Published online Aug 27, 2022. doi: 10.4254/wjh.v14.i8.1598
Peer-review started: March 7, 2022
First decision: June 8, 2022
Revised: June 30, 2022
Accepted: August 10, 2022
Article in press: August 10, 2022
Published online: August 27, 2022
Processing time: 171 Days and 17 Hours
There is an urgent need to risk stratify patients with suspected nonalcoholic fatty liver disease (NAFLD) and identify those with fibrotic nonalcoholic steatohepatitis. This study aims to apply a simple diagnostic algorithm to identify subjects with at-risk NAFLD in the general population.
To apply a simple diagnostic algorithm to identify subjects with at-risk NAFLD in the general population.
Adult subjects were included from the National Health and Nutrition Exam
Three thousand six hundred and sixty-nine patients were identified who met all inclusion and exclusion criteria. From this cohort, 911 (28.6%) patients had elevated ALT of which 236 (22.9%) patients had elevated FIB4 scores ≥ 1.3. Among patients with elevated FIB4 score, 75 (24.4%) had elevated FAST scores, ruling in advanced fibrosis. This accounts for 2.0% of the overall study population. Applying this algorithm to 737 patients with T2DM, 213 (35.4%) patients had elevated ALT, 85 (37.9%) had elevated FIB4, and 42 (46.1%) had elevated FAST scores. This accounts for 5.7% of the population with T2DM.
The application of this algorithm to identify at-risk NAFLD patients in need for specialty care is feasible and demonstrates that the vast majority of patients do not need subspecialty referral for NAFLD.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis represent a major public health threat as the incidence and prevalence continues to rise. This patient population has the potential to overwhelm hepatology clinics if not appropriately triaged by those physicians making referrals. This manuscript presents a simple diagnostic algorithm that outlines how physicians can approach an undifferentiated patient with findings concerning for NAFLD.
- Citation: Alkhouri N, Aggarwal P, Le P, Payne J, Sakkal C, Polanco P, Harrison S, Noureddin M. Simple diagnostic algorithm identifying at-risk nonalcoholic fatty liver disease patients needing specialty referral within the United States. World J Hepatol 2022; 14(8): 1598-1607
- URL: https://www.wjgnet.com/1948-5182/full/v14/i8/1598.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i8.1598
Nonalcoholic fatty liver disease (NAFLD) undoubtedly presents a significant public health crisis in the coming years. The estimated global prevalence of NAFLD is about 25% with higher prevalence in middle-aged American adults at 38%[1,2]. As projected by Census.gov, the population of the United States is expected to surpass 350 million by 2030[3]. While the demand will continue to increase for specialty referral, it is likely that the triaging of these patients into low-risk and high-risk will increasingly fall on the shoulders of primary care physicians (PCPs).
NAFLD has a spectrum ranging from nonalcoholic fatty liver (NAFL) or simple steatosis to nonalcoholic steatohepatitis (NASH) which is characterized by hepatic inflammation and hepatocyte ballooning leading to progressive fibrosis (F1-F3) and eventually cirrhosis (F4)[4-7]. Several studies have shown that patients with NASH and significant fibrosis (³F2) have increased risk for developing major adverse liver outcomes such as ascites, encephalopathy, and variceal bleeding[8,9].
There are several different methods for determining the presence and severity of NAFLD in the general and at-risk population. Checking alanine aminotransferase (ALT) levels has been recommended by several professional societies as a simple screening tool for NAFLD in at-risk populations such as those with metabolic syndrome and type 2 diabetes mellitus (T2DM)[10] {, 2016 #538}{, 2016 #538}. The fibrosis-4 (FIB4) index was developed and validated to identify patients with advanced fibrosis. Its use has been validated in patients with NAFLD; however, there has been some debate about the optimal cutoff[11-13]. By choosing a cutoff of 1.3 to differentiate low risk from high risk patients, our aim was to optimize the sensitivity to ensure patients with advanced fibrosis are not excluded prematurely. This cutoff point has been validated in patients with NAFLD and NASH and is generally regarded as the optimal benchmark for ruling out patients who are low risk for NASH[14,15].
Vibration controlled transient elastography (VCTE) is a more direct measure of liver stiffness. In a validation cohort of nearly 400 patients, VCTE performed well in identifying patients with advanced fibrosis and cirrhosis with areas under the receiver operating curve of 0.83 and 0.93, respectively[16]. Measuring the liver stiffness measure (LSM) and controlled attenuated parameter (CAP), VCTE or FibroScan® is a relatively inexpensive way to identify patients at risk for advanced liver disease. The FAST score, which is calculated from combining LSM, CAP and aspartate aminotransferase data, is a noninvasive metric that has improved sensitivity and specificity compared to FibroScan® alone in terms of identifying patients with NASH and fibrosis stage F2 or higher (at-risk NAFLD). In a validation cohort, the cutoff of 0.35 was chosen for its 90% sensitivity in identifying at-risk NAFLD patients[17].
Given that this disease process can take several decades to become clinically significant and that there is significant phenotypic variation, identifying patients who may qualify for therapeutic interventions has become increasingly important. Often, patients are referred to hepatology after their disease has progressed to cirrhosis when there is more limited opportunity to reverse disease course compared to earlier therapy. Conversely, patients can be referred for evaluation extremely early in their disease course at which point they are not candidates for pharmacologic therapy. Currently, there are no FDA-approved therapies for NAFLD or NASH. However, patients with advanced or bridging fibrosis (F2-F3) and cirrhosis (F4) are the targets for several ongoing clinical trials[18]. Catching patients in this therapeutic window presents a significant obstacle in the efficacious management of NASH. Presently, there are no unifying diagnostic algorithms to help these providers differentiate between which patients need referral to hepatology. Several algorithms that combined serologic tests and VCTE have been proposed but their implications for the US healthcare system in terms of resource utilization and need for specialty providers have not been evaluated. The aim of this study was to assess the application of a simple diagnostic algorithm that combined ALT, FIB4 and the FAST score to identify subjects with at-risk NAFLD in the US general population and in those with T2DM.
The National Health and Nutrition Examination Survey (NHANES) is a deidentified database created in partnership with the Center for Disease Control and Prevention. This database catalogs patient information from patient-completed surveys and objective medical data obtained from physical exams. In addition to demographic, socioeconomic, dietary, and health-related questions, the NHANES database also contains medical, dental, physiologic, and laboratory measurements[19].
We identified all adult subjects from the NHANES database between 2017 and 2018 who had valid VCTE data. Patients were excluded if they had any history or evidence of viral hepatitis (B or C), or significant alcohol consumption as defined by an average of ≥ 2 alcoholic beverages per day in men and ≥ 1 alcoholic beverage in women. Elevated ALT was defined as having values > 19 IU/L in women and > 30 IU/L in men.
Patients were considered for be at risk for NAFLD if they had elevated ALT score (> 19 U/L for female and > 30 U/L for male) in the absence of excessive alcohol consumption or viral hepatitis. FIB4 scores were calculated on all patients. Those with FIB4 < 1.3 were deemed low-risk for at-risk NAFLD while those with FIB4 ≥ 1.3 were subject to further evaluation with the FAST score. Subjects with a FAST score < 0.35 were also deemed low risk. If patients had both an elevated FIB4 and elevated FAST score, they were considered high risk for advanced liver disease and warranted further evaluation by a specialist. The same algorithm was applied to patients with T2DM. Appropriate survey weights were applied for all analyses which were performed using Stata version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.).
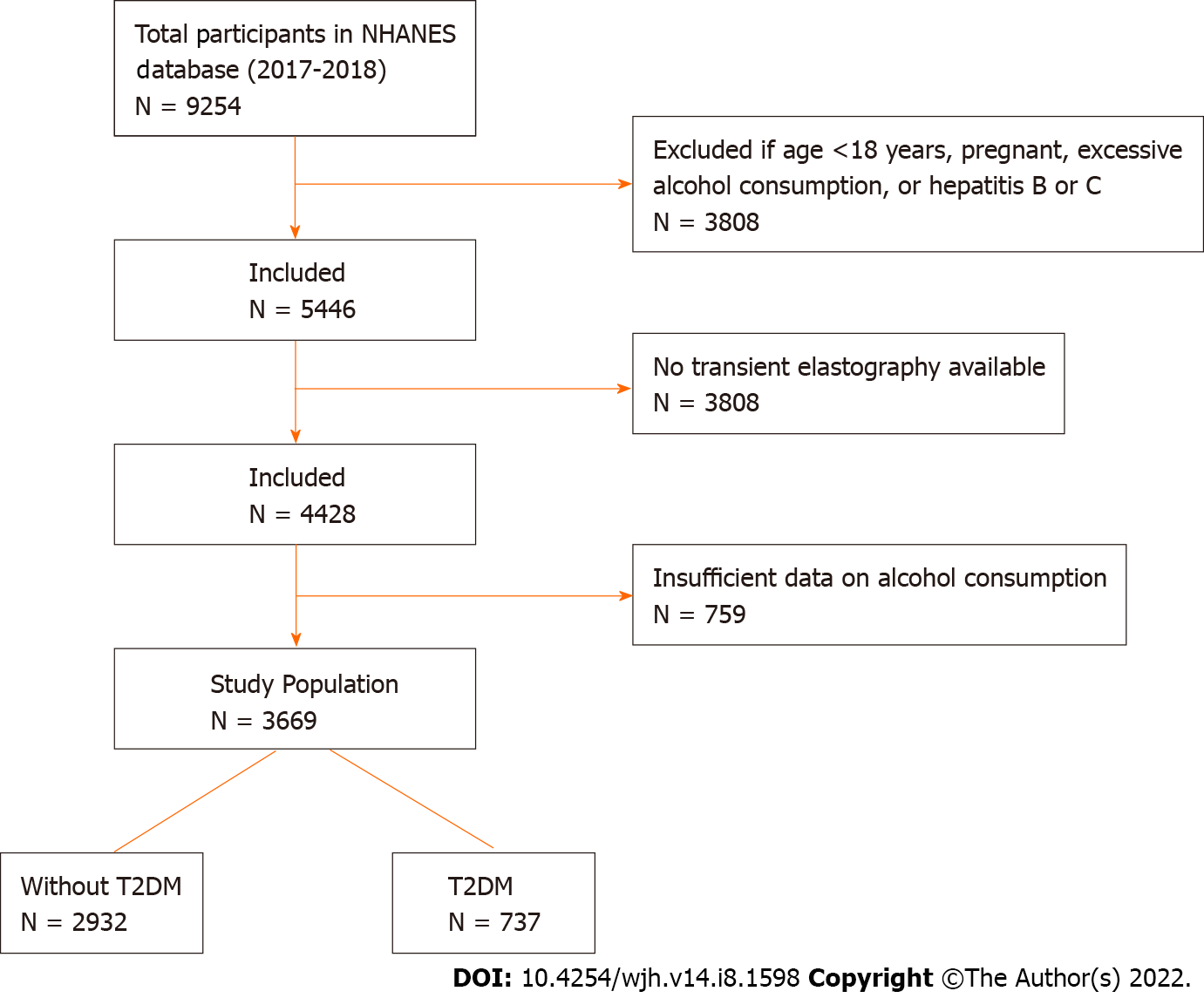
A total of 9254 patients were identified in the NHANES database between 2017 and 2018. From this list, 3808 patients (41.1%) were excluded based on the aforementioned exclusion criteria. Another 1018 patients (11.0%) had not completed transient elastography and 759 patients (8.2%) did not have satisfactory record of alcohol consumption. The final study population included 3669 patients meeting all inclusion and exclusion criteria. Among these patients, 737 patients (7.9%) had T2DM (Figure 1).
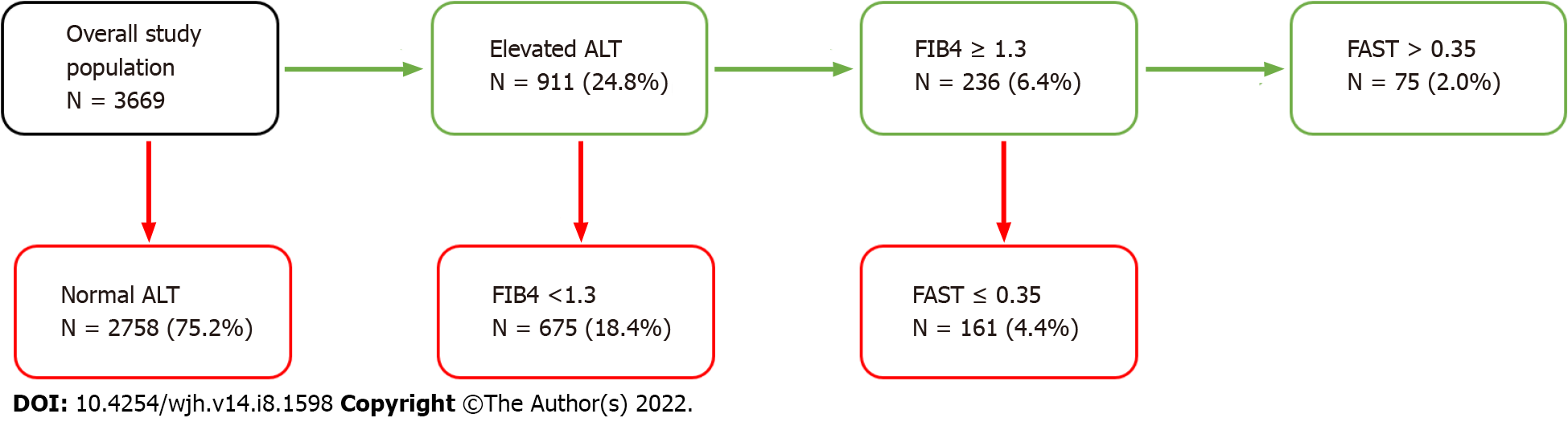
In the overall study population, 911 patients (28.6%) had elevated ALT per our inclusion criteria. Among these patients with elevated ALT, 236 patients (22.9%) had an elevated FIB4 score ≥ 1.3. Among the 236 patients with an elevated FIB4 score, 75 patients (24.4%) had an elevated FAST score ≥ 0.35. This accounts for 2.0% of the overall study population (Figure 2).
When comparing the patients at high risk for clinically significant NASH to their low-risk counterparts, several variables were clinically significant. Patients with at-risk NAFLD were more likely to be older (57.6% vs 47.3%, P < 0.01), of male gender (71.6% vs 49.6%, P < 0.01), obese (33.1% vs 29.6%, P < 0.01), and Hispanic (29.2% vs 16.6%, P < 0.01). When assessing their comorbidities, high risk patients were more likely to have T2DM (55.7% vs 14.1%, P < 0.01) and hypertension (86.5% vs 45.2%, P < 0.01). These data are summarized in Table 1.
| Study population | High risk patients | P value1 | |
| N = 3669 | N = 75 | ||
| Age (yr) — mean (95%CI) | 47.3 (45.7-48.9) | 57.6 (54.6-60.6) | 0.005 |
| Male — % (95%CI) | 49.6 (46.8-52.5) | 71.6 (51.9-85.5) | 0.005 |
| BMI (kg/m2) — mean (95%CI) | 29.6 (28.9-30.3) | 33.1 (30.5-35.7) | 0.003 |
| Race/Ethnicity — % (95%CI) | |||
| Non-Hispanic White | 63.6 (58.3-68.6) | 45.4 (29.6-62.2) | < 0.001 |
| Non-Hispanic Black | 10.8 (7.9-14.6) | 10.7 (4.1-25.4) | |
| Hispanic | 16.6 (12.8-21.1) | 29.2 (17.3-44.9) | |
| Non-Hispanic Asian | 4.4 (3.1-6.4) | 4.2 (1.6-10.7) | |
| Other | 4.7 (3.5-6.2) | 10.4 (2.4-35.8) | |
| Comorbidities — % (95%CI) | |||
| T2DM | 14.1 (12.4-15.9) | 55.7 (39.2-71.0) | 0.001 |
| Hypertension | 45.2 (42.0-48.5) | 86.5 (75.4-93.1) | 0.001 |
| Lab values — mean (95%CI) | |||
| Total bilirubin (mg/dL) | 0.47 (0.46-0.49) | 0.60 (0.50-0.70) | 0.89 |
| AST (IU/L) | 21.34 (20.87-21.82) | 52.63 (44.03-61.24) | < 0.001 |
| ALT (IU/L) | 22.55 (21.77-23.32) | 54.88 (46.57-63.18) | < 0.001 |
| GGT (IU/L) | 28.05 (26.78-29.32) | 92.98 (46.70-139.26) | 0.02 |
| Albumin (g/dL) | 4.11 (4.07-4.14) | 4.10 (4.02-4.18) | 0.50 |
| Alkaline (IU/L) | 76.04 (74.33-77.76) | 89.72 (66.07-113.36) | 0.46 |
| LSM (kPa) | 5.65 (5.43-5.86) | 11.83 (7.99-15.67) | 0.002 |
| CAP (dB/m) | 261.59 (257.55-265.62) | 324.59 (310.78-338.40) | < 0.001 |
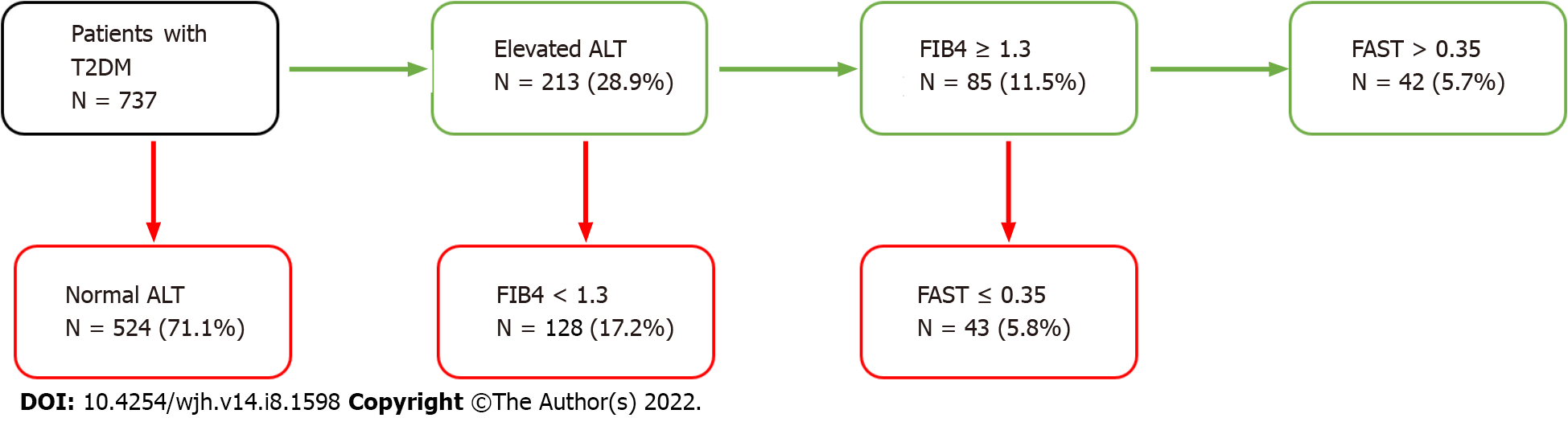
In the subset of 737 patients with T2DM, 213 patients (35.4%) had an elevated ALT. Among these patients, 85 patients (37.9%) had an elevated FIB4 score ≥ 1.3. Among these patients, 42 patients (46.1%) had an elevated FAST score. This accounts for 5.7% of the diabetes cohort (Figure 3) being in the category of at-risk NAFLD. Patients with diabetes are nearly three times more likely to have fibrotic NASH compared to the overall study population (OR 2.89 [1.96-4.26, P < 0.01]).
Clinical parameters were similarly evaluated in the diabetes cohort and compared between high risk and low risk patients. These data are summarized in Table 2. When compared to their low-risk counterparts, diabetic patients at high risk for clinically significant NASH were not found to be significantly different in any demographic category. In this cohort, high risk patients were found to have an elevated GGT value compared to the low-risk patients (69.2 IU/L vs 34.6 IU/L, P < 0.01).
| T2DM population | High risk patients | P value1 | |
| N = 737 | N = 42 | ||
| Age (yr) — mean (95%CI) | 60.3 (58.3-62.5) | 62.7 (57.4-68.0) | 0.48 |
| Male — % (95%CI) | 55.6 (47.2-63.8) | 77.1 (55.6-90.0) | 0.67 |
| BMI (kg/m2) — mean (95%CI) | 33.5 (32.2-34.8) | 34.5 (30.9-38.1) | 0.13 |
| Race/Ethnicity — % (95%CI) | |||
| Non-Hispanic White | 60.5 (53.6-66.9) | 45.7 (25.5-67.4) | 0.07 |
| Non-Hispanic Black | 13.2 (8.8-19.3) | 5.7 (1.8-16.9) | |
| Hispanic | 15.2 (11.5-19.7) | 26.5 (14.7-42.9) | |
| Non-Hispanic Asian | 4.9 (3.2-7.4) | 3.7 (1.1-12.2) | |
| Other | 6.2 (4.1-9.4) | 18.4 (4.3-53.4) | |
| Comorbidities — % (95%CI) | |||
| T2DM | NA | NA | NA |
| Hypertension | 80.5 (76.6-83.9) | 95.7 (80.1-99.2) | 0.64 |
| Lab values — mean (95%CI) | |||
| Total bilirubin (mg/dL) | 0.48 (0.43-0.53) | 0.56 (0.42-0.70) | 0.18 |
| AST (IU/L) | 21.74 (20.58-22.89) | 47.49 (38.36-56.61) | < 0.001 |
| ALT (IU/L) | 24.69 (22.83-26.56) | 53.58 (43.19-63.98) | 0.004 |
| GGT (IU/L) | 34.66 (32.51-36.81) | 69.21 (47.44-90.98) | 0.005 |
| Albumin (g/dL) | 3.99 (3.94-4.05) | 4.11 (4.01-4.20) | 0.46 |
| Alkaline (IU/L) | 83.36 (79.97-86.75) | 82.85 (71.44-94.26) | 0.46 |
| LSM (kPa) | 7.32 (6.47-8.18) | 14.45 (8.62-20.27) | 0.005 |
| CAP (dB/m) | 308.21 (302.05-314.37) | 333.66 (320.14-347.17) | 0.02 |
The main findings of the current study are the following: (1) In a nationally representative cohort of adult Americans, the implementation of a simple diagnostic algorithm to identify patients with at-risk NAFLD (fibrotic NASH) in need for pharmacologic intervention was feasible; and (2) by using this algorithm, only 2% of the general adult population and 6% of diabetics would have needed referral to a subspecialist providing reassurance that the implementation of this algorithm on a large scale will not lead to overwhelming subspecialists with unnecessary referrals.
Given a burdened healthcare system with significant logistic constraints, there is room for improvement in the subspecialty referral process to gastroenterology and hepatology for patients with NAFLD. These data add to a growing body of literature that supports that the vast majority of patients who are referred require clinical observation and serial monitoring. In a recent study of an Australian cohort, the authors found that 75% of patients referred to hepatology had low risk of advanced fibrosis and more than 2/3 (68%) of patients could be discharged back to their primary care or referring physician[20].
Our approach provides a more nuanced method of screening and identifying patients with NAFLD. In a survey of more than one hundred primary care physicians, the vast majority (70%) reported they would be unlikely to refer a patient to hepatology unless the patient had abnormal liver enzymes[21]. In a survey of eleven laboratories, it was found that the upper limit of normal for ALT values varied from 35 U/L to 79 U/L for men and 31 U/L to 55 U/L for women[22]. Thus, our more sensitive cutoff of 30 U/L for men and 19 U/L for women could be misinterpreted as within normal range by providers[23]. The circumstance may arise where patients have elevated FAST scores, but normal FIB-4 scores or vice versa. In these situations with equivocal findings in which patients did not follow the stepwise algorithm, the authors would recommend subspecialty referral for further risk stratification and diagnostics. This algorithm provides a more effective method of triaging patients for subspecialty referral by relying less on one sole biomarker. Rather, it employs a more comprehensive set of tools for assessment that account for other liver specific variables better than transaminases alone.
Notably, several demographic variables lost statistical significance in the cohort of patients with T2DM, for which there may be several explanations. It’s possible that our sample size was too small to achieve statistical significance, which necessitates further investigation into this vulnerable population. However, it is more likely that consistent with previous literature, insulin resistance and the associated T2DM is one of the most significant independent risk factors for the development of NAFLD and NASH[24-26]. This association can make triaging patients with T2DM more challenging in the clinical setting. Rather, clinicians should rely on objective metrics such as the FIB4 score and FibroScan® data to better understand these patients’ disease states.
A significant challenge for PCPs, endocrinologists, and other non-liver providers is likely access to FibroScan®. As a relatively new technology that has yet to become ubiquitous both in awareness and availability, referring providers rely on subspecialists to distinguish low-risk patients from high-risk patients and subsequently order the appropriate diagnostic imaging. However, even in the absence of Fibroscan®, meaningful triage can be accomplished with the FIB4 score. The FIB4 score is an extremely cost-efficient starting point for providers that requires measurement of a patient’s blood count and serum chemistry. The implementation of this inexpensive screening modality alone would increase the specificity of referral for NAFLD significantly.
In a recently published clinical care pathway, the authors generated a similar pathway for categorizing patients at high risk for advanced fibrosis. Their pathway starts with identifying patients at risk for NAFLD, including those with metabolic risk factors, T2DM, or imaging that shows steatosis or fibrosis. Subsequently, these patients are stratified into low, intermediate, or high risk based on FIB-4 cutoffs of < 1.3, 1.3-2.67, and > 2.67 respectively. Low risk patients are referred back to their PCPs for clinical observation, while high-risk patients are referred forward to hepatology. Intermediate risk patients are recommended to undergo FibroScan® and are further stratified in to low, intermediate, or high risk based on LSM cutoffs of < 8, 8-12, and > 12 kPa. Both intermediate and high-risk patients based on LSM value (> 8 kPa) are recommended for referral to hepatology[27]. The authors estimated that roughly 10% of patients in this pathway will have high-risk disease. The implementation of our complementary pathway showed that this may be a slight overestimate given that 2% of the general population and 6% of diabetics were found to be high-risk based on our algorithm. To our knowledge, this is the first study of its kind to implement this type of algorithm on a large scale.
Due to a multitude of factors including indolent disease course and phenotypic variation, identifying patients at high risk for advanced fibrosis presents a significant challenge and leads to an excess of referrals to subspecialists. The creation and implementation of a novel diagnostic algorithm that stratifies patients into low-risk and high-risk demonstrates that less than 5% of the general population would need subspecialty referral and the overwhelming majority of patients can be managed with clinical observation and subsequent non-invasive testing by their primary care and referring physicians.
Nonalcoholic fatty liver disease (NAFLD) presents a significant public health crisis to primary care physicians and endocrinologists. This growing need necessitates a simple and efficient algorithm that can streamline the process of subspecialty referral to hepatology.
More than half of all patients with NAFLD are at low risk for advanced fibrosis. Though there are no Food and Drug Administration -approved agents for nonalcoholic steatohepatitis (NASH) presently, the efficient identification of patients with NASH with advanced fibrosis will be paramount in the care of these patients.
This study aims to create and enact a diagnostic algorithm for all patients with suspected NAFLD to identify the patients at high risk for advanced fibrosis.
Patients with suspected NAFLD were identified in the NHANES database who had historical FibroScan data. FIB4 and FAST scores were calculated for these patients. Those with FIB4 > 1.3 and/or FAST score > 0.67 were deemed high risk for advanced fibrosis.
Of the 3669 patients meeting the inclusion and exclusion criteria, only 75 patients had both an elevated FIB4 and an elevated FAST score which represents roughly 2.0% of the overall population. Among the 737 patients with type 2 diabetes mellitus, 42 patients (5.1%) were found to have both elevated FIB4 and FAST scores.
Given an overwhelming number of patients are referred to hepatology who are most likely at low risk for advanced fibrosis, the utilization of this algorithm by referring providers would help to streamline the process for referrals and eventually more seamlessly identify patients at risk for advanced fibrosis who may need therapy for NASH.
As novel therapeutic agents are currently being studied in patients with NASH with advanced fibrosis, the creation and implementation of a diagnostic algorithm to efficiently identify patients needing therapy becomes increasingly important. Given the wide range of noninvasive tests, this algorithmic approach using two popular tests helps to capture patients at risk for advanced fibrosis while reassuring low-risk patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu ZW, China; Pham TTT, Viet Nam S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7528] [Article Influence: 836.4] [Reference Citation Analysis (0)] |
| 2. | Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, Paradis V, Bedossa P, Aldridge Whitehead JM, Labourdette A, Miette V, Neubauer S, Fournier C, Paredes AH, Alkhouri N. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 3. | Vespa J, Medina L, Armstrong D. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. Current Population Reports. 2018;1144. [DOI] [Full Text] |
| 4. | Ekstedt M, Nasr P, Kechagias S. Natural History of NAFLD/NASH. Curr Hepatol Rep. 2017;16:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 6. | Pais R, Maurel T. Natural History of NAFLD. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2227] [Article Influence: 222.7] [Reference Citation Analysis (0)] |
| 9. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1701] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 10. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3176] [Article Influence: 352.9] [Reference Citation Analysis (4)] |
| 11. | Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 12. | Takeuchi H, Sugimoto K, Oshiro H, Iwatsuka K, Kono S, Yoshimasu Y, Kasai Y, Furuichi Y, Sakamaki K, Itoi T. Liver fibrosis: noninvasive assessment using supersonic shear imaging and FIB4 index in patients with non-alcoholic fatty liver disease. J Med Ultrason (2001). 2018;45:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, Fujii H, Eguchi Y, Suzuki Y, Takahashi H, Nakahara T, Seko Y, Mori K, Kanemasa K, Shimada K, Imai S, Imajo K, Kawaguchi T, Nakajima A, Chayama K, Saibara T, Shima T, Fujimoto K, Okanoue T, Itoh Y; Japan Study Group of Non-Alcoholic Fatty Liver Disease (JSG-NAFLD). The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, Hall R, Harrower U, Hudson M, Langford A, Mackie A, Mitchell-Thain R, Sennett K, Sheron NC, Verne J, Walmsley M, Yeoman A. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (1)] |
| 15. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 648] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 16. | Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Brandman D, Doo E, Tonascia JA, Kleiner DE, Chalasani N, Sanyal AJ; NASH Clinical Research Network. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:156-163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 450] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 17. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 548] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 18. |
Stonehill M, The race is on for medical treatment of NASH.
|
| 19. | About the National Health and Nutrition Examination Survey. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. |
| 20. | Elangovan H, Rajagopaul S, Williams SM, McKillen B, Britton L, McPhail SM, Horsfall LU, Valery PC, Hayward KL, Powell EE. Nonalcoholic Fatty Liver Disease: Interface Between Primary Care and Hepatology Clinics. Hepatol Commun. 2020;4:518-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Patel PJ, Banh X, Horsfall LU, Hayward KL, Hossain F, Johnson T, Stuart KA, Brown NN, Saad N, Clouston A, Irvine KM, Russell AW, Valery PC, Williams S, Powell EE. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J. 2018;48:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Neuschwander-Tetri BA, Unalp A, Creer MH; Nonalcoholic Steatohepatitis Clinical Research Network. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1049] [Article Influence: 45.6] [Reference Citation Analysis (4)] |
| 24. | Li Y, Wang J, Tang Y, Han X, Liu B, Hu H, Li X, Yang K, Yuan J, Miao X, Yao P, Wei S, Wang Y, Liang Y, Zhang X, Guo H, Pan A, Yang H, Hu FB, Wu T, He M. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: Evidence from the Dongfeng-Tongji cohort study. PLoS One. 2017;12:e0174291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224-1229, 1229.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Lu H, Liu H, Hu F, Zou L, Luo S, Sun L. Independent Association between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Int J Endocrinol. 2013;2013:124958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 376] [Article Influence: 94.0] [Reference Citation Analysis (0)] |