Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1512
Peer-review started: March 29, 2022
First decision: May 12, 2022
Revised: May 25, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: July 27, 2022
Processing time: 120 Days and 1.7 Hours
Hepatitis B virus (HBV) is a known carcinogen that may be involved in pancreatic cancer development. Detection of HBV biomarkers [especially expression of HBV regulatory X protein (HBx)] within the tumor tissue may provide direct support for this. However, there is still a lack of such reports, particularly in non-endemic regions for HBV infection. Here we present two cases of patients with pancreatic ductal adenocarcinoma, without a history of viral hepatitis, in whom the markers of HBV infection were detected in blood and in the resected pancreatic tissue.
The results of examination of two patients with pancreatic cancer, who gave informed consent for participation and publication, were the source for this study. Besides standards of care, special examination to reveal occult HBV infection was performed. This included blood tests for HBsAg, anti-HBc, anti-HBs, HBV DNA, and pancreatic tissue examinations with polymerase chain reaction for HBV DNA, pregenomic HBV RNA (pgRNA HBV), and covalently closed circular DNA HBV (cccDNA) and immunohistochemistry staining for HBxAg and Ki-67. Both subjects were operated on due to pancreatic ductal adenocarcinoma and serum HBsAg was not detected. However, in both of them anti-HBc antibodies were detected in blood, although HBV DNA was not found. Examination of the resected pancreatic tissue gave positive results for HBV DNA, expression of HBx, and active cellular proliferation by Ki-67 index in both cases. However, HBV pgRNA and cccDNA were detected only in case 1.
These cases may reflect potential involvement of HBV infection in the development of pancreatic cancer.
Core Tip: Hepatitis B virus (HBV) is a known carcinogen that may be involved in pancreatic cancer development. Detection of HBV biomarkers (especially expression of HBV regulatory X protein) within the tumor tissue may provide direct support for this. However, there is still a lack of such reports, particularly in non-endemic regions for HBV infection. We present two cases of HBsAg-negative patients with pancreatic ductal adenocarcinoma, in whom the markers of HBV were detected in blood and in the tumor tissue. This reflects potential role of the virus in the etiology and pathogenesis of pancreatic ductal adenocarcinoma.
- Citation: Batskikh S, Morozov S, Kostyushev D. Hepatitis B virus markers in hepatitis B surface antigen negative patients with pancreatic cancer: Two case reports . World J Hepatol 2022; 14(7): 1512-1519
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1512.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1512
Pancreatic cancer (PC) is one of the most prevalent cancers worldwide and its incidence rate is growing[1]. Among different types of pancreatic cancer, pancreatic ductal adenocarcinoma represents 90% of cases[2]. Despite difference in epidemiology observed across regions (incidence rates of 0.5-9.7 per 100000 people), it causes about 4% of all deaths per year globally[2]. PC is known for its aggressive nature with a low 5-year survival rate that does not exceed 9%[3].
Early detection of PC remains a challenge. Therefore, stratification of risk factors and identification of subjects at risk are actual. The known risk factors for PC are male sex, non-O (I) blood group, cigarette smoking, low physical activity, genetics and positive family history, presence of diabetes mellitus, obesity, dietary factors (high levels of red and processed meat, low fruits and vegetables consumption, and alcohol intake), and history of pancreatitis[4]. Association of PC with some infections, including hepatitis B virus (HBV) infection, has been described[5,6]. However, the results of these reports are controversial, and the mechanisms of HBV involvement in pathogenesis of PC are not fully clear.
HBV is a known carcinogen that causes up to 80% of cases of hepatocellular carcinoma in endemic regions[7]. Also, the virus may be involved in non-liver oncogenesis due to its ability to integrate into the genome of infected cells, to cause genomic aberrations and enhance expression of oncogenes or inhibit tumor suppressors[8]. Several reports have shown that replication of the virus may occur not only in the liver, but also in other organs, including the pancreas[9-11]. Moreover, pancreatic beta cells and hepatocytes develop from the ventral foregut endoderm during ontogenesis and thus may share characteristics favorable for HBV-induced tumor development[12]. Markers of previous or current HBV infection are commonly found in patients with PC, while HBV DNA and viral antigens have been detected in the pancreatic tumor tissues, suggesting a potential role of the infection in the etiology of this cancer[13-15]. However, most of these reports came from Asian countries, where HBV infection is prevalent, and most of subjects were HBsAg-positive. In contrast, uncertain results of the cohort studies performed in Europe (1 from Denmark and 2 from Sweden) make an association of the PC and HBV infection questionable[5,16-18]. Although the data of epidemiological studies are important, direct support of the involvement of HBV infection in PC development may be provided with the detection of HBV biomarkers [especially expression of HBV regulatory X protein (HBx)] within the tumor tissue. However, there is still a lack of such reports, especially in non-endemic regions for HBV infection.
Here we report two cases of patients with no history of HBV infection, admitted to the Moscow Clinical Research Center named after A.S. Loginov for pancreatic cancer treatment, who gave their consent for special examination and the use of the obtained data for scientific purposes, including publication of images.
Case 1: The patient was a 61-year-old white/Caucasian man, with blood type O (I). His complaints were non-remarkable.
Case 2: The patient was a 60-year-old white/Caucasian man, with blood type A (II) with no remarkable complaints.
Case 1: The patient was admitted for planned surgery in June 2019. Previous repeated screening blood tests on HBsAg were negative.
Case 2: The patient was admitted in February 2020 for planned surgical treatment due to previously diagnosed pancreatic cancer involving the superior mesenteric vein. Before surgery, he received seven courses of neoadjuvant chemotherapy according to the FOLFIRINOX scheme with no progression of the tumor.
Case 1: The patient’s history of past illness was non-remarkable.
Case 2: The patient had a known history of chronic pancreatitis, type 2 diabetes mellitus, and obesity (body mass index 34.5 kg/m2).
Case 1: The patient had a history of alcohol abuse.
Case 2: The patient had a personal history of alcohol abuse and smoking experience for more than 20 years.
Cases 1 and 2: No notable deviations.
Case 1: At admission, blood tests revealed signs of previous hepatitis B, but no markers of current HBV infection (Table 1). Methods used for standard and special examinations are described in Supple
| Subject 1 | Subject 2 | |
| HBsAg (blood) | Negative | Negative |
| Anti-HBc (blood) | Positive | Positive |
| Anti-HBs (blood) | Positive | Negative |
| HBV DNA, IU/mL (blood) | Not detected | Not detected |
| HBV DNA, IU/mL (pancreatic tissue) | 364 | 1183 |
| pgRNA HBV, IU/mL (pancreatic tissue) | 520 | Not detected |
| cccDNA, copies/cell x 10-6 (pancreatic tissue) | 314 | Not detected |
| HBxAg (pancreatic tissue) | Positive | Positive |
| HBx - positive cells1, % (pancreatic tissue) | 3.4 | 3.7 |
Histological assessment of the resected tissue revealed ductal adenocarcinoma of the pancreas (pT1 G2 R0 N0 V0 Pn0)[21,22].
Case 2: At admission, no markers of current HBV infection were detected by blood tests. However, serum anti-HBc test was positive, suggesting that the patient had a previous hepatitis B (Table 1).
Morphological examination of the resected tissue identified pancreatic ductal adenocarcinoma with involvement of the duodenal wall (pT2 R0 N0 V0 Pn1 TRS 3)[21,22].
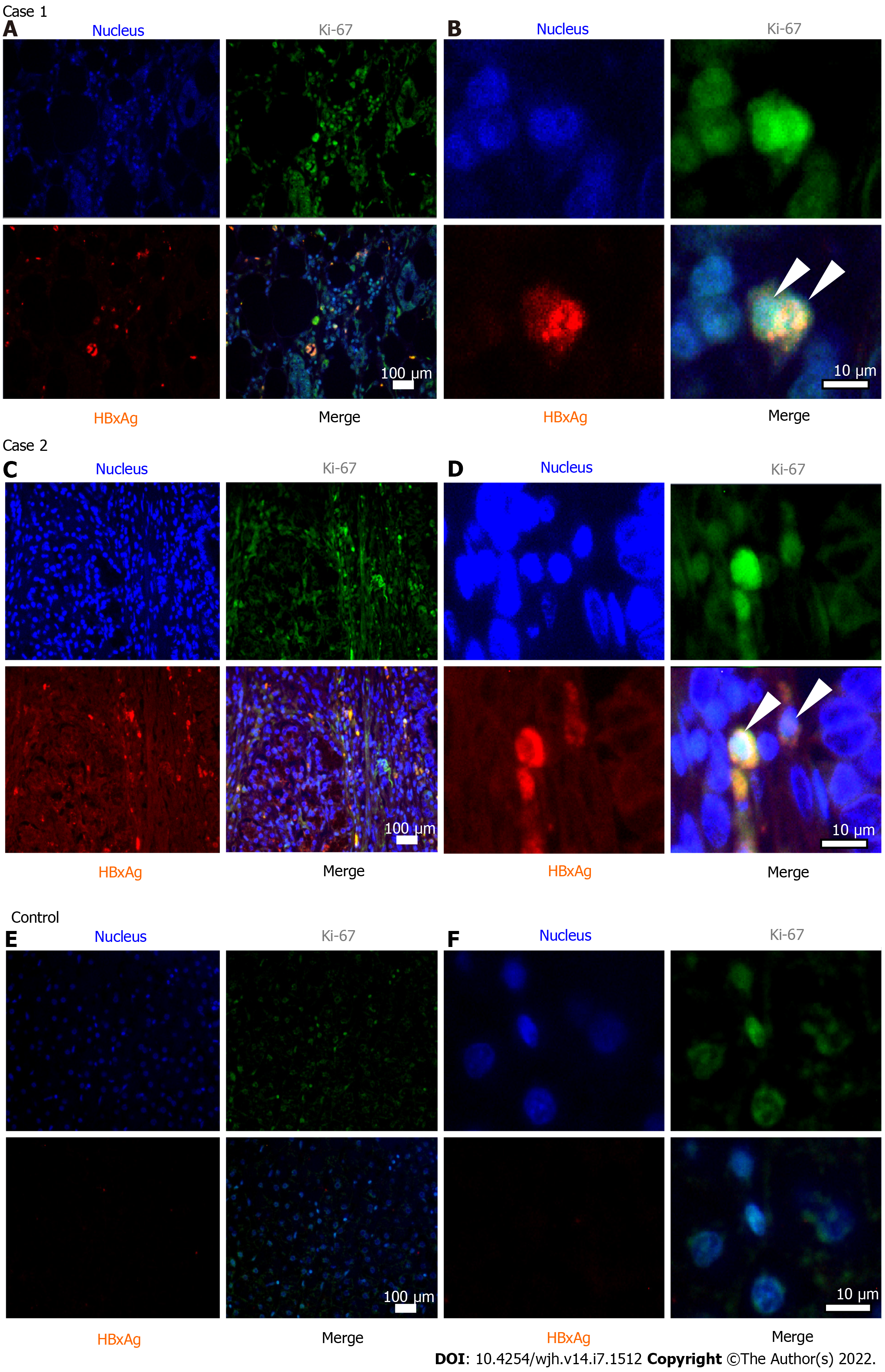
Case 1: Special examination of the resected pancreatic tissue in this case revealed markers of HBV replication and active cellular proliferation, as well as expression of HBx (shown in Table 1 and Figure 1).
Case 2: Examination of the resected pancreatic tissue gave positive result for HBV DNA, with no other markers of active viral replication (Table 1). However, immunohistochemistry revealed expression of HBx and high level of cellular proliferation by Ki-67 index (Table 1 and Figure 1).
In both cases, based on result of a complex examination, cancer of the head of the pancreas was diagnosed.
Case 1: The patient underwent laparoscopic distal subtotal pancreatic resection with resection of the splenic vessels using the Warshaw technique.
Case 2: The patient underwent gastropancreatoduodenal resection.
Cases 1 and 2: After discharge, both patients continued treatment offered by a local oncologist. No special treatment for silent HBV infection was required. The patients were advised to undergo regular check-ups to exclude reactivation of HBV infection: Alanine aminotransferase, HBsAg, and HBV DNA (in blood) at least once in 3 mo.
These two cases demonstrate the presence of HBV markers in HBsAg-negative patients with pancreatic cancer in non-endemic regions for the infection. Both of our patients had several known risk factors for PC development. We suppose that previous HBV infection could be an additional risk factor for PC. It is known that HBV infection, even resolved, may present a molecular basis for carcinogenesis. Carcinogenic mechanisms in HBsAg-negative persons with previous HBV infection may be related to transcriptional activity of episomal HBV genomes (cccDNA), which remains in the cell nucleus as a matrix for the life-long synthesis of new virions. In case 1, detection of not only HBV DNA but also cccDNA and pgRNA HBV (transcribed exclusively from cccDNA) suggests that this patient had a silent low-level replication of the virus in the pancreatic tissue. In case 2, pgRNA HBV and cccDNA were not detected despite a significant amount of HBV DNA in the pancreatic tissue. While no HBV replication in this patient was found, integrated HBV DNA could evidently cause the expression of HBx, which is similar to that observed in hepatocellular cancer[23]. This protein, detected in pancreatic tissue of both of our subjects, is considered to be the most pro-oncogenic[24]. It is assumed that HBx plays a major role in pathogenesis of liver cancer through nuclear translocation, protein-protein interactions, influence on transcription regulation, induction of chromosomal instability, control of proliferation, and transformation, invasion, and metastasis of tumor cells even in cases when HBV replication is absent[23,24]. These mechanisms may also play a role in extra-hepatic cancer development. To our knowledge, there are only two studies that described HBx expression in pancreatic cancer tissues, both performed in a cohort of Asian patients in HBV endemic regions[11,25]. Song et al[11] reported that HBx expression was detected in ten out of ten subjects with PC and only three were HBsAg-negative.
Although the presence of HBV biomarkers in pancreatic adenocarcinoma tissue detected by PCR and immunohistochemistry does not allow proving causal relationship between the two conditions, it reflects potential involvement of the virus in the etiology and pathogenesis of pancreatic cancer. It may be important that Ki-67 proliferative index was more than 50% in both subjects. According to the literature, such values are relatively rare among PC patients (approximately 12%), and associated with more aggressive grade and poorer prognosis[20].
Together with data of the cohort studies, our cases may be important for the clinical practice. It is not yet clear whether universal testing of all patients with PC for anti-HBc and HBV DNA is necessary. However, these tests are reasonable when chemotherapy is planned, and when blood transaminases flare on the mentioned treatment occurs[26,27].
Detection of HBV cccDNA in pancreatic tissue in HBsAg-negative subject in our report may support the need for revision of the statements of the Taormina Workshop (2018), which defines occult HBV infection as the presence of replication-competent HBV DNA in the liver and/or HBV DNA in the blood of people who test negative for HBsAg[28]. As extrahepatic replication of HBV DNA may occur in HBsAg-negative subjects (as shown in a number of studies and in our case 1), skipping a mention of specific organ for HBV DNA (cccDNA) detection seems reasonable.
The described cases may reflect potential involvement of HBV infection in the development of pancreatic ductal adenocarcinoma. Larger studies are necessary to assess the risk of pancreatic ductal adenocarcinoma in subjects with previous HBV infection and define HBV-associated mechanisms of cancerogenesis in them.
The authors acknowledge the subjects who gave their consent for participation and preparation of these cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan; Wang CY, Taiwan S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019;48:e113-e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (24)] |
| 3. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1520] [Article Influence: 253.3] [Reference Citation Analysis (1)] |
| 4. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 694] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 5. | Liu X, Zhang ZH, Jiang F. Hepatitis B virus infection increases the risk of pancreatic cancer: a meta-analysis. Scand J Gastroenterol. 2021;56:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Desai R, Patel U, Sharma S, Singh S, Doshi S, Shaheen S, Shamim S, Korlapati LS, Balan S, Bray C, Williams R, Shah N. Association Between Hepatitis B Infection and Pancreatic Cancer: A Population-Based Analysis in the United States. Pancreas. 2018;47:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001;82:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 8. | Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 422] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984;65 ( Pt 3):651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Jin Y, Gao H, Chen H, Wang J, Chen M, Li G, Wang L, Gu J, Tu H. Identification and impact of hepatitis B virus DNA and antigens in pancreatic cancer tissues and adjacent non-cancerous tissues. Cancer Lett. 2013;335:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Song C, Lv J, Liu Y, Chen JG, Ge Z, Zhu J, Dai J, Du LB, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Chen Z, Liu J, Jiang J, Zhu L, Zhai X, Jiang Y, Ma H, Jin G, Shen H, Li L, Hu Z; China Kadoorie Biobank Collaborative Group. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw Open. 2019;2:e195718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Hohenberger P. The pancreas as target organ for hepatitis B virus--immunohistological detection of HBsAg in pancreatic carcinoma and chronic pancreatitis. Leber Magen Darm. 1985;15:58-63. [PubMed] |
| 14. | Yoshimura M, Sakurai I, Shimoda T, Abe K, Okano T, Shikata T. Detection of HBsAg in the pancreas. Acta Pathol Jpn. 1981;31:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:4234-4241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Andersen ES, Omland LH, Jepsen P, Krarup H, Christensen PB, Obel N, Weis N; DANVIR Cohort Study. Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat. 2015;22:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Huang J, Magnusson M, Törner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer. 2013;109:2917-2923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Sundquist K, Sundquist J, Ji J. Risk of hepatocellular carcinoma and cancers at other sites among patients diagnosed with chronic hepatitis B virus infection in Sweden. J Med Virol. 2014;86:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Qu B, Ni Y, Lempp FA, Vondran FWR, Urban S. T5 Exonuclease Hydrolysis of Hepatitis B Virus Replicative Intermediates Allows Reliable Quantification and Fast Drug Efficacy Testing of Covalently Closed Circular DNA by PCR. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Pergolini I, Crippa S, Pagnanelli M, Belfiori G, Pucci A, Partelli S, Rubini C, Castelli P, Zamboni G, Falconi M. Prognostic impact of Ki-67 proliferative index in resectable pancreatic ductal adenocarcinoma. BJS Open. 2019;3:646-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Bertero L, Massa F, Metovic J, Zanetti R, Castellano I, Ricardi U, Papotti M, Cassoni P. Eighth Edition of the UICC Classification of Malignant Tumours: an overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch. 2018;472:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Amin MB, Edge SB, Greene FL. AJCC Cancer Staging Manual. 8th edition. New York, NY: Springer, 2017. [cited 20 April 2022]. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21388. |
| 23. | Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Geng M, Xin X, Bi LQ, Zhou LT, Liu XH. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World J Gastroenterol. 2015;21:10732-10738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Bai X, Zhang Q, Wen L, Su W, Fu Q, Sun X, Lou Y, Yang J, Zhang J, Chen Q, Wang J, Liang T. The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett. 2016;380:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Myint A, Tong MJ, Beaven SW. Reactivation of Hepatitis B Virus: A Review of Clinical Guidelines. Clin Liver Dis (Hoboken). 2020;15:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Koffas A, Dolman GE, Kennedy PT. Hepatitis B virus reactivation in patients treated with immunosuppressive drugs: a practical guide for clinicians. Clin Med (Lond). 2018;18:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |