Hepatology. 2021;74:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 24. | Orr JG, Homer T, Ternent L, Newton J, McNeil CJ, Hudson M, Jones DE. Health related quality of life in people with advanced chronic liver disease. J Hepatol. 2014;61:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3785] [Article Influence: 540.7] [Reference Citation Analysis (2)] |
| 26. | Llywodraeth Cymru Welsh Government. National Survey for Wales. [cited 20 July 2022]. Available from: https://gov.wales/national-survey-wales. |
| 27. | Health in Wales. Welsh Health Survey 2004/5. [cited 20 July 2022]. Available from: https://www.wales.nhs.uk/news/5137. |
| 28. | Diabetes UK. The state of the nation 2019. A review of diabetes services in Wales. [cited 20 July 2022]. Available from: https://www.diabetes.org.uk/resources-s3/public/2020-06/SOTN%20report%20ENGLISH%5BW%5D_0.pdf. |
| 29. | Office for National Statistics. Adult drinking habits in Great Britain 2017. [cited 20 July 2022]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/bulletins/opinionsandlifestylesurveyadultdrinkinghabitsingreatbritain/2017. |
| 30. | [UK] HM Government. The Government’s Alcohol Strategy. 2012. [cited 20 July 2022]. Available from: https://findings.org.uk/PHP/dl.php?f=HM_Government_4.txt#:~:text=The%20strategy%20reminds%20readers%20that%20already%20the%20government,lower%20rate%20for%20beer%20at%20or%20below%202.8%25. |
| 31. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D; BEHoLD AI463022 Study Group. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1089] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 32. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 909] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 33. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 34. | Colussi G, Donnini D, Brizzi RF, Maier S, Valenti L, Catena C, Cavarape A, Sechi LA, Soardo G. Sustained virologic response to direct-acting antiviral agents predicts better outcomes in hepatitis C virus-infected patients: A retrospective study. World J Gastroenterol. 2019;25:6094-6106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 36. | Ratib S, Fleming KM, Crooks CJ, Aithal GP, West J. 1 and 5 year survival estimates for people with cirrhosis of the liver in England, 1998-2009: a large population study. J Hepatol. 2014;60:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |