Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1504
Peer-review started: January 30, 2022
First decision: March 7, 2022
Revised: April 10, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: July 27, 2022
Processing time: 177 Days and 19.1 Hours
Liver transplantation has evolved into a safe life-saving operation and remains the golden standard in the treatment of end stage liver disease. The main limiting factor in the application of liver transplantation is graft shortage. Many strategies have been developed in order to alleviate graft shortage, such as living donor partial liver transplantation and split liver transplantation for adult and pediatric patients. In these strategies, liver volume assessment is of paramount importance, as size mismatch can have severe consequences in the success of liver trans
To evaluate the safety, feasibility, and accuracy of light detection and ranging (LIDAR) 3D photography in the prediction of whole liver graft volume and mass.
Seven liver grafts procured for orthotopic liver transplantation from brain deceased donors were prospectively measured with an LIDAR handheld camera and their mass was calculated and compared to their actual weight.
The mean error of all measurements was 17.03 g (range 3.56-59.33 g). Statistical analysis of the data yielded a Pearson correlation coefficient index of 0.9968, indicating a strong correlation between the values and a Student’s t-test P value of 0.26. Mean accuracy of the measurements was calculated at 97.88%.
Our preliminary data indicate that LIDAR scanning of liver grafts is a safe, cost-effective, and feasible method of ex vivo determination of whole liver volume and mass. More data are needed to determine the precision and accuracy of this method.
Core Tip: Liver transplantation (LT) is the golden standard in the treatment of end stage liver disease. The main limiting factor in the application of LT is graft shortage and over the years, many strategies have been developed in order to increase graft availability, such as living donor liver transplantation and split liver transplantation. In these strategies, liver volume assessment is of paramount importance in the success of LT. In this preliminary proof-of-concept study, we evaluated the use of light detection and ranging (LIDAR) technology for ex vivo measurement of hepatic grafts. Preliminary data indicate that LIDAR scanning of liver grafts is a safe, cost-effective, and feasible method of ex vivo determination of whole liver volume and mass.
- Citation: Katsanos G, Karakasi KE, Karolos IA, Kofinas A, Antoniadis N, Tsioukas V, Tsoulfas G. Volumetric assessment of hepatic grafts using a light detection and ranging system for 3D scanning: Preliminary data. World J Hepatol 2022; 14(7): 1504-1511
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1504.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1504
Liver transplantation (LT) has evolved into a safe life-saving operation and remains the golden standard in the treatment of end stage liver disease[1]. The main limiting factor in the application of LT in the vast range of diseases that progress to end stage liver failure, as well as in the developing transplant oncology, is graft shortage, affecting thousands of adult and pediatric patients[2].
Over the years, many strategies have been developed in order to alleviate graft shortage, such as living donor liver transplantation[3] and split liver transplantation[4]. In these strategies, liver volume assessment is of paramount importance, as size mismatch can have severe consequences in the success of LT[5].
Although several techniques have been developed in order to assess liver graft volumes, few data and methods can accurately calculate partial split graft volumes in split liver transplantation[6], especially in the scenario of donors that have not been subjected to abdominal imaging studies.
Reality capture, on the other hand, is the use of various technical means to capture a digital 3D model representation of a subject from the real world. Recent technological advancements have made reality capture hardware such as light detection and ranging (LIDAR) 3D technology available to the public at reasonable prices. This technology has a multitude of applications and its value has not been extensively explored in liver surgery and liver transplantation[7,8]. We conducted a preliminary proof-of-concept study in order to evaluate the feasibility, safety, and accuracy of 3D LIDAR scanning photography of whole liver grafts and the prediction of liver volume and mass.
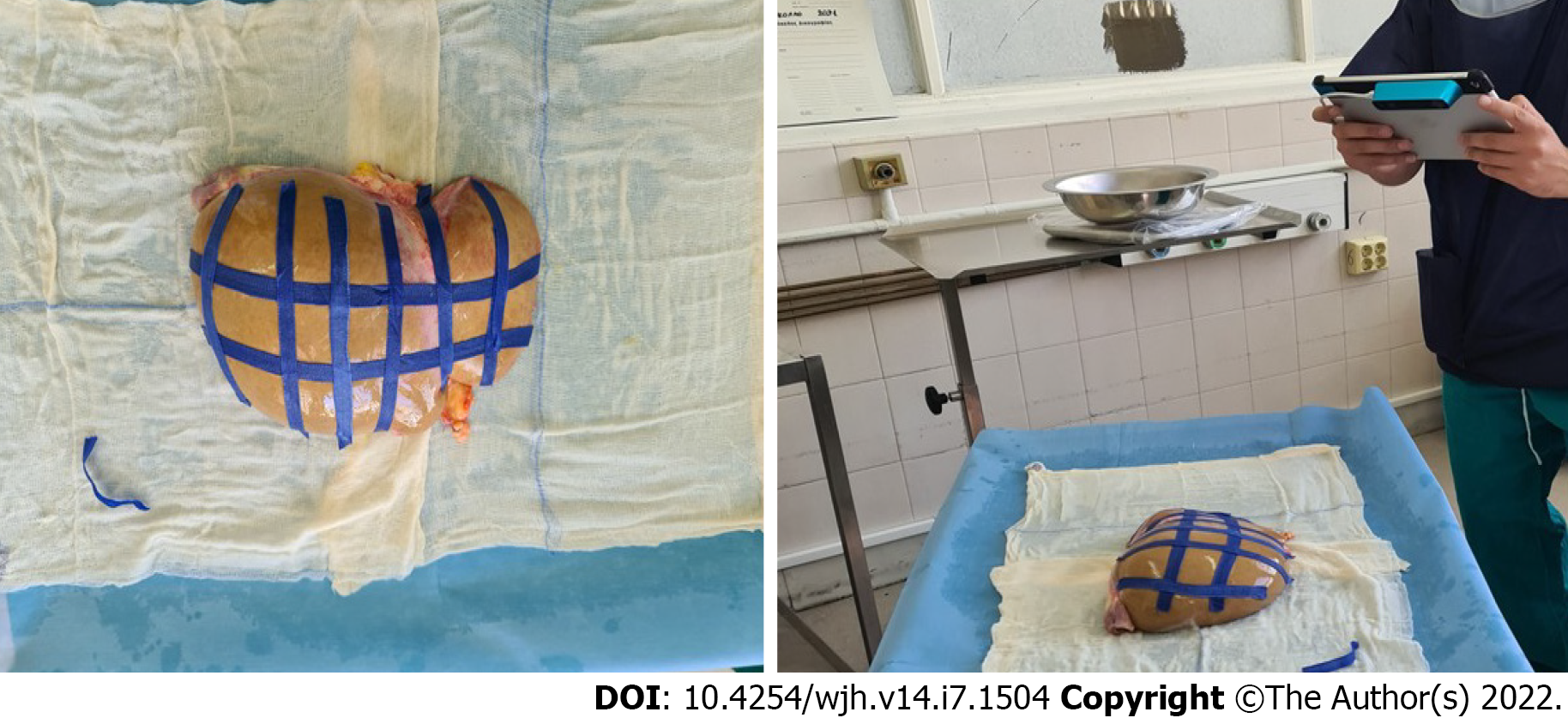
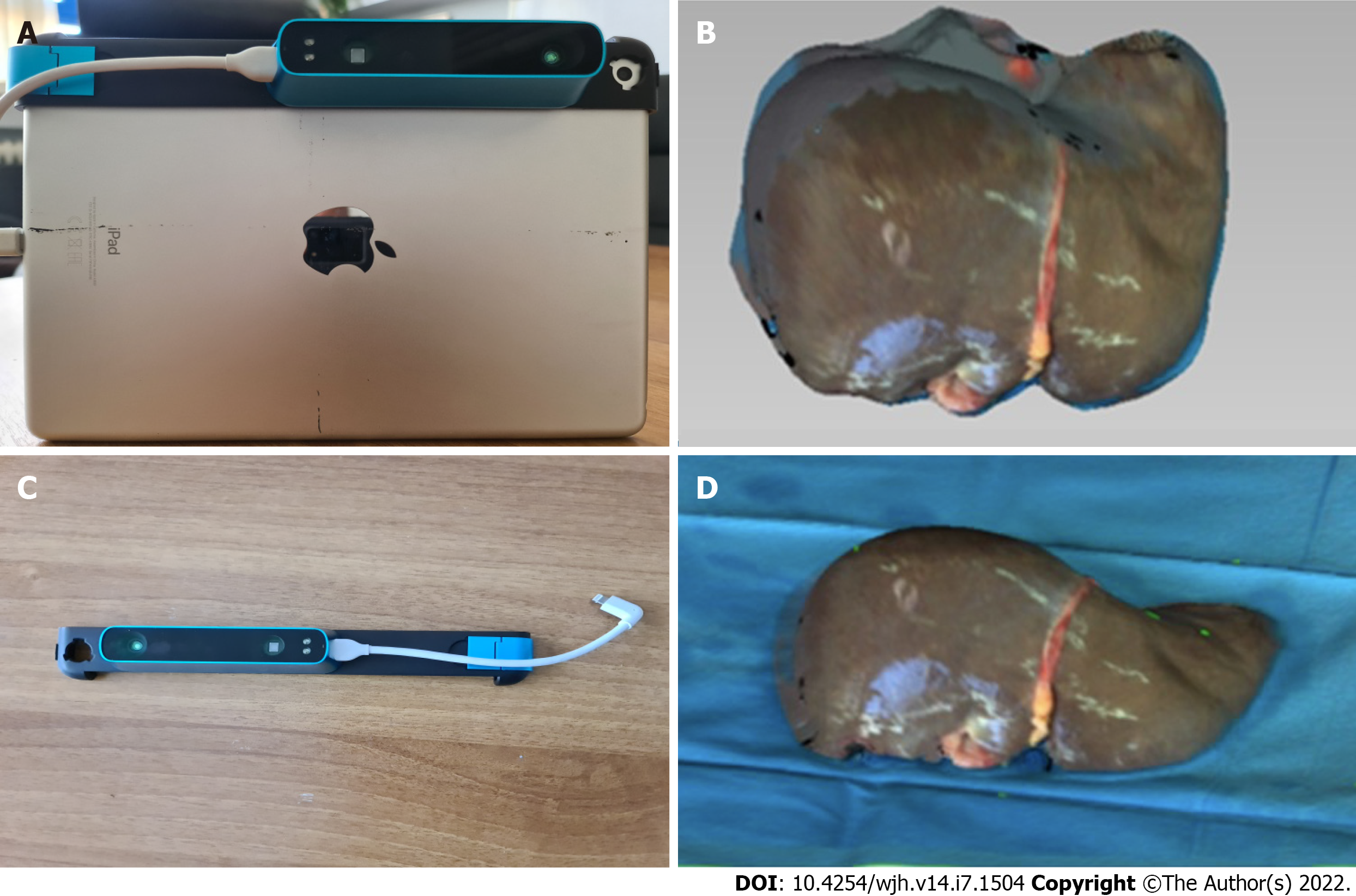
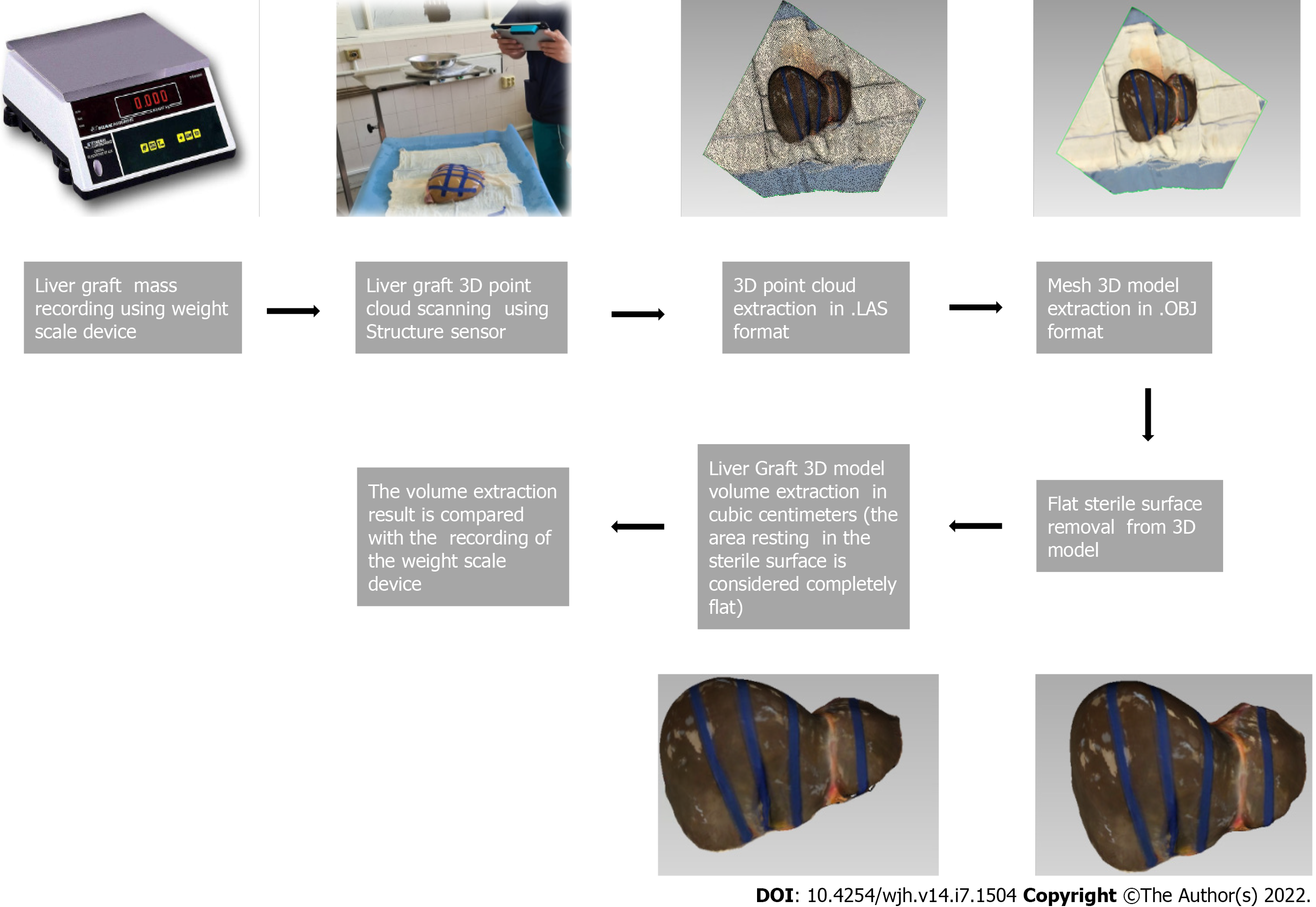
Seven liver grafts procured for orthotopic liver transplantation from brain deceased donors were prospectively measured in this single blind, ongoing study. During the standard back table procedure, grafts were weighed and their mass in grams was recorded using a DSW200D weight scale (DELMAC Group, Athens, Greece). Before graft storage in the traditional nylon bags, the graft was placed on a flat sterile surface and photographed using an Original Structure 3D Scanning Sensor from the Occipital company (Occipital inc., Boulder, United States) (Figure 1). This particular sensor can be adapted to any device with the iOS and iPadOS operating system (Figure 2A), using a special bracket suitable for each corresponding model of iPhone or iPad of the end user. For the purposes of this study, an iPad (6th generation; Apple Inc., California, United States) was used (Figure 2B). The structure sensor communicates with the iPad via a USB to a lightning cable, while the 3D scanning process is done using a suitable iPadOS compatible application provided by Occipital. This application provides the user with the ability to convert the point cloud resulting from the scanning process into a Mesh 3D digital .obj format. The Occipital structure sensor is a mobile based structure light system (SLS). This SLS consists of a laser-emitting diode, an infrared radiation range projector, and an infrared sensor and the iPad’s RGB sensor that provide measuring data to an included system on a chip (SOC) for processing. The output stream from the structure sensor alone consists of a point dataset, with a VGA resolution (640 × 480 pixels), where every pixel records the distance from sensor to the target. The infrared sensor records the reflectance intensity of the infrared (IR) light pattern projected by the IR projector onto the target while its SOC triangulates the 3D scene using specific algorithmic patterns. The main advantage of the above procedure is that the extraction of the 3D model does not require any kind of contact with the physical object (in our case the liver transplant). All measurements were conducted in fully sterile conditions with no contact with the grafts. All measurements lasted less than 3 min.
After completing the 3D reconstruction of the liver graft, the final .obj model is imported into the 3D Mesh and Point Cloud management and editing software, the 3D Slicer, a free, open source and multi-platform software package used for medical, biomedical, and related imaging research. A detailed view of an exported model participating in this study is shown in Figure 2C.
To extract the final volume of the liver model, the part of the surface on which the implant is placed (blue background) is removed from the model. The side of the graft that is in contact with the table is considered as a completely flat surface (Figure 2D). The complete flowchart of the procedure is presented on Figure 3.
In this study, mass and volume calculations were conducted by two separate teams that were blinded as to the other team’s results and measurements.
LIDAR calculated volume was converted into mass using a fixed value of liver density defined by convention at 1.07 gr/mL[9,10] .
Calculated liver mass was compared to the actual weighted liver mass of each graft.
R studio for windows (R studio, Boston MA, United States) version 4.1.1 was used to perform all the statistical analyses employing packages “rstatix” and “tidyverse”.
From June 2021 until January 2022, seven liver grafts from deceased donors were included in the study. The average donor age was 52.4 years, and the men-to-women ratio was 3:4. Apart from gender and age, we recorded weight, height, body mass index, and body surface area (BSA). Liver core biopsy was performed for all liver grafts as a standard practice in our department. Donor demographics are presented in Table 1. Graft weight was measured in grams (g). LIDAR imaging analysis provided the calculated graft volumes expressed in millilitres (mL). Considering the mean human liver density at 1.07 g/mL, calculated LIDAR volumes were converted to mass in grams by multiplying the volumes by 1.07. The theoretical volume of the grafts was also recorded using the Vauthey-Abdalla formula[11] [total liver volume = -794.41 + 1267.28 × body surface area). Table 2 depicts the results.
| N | Gender | Age (yr) | Cause of death | Graft steatosis (%) | Weight (kg) | Height (m) | BMI (kg/m2) | BSA (m2) |
| 1 | Female | 59 | IBI | > 10 | 60 | 1.6 | 23.43 | 1.76 |
| 2 | Male | 32 | IBI | > 10 | 75 | 1.7 | 25.95 | 1.88 |
| 3 | Male | 64 | SH | > 10 | 85 | 1.75 | 27.75 | 2.03 |
| 4 | Female | 63 | ICH | 60 | 70 | 1.6 | 27.34 | 2.06 |
| 5 | Female | 46 | ICH | 5 | 90 | 1.7 | 31.14 | 2.41 |
| 6 | Female | 54 | ICH | 20 | 120 | 1.75 | 39.18 | 1.63 |
| 7 | Male | 49 | IBI | > 10 | 75 | 1.83 | 22.39 | 1.95 |
| N | Graft weight (g) | LIDAR volume (mL) | Vauthey volume (mL) | LIDAR estimated graft mass (g) | LIDAR error (g) | LIDAR error (%) |
| 1 | 1202 | 1179 | 1275.04 | 1261.53 | 59.53 | 4.95 |
| 2 | 1623 | 1490 | 1590.52 | 1594.30 | -28.70 | -1.77 |
| 3 | 2201 | 2090 | 1781.61 | 2236.30 | 35.30 | 1.60 |
| 4 | 1332 | 1248 | 1440.86 | 1335.36 | 3.36 | 0.25 |
| 5 | 1227 | 1141 | 1818.15 | 1220.87 | -6.13 | -0.50 |
| 6 | 1074 | 1040 | 2266.36 | 1112.80 | 38.80 | 3.61 |
| 7 | 1623 | 1482 | 1680.03 | 1585.74 | -37.26 | -2.30 |
The mean duration of the measurement was 123 (74-171) s. No incidence was recorded during the procedure, which was conducted during the usual graft preparation by the surgical team. One graft was discarded due to severe steatosis. In the other six grafts, no cases of graft dysfunction or non-function were recorded in the subsequent transplantation.
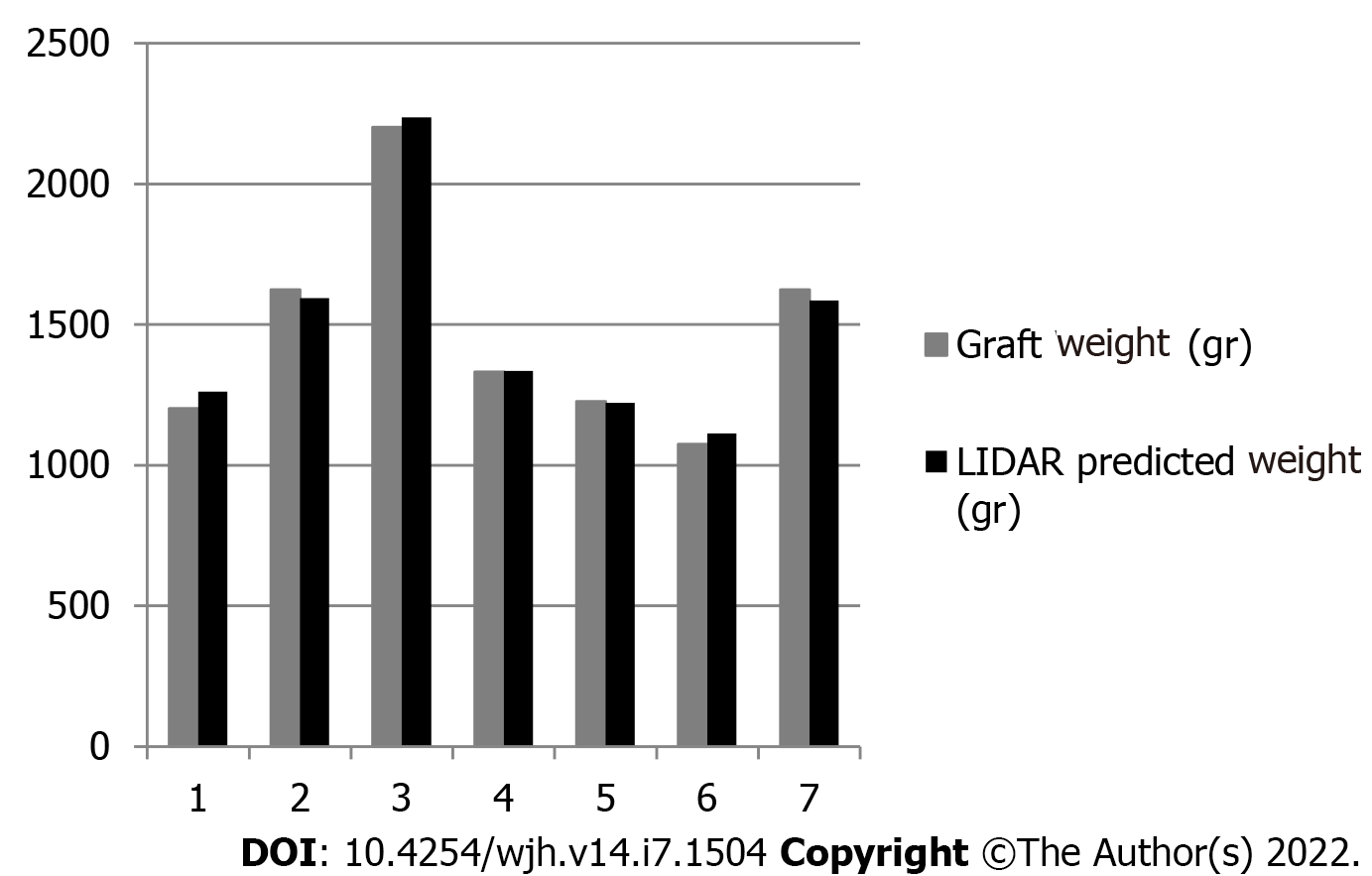
LIDAR assisted graft volume and mass calculation results were compared with the actual weighed mass of the grafts. The mean error of all measurements was 17.03 g (range 3.56-59.33 g). Initially, data fluctuation analysis was performed for one factor (ANOVA). Average values, fluctuation, and degrees of freedom were calculated, and the null hypothesis (F < Fcit) was confirmed (Table 3). Statistical analysis of the data yielded a Pearson correlation coefficient index of 0.9968, indicating a strong correlation between the values and a Student’s t-test P value of 0.26. Mean accuracy of the measurements was calculated at 97.88%. Results are depicted in Figure 4.
| Source of Variation | SD | df | MS | F | F crit |
| Between groups | 225616.3945 | 2 | 112808.1972 | 0.861884735 | 3.554557 |
| Within groups | 2355938.641 | 18 | 130885.4801 | ||
| Total | 2581555.035 | 20 |
Liver graft mass and volume and their relations to recipient somatometric characteristics are essential factors for the outcome of LT. Although in standard whole liver adult to adult orthotopic LT, size is usually not an issue and the already existing methods of graft volume evaluation might be sufficient, accurate prediction of partial liver volumes in living donor[12] and split liver transplantation presents a more complex challenge[13]. Up to date, the main methods for partial liver volume calculation rely on preoperative imaging studies[14,15], which present their own set of challenges[16]. In the present work, we conducted a preliminary proof-of-concept study for the evaluation of the available handheld LIDAR technology for the evaluation of hepatic graft volume, as the first step in the development of a method that could eventually accurately estimate partial split liver volumes of grafts evaluated for split liver transplantation. The use of whole grafts aimed at calibrating the method and detecting eventual technical issues, as well as overcoming the technical issues associated with the split liver surgical technique and the fact that split liver transplantation is not currently performed in Greece. Our preliminary data tend to validate the concept of the study; however, it does not have a valuable clinical application per se, as whole liver mass and volume can be easily calculated by simply weighing the graft or by the water displacement method. However, due to the asymmetric structure of the liver, the calculation of partial liver volumes is more complex, and the existing mathematic formulas cannot accurately predict the segmental hepatic volumes that can vary considerably between patients[17], leaving the preoperative imaging studies of the graft in the form of either a computed tomography (CT) or magnetic resonance imaging (MRI) scan as the most used and valuable option. LIDAR assisted liver volumetry could add a useful tool for ex vivo partial liver volume calculation mainly in cases of split liver transplantation for donors that for various reasons did not have a pre-procurement CT or MRI study. Compared to traditional methods for liver volumetry such as CT and MRI, LIDAR volumetric assessment is more cost-effective, less time-consuming, and less operator-dependent. Triple phase liver CT scans or MRI scans can be difficult to obtain even in tertiary hospitals, let alone in the setting of a small rural donor hospital. Moreover, the multi-organ donor is not burdened with intravenous contrast media administration, which may affect kidney function. Liver 3D model capture using the LIDAR camera is performed ex vivo, just after backtable liver preparation, in less than 3 min and under sterile conditions. Actual volume measurement is done utilizing an open, free software package without the need of an expert radiologist. One obvious drawback in comparison to preoperative donor imaging is that the internal anatomy of the liver cannot be assessed and surgical plane planning is not possible. Another issue is that liver volume is measured during a state of non-perfusion, so liver mass and volume may differ if compared to a perfused organ in vivo[10]. LIDAR assisted volumetry showed a better accuracy than the theoretical volume calculation using the VAUTHEY formula. This is probably mainly due to the lack of precise donor data (mainly donor weight), as many rural hospitals do not have the ability to weigh bedridden patients and the donor weight data derive from crude estimation or medical records. Finally, the main flaw of the present study is the inability to scan the inferior surface of the liver and segment I, which lie against a flat surface, and by convention this surface is considered completely flat in our calculations. The subsequent steps in this ongoing study will be the refinement of the measuring technique, and the evaluation of the method in cadaveric livers with simulation of the ex situ splitting procedure and measurement of partial liver volumes (mainly left lateral section volumes), before moving in the actual setting of real world split liver transplantation.
Our preliminary data indicate that LIDAR scanning of liver grafts is a safe, cost-effective, and feasible method of ex vivo determination of whole liver volume and mass. More data are needed to determine the precision and accuracy of this method.
Split liver transplantation is a viable option of increasing the number of available grafts, as one liver graft can yield two partial grafts for two donors. In this procedure, partial liver volume estimation, particularly left lateral segment volume estimation, is critical to the outcome of the procedure.
To assess the application of light detection and ranging technology in the ex vivo estimation of whole liver grafts.
To evaluate the feasibility, safety, and accuracy of 3D light detection and ranging (LIDAR) scanning photography of whole liver grafts and the prediction of liver volume and mass.
Seven liver grafts procured for orthotopic liver transplantation from brain deceased donors were prospectively measured in this single blind, ongoing study. All measurements were conducted in fully sterile conditions with no contact with the grafts. LIDAR calculated volume was converted into mass using a fixed value of liver density defined by convention at 1.07 gr/mL. Calculated liver mass was compared to the actual weighted liver mass of each graft.
From June 2021 until January 2022, seven liver grafts from deceased donors were included in the study. Graft weight was measured in grams (g). LIDAR imaging analysis provided the calculated graft volumes expressed in millilitres (mL). Considering the mean human liver density at 1.07 g/mL, calculated LIDAR volumes were converted to mass in grams by multiplying the volumes by 1.07. Statistical analysis of the data yielded a Pearson correlation coefficient index of 0.9968, indicating a strong correlation between the values, and a Student’s t-test P value of 0.26. Mean accuracy of the measurements was calculated at 97.88%.
Our preliminary data indicate that LIDAR scanning of liver grafts is a safe, cost-effective, and feasible method of ex vivo determination of whole liver volume and mass. More data are needed to determine the precision and accuracy of this method.
LIDAR assisted liver volumetry could add a useful tool for ex vivo partial liver volume calculation mainly in cases of split liver transplantation for donors that for various reasons did not have a pre-procurement computed tomography (CT) or magnetic resonance imaging (MRI) study. Compared to traditional methods for liver volumetry such as CT and MRI, LIDAR volumetric assessment is more cost-effective, less time-consuming, and less operator-dependent.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang P, China; Yao Y, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Müller PC, Kabacam G, Vibert E, Germani G, Petrowsky H. Current status of liver transplantation in Europe. Int J Surg. 2020;82S:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | de Ville de Goyet J, Baumann U, Karam V, Verkade HJ. Letter to the editor: Organ shortage and pediatric liver transplantation: David against Goliath…. Hepatology. 2022;75:1342-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Pomfret EA, Sung RS, Allan J, Kinkhabwala M, Melancon JK, Roberts JP. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons' State-of-the-Art Winter Symposium. Am J Transplant. 2008;8:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol. 2018;24:5312-5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Reyes J, Perkins J, Kling C, Montenovo M. Size mismatch in deceased donor liver transplantation and its impact on graft survival. Clin Transplant. 2019;33:e13662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, Saner F, Valentin-Gamazo C, Frilling A, Schenk A, Broelsch CE, Malagó M. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant. 2007;7:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Zalevsky Z, Buller GS, Chen T, Cohen M, Barton-Grimley R. Light detection and ranging (lidar): introduction. J Opt Soc Am A Opt Image Sci Vis. 2021;38:LID1-LID2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Svanberg S, Zhao G, Zhang H, Huang J, Lian M, Li T, Zhu S, Li Y, Duan Z, Lin H, Svanberg K. Laser spectroscopy applied to environmental, ecological, food safety, and biomedical research. Opt Express. 2016;24:A515-A527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Overmoyer BA, McLaren CE, Brittenham GM. Uniformity of liver density and nonheme (storage) iron distribution. Arch Pathol Lab Med. 1987;111:549-554. [PubMed] |
| 10. | Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A, Herron D, Mathey C, Ferrari G, Charnsangavej C, Do KA, Denys A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 465] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Radtke A, Sgourakis G, Molmenti EP, Beckebaum S, Cicinnati V, Broelsch CE, Peitgen HO, Malagó M, Schroeder T. Computer-assisted surgical planning in adult-to-adult live donor liver transplantation: how much does it help? Transplantation. 2012;94:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lauterio A, Di Sandro S, Concone G, De Carlis R, Giacomoni A, De Carlis L. Current status and perspectives in split liver transplantation. World J Gastroenterol. 2015;21:11003-11015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, D'Angelica MI, Dumpuri P, Gönen M, Zendejas I, Miga MI, Stefansic JD. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 15. | Reichman TW, Fiorello B, Carmody I, Bohorquez H, Cohen A, Seal J, Bruce D, Loss GE. Using on-site liver 3-D reconstruction and volumetric calculations in split liver transplantation. Hepatobiliary Pancreat Dis Int. 2016;15:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |