Copyright
©The Author(s) 2022.
World J Hepatol. Jul 27, 2022; 14(7): 1344-1356
Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1344
Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1344
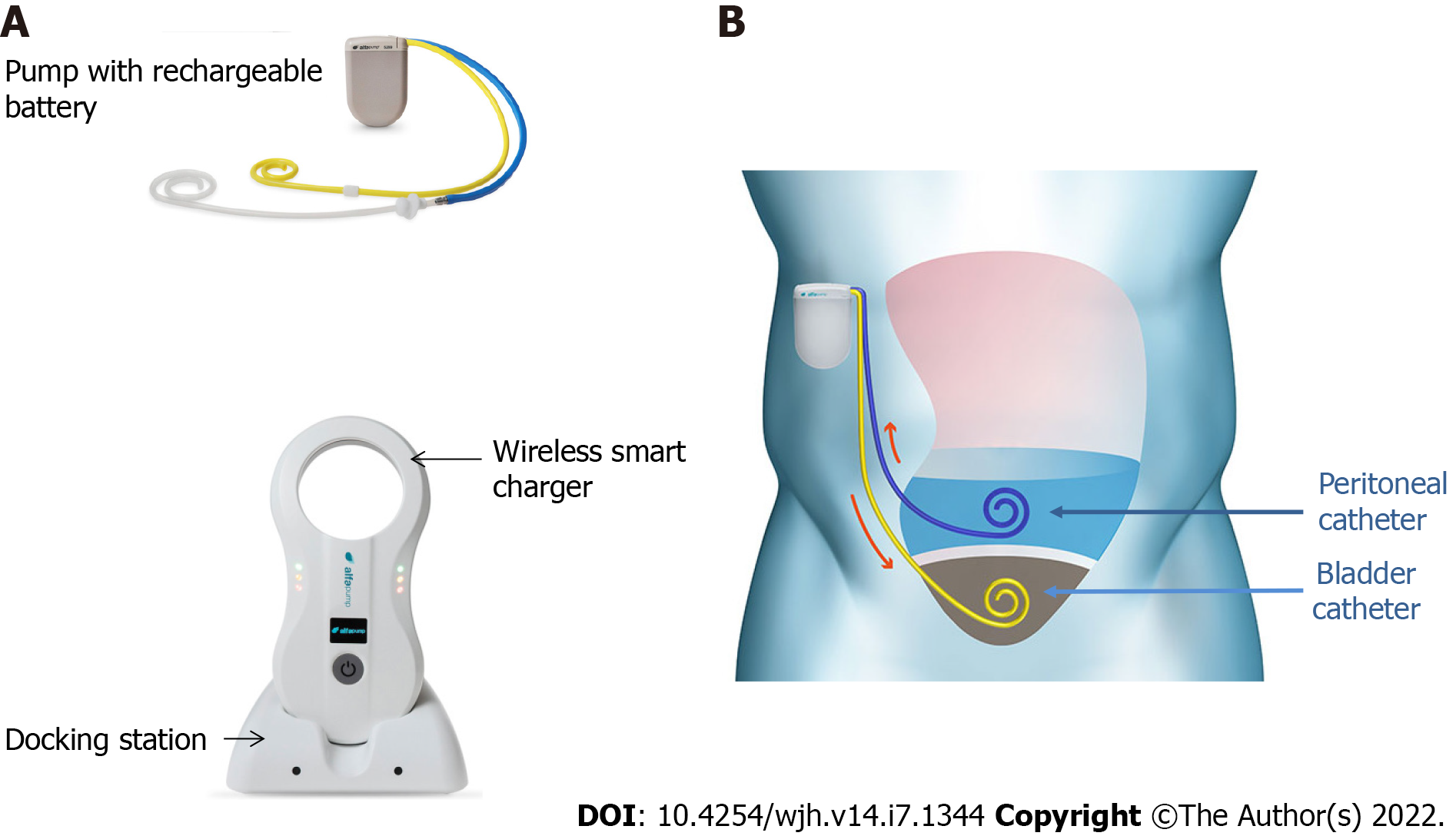
Figure 1 Alfapump® device and principles of its implantation.
A: The system consists of: (1) A pump, which contains a rechargeable battery and is connected to a peritoneal catheter and a bladder catheter; and (2) Charging accessories. The charger collects information and charges the pump through transduction; the docking station must be connected to the electrical network; B: The pump is positioned subcutaneously, under the costal margin (preferably on the right side), so that the patient is not hindered when sitting. The bladder must be full at the time of insertion of the bladder catheter; conversely, only a small amount of ascites is left in place for insertion of the peritoneal catheter, so that the pump can be tested before parietal closure. Images courtesy of Sequana Medical.
- Citation: Weil-Verhoeven D, Di Martino V, Stirnimann G, Cervoni JP, Nguyen-Khac E, Thévenot T. Alfapump® implantable device in management of refractory ascites: An update. World J Hepatol 2022; 14(7): 1344-1356
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1344.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1344