Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1365
Peer-review started: March 26, 2022
First decision: May 1, 2022
Revised: May 9, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 27, 2022
Processing time: 122 Days and 20.6 Hours
Lipid metabolism disorder and inflammatory-immune activation are vital triggers in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Various studies have shown that PPAR-γ exerts potent anti-inflammatory and immunomodulatory properties. However, little is known about the regulation of PPAR-γ activity in modulating cell crosstalk in NAFLD.
To investigate whether the regulation of PPAR-γ activity in lipid-laden hepat
Primary hepatocytes were isolated from wild-type C57BL6/J mice or hepatocyte-specific PPAR-γ knockout mice and incubated with free fatty acids (FFAs). Macrophages were incubated with conditioned medium (CM) from lipid-laden hepatocytes with or without a PPAR-γ agonist. Wild-type C57BL/6J mice were fed a high-fat (HF) diet and administered rosiglitazone.
Primary hepatocytes exhibited significant lipid deposition and increased ROS production after incubation with FFAs. CM from lipid-laden hepatocytes promoted macrophage polarization to the M1 type and activation of the TLR4/NF-κB pathway. A PPAR-γ agonist ameliorated oxidative stress and NLRP3 inflammasome activation in lipid-laden hepatocytes and subsequently prevented M1 macrophage polarization. Hepatocyte-specific PPAR-γ deficiency aggravated oxidative stress and NLRP3 inflammasome activation in lipid-laden hepatocytes, which further promoted M1 macrophage polarization. Rosiglitazone administration improved oxidative stress and NLRP3 inflammasome activation in HF diet-induced NAFLD mice in vivo.
Upregulation of PPAR-γ activity in hepatocytes alleviated NAFLD by modulating the crosstalk between hepatocytes and macrophages via the reactive oxygen species-NLRP3-IL-1β pathway.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is currently one of the most endemic chronic liver diseases worldwide. We aimed to investigate whether the regulation of PPAR-γ activity in lipid-laden hepatocytes affects macrophage polarization and to explore the underlying mechanism. Our study revealed that lipid-laden hepatocytes skewed macrophage polarization to the M1 phenotype. Regulation of PPAR-γ activity alleviates NAFLD by modulating the crosstalk between hepatocytes and macrophages via the reactive oxygen species-NLRP3-IL-1β signaling pathway. Strategies that manipulate PPAR-γ activity to regulate cell crosstalk will be beneficial for treating NAFLD.
- Citation: Li XY, Ji PX, Ni XX, Chen YX, Sheng L, Lian M, Guo CJ, Hua J. Regulation of PPAR-γ activity in lipid-laden hepatocytes affects macrophage polarization and inflammation in nonalcoholic fatty liver disease. World J Hepatol 2022; 14(7): 1365-1381
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1365.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1365
Nonalcoholic fatty liver disease (NAFLD) is currently one of the most common liver diseases, with a high morbidity presently exceeding 25% worldwide[1,2]. Manifesting from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) and even to cirrhosis and hepatocellular carcinoma, NAFLD has already posed heavy public and financial burdens worldwide[3]. Given the lack of effective medications for treatment, there is a need to deeply explore the pathogenesis of NAFLD and to look for potential therapeutic targets for alleviating NAFLD[4].
Lipid metabolism disorder and inflammatory immune activation are two main triggers in the pathogenesis of NAFLD[5]. The widely accepted “two-hit theory” suggests that excess free fatty acids (FFAs) act as the first hit, causing abnormal lipid accumulation and insulin resistance and increasing the susceptibility of the liver to inflammatory damage[5,6]. Based on the first hit, the second hit involves activation of immune cells and oxidative metabolite production, leading to oxidative stress and an inflammatory response[7-9]. Hence, the accumulation of lipotoxic agents in hepatocytes is key to the onset and progression of NAFLD. Lipotoxicity can directly induce endoplasmic reticulum stress and pyroptosis in steatotic hepatocytes[10,11]. Among the many factors that trigger the progression of NAFLD to NASH, activation of immune cells plays a prominent and indispensable role[12].
Activation of macrophages, including hepatic resident Kupffer cells and peripherally recruited monocytes, plays an important role in the progression of NAFLD[12,13]. It is now widely considered that macrophages can be classified into two types: the classically activated M1 phenotype and the alternatively activated M2 phenotype. M1 phenotype macrophages are mainly induced by interferon-γ and lipopolysaccharide and secrete proinflammatory factors (IL-1, 6, 12, 23, CXCL 10, NO, peroxides, etc.), which participate in the Th1 immune response and exert proinflammatory, bactericidal and antitumor effects. M2 macrophages are mainly induced by IL-4 and IL-10 and participate in the Th2 immune response with anti-inflammatory and tissue remodeling effects[14,15]. Under normal conditions, macrophages in the liver predominantly exhibit an M2 phenotype[16]. However, in NAFLD mice induced by a high-fat diet, the number of macrophages increases dramatically, and the polarity of macrophages appears to shift toward the M1 type[17]. These M1 phenotype macrophages contribute to the progression and prolongation of liver inflammation[13]. However, there is no certainty as to what exactly drives the activation of macrophages. Recent studies have revealed that various factors, including high levels of free fatty acids and the gut microbiota, may lead to macrophage activation[18,19]. Furthermore, the crosstalk or interaction between parenchymal and nonparenchymal cells in the liver may reciprocally regulate macrophage phenotype or function.
PPAR-γ is a ligand-activated nuclear transcription receptor that mainly participates in adipocyte differentiation, lipogenesis, and insulin resistance[20]. Recently, much attention has been focused on the immunomodulatory and anti-inflammatory properties of PPAR-γ[21]. It has been demonstrated that activation of PPAR-γ synergistically upregulates the NRF2/HO-1 signaling pathway, thereby ameliorating methotrexate-induced hepatotoxicity[22]. Our previous study demonstrated that regulation of PPAR-γ activity in macrophages and HSCs could modulate their activation and alleviate the development of NAFLD/NASH[17,23]. Most previous studies have focused on the anti-inflammatory properties of PPAR-γ in nonparenchymal cells, such as macrophages and HSCs, in NASH; thus, the role of PPAR-γ in hepatocytes and the interaction between hepatocytes and macrophages remain to be explored.
In the current study, we aimed to investigate whether the regulation of PPAR-γ activity in lipid-laden hepatocytes affects macrophage polarization and explore the underlying mechanism. We found that upregulation of PPAR-γ activity could alleviate NAFLD through modulation of the crosstalk between hepatocytes and macrophages via the ROS-NLRP3-IL-1β signaling pathway.
Primary hepatocytes were isolated from wild-type C57BL/6 mice or hepatocyte-specific PPAR-γ knockout mice via two-step collagenase in situ perfusion of the liver[24] and then cultured in DMEM containing 10% FBS (Gibco, Waltham, MA, United States) with 100 U/mL penicillin G and 100 U/mL streptomycin sulfate at 37 °C with 5% CO2 on collagen I-coated plates. The viability of primary hepatocytes was assessed using a trypan blue exclusion test and was greater than 95%. Mixed free fatty acids (FFAs) with a final concentration of 1 mmol/L were prepared with palmitic acid (PA, 0.66 mmol/L, Sigma Aldrich) and oleic acid (OA, 0.33 mmol/L, Sigma Aldrich)[25]. After overnight culture, primary hepatocytes were treated with FFAs for 24 h to induce a cell model of NAFLD in vitro. In some experiments, primary hepatocytes were pretreated with the PPAR-γ agonist GW1929 (20 μmol/L, Sigma Aldrich) for 3 h, followed by incubation with FFAs for 6 h or 24 h. Cell lysates were collected for RT–PCR and western blot analyses.
RAW264.7 macrophages were purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in DMEM containing 10% fetal bovine serum with 100 U/mL penicillin G and 100 U/mL streptomycin sulfate at 37 °C with 5% CO2. All experimental interventions were conducted on the third passage of cells.
As mentioned above, primary hepatocytes were incubated with FFAs or with GW1929 for 3 h followed by FFAs. Then, the cell culture supernatants were collected, centrifuged, and filtered to remove impurities. RAW264.7 macrophages were incubated with different types of conditioned medium (CM) from primary hepatocytes to establish conditional coculture systems for 6 h or 24 h, which were called CM-NC, CM-FFA, and CM-GW1929+FFA.
The animal protocol was designed to minimize pain or discomfort to the animals. Ppargfl/fl mice and Alb-cre mice on the C57BL6/J background were purchased from GemPharmatech (Nanjing, China) to breed and obtain hepatocyte-specific PPAR-γ knockout (PPAR-γ▲hep) male mice (Supplemen
Free fatty acid-treated hepatocytes were fixed with 4% paraformaldehyde for 1 h and then stained with 5 mg/mL Oil Red O (Sigma Aldrich) for 60 min to examine lipid accumulation.
The cell culture supernatant of primary hepatocytes was collected for further analysis. Triglyceride (TG) and total cholesterol (T-CHO) were measured using a triglyceride assay kit and a total cholesterol assay kit, respectively (Nanjing Jiancheng Bioengineering Institute, China). The IL-1β concentration was measured using an IL-1β ELISA kit (Lianke Biotechnology Company, China). Plasma from mice was centrifuged, separated and stored at -80 °C for further analysis. Plasma levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) were measured using MDA, SOD and GSH assay kits, respectively (Nanjing Jiancheng Bioengineering Institute, China). The plasma level of reactive oxygen species (ROS) was measured using an ROS ELISA kit (Nanjing JiaBeiSen Biotechnology, China). ROS generation in the cell culture supernatant was assayed using a DCFH-DA fluorescent probe kit (Beyotime Biotechnology, China). Total protein was extracted from mouse liver tissues. Caspase-1 activity in liver tissues was assessed with a Caspase-1 activity assay kit (BioVision, Milpitas, CA, United States). All procedures were performed according to the manufacturers’ instructions.
Total RNA was extracted from mouse liver tissues, RAW264.7 macrophages and primary hepatocytes using TRIzol reagent (TaKaRa, Kusatsu, Japan). Complementary DNA was generated from 1 µg of RNA using a cDNA synthesis kit (Nanjing Vazyme Biotech, China). For real-time PCR, 10 ng of template was added to a 10-μL reaction system containing each primer and SYBR Green PCR Master Mix (TaKaRa, Kusatsu, Japan). The PCR thermocycling parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, performed with an ABI Prism 7300 system (Applied Biosystems, Foster City, CA). All reactions were performed in triplicate. The expression levels of target genes were quantified using the double-delta method (2-ΔΔCt). The murine primers (provided by Sangon Biotech Co., Shanghai, China) are shown in Table 1.
| Primer | Forward (5′-3′) | Reverse (5′-3′) |
| Nos2 | GTGTTCCACCAGGAGATGTTG | CTCCTGCCCACTGAGTTCGTC |
| Tnf | TCTTCTCATTCCTGCTTGTGG | GGTCTGGGCCATAGAACTGA |
| Il-6 | GTTCTCTGGGAAATCGTGGA | GGAAATTGGGGTAGGAAGGA |
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Mrc2 | TACAGCTCCACGCTATGGATT | CACTCTCCCAGTTGAGGTACT |
| Il-10 | GTTACTTGGGTTGCCAAG | TTGATCATCATGTATGCTTC |
| Acox1 | ACCAGCCCAACTGTGACTTC | ACAAAGGCATGTAACCCGTA |
| Cpt1a | CTTCCCATTTGACACCTTTG | ATACGTGAGGCAGAACTTGC |
| Srebp1c | ACAGCAACCAGAAGCTCAAG | TGCCCTCCATAGACACATCT |
| Fasn | TTGGGTGCTGACTACAACCT | TGGATGATGTTGATGATGGA |
| Keap1 | AGAGCGGGATGAGTGGCA | GCTGAATTAAGGCGGTTTGTC |
| Nrf2 | CTTTAGTCAGCGACAGAAGGAC | AGGCATCTTGTTTGGGAATGTG |
| Ho-1 | AGACCGCCTTCCTGCTCAACAT | TCTGACGAAGTGACGCCATCTGT |
| Nlrp3 | GAGTTCTTCGCTGCTATGT | ACCTTCACGTCTCGGTTC |
| Caspase-1 | TGGAGAGAAACAAGGAG | TTGAAGAGCAGAAAGCAAT |
| Il-1β | TCTTTGAAGTTGACGGACCC | TGAGTGATACTGCCTGCCTG |
| Ppar-γ | GCCCTTTACCACAGTTGATTTCT | GTGATTTGTCCGTTGTCTTTCCT |
| β-actin | TGTTACCAACTGGGACGACA | CTGGGTCATCTTTTCACGGT |
Total proteins extracted from mouse liver tissues, RAW264.7 macrophages and primary hepatocytes were assessed using a Pierce BCA protein assay kit (Thermo Fisher Scientific). The proteins were separated by SDS–PAGE (Epizyme Biotech), transferred to polyvinylidenedifluoride membranes (Bio-Rad, Hercules, CA) and incubated with primary antibody in TBST containing 5% (wt/vol) BSA at 4 °C overnight. The blots were then incubated with HRP-conjugated secondary antibody (1:10000, KangChen Biotech, Shanghai, China) at room temperature for 1 h. Immunoreactive bands were detected with an ECL chemiluminescence kit (Thermo Scientific Pierce, Waltham, MA). The density of the bands on the immunoblots was measured using ImageJ software (National Institutes of Health) and was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (1:10000, KangChen Biotech, Shanghai, China) or β-actin (1:5000, Cell Signaling Technology). In this study, the total expression levels of TLR4, IκBα, p-IκBα, NF-κB and p-NF-κB (1:1000, all from Cell Signaling Technology) in macrophages were measured and normalized to the β-actin (1:5000, Cell Signaling Technology) expression level. The total expression levels of NLRP3, IL-1β, Caspase-1, Nrf2, Keap1 and HO-1 (1:1000, all from Cell Signaling Technology) in hepatocytes were measured and normalized to the GAPDH (1:10000, KangChen Biotech, Shanghai, China) expression level.
All statistical analyses were carried out with GraphPad Prism v7.03 software (GraphPad, La Jolla, CA, United States). All the data are expressed as the mean ± SE of the mean. Statistical differences among multiple groups were determined by one- or two-way analysis of variance. Differences between two groups were analyzed using Student’s t test. A P value < 0.05 was considered statistically significant.
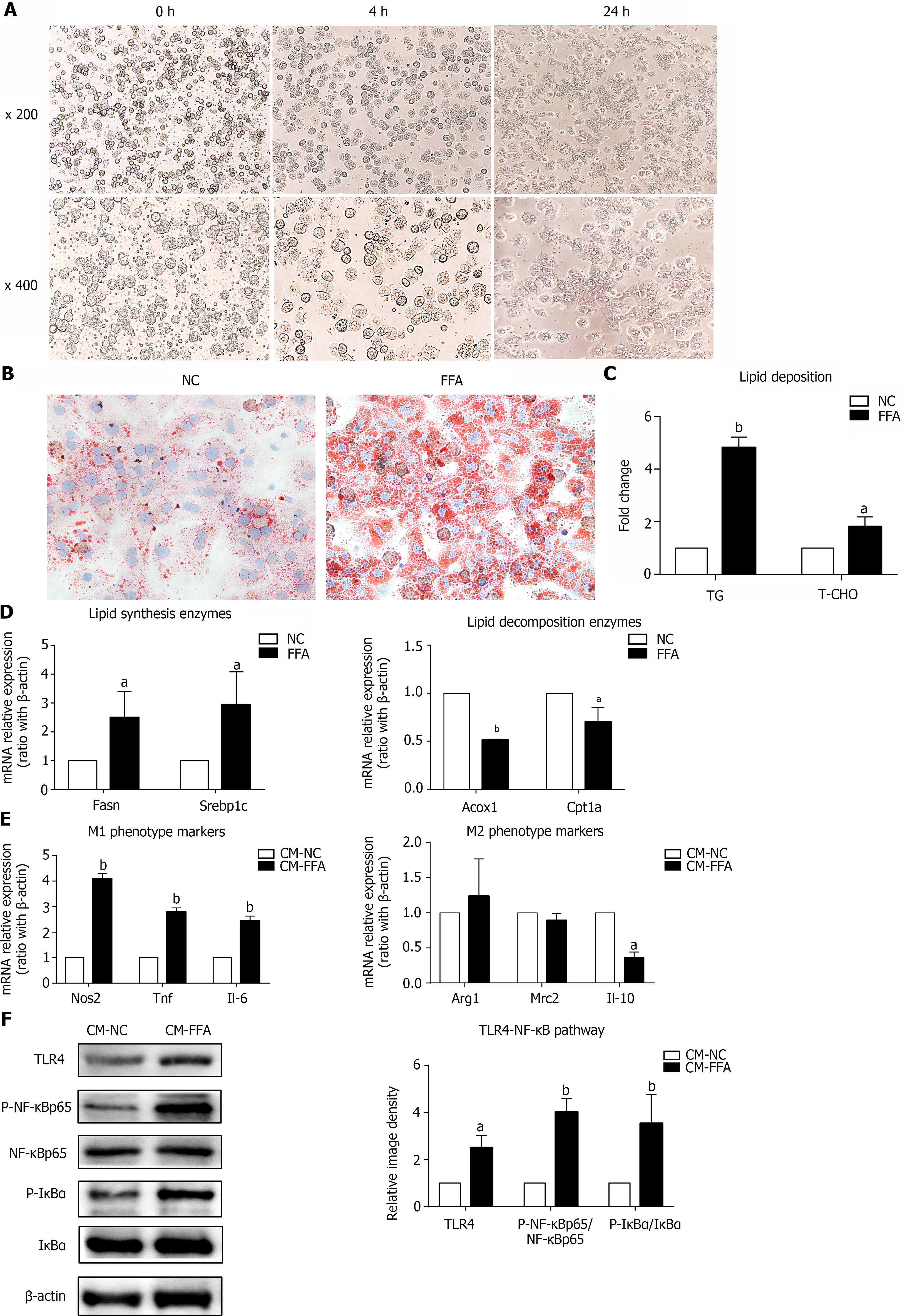
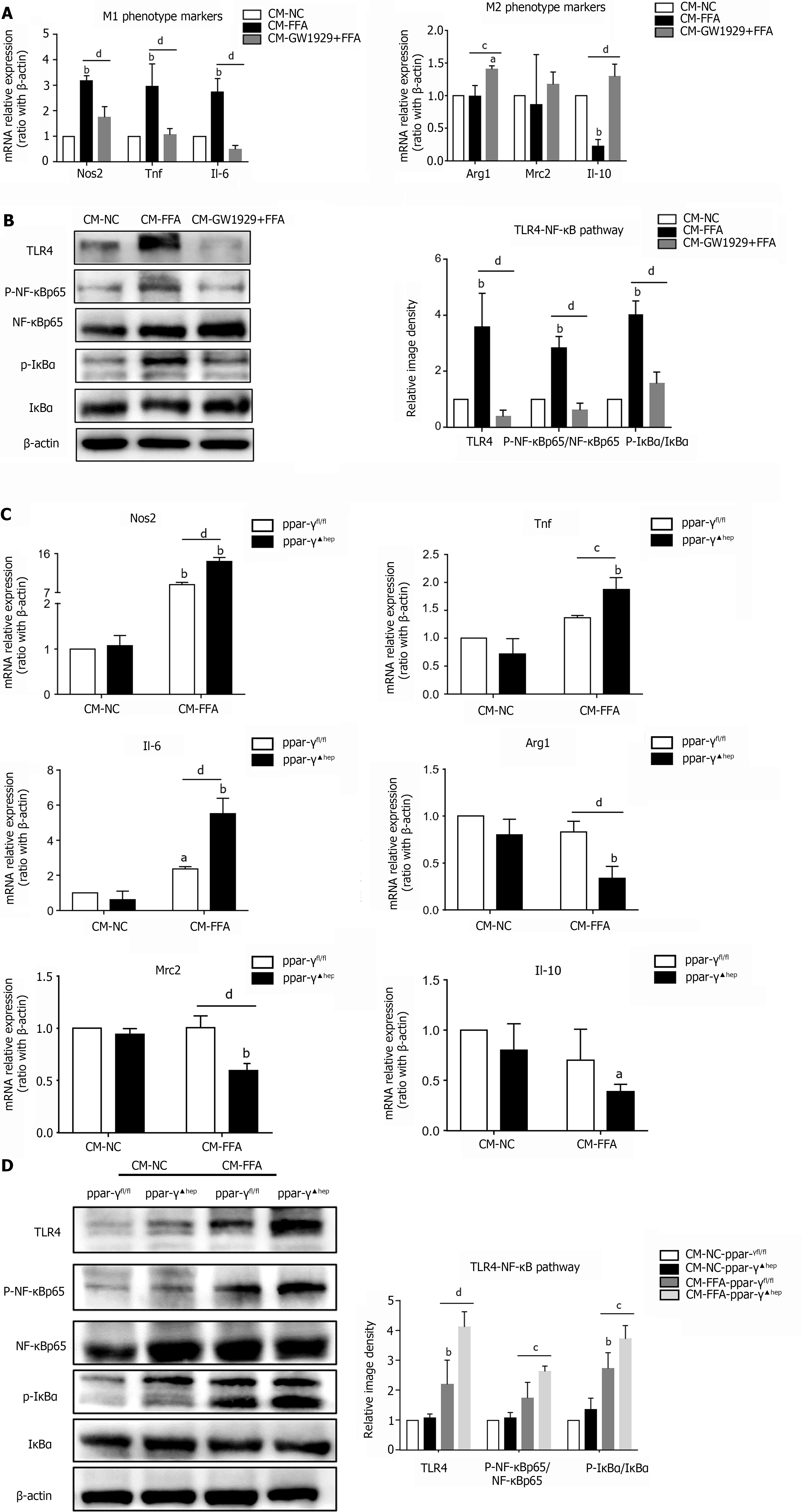
Because of their high plasticity and heterogeneity, macrophages can be skewed into the M1 phenotype or M2 phenotype under different microenvironments[26]. However, whether lipid-laden hepatocytes can affect macrophage polarization is uncertain. Here, we isolated primary hepatocytes and incubated them with FFAs. After 24 h of culture, most primary hepatocytes adhered to the plate and exhibited centered dual nuclei and a polyhedral shape under the microscope, indicating that the primary hepatocytes were in good condition (Figure 1A). Moreover, the hepatocytes displayed excess lipid accumulation after incubation with FFAs, as shown by Oil Red O staining (Figure 1B). At the same time, the TG and T-CHO contents generated in hepatocytes were significantly increased (Figure 1C). The mRNA expression levels of the lipid synthesis genes Fasn and Srebp1c were upregulated, and the mRNA expression levels of the lipid decomposition genes Acox1 and Cpt1a were downregulated (Figure 1D). These results suggested that the NAFLD hepatocyte model was successfully established. Next, a supernatant transfer experiment between lipid-laden hepatocytes and macrophages was established. We found that CM from FFA-treated hepatocytes induced M1-polarized macrophages with significant upregulation of all M1 markers, including Nos2, Tnf and Il-6, and partial downregulation of M2 markers, such as Il-10 (Figure 1E). In addition, the NF-κB signaling pathway in macrophages was activated by CM-FFA, as demonstrated by significant increases in the protein expression levels of TLR4, p-NF-κB and p-IκBα (Figure 1F). These results demonstrate that lipid-laden hepatocytes exert direct roles in M1 macrophage polarization and inflammation.
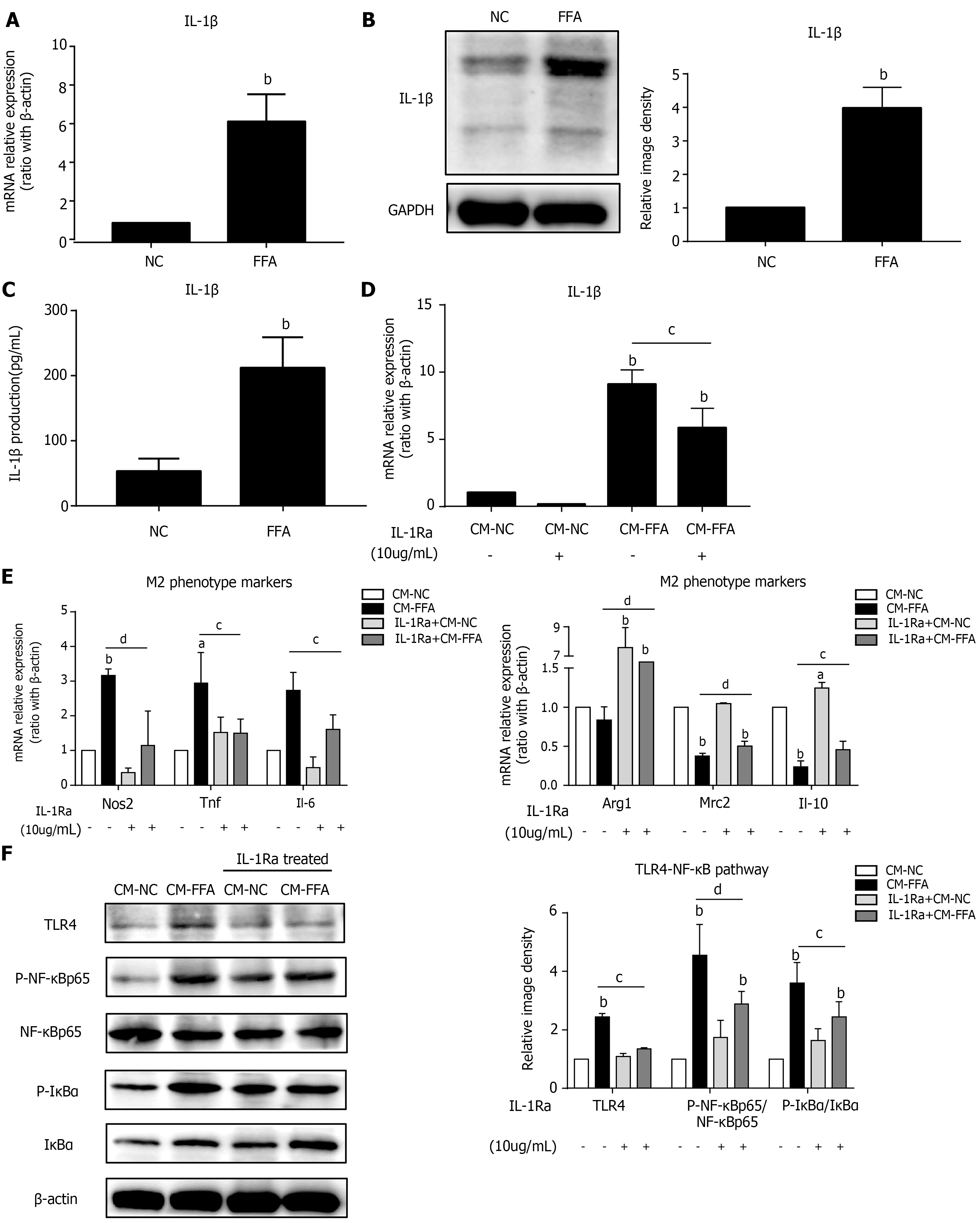
Lipid-laden hepatocytes promoted M1 macrophage polarization and inflammation; however, the possible pathways of signal exchange between the primary hepatocytes and macrophages were unclear. We found that incubation with FFAs obviously increased the mRNA and protein expression levels of the inflammatory factor IL-1β in hepatocytes (Figure 2A and B). Similarly, lipid-laden hepatocytes secreted a high level of IL-1β into the cell culture supernatant (Figure 2C). To further investigate whether IL-1β participates in the signaling between hepatocytes and macrophages, we pretreated macrophages with an interleukin-1 receptor antagonist (IL-1Ra) to block IL-1β receptors. Then, a supernatant transfer experiment between lipid-laden hepatocytes and macrophages was conducted. The results showed that IL-1β expression in macrophages was significantly decreased with IL-1Ra pretreatment (Figure 2D). As expected, we found that inhibition of IL-1β signaling with IL-1Ra significantly prevented macrophage M1 polarization induced by CM-FFAs, as shown by the downregulation of M1-type markers and the upregulation of M2-type markers (Figure 2E). Simultaneously, IL-1Ra suppressed the protein expression levels of TLR4, p-NF-κB and p-IκBα in macrophages induced by CM-FFA (Figure 2F). These results indicate that NAFLD hepatocytes induce M1 macrophage polarization and inflammatory signal activation via IL-1β signaling.
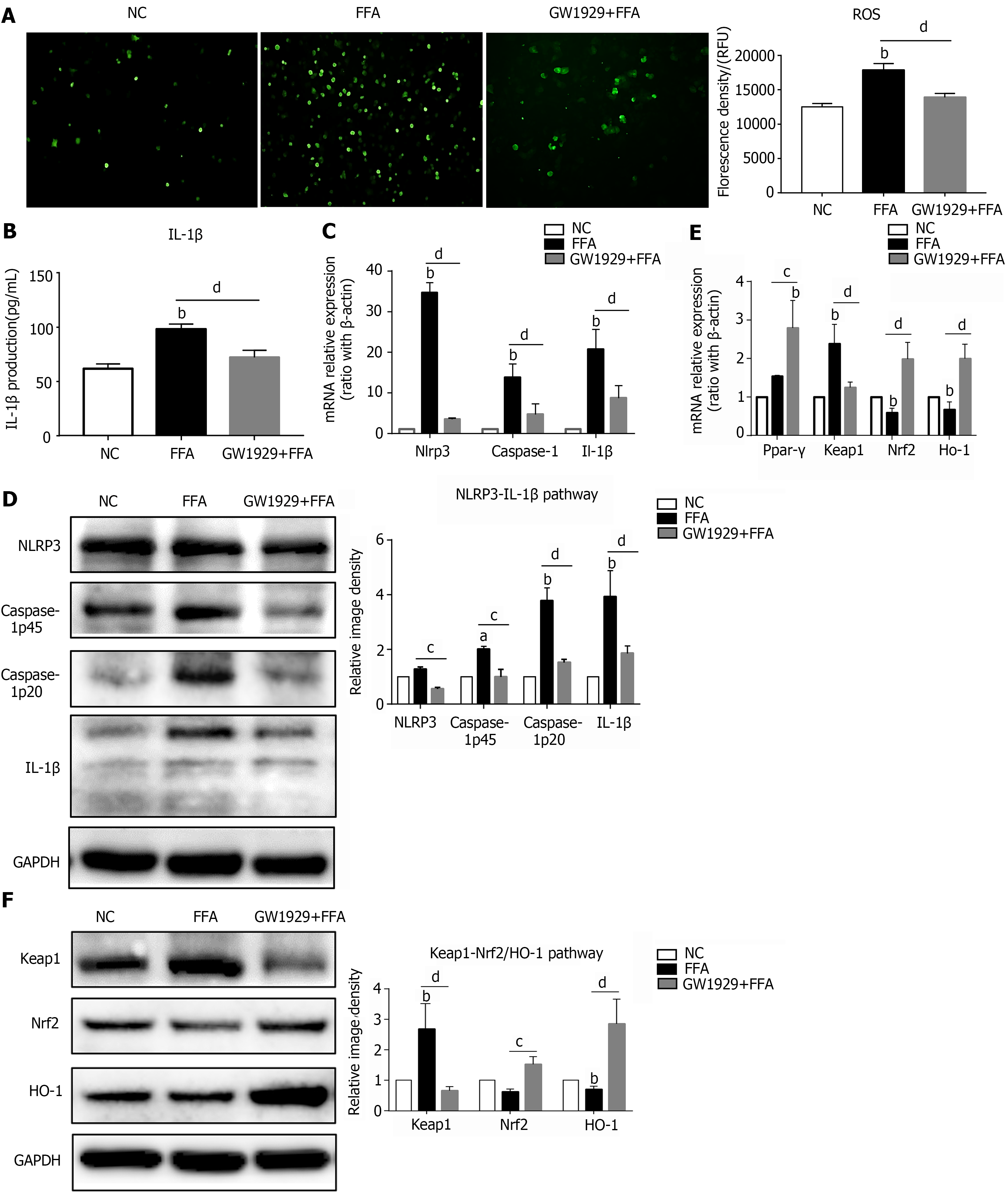
PPAR-γ is a nuclear receptor that is firmly involved in lipid metabolism and the inflammatory-immune response[27]. To further explore the role and properties of PPAR-γ in lipid-laden primary hepatocytes, the PPAR-γ agonist GW1929 was added to the hepatocyte culture system for 3 h before incubation with FFAs. The results showed that GW1929 administration significantly decreased the ROS content and IL-1β secretion level in hepatocytes treated with FFAs (Figure 3A and B). In addition, GW1929 significantly downregulated both the mRNA and protein expression levels of NLRP3 inflammasome-related genes, including Nlrp3, Caspase-1 and IL-1β, in lipid-laden hepatocytes (Figure 3C and D). Furthermore, GW1929 markedly reduced the mRNA and protein expression levels of the oxidative injury marker Keap1 but enhanced the mRNA and protein expression levels of the antioxidant-related genes Nrf2 and Ho-1 (Figure 3E and F). These results indicate that upregulation of PPAR-γ activity in NAFLD hepatocytes can ameliorate oxidative stress and NLRP3-IL-1β pathway activation.
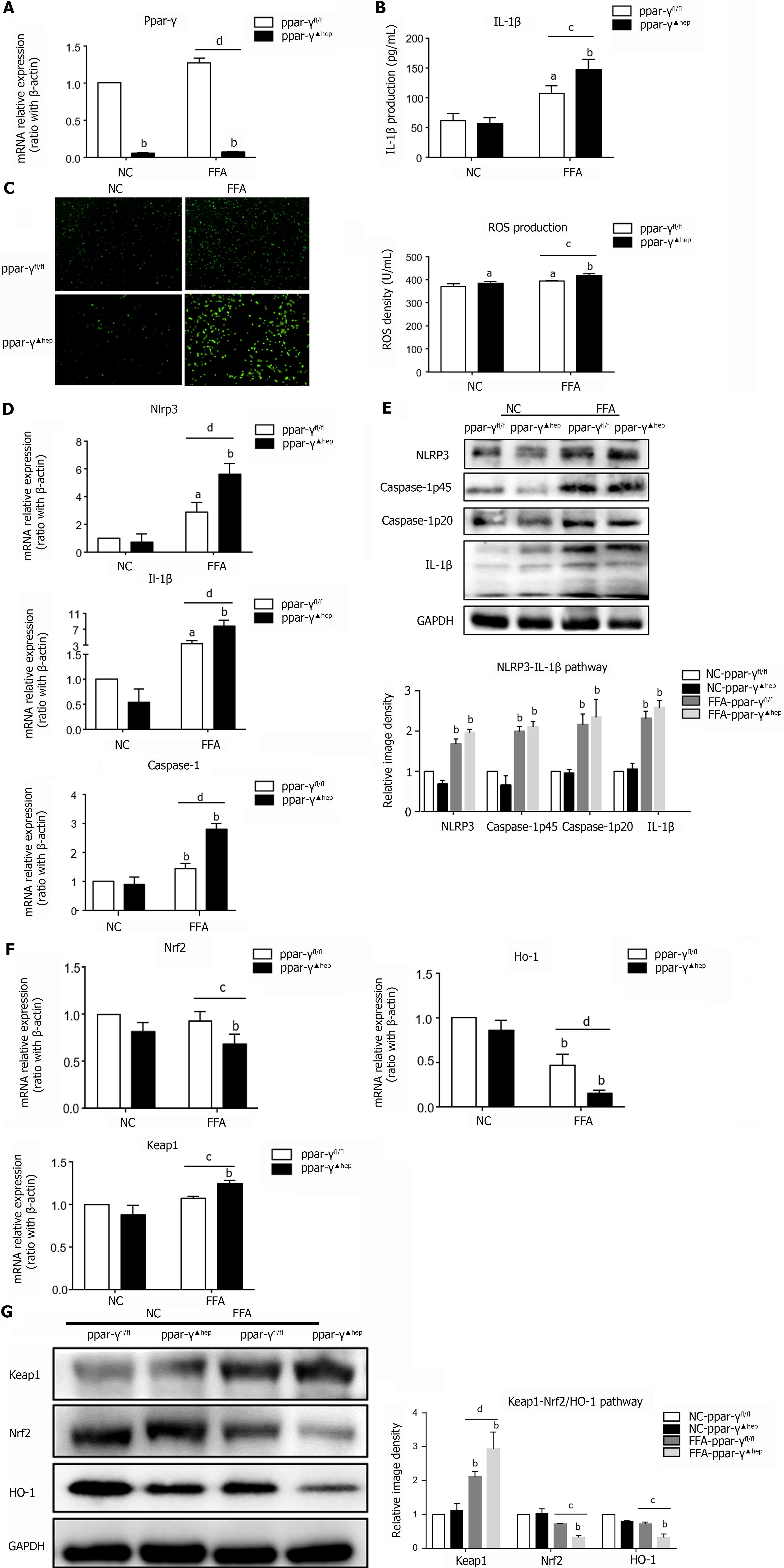
To further confirm the anti-inflammatory and antioxidant effects of PPAR-γ on lipid-laden hepatocytes, we isolated primary hepatocytes from hepatocyte-specific PPAR-γ knockout mice and treated them with FFAs in vitro. The mRNA expression level of Ppar-γ in primary hepatocytes from hepatocyte-specific PPAR-γ knockout mice was fully knocked out (Figure 4A). As expected, the loss of PPAR-γ in hepatocytes enhanced IL-1β secretion and ROS generation after incubation with FFAs (Figure 4B and C). In addition, PPAR-γ deficiency in lipid-laden hepatocytes increased the mRNA and protein expression levels of the Nlrp3, Caspase-1 and IL-1β genes (Figure 4D and E). Furthermore, PPAR-γ deficiency markedly increased the mRNA and protein expression levels of Keap1 but decreased the mRNA and protein expression levels of Nrf2 and Ho-1 (Figure 4F and G). These results further confirm that PPAR-γ exerts a protective effect against lipid peroxidation and inflammation in lipid-laden hepatocytes.
Next, we further explored whether regulation of PPAR-γ activity in lipid-laden hepatocytes would subsequently affect macrophage polarization shifts and inflammation. Macrophages were incubated with CM derived from hepatocytes that were pretreated with GW1929 or FFAs alone or with CM from PPAR-γ-deficient hepatocytes treated with FFAs. Interestingly, the increase in M1 marker expression was significantly downregulated and the expression levels of the M2 markers Arg1 and Il-10 were markedly upregulated in macrophages incubated with CM from lipid-laden hepatocytes that were pretreated with GW1929 (CM-GW1929+FFA) compared with the CM-FFA group (Figure 5A). In addition, the protein expression levels of TLR4, p-NF-κB and p-IκBα were significantly decreased in macrophages from the CM-GW1929+FFA group (Figure 5B). In contrast, depletion of PPAR-γ in lipid-laden hepatocytes significantly upregulated the mRNA expression levels of all M1 markers but decreased the mRNA expression levels of Arg1 and Mrc2 in macrophages (Figure 5C). Furthermore, depletion of PPAR-γ in lipid-laden hepatocytes further promoted activation of the TLR4/NF-κB signaling pathway in macrophages (Figure 5D). These results illustrate that regulation of PPAR-γ activity in NAFLD hepatocytes can modulate macrophage polarization and inflammation.
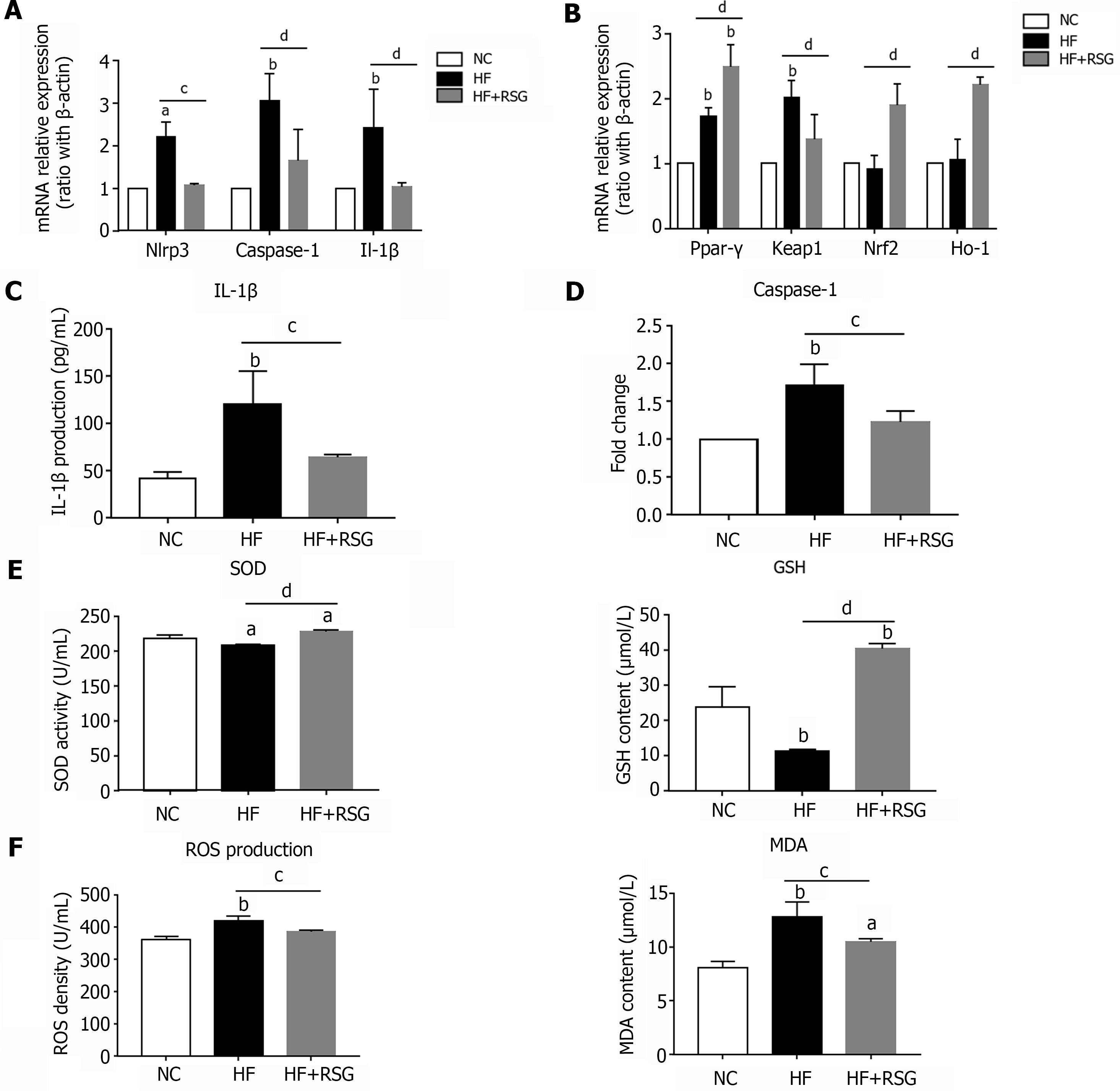
Our in vitro experiments demonstrated that PPAR-γ exerts anti-inflammatory and antioxidant effects in lipid-laden hepatocytes, which can further affect macrophage polarization and inflammation. Next, we further explored whether these effects also occur in vivo. Our previous studies showed that rosiglitazone administration improves hepatic steatosis and Kupffer cell activation in high-fat diet-induced NAFLD mice. Here, we found that rosiglitazone significantly decreased the mRNA expression levels of the Nlrp3, Caspase-1 and Il-1β genes in bulk cells from the livers of high-fat diet-induced NAFLD mice (Figure 6A). Furthermore, rosiglitazone markedly downregulated the mRNA expression level of Keap1 but increased the mRNA expression levels of Ppar-γ, Nrf2 and Ho-1 (Figure 6B). The IL-1β level in plasma and the Caspase-1 activity in the liver both declined after rosiglitazone administration (Figure 6C and D), while the SOD activity and GSH content were significantly enhanced in rosiglitazone-treated mouse plasma (Figure 6E). In contrast, the levels of oxidative injury metabolites, such as ROS and MDA, in plasma were decreased after rosiglitazone intervention (Figure 6F). Therefore, we concluded that rosiglitazone not only alleviated hepatic steatosis and Kupffer cell activation but also improved NLRP3 inflammasome activation and oxidative stress in HF diet-fed NAFLD mice.
Lipid metabolism disorder is a critical initiator of the inflammatory response and immune activation in NAFLD[10]. How lipids lead to innate immune activation is poorly understood. Here, we demonstrated that lipid-laden hepatocytes can transmit inflammatory signals to macrophages through the release of IL-1β, directly inducing M1 macrophage polarization and inflammatory activation. Regulation of PPAR-γ activity in lipid-laden hepatocytes further affected macrophage polarization and inflammation. In addition, an in vivo study showed that a PPAR-γ agonist improved NLRP3 inflammasome activation and oxidative stress in high-fat diet-induced NAFLD mice, expanding our understanding of the underlying mechanisms of PPAR-γ in NAFLD.
Macrophage activation is considered to be a prominent hallmark of NASH[28]. The polarization of macrophages is firmly related to the inflammatory state. Previous studies have suggested that macrophages are more likely to transform from the M1 to the M2 phenotype if activation of the NF-κB signaling pathway is inhibited[15]. TLR4 deficiency directly alters the polarization of adipose tissue macrophages toward alternative activation[29]. Upon activation, macrophages not only release inflammatory cytokines and chemokines but also regulate the status or function of surrounding cells[13,30]. Recent studies have demonstrated that selective depletion of Kupffer cells reduced liver steatosis and monocyte infiltration in NASH[31-33]. Our previous study confirmed that macrophages/Kupffer cells polarized by fatty acids can regulate lipid metabolism in hepatocytes[30]. This result suggests a potential interaction between macrophages and hepatocytes in NAFLD. In the current study, our results revealed that lipid-laden hepatocytes directly induced macrophage M1 polarization and TLR4/NF-κB pathway activation. Through an in-depth study, we found that IL-1β secreted by lipid-laden hepat
PPAR-γ is a ligand-activated nuclear receptor with potent anti-inflammatory properties and is involved in the regulation of immune and inflammatory responses[34]. A recent study demonstrated that modulation of PPAR-γ activity attenuated HFD-induced NAFLD by regulating lipid metabolism and oxidative stress in hepatocytes via Nrf2 activation[35]. Due to the low expression of PPAR-γ in hepatocytes, the role of PPAR-γ in hepatocytes is not fully understood[36]. In the current study, we found that ROS generation and IL-1β secretion in lipid-laden hepatocytes were significantly reduced when cells were pretreated with the PPAR-γ agonist GW1929. Recently, a study reported that PPAR-γ exerted an anti-inflammatory effect by suppressing NLRP3 inflammasome activation in macrophages[37]. Here, we revealed that a PPAR-γ agonist exerted an anti-inflammatory effect by inhibiting NLRP3 inflammasome activation in lipid-laden hepatocytes. In contrast, PPAR-γ deficiency in hepatocytes enhanced ROS generation, NLRP3 inflammasome activation, and IL-1β secretion after FFA treatment in vitro.
Interestingly, we further found that upregulation of PPAR-γ activity in lipid-laden hepatocytes subsequently reversed macrophage M1 polarization and reduced activation of the TLR4/NF-κB pathway. Conversely, PPAR-γ depletion in lipid-laden hepatocytes exacerbated macrophage M1 polarization and TLR4/NF-κB pathway activation. This result suggests that the regulation of PPAR-γ activity in hepatocytes plays an important role in the interaction between lipid-laden hepatocytes and macrophages. Our previous study revealed that rosiglitazone, a PPAR-γ agonist, significantly alleviated hepatic lipid deposition and Kupffer cell activation in HFD-induced NAFLD mice[17]. In the present study, our results clearly demonstrated that rosiglitazone mitigated NLRP3 inflammasome activation and oxidative stress in HFD-induced NAFLD mice.
A recent study showed that hepatocyte-specific PPAR-γ disruption reduced hepatic steatosis but increased hepatic neutrophil infiltration after HFD feeding plus binge ethanol[38]. PPAR-γ deletion in hepatocytes highly augmented PA- or TNF-α-induced production of Cxcl1. This result indicates that PPAR-γ activation in hepatocytes exerted an anti-inflammatory effect, which was consistent with our findings. Another study found that hepatocyte-specific loss of PPAR-γ reduced the progression of high fat, cholesterol, and fructose (HFCF)-induced NASH in mice[39]. These findings seem to suggest that hepatocyte PPAR-γ contribute to the development of inflammation and fibrosis in NASH. However, the hepatocyte-specific loss of PPAR-γ did not reduce hepatic steatosis in HFCF- or MCD-induced NASH[39]. These contradictory experimental results may be due to the use of different animal models in the studies. In the current study, lipid-laden hepatocytes were established by incubation excess FFAs in vitro. The classical lipogenic property of PPAR-γ is significantly involved in this condition[40]. In addition, we further found that PPAR-γ activation exerted obvious antioxidant and anti-inflammatory effects in lipid-laden hepatocytes. We assumed that FFA overload directly led to lipotoxicity in hepatocytes, which contributed to lipid peroxidation. Thus, PPAR-γ was upregulated and exerted antioxidant effects against ROS to mitigate cell injury and downstream inflammatory events. Therefore, the role of PPAR-γ in hepatocytes is firmly related to different models, diet patterns and cellular stimulations. PPAR-γ is a complex nuclear receptor with specific dominant properties in various disease courses.
In conclusion, our study revealed that lipid-laden hepatocytes significantly skewed macrophage polarization to the M1 phenotype. Upregulation of PPAR-γ activity in lipid-laden hepatocytes improved macrophage M1 polarization and inflammation by attenuating hepatocyte oxidative stress and NLRP3 inflammasome activation. Strategies that target the regulation of PPAR-γ activity to modulate cell crosstalk will be beneficial for treating NAFLD.
Lipid metabolism disorder and inflammatory-immune activation are two vital triggers in nonalcoholic fatty liver disease (NAFLD). Little is known about the regulation of PPAR-γ activity in modulating cell crosstalk in NAFLD.
The role of PPAR-γ in hepatocytes and in the interaction between hepatocytes and macrophages in NAFLD remain unknown.
To investigate whether the regulation of PPAR-γ activity in lipid-laden hepatocytes affects macrophage polarization and inflammation and explore the potential mechanisms.
Primary hepatocytes were isolated from wild-type C57BL6/J mice or hepatocyte-specific PPAR-γ knockout mice and incubated with free fatty acids (FFAs). Macrophages were incubated with conditioned medium from lipid-laden hepatocytes with or without the PPAR-γ agonist. Wild-type C57BL/6J mice were fed a high-fat diet and administered rosiglitazone.
Primary hepatocytes exhibited significant lipid deposition and increased ROS production after incubation with FFAs. Conditioned medium from lipid-laden hepatocytes promoted macrophage polarization to the M1 type and activation of the TLR4/NF-κB pathway. A PPAR-γ agonist ameliorated oxidative stress and NLRP3 inflammasome activation in lipid-laden hepatocytes and subsequently prevented M1 macrophage polarization. Hepatocyte-specific PPAR-γ deficiency aggravated oxidative stress and NLRP3 inflammasome activation in lipid-laden hepatocytes, which further promoted M1 macrophage polarization. Rosiglitazone administration improved oxidative stress and NLRP3 inflammasome activation in HF diet-induced NAFLD mice in vivo.
Upregulation of PPAR-γ activity alleviated NAFLD through modulation of the crosstalk between hepatocytes and macrophages via the ROS-NLRP3-IL-1β signaling pathway.
To elaborate the underlying pathogenesis of NAFLD from the perspective of inflammation and immune activation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Boscá L, Spain; Kulkeaw K, Thailand S-Editor: Wang LL L-Editor: A P-Editor: Cai YX
| 1. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2917] [Article Influence: 416.7] [Reference Citation Analysis (1)] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3792] [Article Influence: 541.7] [Reference Citation Analysis (2)] |
| 3. | Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 4. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 925] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 5. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2117] [Article Influence: 235.2] [Reference Citation Analysis (1)] |
| 6. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [PubMed] |
| 7. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1457] [Article Influence: 242.8] [Reference Citation Analysis (1)] |
| 8. | Abdelmalek MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. 2021;18:85-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 9. | Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69:927-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 657] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 10. | Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 608] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 11. | Sui YH, Luo WJ, Xu QY, Hua J. Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation. World J Gastroenterol. 2016;22:2533-2544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 13. | Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 733] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 14. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2077] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 15. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3119] [Article Influence: 207.9] [Reference Citation Analysis (0)] |
| 16. | Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol. 2017;14:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 18. | Obara N, Fukushima K, Ueno Y, Wakui Y, Kimura O, Tamai K, Kakazu E, Inoue J, Kondo Y, Ogawa N, Sato K, Tsuduki T, Ishida K, Shimosegawa T. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol. 2010;53:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Li R, Zhou R, Wang H, Li W, Pan M, Yao X, Zhan W, Yang S, Xu L, Ding Y, Zhao L. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26:2447-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 20. | Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1694] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 21. | Nakajima A, Wada K, Miki H, Kubota N, Nakajima N, Terauchi Y, Ohnishi S, Saubermann LJ, Kadowaki T, Blumberg RS, Nagai R, Matsuhashi N. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120:460-469. [PubMed] |
| 22. | Hussein OE, Hozayen WG, Bin-Jumah MN, Germoush MO, Abd El-Twab SM, Mahmoud AM. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ Sci Pollut Res Int. 2020;27:20725-20735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Ni XX, Li XY, Wang Q, Hua J. Regulation of peroxisome proliferator-activated receptor-gamma activity affects the hepatic stellate cell activation and the progression of NASH via TGF-β1/Smad signaling pathway. J Physiol Biochem. 2021;77:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Severgnini M, Sherman J, Sehgal A, Jayaprakash NK, Aubin J, Wang G, Zhang L, Peng CG, Yucius K, Butler J, Fitzgerald K. A rapid two-step method for isolation of functional primary mouse hepatocytes: cell characterization and asialoglycoprotein receptor based assay development. Cytotechnology. 2012;64:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O'Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106-116. [PubMed] |
| 26. | Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 27. | Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 570] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 28. | Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 372] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 29. | Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61:2718-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Wu HM, Ni XX, Xu QY, Wang Q, Li XY, Hua J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway. J Gastroenterol Hepatol. 2020;35:1998-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161-40172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 32. | Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 33. | Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 420] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 34. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79-82. [PubMed] |
| 35. | Feng X, Yu W, Li X, Zhou F, Zhang W, Shen Q, Li J, Zhang C, Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol. 2017;136:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Morán-Salvador E, Titos E, Rius B, González-Périz A, García-Alonso V, López-Vicario C, Miquel R, Barak Y, Arroyo V, Clària J. Cell-specific PPARγ deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol. 2013;59:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Yang CC, Wu CH, Lin TC, Cheng YN, Chang CS, Lee KT, Tsai PJ, Tsai YS. Inhibitory effect of PPARγ on NLRP3 inflammasome activation. Theranostics. 2021;11:2424-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 38. | Wang W, Xu MJ, Cai Y, Zhou Z, Cao H, Mukhopadhyay P, Pacher P, Zheng S, Gonzalez FJ, Gao B. Inflammation is independent of steatosis in a murine model of steatohepatitis. Hepatology. 2017;66:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Lee SM, Pusec CM, Norris GH, De Jesus A, Diaz-Ruiz A, Muratalla J, Sarmento-Cabral A, Guzman G, Layden BT, Cordoba-Chacon J. Hepatocyte-Specific Loss of PPARγ Protects Mice From NASH and Increases the Therapeutic Effects of Rosiglitazone in the Liver. Cell Mol Gastroenterol Hepatol. 2021;11:1291-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Grasselli E, Voci A, Canesi L, De Matteis R, Goglia F, Cioffi F, Fugassa E, Gallo G, Vergani L. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J Hepatol. 2011;54:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |