Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1608
Peer-review started: April 15, 2022
First decision: May 12, 2022
Revised: May 27, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: August 27, 2022
Processing time: 132 Days and 20.5 Hours
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide. The landscape of the systemic treatment for advanced HCC is changing quickly, and recently, the standard of care became either atezolizumab plus bevacizumab or tremelimumab plus durvalumab in the single tremelimumab regular interval durvalumab regimen. Nivolumab monotherapy has proven to be effective sometimes for advanced HCC and could be a valuable treatment option for patients outside current treatment indications and reimbursement criteria for the standard of care. This is a particular population of interest.
To evaluate the real-world effectiveness of nivolumab monotherapy in patients with advanced HCC who are not eligible for other treatment.
We conducted a retrospective, multicentric study including 29 patients with advanced HCC from 3 Belgian tertiary hospitals. All patients had had prior chemotherapy or were intolerant or ineligible for treatments. All study subjects received nivolumab 3 mg/kg in monotherapy, administered once every two weeks intravenously. Treatment continued until disease progression, severe adver
The radiological overall response rate (defined as complete or partial response according to the immune RECIST and modified RECIST criteria) to nivolumab monotherapy was 24.1%. The biological overall response rate (defined as a decrease of ≥ 25% in AFP blood level) was 20.7%. Radiological and biological responses were significantly associated both with each other (P < 0.001) and with overall survival (P < 0.005 for radiological response and P < 0.001 for biological response). Overall survival was 14.5 mo (+/- 2.1), and progression-free survival was 10.9 mo (+/- 2.3). After 4 mo of treatment, 78.3% of patients remained clinically stable or even showed improvement in WHO PS. Grade 3 adverse events occurred in 17.2% of patients, none had grade 4 adverse events, and no patients ceased nivolumab due to adverse events.
Nivolumab monotherapy is a good treatment choice in frail patients with HCC who are ineligible for the standard of care or other validated systemic treatments.
Core Tip: We conducted a study on the real-world effectiveness of nivolumab (immunotherapy) in patients with advanced liver cancer who were ineligible for the standard of care or other validated treatments, including patients with impaired liver function and a poor general condition, a population that is usually not included in studies. We showed a reduction of tumor mass in 24.1% of patients, with a disappearance of tumor mass in 13.9% of patients, which is better than that reported in the literature. Furthermore, we confirmed the favorable safety profile of nivolumab. Hence, nivolumab should be considered as a valuable treatment option in selected patients who are otherwise not eligible for treatment.
- Citation: De Wilde N, Vonghia L, Francque S, De Somer T, Bagdadi A, Staub E, Lambrechts J, Bucalau AM, Verset G, Van Steenkiste C. Real-life multi-center retrospective analysis on nivolumab in difficult-to-treat patients with advanced hepatocellular carcinoma. World J Hepatol 2022; 14(8): 1608-1620
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1608.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1608
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and a leading cause of cancer-related death worldwide, with over 826000 deaths in 2020. The worldwide incidence is 14.1 and 5.2 per 100000 men and women, respectively, and the incidence is still increasing[1]. HCC develops mainly in the context of underlying chronic liver disease, mostly in the cirrhotic stage. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, excessive alcohol consumption and metabolic syndrome are the most important risk factors[1-3].
Over the past years, therapeutic options for advanced HCC have changed remarkably. Advanced HCC is defined as a liver tumor not eligible for local therapies given the extent of disease or liver tumors that recurred after local therapies[4]. Sorafenib, a tyrosine kinase inhibitor (TKI) that showed survival benefits as a first-line systemic treatment for advanced HCC, was the only available therapy for more than a decade. Thereafter, other TKIs have become available as first-and second-line treatments for advanced HCC. The introduction of immunotherapy, however, has caused a major shift in the therapeutic landscape of advanced HCC. Immune checkpoint inhibitors (ICIs), such as anti-progr
Recent and ongoing trials of ICI in advanced HCC show encouraging results, with some excellent responders among the treated patients. However, there is great heterogeneity in response to treatment[5,8]. Recently, the Keynote-394 trial presented positive results at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium for pembrolizumab as a second-line monotherapy treatment in advanced HCC; pembrolizumab, an anti-PD-1 blocker equal to nivolumab, showed significant improvements in OS, PFS and overall response rate (ORR) compared with placebo[8].
Nivolumab as monotherapy for advanced HCC has shown promising results in the phase I/II trial Checkmate 040[9] with some durable responses but failed to show statistically significant benefit vs sorafenib in the primary endpoint of overall survival in the phase III trial Checkmate 459[10]. Both studies confirmed the good tolerability and favorable safety profile of nivolumab. The studies enrolled patients with unresectable HCC who were naïve to any systemic treatment and limited to CP class A liver function and World Health Organization (WHO) performance status (PS) 0 or 1[9,10].
In the current retrospective study, we analyzed a population of patients with advanced HCC who showed disease progression under one or more TKIs or were intolerant or ineligible for TKIs. Some of these patients also had decreased liver function with a CP score of B. This study aims to assess the effect of nivolumab monotherapy in a difficult-to-treat patient population with advanced HCC after multiple lines of treatment and for whom best supportive care was the only alternative option. In contrast to the IMbrave150[6] and HIMALAYA trials[7], our study patients were ineligible for the standard of care because they were no longer in a first-line setting because of the more advanced stage of disease, more severely impaired liver function, worse WHO PS and/or underlying comorbidities.
This is a retrospective, multicentric study including 29 patients with advanced-stage Barcelona clinic liver cancer stage C (BCLC-C) or intermediate stage (BCLC-B) HCC for whom no other validated therapeutic option at that time was available[11]. All study subjects were diagnosed with HCC between September 2014 and February 2019. Follow-up and data collection continued until May 2021. Patients were ineligible for curative options, locoregional or systemic TKI therapies, due to disease progression, more advanced cirrhosis, worse WHO PS, underlying comorbidities (mostly cardiovascular) or intolerability to TKIs because of side effects. All patients received nivolumab 3 mg/kg as monotherapy. Nivolumab was administered once every 2 wk intravenously. Treatment continued until progression (as defined below), severe adverse events or death. Patients were enrolled in 3 Belgian centers as follows: Hôpital Erasme, Université Libre de Bruxelles (ULB), Brussels (18 patients), University Hospital of Antwerp (UZA, 6 patients) and Maria Middelares Hospital Ghent (MMG, 5 patients). Approval from the ethics committee was obtained (EC number 21/06/080).
Baseline characteristics and demographics were retrospectively retrieved from the patients’ medical records. The tumor stage was defined by the BCLC staging system. Hepatic function was expressed by the CP system in case cirrhosis was present. Response to nivolumab was evaluated through 3 different outcome parameters. First, the radiological response was evaluated according to the RECIST1.1 (Response Evaluation Criteria In Solid Tumors) criteria in all three centers and additionally iRECIST in ULB and mRECIST in UZA and MMG. Second, the biological response was measured through the evolution of the alpha-fetoprotein (AFP) level, with response defined as a ≥ 25% decrease in the AFP blood level and disease progression defined as a ≥ 25% increase in the AFP blood level. Third, clinical response was assessed considering both the CP score and the World Health Organization PS at baseline and after 2 and 4 mo of therapy.
The primary endpoints were overall and progression-free survival. OS was counted in months from the start of nivolumab treatment until the patient died or until the last follow-up. Seven patients were still alive at the time of enrollment of this study. Progressive disease leading to the decision of treatment cessation was defined by the treating physician considering radiological progression or progression of the AFP level, and patients with progression of one of both were considered as progressive. One patient died before progression was reached; in this case, PFS was equal to overall survival. Adverse events were recorded and graded according to the National Comprehensive Cancer Network severity scale (grades 1-4).
Statistical analysis was performed using the SPSS Statistics 27 statistical software package. Descriptive statistics were used to express categorical variables in numbers and percentages and continuous variables in the mean and standard error of the mean. Survival (OS and PFS) was calculated and graphically represented using a Kaplan–Meier survival curve, and comparative chi-square analysis was performed. Comparative analyses were performed with chi-square testing; Breslow, long rank and Pearson chi-square coefficients were used for correlations. Conventionally, the cutoff for statistical significance was a P value < 0.05.
The patient population consisted of 29 patients (see Table 1). The male/female ratio was 21/8 (72.4%/27.6%), which is a good representation of the real-life HCC context[12]. The mean age was 69.1 (2.1) years, and the mean body mass index was 26.6 (+/- 1.0) kg/m².
| Characteristic | Case subjects (n = 29) |
| Sex | |
| Male (n) | 21 |
| Female (n) | 8 |
| Age at diagnosis, yr (mean ± SEM) | 69.1 ± 2.1 |
| BMI, kg/m² (mean ± SEM) | 26.6 ± 1.0 |
| BCLC stage (n) | |
| BCLB-B | 1 |
| BCLB-C | 28 |
| HCC characteristics (n) | |
| Bilobar | 18/28 |
| Multifocal | 15/26 |
| Vascular invasion | 8/29 |
| UP-TO-7-Criteria | 16/29 |
| Metastasis (n) | |
| No metastases | 16 |
| 1 meta location | 8 |
| 2 meta locations | 3 |
| 4 meta locations | 2 |
| AFP at baseline, ng/mL (mean ± SEM) | 4375.6 ± 2566.6 |
| Cirrhosis (n) | |
| No Cirrhosis | 10 |
| CP A | 10 |
| CP B | 8 |
| Unknown | 1 |
| Origin cirrhosis (n) | |
| HBV | 4 |
| HCV | 2 |
| Ethyl | 7 |
| NAFLD | 3 |
| Other | 2 |
| Missing | 1 |
| WHO performance status (n) | |
| 0 | 5 |
| 1 | 21 |
| 2 | 3 |
| Previous treatment (n) (Resection, radiofrequency ablation, transarterial radioembolization, transarterial chemoembolization, selective internal radiation therapy, sorafenib, capecitabine, GEMOX, doxorubicine, FOLFOX, regorafenib, cabozantinib) | |
| Yes | 27 |
| No | 2 |
One patient had an HCC stage BCLC-B, and all 28 others were advanced stage BCLC-C. The disease was bilobar in 64.3% of patients, multifocal in 57.7% and macrovascular invasion in 27.6%. Up-to-7 criteria were met in 55.2% of patients[13]. Thirteen patients (44.8%) had metastatic disease, of which 8 patients (27.6%) had only one metastatic location. Seventy-five percent of patients had increased levels of AFP at baseline, and the other 25% of patients were AFP negative. The mean baseline AFP was 4375.6 ± 2566.6 µg/L.
Eighteen patients had underlying cirrhosis, 10 patients had no underlying cirrhosis, and in 1 patient, there were no data recorded about underlying liver disease. Of the 18 cirrhotic patients, 10 had CP score A, and 8 had CP score B at baseline. The origin of cirrhosis in our study cohort was heterogeneously divided into HBV-, HCV-, alcoholic- and nonalcoholic steatohepatitis-induced cirrhosis.
Before the start of nivolumab, the WHO PS was 0 in 5 patients (17.2%), 1 in 21 patients (72.4%) and 2 in 3 patients (10.3%). Twenty-seven patients (93.1%) had received 1 or more previous treatments, and 2 patients (6.9%) had not received previous treatments because of contraindications due to comorbidities. For a detailed overview of the different previous treatment lines for each of the study subjects, we refer to Supplementary Table 1.
Radiological response: Serial radiological evaluation was performed according to the criteria RECIST 1.1, iRECIST and mRECIST; to calculate the response rate, we used iRECIST (ULB) and mRECIST (UZA and MMG), as these criteria are more specific in the current context. While the RECIST 1.1 criteria are the most commonly used criteria to evaluate response to conventional chemotherapies in solid tumors, iRECIST criteria are developed specifically to evaluate the response of novel immunomodulating agents, and mRECIST criteria are developed specifically for evaluation of HCC treatment[14].
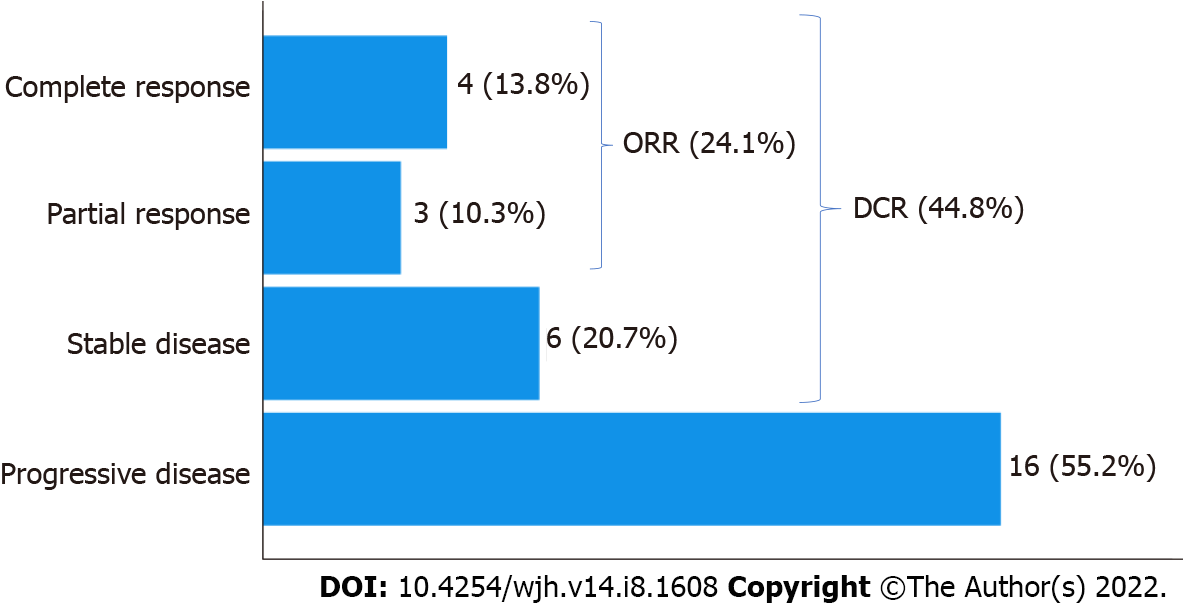
Four patients (13.9%) showed a complete response, 3 patients (10.3%) showed a partial response, and 6 patients (20.7%) showed stable disease following nivolumab therapy. As a result, the radiological overall response rate (defined as complete or partial response) was 24.1%. The radiological disease control rate (defined as complete or partial response or stable disease) was 44.8% (Figure 1).
One patient died before the radiological evaluation was possible.
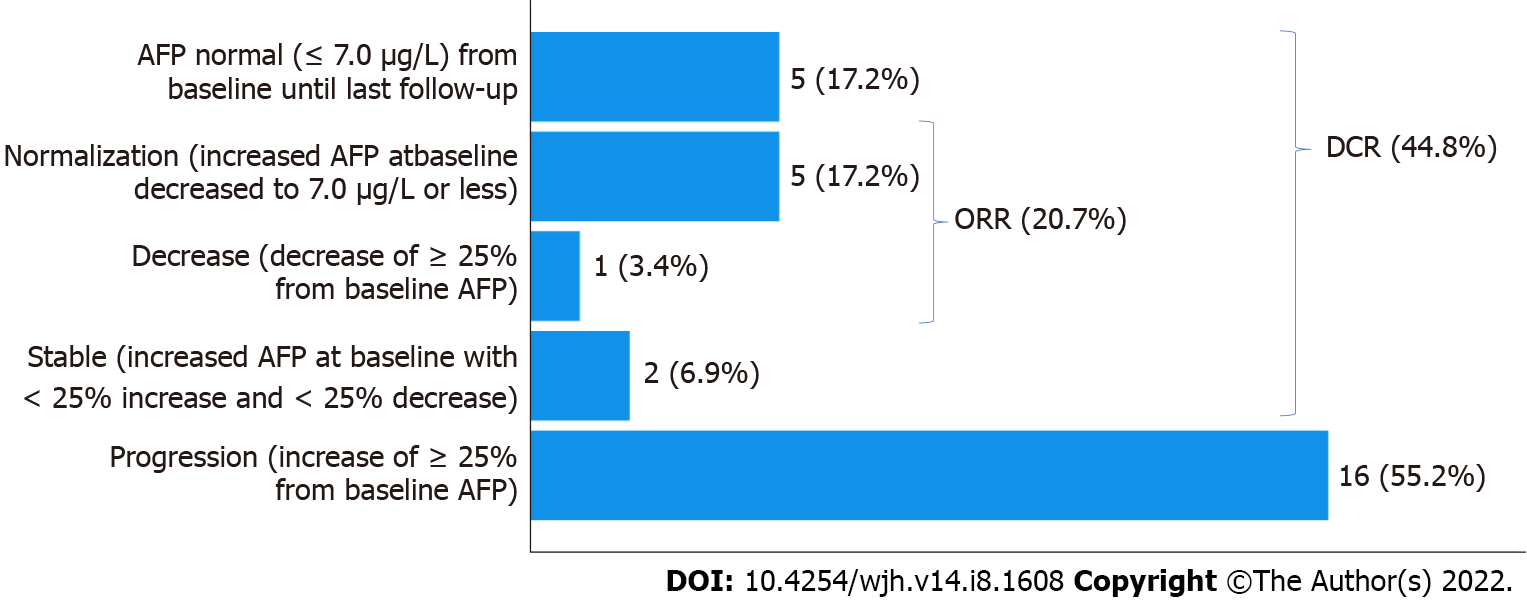
Biological (AFP) response: Of the 29 patients, 7 had no increased AFP level at diagnosis, of whom 2 developed AFP progression during nivolumab therapy. Overall, 16/29 study subjects experienced AFP progression (55.2%). AFP remained stable in 7 patients (24.1%), including the 5 AFP-negative patients at baseline. There was a 25% decrease without normalization of AFP in 1 patient (3.4%) and a normalization of AFP in 5 patients (17.2%). In our results, we differentiated between AFP decrease without normalization and complete AFP normalization.
Altogether, this accounts for a biological disease control rate (defined as AFP normalization, AFP decrease of ≥ 25% or stable AFP, including those with negative AFP from baseline until last follow-up) of 44.8% and a biological overall response rate (defined as AFP normalization or AFP decrease of ≥ 25%) of 20.7% (Figure 2).
Correlation between the radiological and biological response: The radiological and biological responses were significantly correlated (Pearson chi-square coefficient 42.28 with a P value < 0.001). All 4 patients with complete radiological remission had AFP normalization. Among the 3 patients with partial response, the results were mixed: 1 showed AFP normalization, 1 a > 25% decrease and 1 remained AFP-negative from baseline until the last follow-up. Conversely, among the 5 patients with AFP normalization, 4 had a complete radiological response, and 1 had a partial radiological response.
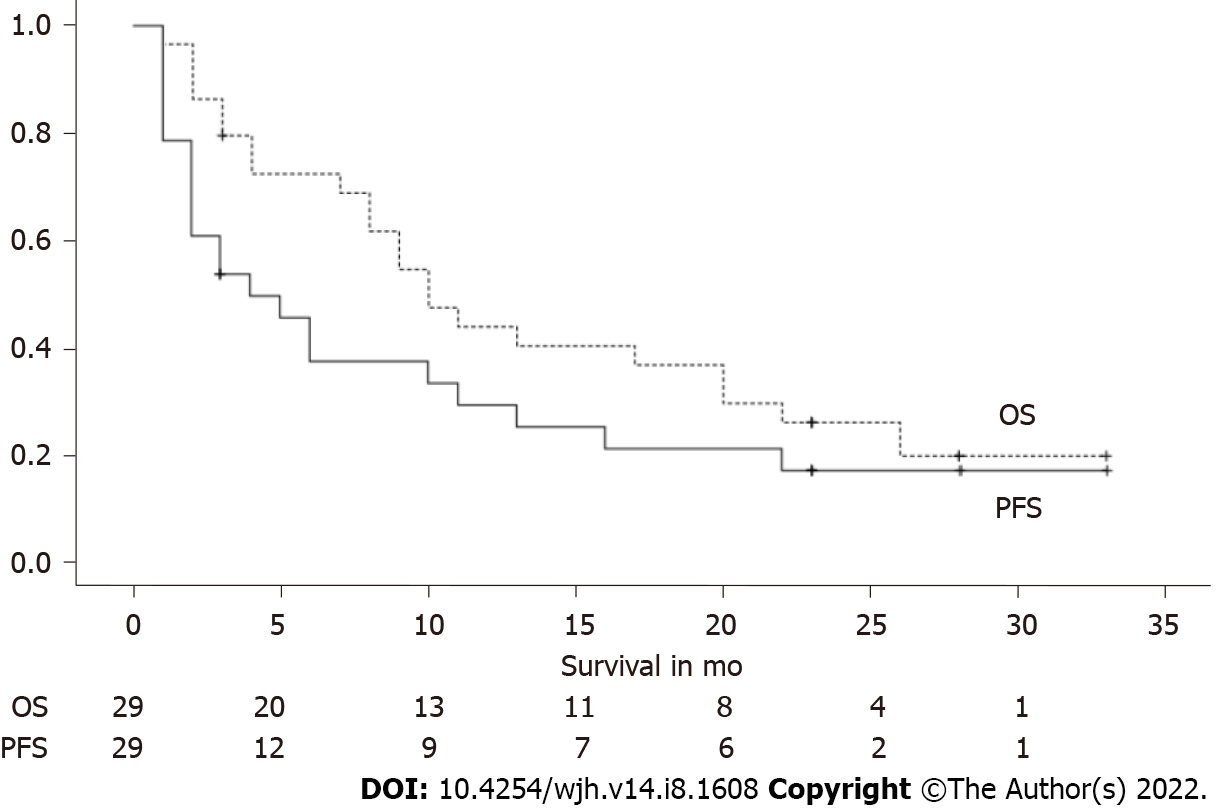
The mean OS after the start of nivolumab was 14.5 mo (+/- 2.1). Seven patients were still alive at the time of completion of this study. The patient who was censored at 3 mo underwent liver transplantation at that time (Figure 3).
Progression-free survival (with progression defined as progression radiologically or progression of AFP level, CFR. Methods) was 10.9 mo (+/- 2.3). There were 6 patients (20.7%) in the study cohort who did not show disease progression and were still alive at the time of study closure; they were censored at 3, 23 (3 patients), 28, and 33 mo (Figure 3).
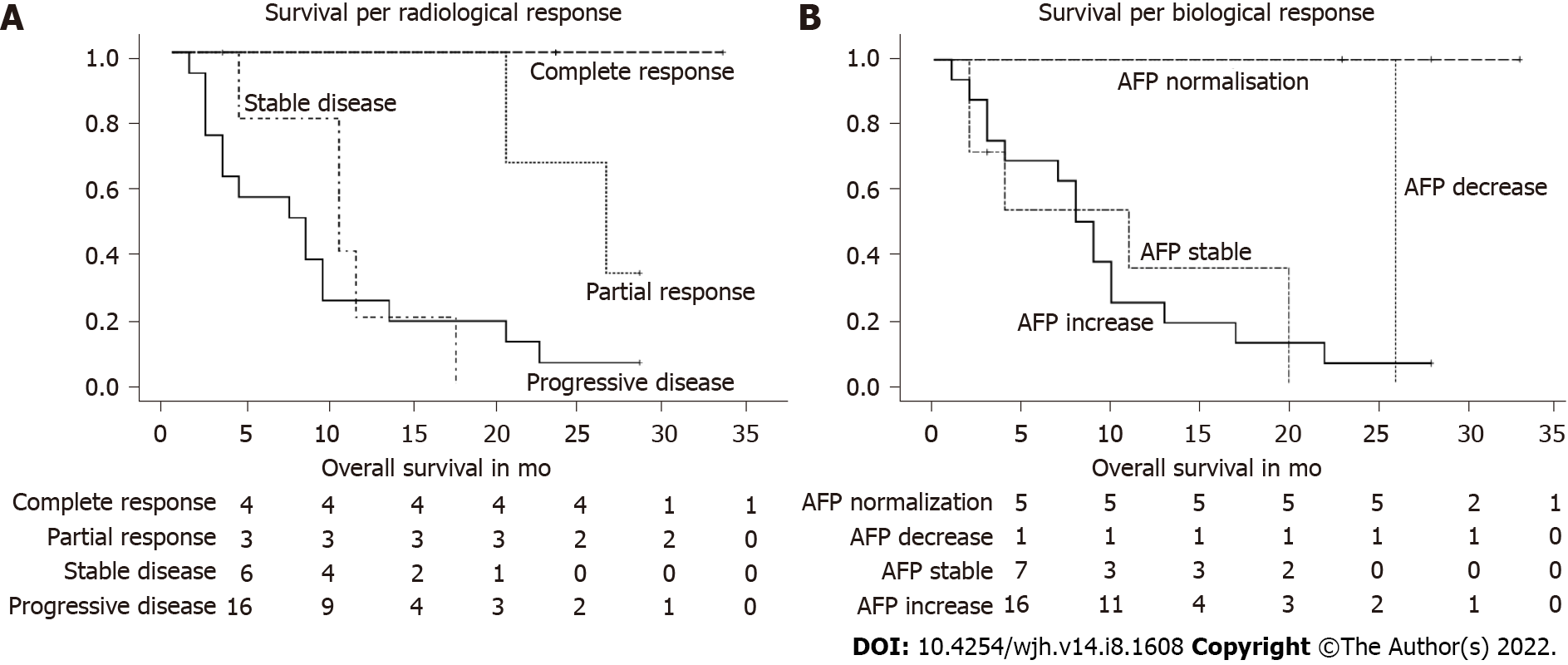
Overall survival per radiological response category: There was a statistically significant correlation between the overall survival and the radiological response to nivolumab, with a P value < 0.005 (Breslow 12.85). In the (radiological) responder group (partial and complete), all 7 patients showed a durable treatment response (1 patient for 20 mo, 1 patient for 26 mo, and 5 patients were still alive at the time of study closure with a sustained tumor response). All 4 patients with complete responses were among those 5 survivors. In the group with stable disease, the mean survival was 10.4 mo (+/- 2.1). One patient in this group received a liver transplant after 3 mo and is still alive without HCC recurrence; the patient was censored at the time of transplantation. Patients with progressive disease had a survival of 8.8 mo (+/- 2.0). One patient in this group was switched to cabozantinib after 6 mo of nivolumab treatment and was still alive at study closure (Table 2, Figure 4A). In Table 2 we presented the survival per radiological response category; total number of patients in each response category, mean overall survival in months, and the number of patients death and alive at study closure are depicted.
| Radiological response | Total n | Overall survival, mo (mean ± SEM) | n of deaths (%) | n alive at study closure (%) |
| Progressive disease | 16 | 8.8 ± 2.0 | 15 (93.7) | 1 (6.3) |
| Stable disease | 6 | 10.4 ± 2.1 | 5 (83.3) | 1 (16.7) |
| Partial response | 3 | Not assessable | 2 (66.7) | 1 (33.3) |
| Complete response | 4 | Not assessable | 0 (0) | 4 (100) |
| Overall | 29 | 14.5 ± 2.1 | 22 (75.9) | 7 (24.1) |
Overall survival per biological (AFP) response category: There was a statistically significant correlation (Breslow coefficient 21.5) between overall survival and biological AFP response, with a P value < 0.001. If we excluded the group of patients with negative AFP levels at baseline until death or last follow-up from the correlation, it remained significant with a Breslow coefficient of 10.27 (P value 0.016).
All 5 patients who showed AFP normalization were still alive at the time of elaboration of this paper, with survival of 100% at 23 mo since the start of nivolumab. One patient with a > 25% AFP decrease died at 26 mo after the start of nivolumab. Two patients with an increased AFP level at baseline showed a stable AFP level during treatment, and both died 2 mo after nivolumab was started. All but 1 of the 16 patients who showed AFP progression died. In this group, survival was 9.6 mo (+/- 1.8). In the group of patients (n = 5) with negative AFP levels at baseline until death or last follow-up, all patients but 1 died; the mean survival was 13.8 mo (+/- 3.9). In Table 3 we presented the survival per biological response category; total number of patients in each response category, mean overall survival in months, and the number of patients death and alive at study closure are depicted. For a visual representation, the categories ‘stable AFP’ and ‘AFP remaining negative from baseline until death/Last follow-up’ were merged in the graph (Table 3, Figure 4B).
| Biological (AFP) response | Total n | Overall survival, mo (mean ± SEM) | n of deaths (%) | n alive at study closure (%) |
| Increase ≥ 25% | 16 | 9.6 ± 1.8 | 15 (93.7) | 1 (6.3) |
| Stable | 2 | 2 | 2 (100) | 0 (0) |
| Decrease ≥ 25% without normalization | 1 | 26 | 1 (100) | 0 (0) |
| Normalization of AFP (< 7 μg/L) | 5 | Not assessable | 0 (0) | 5 (100) |
| AFP remains negative | 5 | 13.8 ± 3.9 | 4 (80) | 1 (20) |
| Overall | 29 | 14.5 ± 2.1 | 22 (75.9) | 7 (24.1) |
Overall survival according to baseline AFP comparing patients with an AFP level > 200 ng/mL and patients with an AFP level < 200 ng/mL was not significantly different (P = 0.517).
We evaluated the clinical response using the WHO PS and the CP score at baseline and at 2 mo and 4 mo.
WHO PS: Before the start of nivolumab treatment, the WHO PS was 0 in 17.2% of patients (n = 5), 1 in 72.4% of patients (n = 21) and 2 in 10.3% of patients (n = 3) (Table 1). We looked at the WHO PS before the start of treatment, after 2 mo, and after 4 mo of treatment to examine the evolution of the WHO PS over time for each patient individually.
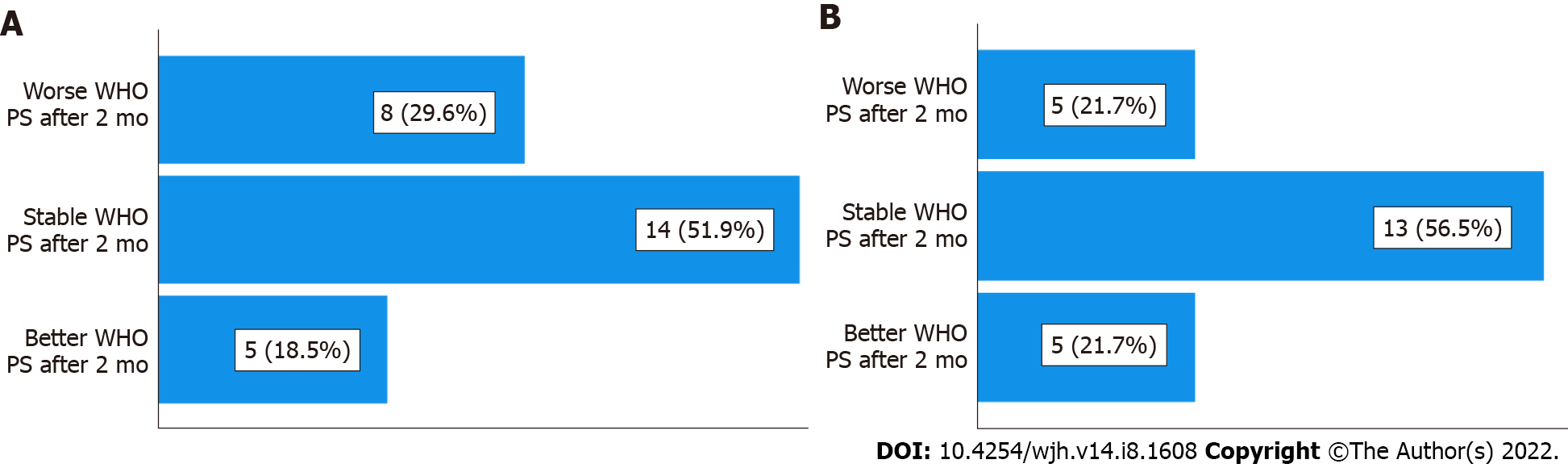
After 2 mo of nivolumab treatment, 29.6% (8/27) of the remaining patients had a worse WHO PS compared to the start of treatment; 51.9% (14/27) of patients had a stable WHO PS; and 18.5% (5/27) of patients had a better WHO PS (Table 4, Figure 5A).
| WHO PS | 2 mo | 4 mo |
| Worse PS | 8 (29.6%) | 5 (21.7%) |
| Stable PS | 14 (51.9%) | 13 (56.5%) |
| Better PS | 5 (18.5%) | 5 (21.7%) |
| Total | 27 (100%) | 23 (100%) |
| Death | 2 | 6 |
After 4 mo of treatment, in the 23 remaining patients (6 died), 21.7% (5/23) had a worse WHO PS, 56.5% (13/23) had a stable WHO PS, and 21.7% (5/23) had a better WHO PS compared to the start of treatment (Table 4, Figure 5B).
Thus, we can say that 70.4% of patients at 2 mo and 78.3% of patients at 4 mo remained clinically stable or even showed improvement.
These data illustrate the favorable tolerability of nivolumab and can support the finding that a subgroup of patients responds well to nivolumab clinically.
Before the start of nivolumab treatment, 18/29 patients had cirrhosis, of which 10 (35.7%) had CP score A and 8 (28.6%) had CP score B.
We looked at the CP before the start of treatment, after 2 mo, and after 4 mo of treatment to examine the evolution of the CP over time for each patient individually. The patients who had no cirrhosis at baseline and did not develop cirrhosis were classified in the category ‘stable CP’, assuming that their liver function remained stable.
After 2 mo of nivolumab treatment, 36% (9/25) of the remaining patients had a worse CP compared to the start of treatment; 60% (15/25) of patients had a stable CP; and 4% (1/25) of patients had a better CP (Table 5).
| CP score | 2 mo | 4 mo |
| Worse CP | 9 (36%) | 7 (33.3%) |
| Stable CP | 15 (60%) | 12 (57.1%) |
| Better CP | 1 (4%) | 2 (9.5%) |
| Total | 25 (100%) | 21 (100%) |
| Death | 2 | 6 |
| Missing | 2 | 2 |
After 4 mo of treatment, 33.3% (7/21) had a worse CP, 57.1% (12/21) had a stable CP, and 9.5% (2/21) had a better CP compared to the start of treatment (Table 5).
There were 2 patients for whom there was no information available concerning the evolution of CP scores. Generally, we observed no major impact on liver dysfunction in the first months after the start of nivolumab therapy.
Five out of 29 patients (17.2%) had grade 3 adverse events: 2 cases of dyspnea, 1 asthenia, 1 cholestatic hepatitis, and 1 cerebellar ataxia. None of these grade 3 adverse events were judged to be unequivocally related to the use of nivolumab. No side effects of grade 4 were reported. No patients ceased nivolumab due to adverse events.
Previous studies[15] suggested a more favorable outcome in certain etiologies of underlying liver disease (e.g., viral-mediated) because of improved antiviral immune responses and reduction of viral load after ICI therapy.
In our study cohort, we could not detect an association between a viral vs a non-viral etiology of liver disease and the radiological response to nivolumab (Pearson correlation was -0.330 with P value 0.086). However, it is important to note that this finding is of limited relevance given the small patient numbers (only 6 patients had an underlying viral liver disease) and hence the lack of power for a robust statistical analysis; therefore, it is impossible to draw conclusions based on this small amount of data (Table 6).
| Radiological response | Non-viral liver disease | Viral disease | Total |
| Progressive disease | 10 | 5 | 15 |
| Stable disease | 5 | 1 | 6 |
| Partial response | 3 | 0 | 3 |
| Complete response | 4 | 0 | 4 |
| Total | 22 | 6 | 28 |
The current standard of care for first-line treatment of advanced HCC is either atezolizumab plus bevacizumab, based on the results of the phase III IMbrave 150 trial[6], or tremelimumab plus durvalumab in the STRIDE regimen, based on the results of the phase III HIMALAYA trial[7]. The IMbrave trial included patients with advanced HCC who had an excellent PS, a liver function no worse than CP A, no extended macrovascular invasion, no prior bleeding event due to untreated or incom
Our real-world population also included patients with a worse PS and worse liver function (including CP B). Furthermore, our patients had already received (often multiple) previous treatment lines, while the IMbrave150 trial and the HIMALAYA trial took place in a first-line setting[6,7].
In the present study, 6 of 29 patients (20.7%) showed an impressively good and sustained response (radiologically and biologically) to nivolumab monotherapy, with 4 complete responders. All 6 patients were still alive and had an excellent PS at study closure, while before nivolumab treatment, these patients had a very poor prognosis, since no other treatment options were available at the start of treatment.
Compared to the phase III Checkmate 459 trial[10], the results of the present study in a selected patient population are notably better, with an overall response rate of 24.1% and especially a complete response in 13.9% of patients, compared to 15% and 4%, respectively, in the Checkmate459 trial[10].
The phase III Checkmate459 trial included systemic therapy-naïve patients, while most of our patients had already received (multiple) previous systemic treatments. However, in the entire cohort of the Checkmate trial, durable effects of nivolumab were observed in sorafenib-naïve (ORR 23%) and sorafenib-experienced (ORR 16%-19%) patients[9,10].
Due to the long follow-up (of 33 mo) of the present study, we demonstrated that when there was a good response to treatment, this response was sustained, and the patient remained in a very good condition for a long time (our patient with the longest survival received nivolumab monotherapy at the time of study closure (2 years) and 9 mo) and was in excellent condition.
In conclusion, even though nivolumab monotherapy could not demonstrate a significant survival benefit over sorafenib in the phase III Checkmate 459 trial[10], in our specific population of patients with HCC for whom no other validated therapeutic option is available, this treatment has been shown to be effective and associated with a sustained response and an excellent PS in a subset of patients.
Recently, pembrolizumab (an anti-PD 1 blocker such as nivolumab) showed positive results as a second-line treatment for advanced HCC in the Keynote-394 trial compared with placebo[8].
In the HIMALAYA trial[7], durvalumab (an anti-PD-L1 blocker) in monotherapy showed nonin
These results confirm that blocking the pathophysiologic pathway of PD-1 is an effective strategy in the treatment of advanced HCC.
Interestingly, nivolumab plus ipilimumab (= anti-CTLA-4) as a second-line treatment for advanced HCC demonstrated an ORR of 33% (per RECIST 1.1)[16]. In the HIMALAYA trial[7], on which the latest standard of care was based, the same combination of dual antibody therapy was used, durvalumab, a PD-L1 blocker, plus tremelimumab, a CTLA-4 blocker, and this combination showed good efficacy and safety.
However, dual antibody therapy implies a higher cost and a higher potential of (life-threatening) adverse events, but compared to TKIs in second-line therapy, ICIs might be preferable because of the higher response rate (including some complete responders) and the more favorable safety profile[7,17].
Especially in a second- or third-line setting, in a more frail or ill patient population who have worse liver function or intolerability or contraindications for TKIs (e.g., patients with a higher risk of bleeding varices) or who have already progressed under TKI treatment and for whom there is no validated treatment available (yet), nivolumab in monotherapy is a valuable treatment option[18,19].
Radiological (tumor mass) and biological (AFP) evolution were used as outcome parameters to define the response to nivolumab therapy. They are strongly associated both mutually and with survival, supporting their value as good outcome parameters.
Our study confirmed that nivolumab is generally well tolerated and has a favorable safety profile even in patients with impaired liver function and/or poor PS. Generally, there was no major impact on the WHO PS or on the CP scores following the start of nivolumab treatment. Nevertheless, clinicians should always be aware of potentially life-threatening immune-mediated toxicity when administering ICIs[16].
Today, the field of systemic therapy of advanced HCC is in full development. Future research is warranted to determine the best standard of care in the first-line setting and to develop evidence-based recommendations concerning the treatment sequence after first-line therapy, strategies to select patients who will benefit from one of the currently available treatment modalities, and an adequate therapeutic approach in patients with CP B and/or a poor PS[18].
Nivolumab monotherapy in this real-world retrospective multicenter case series of difficult-to-treat HCC cases – deemed ineligible for other systemic treatments, including patients with impaired liver function and poor PS – is a valuable option with a substantial number of responders with sustained clinical improvement, good OS and PFS and with excellent tolerability and safety.
Nivolumab monotherapy should hence be considered in selected patients otherwise not eligible for systemic treatment, a population that is usually not included in randomized controlled trials.
These results need to be confirmed in a prospective trial targeting the specific population of frail patients with HCC for whom antiangiogenic agents are contraindicated. Further studies are warranted to better define the group of patients who might benefit from this strategy and how to select them.
Hepatocellular carcinoma is one of the leading causes of cancer-related death worldwide. The landscape of the systemic treatment for advanced hepatocellular carcinoma (HCC) is changing quickly. However, frail patients with impaired liver function and poor performance status, who already received multiple treatment lines, are often excluded from randomized controlled trial’s and are ineligible for the standard of care. Nivolumab monotherapy has proven to be effective sometimes for advanced HCC, with a favorable safety profile, and hence nivolumab could be a valuable treatment option for these patients.
Given the recent fast evolutions in the systemic treatment of advanced HCC nowadays, this study is very topical. This article provides interesting information as a starting point for further research, e.g., on patient selection per treatment strategy.
We aimed in this study to evaluate the real-world effectiveness of nivolumab monotherapy in patients with advanced HCC who are not eligible for other treatment.
We conducted a retrospective, multicentric study including 29 patients with advanced HCC. All patients had had prior chemotherapy or were intolerant or ineligible for treatments. Data were retrieved from patients’ medical records. The outcome parameters that we evaluated were radiological response according to RECIST criteria, the biological response through the evolution of the alpha-fetoprotein level, and clinical response considering both the Child–Pugh score and the World Health Organization performance status. A safety profile was also reported. Statistical analysis was performed using the SPSS Statistics 27 statistical software package.
The radiological overall response rate (ORR) to nivolumab monotherapy was 24.1%. The biological ORR was 20.7%. Radiological and biological responses were significantly associated both with each other and with overall survival. Overall survival was 14.5 mo (+/- 2.1), and progression-free survival was 10.9 mo (+/- 2.3). We confirmed the favorable safety profile of nivolumab. We showed notably better results than reported in literature. Hence nivolumab monotherapy should be considered as a valuable treatment option in selected patients otherwise not eligible for systemic treatment. Further research is warranted to confirm these findings.
Nivolumab monotherapy is a good treatment choice in frail patients with HCC who are ineligible for the standard of care or other validated systemic treatments.
This article provides interesting information as a starting point for further research. Future research is warranted to confirm the good treatment response to nivolumab in a subgroup of patients with advanced HCC, and furthermore to define this subgroup of patients, to facilitate patient selection per treatment strategy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt; Yeoh SW, Australia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | World Health Organization. The Global Cancer Observatory 2020. [cited 2022 Apr 15]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1215] [Article Influence: 202.5] [Reference Citation Analysis (1)] |
| 3. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 806] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 4. | Huitzil-Melendez FD, Capanu M, O'Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Lin Z, Lu D, Wei X, Wang J, Xu X. Heterogeneous responses in hepatocellular carcinoma: the achilles heel of immune checkpoint inhibitors. Am J Cancer Res. 2020;10:1085-1102. [PubMed] |
| 6. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39:267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 7. | Ghassan KA, Chan SL, Kudo M, Lau G, Kelley RK. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 8. | Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, Meng Z, Bai Y, Chen X, Liu X, Xiao J, Ho GF, Mao Y, Ye X, Ying J, Li J, Zhong WY, Zhou Y, Siegel AB, Hao C. Pembrolizumab (pembro) plus best supportive care (BSC) vs placebo plus BSC as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol. 40:383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta-Rivera M, Choo SP, El-Khoueiry AB, Kuromatsu R, El-Rayes B, Numata K, Itoh Y, Di Costanzo F, Crysler O, Reig M, Shen Y, Neely J, Tschaika M, Wisniewski T, Sangro B. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 10. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab vs sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 738] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 11. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [PubMed] [DOI] [Full Text] |
| 12. | Liu P, Xie SH, Hu S, Cheng X, Gao T, Zhang C, Song Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget. 2017;8:68131-68137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (1)] |
| 14. | Henze J, Maintz D, Persigehl T. RECIST 1.1, irRECIST 1.1, and mRECIST: How to Do. Curr Radiol Rep. 2016;4:48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Liu X, Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist. 2019;24:S3-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 964] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 17. | Kambhampati S, Bauer KE, Bracci PM, Keenan BP, Behr SC, Gordan JD, Kelley RK. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: Safety and clinical outcomes in a retrospective case series. Cancer. 2019;125:3234-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (1)] |
| 19. | Fang P, Hu JH, Cheng ZG, Liu ZF, Wang JL, Jiao SC. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II trials. PLoS One. 2012;7:e49717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |