Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1382
Peer-review started: March 2, 2022
First decision: April 13, 2022
Revised: April 29, 2022
Accepted: June 14, 2022
Article in press: June 14, 2022
Published online: July 27, 2022
Processing time: 147 Days and 12.8 Hours
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States and globally. The currently understood model of pathogenesis consists of a ‘multiple hit’ hypothesis in which environmental and genetic factors contribute to hepatic inflammation and injury.
To examine the genetic expression of NAFLD and non-alcoholic steatohepatitis (NASH) tissue samples to identify common pathways that contribute to NAFLD and NASH pathogenesis.
We employed the Search Tag Analyze Resource for Gene Expression Omnibus platform to search the The National Center for Biotechnology Information Gene Expression Omnibus to elucidate NAFLD and NASH pathology. For NAFLD, we conducted meta-analysis of data from 58 NAFLD liver biopsies and 60 healthy liver biopsies; for NASH, we analyzed 187 NASH liver biopsies and 154 healthy liver biopsies.
Our results from the NAFLD analysis reinforce the role of altered metabolism, inflammation, and cell survival in pathogenesis and support recently described contributors to disease activity, such as altered androgen and long non-coding RNA activity. The top upstream regulator was found to be sterol regulatory element binding transcription factor 1 (SREBF1), a transcription factor involved in lipid homeostasis. Downstream of SREBF1, we observed upregulation in CXCL10, HMGCR, HMGCS1, fatty acid binding protein 5, paternally expressed imprinted gene 10, and downregulation of sex hormone-binding globulin and insulin-like growth factor 1. These molecular changes reflect low-grade inflammation secondary to accumulation of fatty acids in the liver. Our results from the NASH analysis emphasized the role of cholesterol in pathogenesis. Top canonical pathways, disease networks, and disease functions were related to cholesterol synthesis, lipid metabolism, adipogenesis, and metabolic disease. Top upstream regulators included pro-inflammatory cytokines tumor necrosis factor and IL1B, PDGF BB, and beta-estradiol. Inhibition of beta-estradiol was shown to be related to derangement of several cellular downstream processes including metabolism, extracellular matrix deposition, and tumor suppression. Lastly, we found riciribine (an AKT inhibitor) and ZSTK-474 (a PI3K inhibitor) as potential drugs that targeted the differential gene expression in our dataset.
In this study we describe several molecular processes that may correlate with NAFLD disease and progression. We also identified ricirbine and ZSTK-474 as potential therapy.
Core Tip: Our results from the non-alcoholic fatty liver disease analysis reinforce the role of altered metabolism, inflammation, and cell survival in pathogenesis and support recently described contributors to disease activity, such as altered androgen and lncrna activity. The top upstream regulator was found to be sterol regulatory element binding transcription factor 1 (SREBF1), a transcription factor involved in lipid homeostasis. Downstream of SREBF1, we observed upregulation in CXCL10, HMGCR, HMGCS1, FABP5, PEG10, and downregulation of SHBG and IGF1. These molecular changes reflect low-grade inflammation secondary to accumulation of fatty acids in the liver. Our results from the NASH analysis emphasized the role of cholesterol in pathogenesis. Top upstream regulators included pro-inflammatory cytokines TNF and IL1B, PDGF BB, and beta-estradiol. Inhibition of beta-estradiol was shown to be related to derangement of several cellular downstream processes including metabolism, extracellular matrix deposition, and tumor suppression. Lastly, we found riciribine (an AKT inhibitor) and ZSTK-474 (a PI3K inhibitor) as potential drugs that targeted the differential gene expression in our dataset.
- Citation: Aljabban J, Rohr M, Syed S, Khorfan K, Borkowski V, Aljabban H, Segal M, Mukhtar M, Mohammed M, Panahiazar M, Hadley D, Spengler R, Spengler E. Transcriptome changes in stages of non-alcoholic fatty liver disease. World J Hepatol 2022; 14(7): 1382-1397
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1382.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1382
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease that is characterized by the accumulation of triglycerides within hepatocytes. This process strongly resembles alcohol-induced fatty liver damage but occurs in the absence of excessive alcohol consumption. Akin to obesity, rates of NAFLD are burgeoning and represent a growing health burden; it is estimated that the global disease prevalence is between 20-30%[1]. There is growing evidence that NAFLD is a multisystem disease with both intra- and extra-hepatic manifestations, with strong association between NAFLD and type 2 diabetes mellitus and metabolic syndrome[2].
NAFLD comprises of a spectrum of disease that includes simple steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC). While hepatic steatosis is seen as a generally benign state, NASH is considered a progressive disease state with increased risk of intra- and extra-hepatic disease complications, including cirrhosis[3]. The gold-standard to diagnose NASH is an invasive liver biopsy. As there are no effective non-invasive diagnostic techniques, which makes estimating the true prevalence of NASH difficult; however, it has been estimated that up 25% of patients with NAFLD have concurrent NASH[4]. As rates of NAFLD continue to increase, it is estimated that NAFLD-related cirrhosis will soon surpass chronic hepatitis as the leading indication for liver transplantation[5].
The increasing prevalence and health burden of NAFLD has made it imperative to understand the pathogenesis of this disease process. The most current, best understood model of NAFLD conceptualizes a ‘multiple hit’ hypothesis in which interactions between genetics and environmental factors promote inflammation, cellular injury, and liver damage[6]. These ‘hits’ include lipid accumulation secondary to diet and lifestyle, obesity, and insulin resistance, all of which predispose the liver to inflammation and fibrosis. However, the mechanisms by which these hits promote disease progression are still poorly understood. In this meta-analysis, we aim to use bioinformatics of publicly available data to elucidate the most common genetic pathways involved in NAFLD and identify potential therapeutic targets for intervention.
The National Center for Biotechnology Information Gene Expression Omnibus (GEO) is one of the largest databases available to researchers. The Search, Tag, Analyze, Resource GEO, or STARGEO, was developed to tag samples from the GEO database and produce robust meta-analyses. The GEO database is a genomics repository comprised of all published samples from omics studies. Briefly, STARGEO uses a standard random model for meta-analysis to generate both meta P values and effects size across studies[7]. Study weight percentages were calculated using the inverse variance method via the DerSimonian-Laird estimate[8]. The STARGEO “Tagging” interface was used to gather samples under the “NAFLD,” “NASH,” and “NASH_NAFLD_Control” tag to conduct two separate meta-analyses: one comparing liver biopsies from NAFLD patients to healthy liver controls and the other comparing liver biopsies from NASH to healthy controls.
Series GSE48452, GSE63067, GSE66676, and GSE107231 were used to gather NAFLD, NASH, and healthy liver samples[9-12]. Studies were found by searching NAFLD or NASH under human samples on stargeo.org. Studies selected for analysis had to meet the following criteria: expression analysis was conducted on liver biopsies, the study included contained patients meeting NAFLD or NASH criteria and had matched healthy controls, and biopsies met definitive diagnosis of liver steatosis as below.
In these studies, liver biopsies were performed to diagnose liver disease and healthy liver biopsies were defined as having less than < 5% steatosis and patients with evidence of viral hepatitis, alcoholic consumption, and hemochromatosis were excluded. Standard histopathological analysis by blinded pathologists were used to defined NASH, NAFLD, and healthy liver samples[13]. For example, GSE48452 investigated intra-individual biopsies taken pre and post-bariatric surgery meeting NAFLD, NASH, and healthy liver criteria as above. Only pre-bariatric samples were tagged. For the NAFLD analysis, there was a total of 58 NAFLD liver biopsies and 60 healthy liver biopsies. The NASH analysis featured 187 NASH liver biopsies and 154 healthy liver biopsies.
We were able to extract approximately 20000 genes for each of the meta-analyses conducted using STARGEO. We analyzed gene signature outputs with Ingenuity Pathway Analysis (IPA) to genes showing statistical significance (P < 0.05) and an absolute experimental log ratio greater than 0.1 between case and control samples[14]. The genes included in our analysis are further detailed in Tables 1 and 2. IPA allowed us to define top canonical pathways, disease functions, disease networks, and potential upstream regulators that define NAFLD and NASH pathogenesis. Regulator analysis identifies upstream regulators that best explain the genetic expression in our dataset with P values reflecting the degree of overlap of known effector targets and the gene signature analyzed in IPA. We also used the global molecular network feature of IPA to identify top disease networks. IPA ranks networks from the Global Molecular Network based on the number of focus genes from given networks that match with our analysis. Significance is represented by the p-score, as previously described[14].
| Overlap | P value | |
| Top canonical pathways in NAFLD vs healthy control | ||
| Liver X receptor / retinoid X receptor activation | 5/121 | 4.35E-05 |
| Superpathway of cholesterol biosynthesis | 3/29 | 1.08E-04 |
| Granulocyte adhesion and diapedesis | 5/173 | 2.34E-04 |
| CREB signaling | 8/596 | 6.25E-04 |
| Mevalonate pathway I | 2/14 | 8.96E-04 |
| Top canonical pathways in NASH vs healthy control | ||
| Cholesterol biosynthesis I | 4/13 | 5.48E-05 |
| Cholesterol Biosynthesis II (via 24,25-dihydrolanosterol) | 4/13 | 5.48E-05 |
| Cholesterol biosynthesis III (via desmosterol) | 4/13 | 5.48E-05 |
| IGF-1 signaling | 9/106 | 9.16E-05 |
| Superpathway of cholesterol biosynthesis | 5/28 | 1.05E-04 |
| Top upregulated genes | Top downregulated genes | ||||||
| NAFLD vs Healthy | NASH vs Healthy | NAFLD vs Healthy | NASH vs Healthy | ||||
| XIST | 0.326 | Crystallin alpha A | 1.185 | LINC02535 | -0.198 | MT1L | -0.454 |
| PEG10 | 0.267 | CYP7A1 | 0.409 | GPR88 | -0.194 | CYR61 | -0.386 |
| SUCO | 0.252 | BBOX1 | 0.381 | CYP1A1 | -0.170 | FOSB | -0.339 |
| CBWD5 | 0.239 | TAF4B | 0.355 | IGFBP2 | -0.168 | IGFBP2 | -0.326 |
| TMEM154 | 0.228 | FNDC5 | 0.346 | P4HA1 | -0.166 | FOS | -0.275 |
| HMGCR | 0.225 | MROH2A | 0.293 | TSPAN13 | -0.159 | CAPZA3 | -0.254 |
| LINC00885 | 0.216 | Fc alpha and mu receptor | 0.265 | NR4A2 | -0.148 | CSRNP1 | -0.254 |
| Chitinase 3 Like 1 | 0.186 | IL13RA2 | 0.252 | PER3 | -0.145 | PCDHB19P | -0.252 |
| MEP1B | 0.181 | ABHD1 | 0.250 | SHBG | -0.135 | Nicotinamide phosphoribosyltransferase | -0.240 |
| Phosphodiesterase 11A | 0.180 | Muscular LMNA interacting protein | 0.229 | CENPO | -0.131 | RASD1 | -0.237 |
To find potential drug interactions, we used clue.io to analyze our dataset[15]. We inversed the gene expression pattern from the meta-analysis and used the “list-maker” function to identify drugs (Table 3). We focused on HEPG2 cell lines given they are immortalized HCC cells that relate most closely to the cells studied in our analysis.
| Top disease functions in NAFLD vs healthy control | P values |
| Inflammatory response | 1.67E-03 |
| Liver lesion | 6.59E-05 |
| Cell movement of epithelial cells | 3.88E-04 |
| Activation of cells | 5.30E-04 |
| Synthesis of lipid | 5.49E-08 |
| Accumulation of lipid | 6.12E-04 |
| Concentration of lipid | 2.38E-06 |
| Fibrosis | 3.75E-05 |
| Secretion of lipid | 1.06E-03 |
| Hepatic injury | 1.54E-04 |
| Organismal injury and abnormalities | 5.10E-16 |
| Cancer | 3.47E-15 |
| Dermatologic diseases and conditions | 5.20E-11 |
| Metabolic disease | 6.69E-10 |
| Lipid metabolism | 8.09E-12 |
| Molecular transport | 7.06E-12 |
| Small molecule biochemistry | 9.86E-11 |
| Cell death and survival | 5.05E-8 |
| Cellular movement | 6.28E-8 |
| Adipogenesis | 1.31E-7 |
All data analyzed were taken from Gene Expression Omnibus. There was no interaction or intervention with human subjects and no involvement with access to identifiable private patient information. As such, no Institutional Review Board approval was necessary.
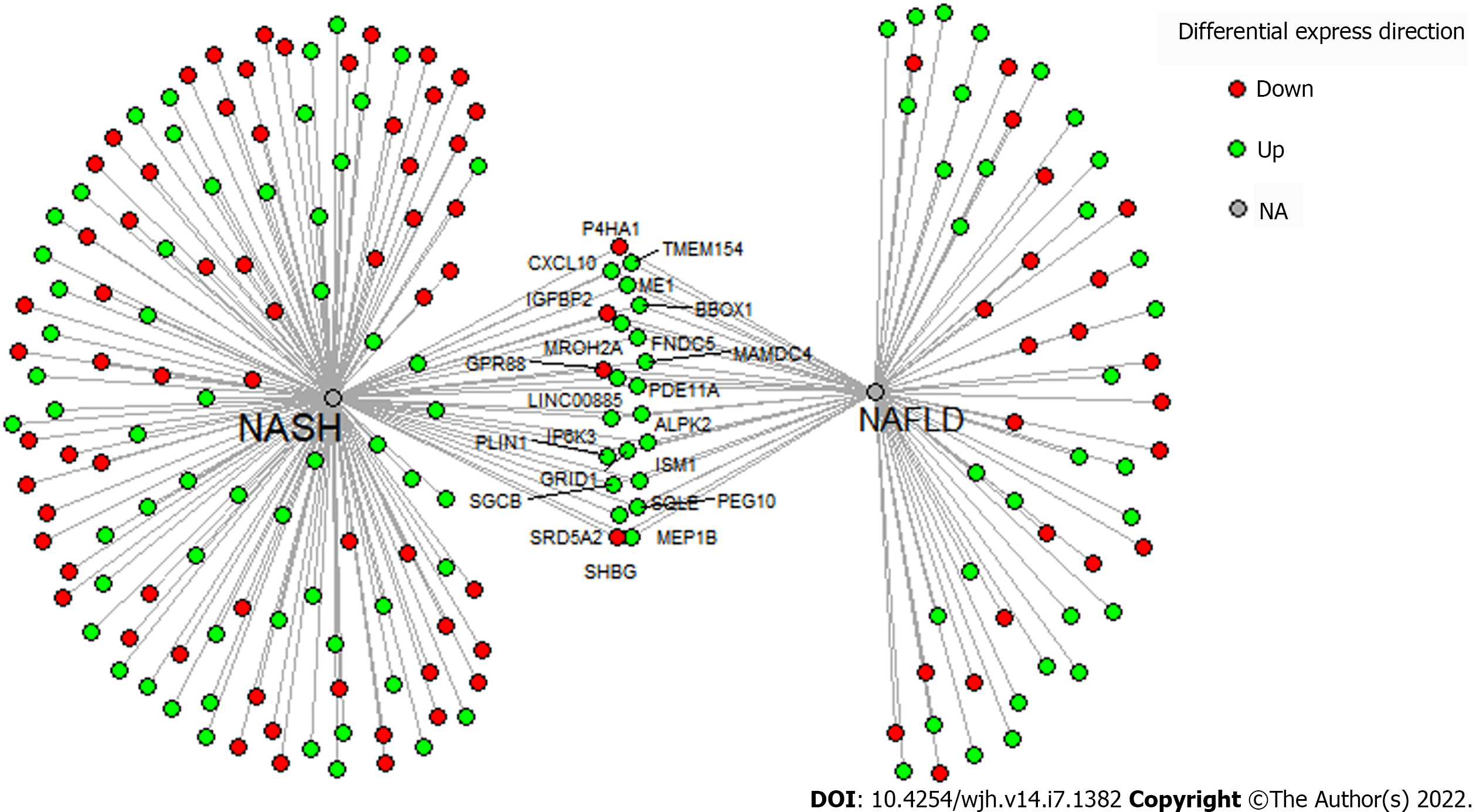
From STARGEO, we were able to extract approximately 20000 genes from our analysis of NAFLD and NASH liver biopsies compared to normal biopsy controls. Table 1 summarizes top upregulated and downregulated genes from the two analyses. Only genes that demonstrated statistically significant (P < 0.05) differences in up-and down-regulation and absolute experimental log ratios of 0.1 were analyzed in IPA. Additionally, we used IPA to classify the top canonical pathways for NAFLD and NASH. P values and experimental log ratios are included in Tables 1 and 2.
For the NAFLD analysis, the genetic changes and top canonical pathways illustrate several disease processes such as dysregulated metabolism, immune cell recruitment, and altered signal transduction. IPA identified liver X receptor/retinoid X receptor activation (P = 4.35E-05), superpathway of chole
Similarly, the gene expression changes and top canonical pathways from the NASH analysis detailed several pathologic processes. IPA identified cholesterol biosynthesis and insulin-like growth factor 1 (IGF-1) signaling as top canonical pathways. We found upregulation of genes involved in bile acid synthesis and carnitine synthesis including cholesterol 7 alpha hydroxylase (CYP7A1) and gamma-butyrobetaine hydroxylase 1 (BBOX1), respectively[26-28]. Notably, we saw upregulation of the novel myokine fibronectin type 3 (FNDC5), which correlated with NAFLD severity and extracellular matrix deposition[29]. Interestingly, we found upregulation of the lamin-associated gene muscular LMNA interacting protein. Lamins and lamin-associated proteins have implications in liver disease[30].We also found upregulation of several pro-inflammatory genes including the interleukin 13 receptor and the immunoglobin receptor Fc alpha and mu receptor[31]. Our genetic analysis also highlighted dysregulated apoptosis through the downregulation of pro-apoptotic regulators such as the matricellular protein cysteine-rish angiogenic inducer 61 (CYR61), FOS protein (modulates JUN signaling), and Ras related dexamethasone induced 1 (RASD1) from the RAS family[32-24]. Lastly, we found downregulation of insulin-like growth factor binding protein-2 (IGFBP2), similar for our NAFLD analysis above, and the nicotinamide phosphoribosyltransferase, a rate-limiting enzyme in the NAD+ pathway[35].
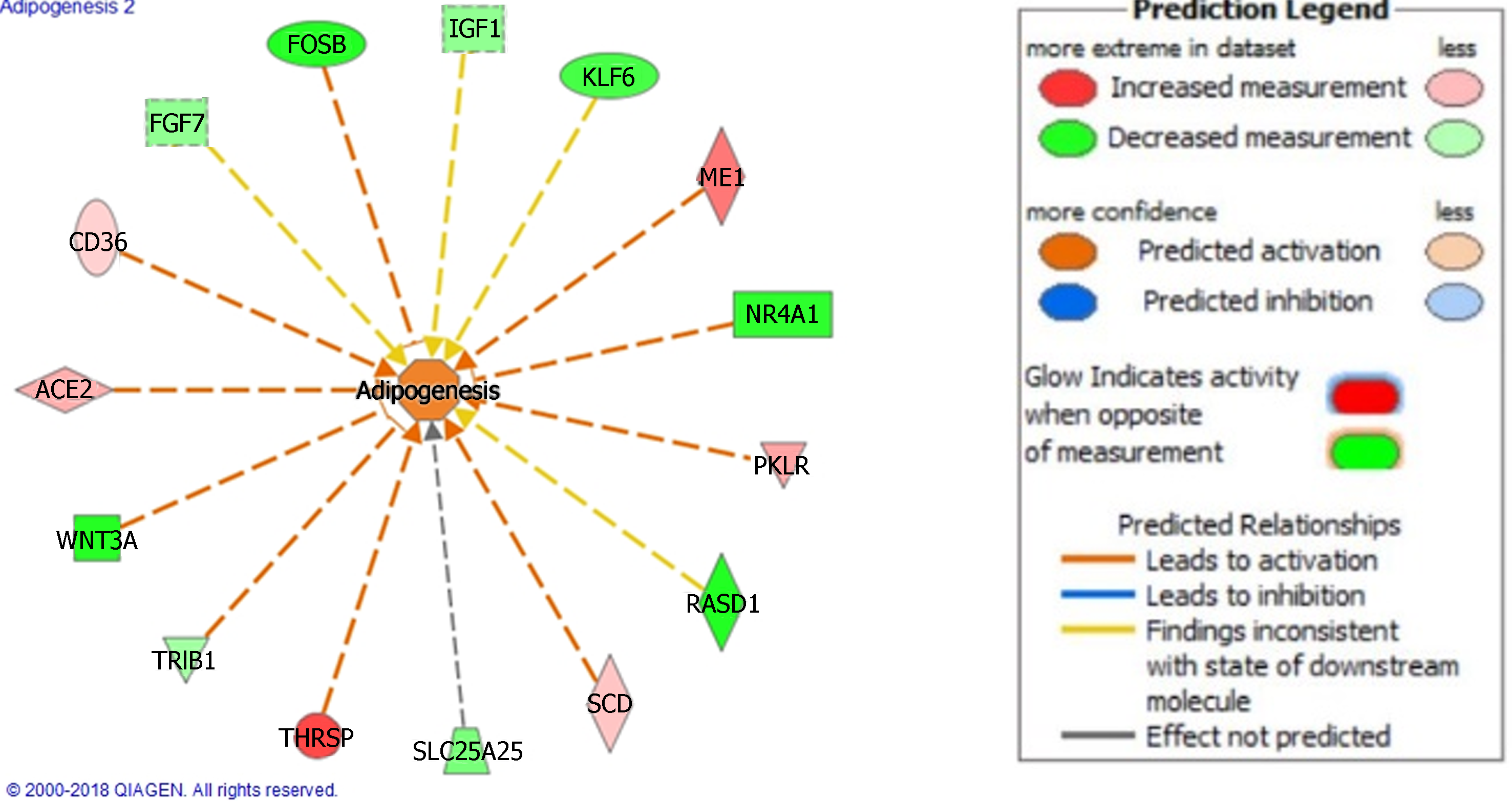
NAFLD and NASH are the result of several complex disease processes in tandem. To define these processes, we used IPA to identify top disease function and networks of interest. In the NAFLD analysis, disease processes were largely related to lipid regulation, inflammation, and hepatic fibrosis and injury (Table 2). Similarly, the disease functions in NASH included processes related to lipid metabolism in addition to other functions such as cancer and cell death and survival. Figure 1 illustrates one of the disease functions, adipogenesis, in the NASH analysis.
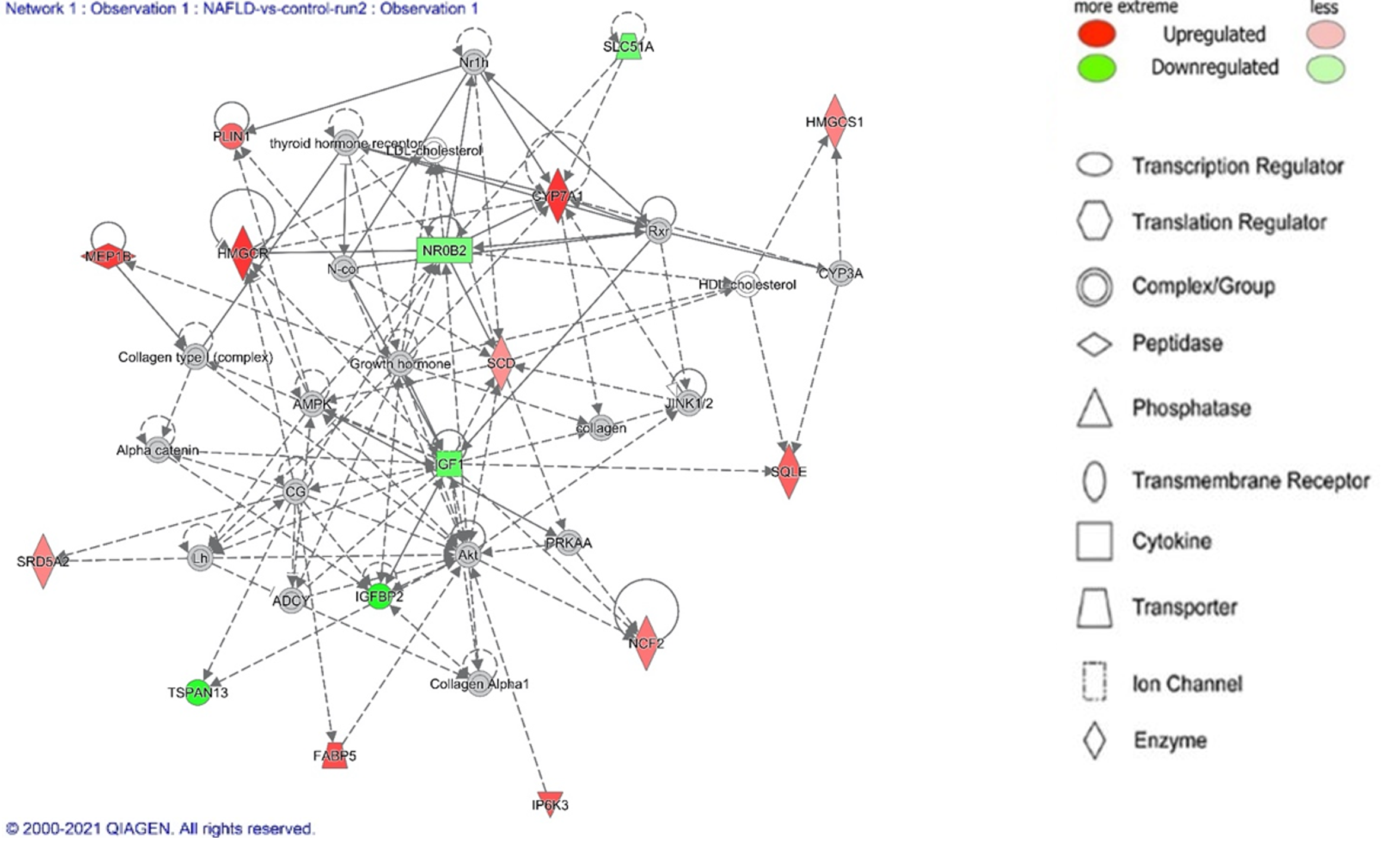
Next, we employed the IPA Disease Network feature to further elucidate the pathologic changes in NAFLD and NASH. IPA takes genes from the analyzed dataset and superimposes it onto curated information from the Ingenuity Knowledge base[14]. In Table 3, we detail the top disease networks identified for NAFLD and NASH. Figure 2 details the lipid metabolism network from the NAFLD analysis.
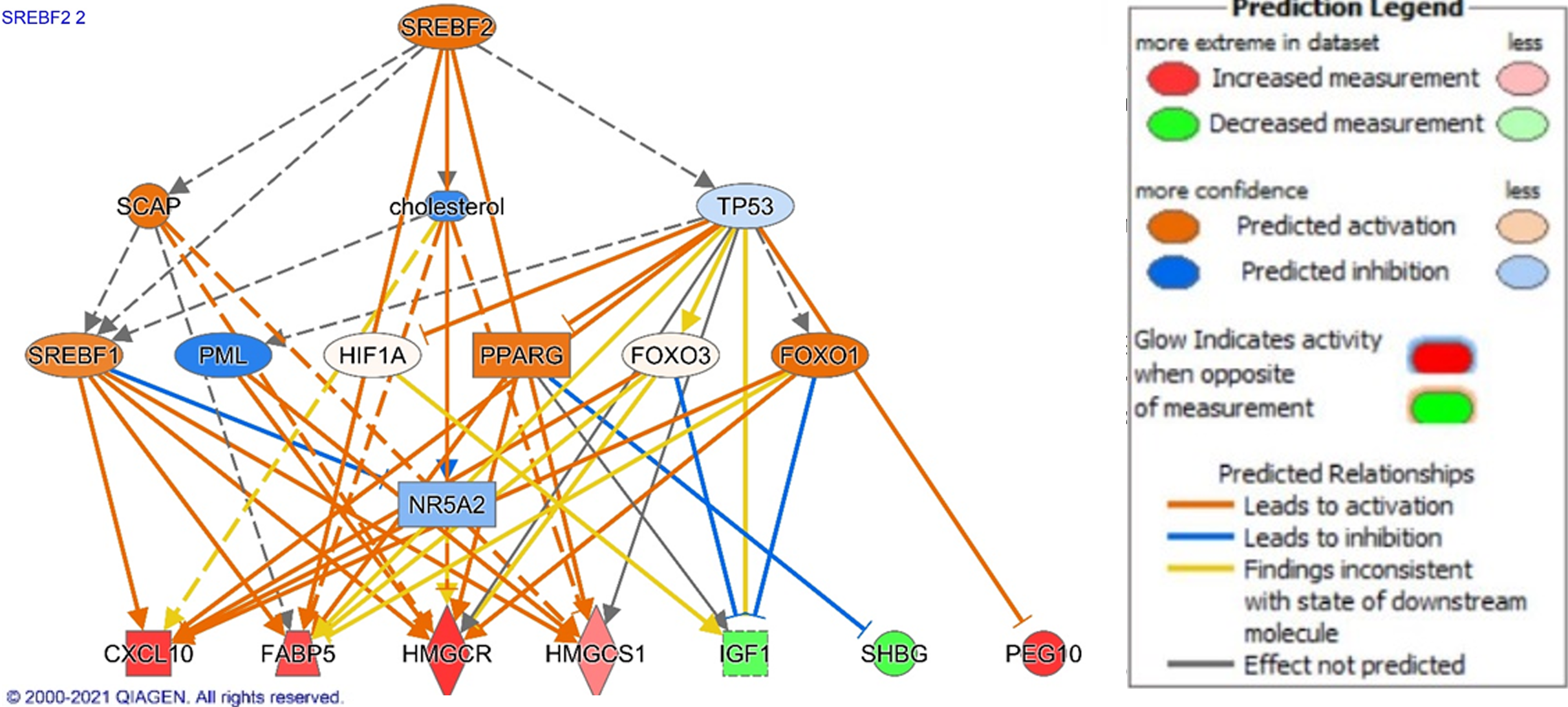
To propose potential drivers of NAFLD and NASH pathogenesis and their downstream effector genes, we used IPA Upstream Regulator analysis[14]. In the NAFLD analysis, beta-estradiol (P = 9.42E-12), cholesterol (P = 1.79E-11), tumor necrosis factor (TNF) (P = 8.73E-10), nuclear receptor coactivator (P = 1.22E-09), and sterol regulatory element binding transcription factor 1 (SREBF1) (P = 12.8E-08) were top upstream regulators. Of these regulators, SREBF1 demonstrated the highest z-score (2.200), demon
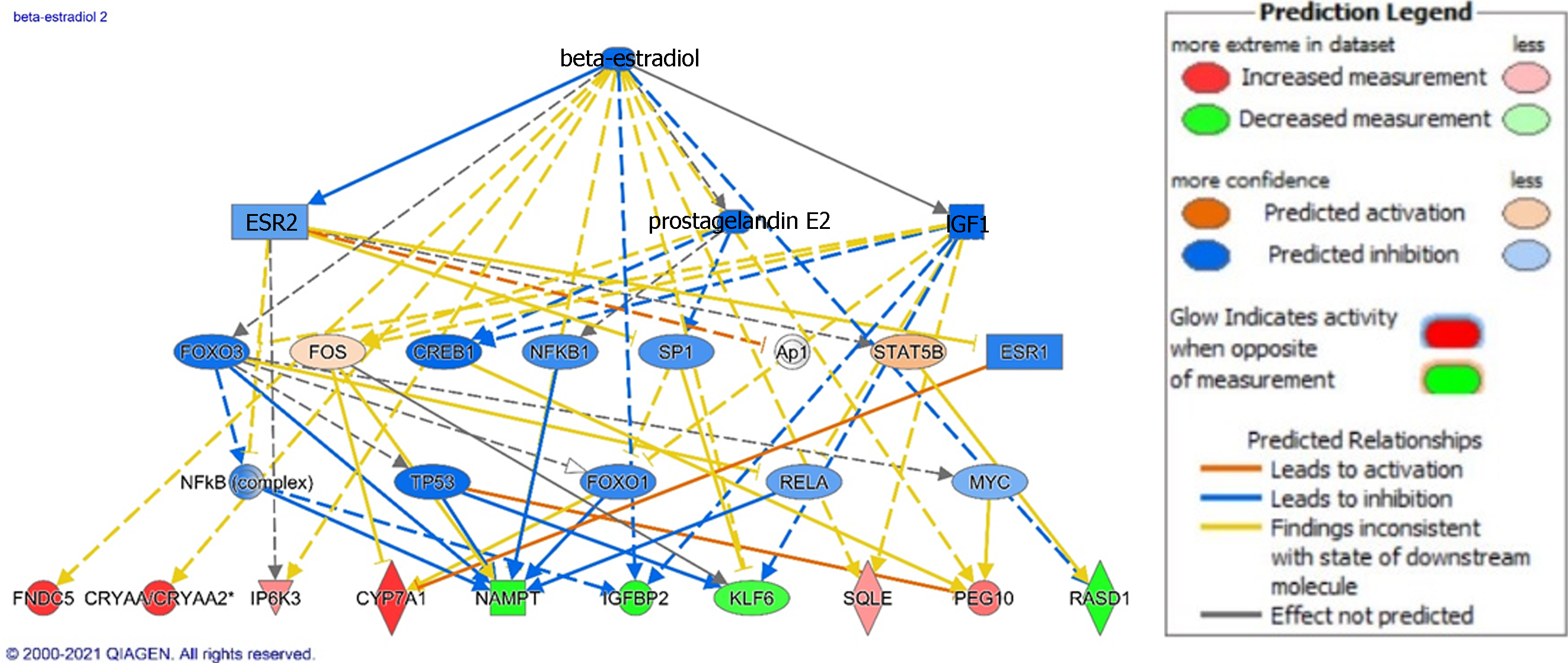
In the NASH analysis, TNF (P = 1.22E-19), lipopolysaccharide or LPS (P = 6.27E-16), beta estradiol (P = 1.42E-15, with predicted inhibition), interleukin 1B or IL1B (P = 1.78E-14), and platelet-derived growth factor BB or PDGF BB (P = 1.90E-14) were top upstream regulators. Beta-estradiol demonstrates anti-fibrotic effects in the liver, so we investigated its downstream effects in our dataset (Figure 4). Inhibition of beta-estradiol activity is reflected by the changes we noted in the top upregulated genes including crystallin alpha A, BBOX1, CYP7A1, and FNDC5 and top downregulated genes including IGFBP2, nicotinamidenphosphoribosyltransferase pseudogene 1 (NAMP1), and RASD1 genes in our dataset described above. In addition, IPA related inhibition of beta-estradiol to other gene expression changes of interest including upregulation of the PEG10, squalene epoxidase (SQLE), IP6K3 and downregulation of the tumor suppressor Kruppel-like factor 6 (KLF6)[36,37].
To investigate potential drug targets from our dataset, we utilized clue.io. We inputted genes that were both upregulated and downregulated in our NAFD and NASH dataset (see Figure 5). We used the query tool from the platform and focused on HEPG2 cell lines, immortalized HCC cells. By looking at compounds that inverse the pathologic expression patterns in our meta-analyses, we identified riciribine (an AKT inhibitor) and ZSTK-474 (a PI3K inhibitor) as potential therapeutic compounds that target the genes in our investigation (see Table 3).
NAFLD represents a growing health burden, with an astonishing prevalence of 25% of the global population[38]. A better understanding of pathogenesis is needed to tackle this herculean disease. Here, we use meta-analysis of public data using our STARGEO platform in search of insights to disease and potential therapeutic targets. The gene expression profiles from our analyses can elucidate function and regulatory patterns to disease[39]. Our results from the NAFLD analysis reinforce the role of altered metabolism, inflammation, and cell survival and supports recently described contributors to disease such as altered androgen and lncrna activity[17,23,40].
Our results demonstrated several changes that are implicated in altered lipid and metabolic homeostasis. It is the accumulation of lipids that lead to several downstream effects that characterized NAFLD development and progression[20,41]. For example, lipid droplets in hepatocytes can lead to hepatic insulin resistance, decreased autophagy, oxidative stress, and interaction with several transcription factors such as SREBF[20]. These lipid droplets can form through the activity of proteins from the perilipin family, such as perilipin 1 (PLN1), which was upregulated in our dataset (Table 2)[20]. “Superpathway of Cholesterol Biosynthesis” was one of the top canonical pathways, and several top disease functions and networks were related to lipid accumulation (Tables 1-3). In addition, cholesterol and SREBF1, a transcription factor involved in lipid homeostasis, were top upstream regulators[42]. SREBF1 stimulates accumulation of lipids in hepatocytes through activation of patatin-like phospholipase domain-containing 3 (PNPAL3)[43]. In our results, we illustrate how downstream signaling of SREBF1 and SREBF2 Leads to fatty acid accumulation and other disease functions. Downstream of SREBF1 and SREBF2 signaling, we noted upregulation of CXCL10, HMGCR, HMGCS1, FABP5, and PEG10 in addition to downregulation of SHBG and IGF1. HMGCR catalyzes the first reaction of cholesterol synthesis and HMGCS1 also contributes to hepatic cholesterol synthesis. Increased activity of HMGCSR and HMGCS1 was associated with NAFLD and with fatty acid accumulation[44]. Additionally, FABP5 is a fatty acid binder normally expressed in adipocytes, but expression in hepatocytes was correlated with fatty acid infiltration in NAFLD[45]. In addition to fatty acid changes, we found downregulation of IGF-1, which leads to hyperglycemia and increases risk for diabetes seen as in NAFLD[46,47]. IGF-1 also has anti-fibrotic effects through attenuation of hepatic stellate cell (HSC) activation in murine models[48]. Furthermore, SHBG was downregulated in analysis, with decreased SHBG levels being associated with increased insulin resistance in NAFLD patients[49]. Higher levels of SHBG are also associated with lower odds for NAFLD and may have some protective effect[50]. In addition to fatty acid accumulation and glycemia, we related SREBF activity to malignant changes through upregulation of PEG10. PEG10 is a transcription factor that was found to be an oncogene in several solid cancers such as HCC, gastric cancer, and breast carcinoma[36]. PEG10 is upregulated in NASH and NAFLD and may be associated with increased risk for HCC seen in this patient population[18]. Furthermore, our results fortified other changes in NAFLD that are implicated in lipolytic changes that may induce NAFLD. CREB signaling was identified as a top canonical pathway. Awaad, et al showed elevated cAMP and CREB levels in a NAFLD murine model and suggest the role of cAMP and CREB as a marker of early NAFLD[51].
Accumulation of fatty acids in the liver induces chronic, low-grade inflammation, and subsequently, progression of NAFLD to NASH. Our results illustrated the inflammatory changes in NAFLD. The inflammatory response was a top disease function in our analysis (Table 3) and the pro-inflammatory cytokine TNF was a top upstream regulator. In murine models, TNF plays an essential role in NAFLD development through upregulation of inflammatory mediators and genes associated with liver fibrosis[52]. TNF also induces hepatic steatosis in murine models through upregulation of SREB proteins[53]. We also noted upregulation of several pro-inflammatory cytokines in our analysis including CXCL10 (Table 1). CXCL10 recruits T cells and macrophages and is an independent risk factor for NASH[54]. Since fatty acids lead to inflammatory changes, it is expected that SREB signaling would lead to downstream pro-inflammatory changes such as upregulation of CXCL10 (Figure 3).
Aside from inflammation and metabolic derangements, our results illustrated several other signaling and cellular processes of interest in NAFLD. One such cellular process is protein prenylation. Protein prenylation is a protein post-translational modification where farnesyl (farnesylation) or geranylgeranyl (geranylgeranylation) side chain is added to a C-terminal cysteine residue[55]. The mevalonate pathway, a top canonical pathway in our analysis, affects the ratio of farnesylation and geranylgeranylation. Alteration in this ratio is implicated in NAFLD and NAFLD-associated fibrosis[56]. In addition to post-translational protein modification, our results suggest a role for lncRNAs in NAFLD. LnRNAs are critical mediators of normal liver physiology, with aberrant expression being observed in metabolic, fibrotic, and malignant hepatic changes[17]. We found upregulation of lncRNAs in our analysis, including XIST and LINC0085. XIST is one of the earliest described lnRNAs and assists in the formation of silenced heterochromatin[57]. While not well-described in NAFLD and NASH, XIST has been shown to promote HCC and colorectal cancer[58,59]. Additionally, LINC0085 is a positive cell growth regulator in breast cancer models and may, alongside XIST, cause proliferative and pathologic changes in hepatocytes in NAFLD and NASH[16]. Lastly, recent research has connected the link between circadian rhythm genes with NAFLD[24]. Asynchronization of circadian rhythms, such as from shift work, are correlated with higher prevalence and NAFLD[60]. Per3 is a circadian rhythm gene that regulates adipogenesis, with deletion leading to increased adipogenesis in animal models[24]. Thus, downregulation of Per3 in our results may suggest dysregulation of circadian rhythm and consequent changes in regulation of adipogenesis.
NASH is a subset of NAFLD characterized by steatosis inflammation and fibrosis[61]. It typically takes years for NAFLD to progress for NASH, and while the mechanisms behind this progression are not clear, our current understanding suggests a “multi-hit hypothesis” where multiple modes of fatty acid accumulation and oxidative stress synergistically induce liver inflammation and fibrosis[61]. Aside from lifestyle modifications, obeticholic acid is the only FDA-approved treatment of NASH[62]. The growing burden of NASH necessitates new therapeutics and our analysis of NASH offers insight into potential treatment.
Ingenuity Pathway Analysis of our NASH dataset reinforces the role of cholesterol. Several of our top canonical pathways, disease network, and disease functions were related to cholesterol synthesis, lipid metabolism, adipogenesis, and metabolic disease (Figure 1, and Tables 1, 3 and 4). The role of lipids in liver injury have been described above[20,40]. Other disease functions and disease networks of note involved cell death and survival, cancer, digestive system disease, and organismal injury (Tables 3 and 4). The top upstream regulators in addition to upregulated and downregulated genes reflect activity related to these disease functions. Among our top upstream regulators were pro-inflammatory cytokines TNF and IL1B, PDGF BB, and beta-estradiol (with predicted inhibition).
| Top molecular networks in NAFLD vs healthy control | |
| Lipid metabolism, small molecule biochemistry, vitamin and mineral metabolism | 34 |
| Cell-to-cell signaling and interaction, cellular movement, hematological system development and function | 23 |
| Connective tissue disorders, inflammatory disease, organismal injury and abnormalities | 19 |
| Cellular development, connective tissue development and function, skeletal and muscular system development and function | 16 |
| Cell death and survival, neurological disease, organismal injury and abnormalities | 16 |
| Amino acid metabolism, molecular transport, small molecule biochemistry | 34 |
| Cellular development, skeletal and muscular system development and function, tissue development | 34 |
| Hereditary disorder, neurological disease, organismal injury and abnormalities | 32 |
| Digestive system development and function, lipid metabolism, small molecule biochemistry | 29 |
| Cell cycle, cell death and survival, cellular movement | 29 |
As already described, inflammation is a major contributor to liver disease. It has been long shown that patients with NASH, and more so those with severe NASH, have elevated levels of TNF[63]. Elevated serum levels of TNF in NASH patients was linked to increased major adverse hepatic events[64]. While TNF inhibition reduces steatosis and fibrosis in murine models, their role in select NAFLD and NASH patient populations has still not been proven effective[52,65-67]. Similarly, IL1B signaling has pro-fibrotic and lipogenic effects in murine models and may have promise as directed therapy in NASH patients[68-70]. Lastly, the cytokine PDGF BB exerts its pro-fibrotic effects through activation of hepatic stellate cells and, consequently, is another potential drug target[71].
Experimental models have shown that estrogen has protective, anti-fibrotic activity through attenuation of HSC activation and generation of reactive oxygen species[72]. Additionally, estrogen receptor agonism in a NASH murine model had therapeutic effects through modulating bile acid receptor signaling and inhibiting fibrosis and adipogenesis[73]. Interestingly, decreased estrogen levels and other hormone changes in menopause may be related to increase risk for NAFLD and NASH[74]. Since beta-estradiol was a top upstream regulator with predicted inhibition in our NASH analysis, we applied IPA to investigate beta-estradiol signaling and its downstream genetic effects (Figure 4).
Our analysis related inhibition of beta-estradiol to derangement of several cellular processes downstream including metabolism, extracellular matrix deposition, and tumor suppression. In regard to metabolism, we related inhibition of estradiol to upregulation of IP6K3, CYP7A1, and SQLE and to downregulation of NAMP1 and IGFBP2. IP6K3 produces inositol pyrophosphates and regulates metabolic control[75]. Deletion in murine models leads to improved glucose tolerance, reduced body weight, and protection from fatty liver disease[75,76]. SQLE is involved in cholesterol synthesis and has been shown in both human and animal studies to promote development of HCC in fatty liver disease[77]. CYP7A1 is a rate-limiting enzyme in the classical pathway of bile acid synthesis with upregulated gene expression in NAFLD and NASH patients alike, but discrepancies exist in post-transcriptional protein levels[27]. The effects of fatty liver disease on CYP7A1 are inconsistent, but bile acid dysregulation is a growing hallmark in this disease[27]. NAMP1 is a critical enzyme in the synthesis of nicotinamide adenine dinucleotide (NAD+). NAD+ functions in mitochondrial oxidative phosphorylation and protection of cells from reactive oxygen species[78]. Depletion of hepatic NAD+ has been shown to be a risk factor for NAFLD in a murine model[35]. There is growing interest in targeting NAD+ in NAFLD[79]. Lastly, IGFBP2 binds to IGF1 and has a positive effects in glucose control[80]. Early epigenetic silencing, via methylation, of IGBFP2 predicts development of fatty liver later in mice[81].
In addition to metabolic changes, our analysis showed pro-oncogenic and fibrotic genetic changes in NASH that may relate to inhibition of beta-estradiol signaling. Through IPA, we correlated inhibition of beta-estradiol signaling to upregulation of PEG10 and FNDC5 and to downregulation of RASD1 and KLF6. Interestingly, we found upregulation of PEG10 in our NAFLD analysis and discussed its pro-oncogenic activity. RASD1 is a member of the Ras superfamily of G proteins that regulate signal transduction through G-protein coupled receptors[82]. RASD1 prevents aberrant cell growth, and its downregulation may lead to increased risk for HCC seen in fatty liver disease.[34,83] Additionally, KFL6 is a zinc finger transcriptional protein with tumor suppressor function that is inhibited in various cancers, including HCC[37]. Lastly, FNDC5 is a novel myokine that controls extracellular matrix deposition. Higher expression of FNDC5 in HSCs correlated to severity of fibrosis in NAFLD patients[29]. Our results illustrate malignant and fibrotic gene expression changes in both NAFLD and NASH stages of disease and its possible relation with inhibition of beta-estradiol signaling.
Lastly, our analysis suggests potential use of riciribine and ZSTK-474 in the treatment of NAFLD. Dysregulation of the PI3KT/AKT pathway in hepatocytes has been described in NAFLD[84]. Such dysregulation is implicated in hepatic steatosis and fibrosis. While the mechanisms underpinning pathogenesis through the PI3KT/AKT pathway are still under investigation, our results add further evidence of targeting this pathway for therapeutic benefit.
Our meta-analysis approach offers insights into NAFLD and NASH, but this approach is not without limitations. Biological samples in Gene Expression Omnibus have limitations in terms of description of samples. Some details that may present confounding variables are the co-morbidities in patients and differing stages in fatty liver disease, including degree of fibrosis. Other patient characteristics may also influence results such as medications, age, gender, and ethnicity. Samples were also taken under different conditions such as diagnosis of undifferentiated liver disease or in bariatric patients, which may lead to further differences between samples. Though there are set diagnostic criteria for hepatic steatosis on biopsy, the diagnoses were made by separate pathologists across these studies and a meta-analysis approach would not be able to account for these differences. Additionally, while transcriptomic and meta-analysis studies can offer a global view of disease function and regulatory signaling using gene expression patterns, causality necessitates more direct functional experimentation[39]. This approach itself does not offer direct experimental or clinical evidence. Nonetheless, our results offer a foundation to future studies in NAFLD and NASH that warrant further investigation with experimental and human models.
We utilized our platform STARGEO to produce genetic signatures from GEO datasets that provide molecular insights to fatty liver disease. We conducted to separate analysis of NAFLD and NASH liver biopsies to investigate genetic changes that define stages of fatty liver disease. Our analyses buttresses how the dysregulation in lipid homeostasis, though such regulators as the transcription factor SREBF1, contribute to steatosis. We also noted upregulation of genes implicated in oncogenesis, such as PEG10, that may partly explain the increased risk of HCC in these patients. We also describe the potential contribution on long noncoding RNAs in NAFLD pathogenesis. From our NASH analysis, we explored how beta-estradiol dysregulation may mechanistically contribute to steatosis and its several consequences such as fibrosis and oncogenesis. Lastly, we used out dataset and clue.io to identify genes that target pathologic genetic changes and signaling, such as PI3KT/AKT signaling, and found ricirbine and ZKST-474 as possible therapeutic targets. Overall, our analysis illustrates several changes that may explain progression of NAFLD pathogenesis and promising directions that warrant further investigation.
Non-alcoholic fatty liver disease (NAFLD) pathogenesis is poorly understood but may result from a mix of exogenous and genetic factors that lead to fatty infiltration and inflammation.
NAFLD is a growing cause for liver transplant with limited therapeutic options.
To define genetic changes that underlie NAFLD and progression to non-alcoholic steatohepatitis (NASH) in pursuit of identifying promising therapeutic targets.
We employed our STARGEO platform to conduct meta-analyses of publicly available liver biopsies from NAFLD and NASH patients.
We identified various genes implicated in inflammation and fatty infiltration, as well as signaling processes that lead to these changes. We also identified riciribine and ZSTK-474 as potential drugs.
NAFLD and its progression to NASH is likely led by several genetic changes detailed in our manuscript. The genetic changes in our dataset are targeted by ricirbine and ZSTK-474 and warrants further study.
As NAFLD becomes an increasing clinical burden, a bioinformatics approach is valuable in understanding causes and elucidating treatment avenues.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du Y, China; Kotlyarov S, Russia A-Editor: Lin FY S-Editor: Wang LL L-Editor: A P-Editor: Cai YX
| 1. | Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 3. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2128] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 4. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 946] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 5. | Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 7. | Hadley D, Pan J, El-Sayed O, Aljabban J, Aljabban I, Azad TD, Hadied MO, Raza S, Rayikanti BA, Chen B, Paik H, Aran D, Spatz J, Himmelstein D, Panahiazar M, Bhattacharya S, Sirota M, Musen MA, Butte AJ. Precision annotation of digital samples in NCBI's gene expression omnibus. Sci Data. 2017;4:170125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30408] [Article Influence: 779.7] [Reference Citation Analysis (0)] |
| 9. | Ahrens M, Ammerpohl O, von Schönfels W, Kolarova J, Bens S, Itzel T, Teufel A, Herrmann A, Brosch M, Hinrichsen H, Erhart W, Egberts J, Sipos B, Schreiber S, Häsler R, Stickel F, Becker T, Krawczak M, Röcken C, Siebert R, Schafmayer C, Hampe J. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 10. | Frades I, Andreasson E, Mato JM, Alexandersson E, Matthiesen R, Martínez-Chantar ML. Integrative genomic signatures of hepatocellular carcinoma derived from nonalcoholic Fatty liver disease. PLoS One. 2015;10:e0124544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, Brandt ML, Harmon CM, Helmrath MA, Michalsky MP, Courcoulas AP, Zeller MH, Inge TH; Teen-LABS Consortium. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology. 2015;149:623-34.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Guo J, Fang W, Sun L, Lu Y, Dou L, Huang X, Tang W, Yu L, Li J. Ultraconserved element uc.372 drives hepatic lipid accumulation by suppressing miR-195/miR4668 maturation. Nat Commun. 2018;9:612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8234] [Article Influence: 411.7] [Reference Citation Analysis (5)] |
| 14. | Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2902] [Cited by in RCA: 4208] [Article Influence: 350.7] [Reference Citation Analysis (0)] |
| 15. | Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, Gould J, Davis JF, Tubelli AA, Asiedu JK, Lahr DL, Hirschman JE, Liu Z, Donahue M, Julian B, Khan M, Wadden D, Smith IC, Lam D, Liberzon A, Toder C, Bagul M, Orzechowski M, Enache OM, Piccioni F, Johnson SA, Lyons NJ, Berger AH, Shamji AF, Brooks AN, Vrcic A, Flynn C, Rosains J, Takeda DY, Hu R, Davison D, Lamb J, Ardlie K, Hogstrom L, Greenside P, Gray NS, Clemons PA, Silver S, Wu X, Zhao WN, Read-Button W, Haggarty SJ, Ronco LV, Boehm JS, Schreiber SL, Doench JG, Bittker JA, Root DE, Wong B, Golub TR. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171:1437-1452.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 2127] [Article Influence: 265.9] [Reference Citation Analysis (0)] |
| 16. | Abba MC, Canzoneri R, Gurruchaga A, Lee J, Tatineni P, Kil H, Lacunza E, Aldaz CM. LINC00885 a Novel Oncogenic Long Non-Coding RNA Associated with Early Stage Breast Cancer Progression. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Mahpour A, Mullen AC. Our emerging understanding of the roles of long non-coding RNAs in normal liver function, disease, and malignancy. JHEP Rep. 2021;3:100177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Arendt BM, Comelli EM, Ma DW, Lou W, Teterina A, Kim T, Fung SK, Wong DK, McGilvray I, Fischer SE, Allard JP. Altered hepatic gene expression in nonalcoholic fatty liver disease is associated with lower hepatic n-3 and n-6 polyunsaturated fatty acids. Hepatology. 2015;61:1565-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 19. | Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 20. | Carr RM, Ahima RS. Pathophysiology of lipid droplet proteins in liver diseases. Exp Cell Res. 2016;340:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Sun C, Fan JG, Qiao L. Potential epigenetic mechanism in non-alcoholic Fatty liver disease. Int J Mol Sci. 2015;16:5161-5179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Buqué X, Martínez MJ, Cano A, Miquilena-Colina ME, García-Monzón C, Aspichueta P, Ochoa B. A subset of dysregulated metabolic and survival genes is associated with severity of hepatic steatosis in obese Zucker rats. J Lipid Res. 2010;51:500-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Nasiri M, Nikolaou N, Parajes S, Krone NP, Valsamakis G, Mastorakos G, Hughes B, Taylor A, Bujalska IJ, Gathercole LL, Tomlinson JW. 5α-Reductase Type 2 Regulates Glucocorticoid Action and Metabolic Phenotype in Human Hepatocytes. Endocrinology. 2015;156:2863-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Shi D, Chen J, Wang J, Yao J, Huang Y, Zhang G, Bao Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front Physiol. 2019;10:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Wang S, Hu M, Qian Y, Jiang Z, Shen L, Fu L, Hu Y. CHI3L1 in the pathophysiology and diagnosis of liver diseases. Biomed Pharmacother. 2020;131:110680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1244] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 27. | Zhang X, Deng R. Dysregulation of Bile Acids in Patients with NAFLD. In Nonalcoholic Fatty Liver Disease - An Update, 2019. [DOI] [Full Text] |
| 28. | Paul HS, Sekas G, Adibi SA. Carnitine biosynthesis in hepatic peroxisomes. Demonstration of gamma-butyrobetaine hydroxylase activity. Eur J Biochem. 1992;203:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Gao F, Zheng KI, Zhu PW, Li YY, Ma HL, Li G, Tang LJ, Rios RS, Liu WY, Pan XY, Targher G, Byrne CD, Chen YP, Zheng MH. FNDC5 polymorphism influences the association between sarcopenia and liver fibrosis in adults with biopsy-proven non-alcoholic fatty liver disease. Br J Nutr. 2021;126:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Brady GF, Kwan R, Bragazzi Cunha J, Elenbaas JS, Omary MB. Lamins and Lamin-Associated Proteins in Gastrointestinal Health and Disease. Gastroenterology. 2018;154:1602-1619.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Shibuya A, Honda S. Immune regulation by Fcα/μ receptor (CD351) on marginal zone B cells and follicular dendritic cells. Immunol Rev. 2015;268:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, Nevzorova YA, Liedtke C, Weiskirchen R. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-β signaling. Biochim Biophys Acta. 2014;1843:902-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Preston GA, Lyon TT, Yin Y, Lang JE, Solomon G, Annab L, Srinivasan DG, Alcorta DA, Barrett JC. Induction of apoptosis by c-Fos protein. Mol Cell Biol. 1996;16:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, Lanier SM. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23:5858-5863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, Wang P, Miao CY. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173:2352-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 36. | Xie T, Pan S, Zheng H, Luo Z, Tembo KM, Jamal M, Yu Z, Yu Y, Xia J, Yin Q, Wang M, Yuan W, Zhang Q, Xiong J. PEG10 as an oncogene: expression regulatory mechanisms and role in tumor progression. Cancer Cell Int. 2018;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Kremer-Tal S, Reeves HL, Narla G, Thung SN, Schwartz M, Difeo A, Katz A, Bruix J, Bioulac-Sage P, Martignetti JA, Friedman SL. Frequent inactivation of the tumor suppressor Kruppel-like factor 6 (KLF6) in hepatocellular carcinoma. Hepatology. 2004;40:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 39. | Russo G, Zegar C, Giordano A. Advantages and limitations of microarray technology in human cancer. Oncogene. 2003;22:6497-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | Kerr TA, Davidson NO. Cholesterol and nonalcoholic fatty liver disease: renewed focus on an old villain. Hepatology. 2012;56:1995-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Moslehi A, Hamidi-Zad Z. Role of SREBPs in Liver Diseases: A Mini-review. J Clin Transl Hepatol. 2018;6:332-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 43. | Qiao A, Liang J, Ke Y, Li C, Cui Y, Shen L, Zhang H, Cui A, Liu X, Liu C, Chen Y, Zhu Y, Guan Y, Fang F, Chang Y. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology. 2011;54:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015;1852:1765-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 45. | Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Sirén J, Hamsten A, Fisher RM, Yki-Järvinen H. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 46. | Stanley TL, Fourman LT, Zheng I, McClure CM, Feldpausch MN, Torriani M, Corey KE, Chung RT, Lee H, Kleiner DE, Hadigan CM, Grinspoon SK. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J Clin Endocrinol Metab. 2021;106:e520-e533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 47. | Yao Y, Miao X, Zhu D, Li D, Zhang Y, Song C, Liu K. Insulin-like growth factor-1 and non-alcoholic fatty liver disease: a systemic review and meta-analysis. Endocrine. 2019;65:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Nishizawa H, Iguchi G, Fukuoka H, Takahashi M, Suda K, Bando H, Matsumoto R, Yoshida K, Odake Y, Ogawa W, Takahashi Y. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. 2016;6:34605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Ye J, Yao Z, Tan A, Gao Y, Chen Y, Lin X, He R, Tang R, Hu Y, Zhang H, Yang X, Wang Q, Jiang Y, Mo Z. Low Serum Sex Hormone-Binding Globulin Associated with Insulin Resistance in Men with Nonalcoholic Fatty Liver Disease. Horm Metab Res. 2017;49:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Ann Hepatol. 2017;16:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Awaad AK, Kamel MA, Mohamed MM. The role of hepatic transcription factor cAMP response element-binding protein (CREB) during the development of experimental nonalcoholic fatty liver: a biochemical and histomorphometric study. Egypt Liver J. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, Soejima E, Tajiri Y, Yamada K. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm Metab Res. 2018;50:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 53. | Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp Biol Med (Maywood). 2007;232:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Xu Z, Zhang X, Lau J, Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert Rev Mol Med. 2016;18:e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1558] [Cited by in RCA: 1509] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 56. | Zhao Y, Wu TY, Zhao MF, Li CJ. The balance of protein farnesylation and geranylgeranylation during the progression of nonalcoholic fatty liver disease. J Biol Chem. 2020;295:5152-5162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Wutz A, Rasmussen TP, Jaenisch R. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet. 2002;30:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 571] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 58. | Chang S, Chen B, Wang X, Wu K, Sun Y. Long non-coding RNA XIST regulates PTEN expression by sponging miR-181a and promotes hepatocellular carcinoma progression. BMC Cancer. 2017;17:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 59. | Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng ZL, Pan ZZ, Huang P, Wang FH, Li YH, Ju HQ, Xu RH. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8:e3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 60. | Johnston JD. Physiological links between circadian rhythms, metabolism and nutrition. Exp Physiol. 2014;99:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-Alcoholic Steatohepatitis: A Review of Its Mechanism, Models and Medical Treatments. Front Pharmacol. 2020;11:603926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 62. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 64. | Zhu Z, Li S. Association between tumor necrosis factor-α and the risk of hepatic events: A median 3 years follow-up study. Hepat Mon. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Park C, Balaji N, Jung S, Choi J, Ju M, Lee S, Kim J, Bong S, Chung S, Lee YJ, Yi J. Advanced Passivation Technology and Loss Factor Minimization for High Efficiency Solar Cells. J Nanosci Nanotechnol. 2015;15:7699-7705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 66. | Tang KT, Dufour JF, Chen PH, Hernaez R, Hutfless S. Antitumour necrosis factor-α agents and development of new-onset cirrhosis or non-alcoholic fatty liver disease: a retrospective cohort. BMJ Open Gastroenterol. 2020;7:e000349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Wandrer F, Liebig S, Marhenke S, Vogel A, John K, Manns MP, Teufel A, Itzel T, Longerich T, Maier O, Fischer R, Kontermann RE, Pfizenmaier K, Schulze-Osthoff K, Bantel H. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020;11:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 68. | Dobre M, Milanesi E, Mănuc TE, Arsene DE, Ţieranu CG, Maj C, Becheanu G, Mănuc M. Differential Intestinal Mucosa Transcriptomic Biomarkers for Crohn's Disease and Ulcerative Colitis. J Immunol Res. 2018;2018:9208274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, Kim JK, Czech MP. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 2014;9:e107265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 70. | Tilg H, Effenberger M, Adolph TE. A role for IL-1 inhibitors in the treatment of non-alcoholic fatty liver disease (NAFLD)? Expert Opin Investig Drugs. 2020;29:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y, Zhang SZ, Fang J, Yu CH. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol Med Rep. 2017;16:7879-7889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 72. | Itagaki T, Shimizu I, Cheng X, Yuan Y, Oshio A, Tamaki K, Fukuno H, Honda H, Okamura Y, Ito S. Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 2005;54:1782-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Ponnusamy S, Tran QT, Thiyagarajan T, Miller DD, Bridges D, Narayanan R. An estrogen receptor β-selective agonist inhibits non-alcoholic steatohepatitis in preclinical models by regulating bile acid and xenobiotic receptors. Exp Biol Med (Maywood). 2017;242:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Venetsanaki V, Polyzos SA. Menopause and Non-Alcoholic Fatty Liver Disease: A Review Focusing on Therapeutic Perspectives. Curr Vasc Pharmacol. 2019;17:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 75. | Moritoh Y, Oka M, Yasuhara Y, Hozumi H, Iwachidow K, Fuse H, Tozawa R. Inositol Hexakisphosphate Kinase 3 Regulates Metabolism and Lifespan in Mice. Sci Rep. 2016;6:32072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Mukherjee S, Haubner J, Chakraborty A. Targeting the Inositol Pyrophosphate Biosynthetic Enzymes in Metabolic Diseases. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Liu D, Wong CC, Fu L, Chen H, Zhao L, Li C, Zhou Y, Zhang Y, Xu W, Yang Y, Wu B, Cheng G, Lai PB, Wong N, Sung JJY, Yu J. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 78. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8502] [Cited by in RCA: 8902] [Article Influence: 468.5] [Reference Citation Analysis (0)] |
| 79. | Guarino M, Dufour JF. Nicotinamide and NAFLD: Is There Nothing New Under the Sun? Metabolites. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 81. | Kammel A, Saussenthaler S, Jähnert M, Jonas W, Stirm L, Hoeflich A, Staiger H, Fritsche A, Häring HU, Joost HG, Schürmann A, Schwenk RW. Early hypermethylation of hepatic Igfbp2 results in its reduced expression preceding fatty liver in mice. Hum Mol Genet. 2016;25:2588-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Greenwood MP, Greenwood M, Mecawi AS, Antunes-Rodrigues J, Paton JF, Murphy D. Rasd1, a small G protein with a big role in the hypothalamic response to neuronal activation. Mol Brain. 2016;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Pennisi G, Celsa C, Giammanco A, Spatola F, Petta S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 84. | Matsuda S, Kobayashi M, Kitagishi Y. Roles for PI3K/AKT/PTEN Pathway in Cell Signaling of Nonalcoholic Fatty Liver Disease. ISRN Endocrinol. 2013;2013:472432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |