Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1344
Peer-review started: March 31, 2022
First decision: May 12, 2022
Revised: May 30, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: July 27, 2022
Processing time: 118 Days and 5.6 Hours
Refractory ascites (RA) is a frequent and life-threatening complication of cirrhosis. In selected patients with RA, transjugular intrahepatic portosystemic shunt (TIPS) placement and liver transplantation (LT) are currently considered the best therapeutic alternatives to repeated large volume paracentesis. In patients with a contraindication to TIPS or LT, the alfapump® system (Sequana Medical, Ghent, Belgium) has been developed to reduce the need for iterative paracentesis, and consequently to improve the quality of life and nutritional status. We report here recent data on technical progress made since the first implantation, the efficacy and tolerance of the device, the position of the pump in the therapeutic arsenal for refractory ascites, and the grey areas that remain to be clarified regarding the optimal selection of patients who are potential candidates for this treatment.
Core Tip: The alfapump® system (Sequana Medical, Ghent, Belgium) is a subcutaneous implantable device that allows the transfer of ascites from the peritoneal cavity to the bladder. In this review, we describe the practical aspects of the alfapump® device implantation, and discuss its effectiveness and safety as a treatment for refractory ascites in cirrhotic patients, based on the most recently published data.
- Citation: Weil-Verhoeven D, Di Martino V, Stirnimann G, Cervoni JP, Nguyen-Khac E, Thévenot T. Alfapump® implantable device in management of refractory ascites: An update. World J Hepatol 2022; 14(7): 1344-1356
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1344.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1344
Cirrhotic patients may develop a wide range of complications secondary to portal hypertension and/or liver insufficiency. Among them, ascites occurs in nearly 60% of patients with compensated cirrhosis within 10 years, during the course of their disease[1]. Approximately 10% of patients with ascites develop refractory ascites (RA), defined as ascites that cannot be mobilized by appropriate medical therapy (i.e., a low salt diet combined with diuretic therapy), or whose early recurrence cannot satisfactorily be prevented[2]. The prognosis of RA is poor, with a transplant-free survival (TFS) rate of only 50% at 6 mo, notably because of an increased risk of type 2 hepatorenal syndrome (recently renamed HRS-non-acute kidney injury (AKI) by the European Association for the Study of the Liver[3,4]). RA generally leads to severe malnutrition, deteriorated quality of life, and uncomfortable symptoms or complications (in particular anorexia, abdominal hernia, and dyspnea). Liver transplantation (LT) is the ultimate solution for RA and should be considered systematically. In patients who are not eligible for LT because of advanced age and/or comorbidities, or for whom access to LT remains limited [low or intermediate Model for End-stage Liver Disease (MELD) scores], alternative or “bridging” therapies should be proposed. The first-line treatment for RA consists of large volume paracentesis (LVP). This procedure, although easy to perform, is not risk-free (a risk of major complications of around 1%, especially in case of severe liver failure) and LVP does not improve the patient's quality of life because of the repeated hospitalizations[5]. Furthermore, albumin infusions, administered for the prevention of post-paracentesis circulatory dysfunction after each LVP, also contribute to a heavy healthcare burden. Transjugular portosystemic shunt (TIPS) placement reduces portal pressure and improves effective blood volume and renal function within 4 to 6 wk, making this procedure an effective treatment for RA. In the most recent series including patients with recurrent ascites, covered TIPS was associated not only with better control of ascites, but also with a significant improvement in 1-year TFS compared to patients treated with iterative paracentesis (93% vs 52%; P = 0.003) without increasing the incidence of hepatic encephalopathy[6]. However, careful selection of candidates for TIPS placement is necessary to prevent the occurrence of short- and medium-term complications, and TIPS can ultimately be implanted in only 40% of cirrhotic patients with ascites[7]. The Automated Low-Flow Ascites Pump (alfapump®) system is a therapeutic alternative to TIPS and LT for the treatment of RA[2,8]. In this review, we describe the practical aspects of the alfapump® device implantation and discuss its effectiveness and safety as a treatment for RA, according to the current literature.
A search of PubMed and Embase was performed by two independent investigators (D.W.V. and T.T), since inception. The search terms used were “alfapump” AND “ascites”. Additionally, reference lists were manually searched for the relevant literature. The articles identified by the initial search were considered for further analysis if they contained original data relating to alfapump® use in patients with non-malignant ascites related to cirrhosis. The search for the terms “alfapump” AND “ascites” retrieved a total of 72 articles. Of these 72 publications, we excluded papers that were not in English (n = 2), articles not published in full (n = 23), articles that were off-topic (n = 7), as well as letters to the editor (n = 7), editorials (n = 2), errata/corrigenda (n = 2), reviews (n = 11), and guidelines (n = 1). Thus, a final total of 17 original articles reporting data on the use of the alfapump® in patients with refractory ascites related to cirrhosis were included in the review (see flowchart of study selection in Supple
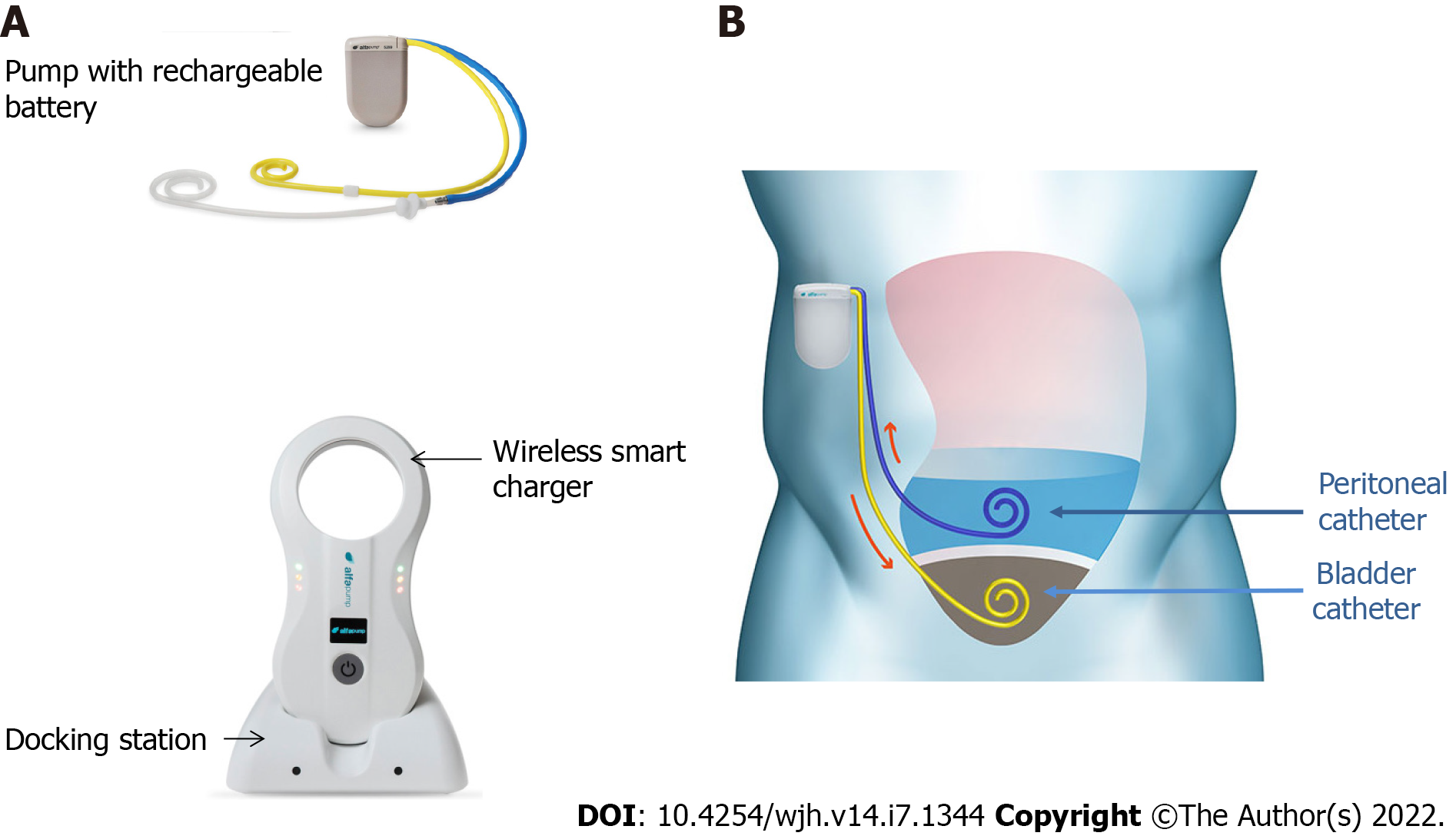
The basic working principle and surgical aspects of the implantation of alfapump® have been described elsewhere[9]. Briefly, the device is manufactured by Sequana Medical (Ghent, Belgium) and obtained the CE mark in July 2011. It comprises a battery-powered pump implanted subcutaneously in the abdominal wall, connected to a first catheter placed in the peritoneal cavity, and to a second catheter that is tunneled under the skin and connected to the bladder, thereby enabling the transfer of ascites to the bladder for elimination via urination (Figures 1 and 2). Sensors are used to adjust the pumping cycles according to the peritoneal and bladder pressures: The cycle is interrupted if the pressure becomes too low in the peritoneal cavity or too high in the bladder.
A consensus statement has recently been published by hepatologists and surgeons experienced in using alfapump®, which provides practical recommendations regarding patient selection, implantation procedure, and post-implantation care[10].
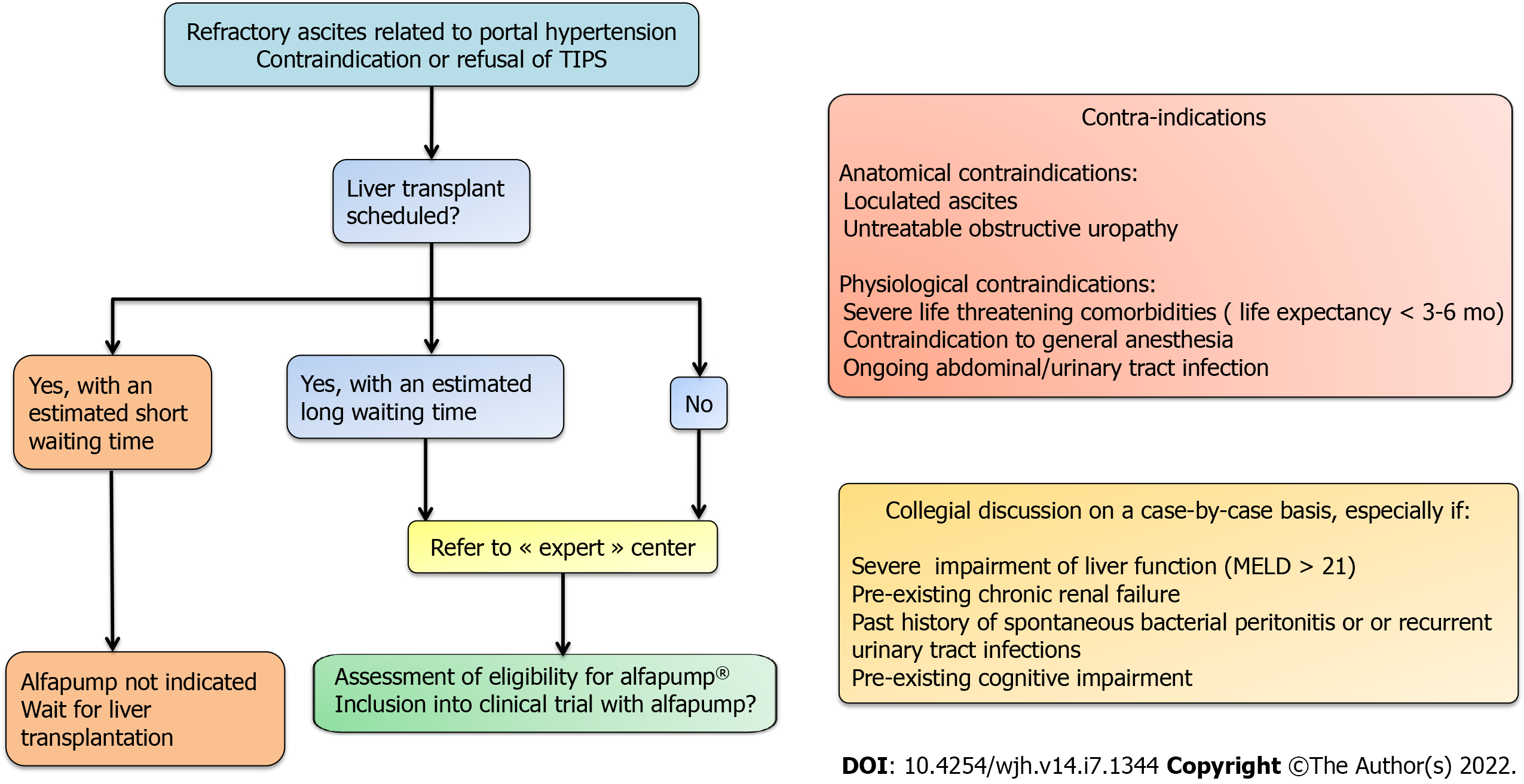
The absolute contraindications for the implantation of the alfapump® device are loculated ascites, untreatable obstructive uropathy, the presence of an active bacterial infection at the time of implantation (spontaneous bacterial peritonitis, urinary infection, or abdominal skin infection in particular), and an expected survival of less than 3 mo. Special caution is advised regarding frail patients, and nutritional status should be considered and optimized before implantation[10]. Once implanted, the patient must charge the pump battery by transcutaneous induction, twice a day for about 20 min, using a user-friendly charging device (Smart Charger) that is placed over the area of the pump. While charging, the charger also collects data from the pump, which are then transmitted anonymously to a central databank of Sequana Medical. The data are transferred to the treating physician by e-mail on a weekly basis and in the event of acute dysfunction. This makes it possible not only to provide an early warning in case of pump dysfunction, but also to adjust the operating time, the frequency of cycles, and the daily volume of ascites to be evacuated, and to check the correct charging of the device[9].
Consistent data are available in the literature and detailed procedures have been published in expert consensus statements[10] and in the article by Dembinsky et al[11]. The manufacturer provides technical instructions regarding the surgical procedure and advice regarding pre- and post-implantation care, that are consistent with expert recommendations. In accordance with these recommendations[10,11], the patient is hospitalized 24-48 h before implantation. Paracentesis is performed to ensure that there is no ongoing spontaneous bacterial peritonitis, and to drain the abdomen. It is mandatory to leave 1-2 liters of ascites prior to implantation in order to check that the pump is functioning adequately before surgical closure and to minimize the risk of ascitic fluid leakage. Intravenous antibiotic prophylaxis is started on the day of the implantation and continued for 48 to 72 h. Prior to the procedure, the daily volume, operating time, and frequency of the pumping cycles are determined and programmed (FlowControl™ software) by the clinician according to the volume and frequency of paracentesis required in the weeks prior to implantation. A target should be set that is 20% higher than the pre-implant rate, because a postoperative increase in ascites production is frequent. Alfapump® works in cycles of very small volumes (5-10 mL) that are pumped every 5-10 min into the bladder, enabling the removal of 500 mL to 4 L of ascites per day. Some inactive periods can be determined for the patient’s comfort (for example to avoid nocturnal urination[9]). A detailed description of the surgical procedure has been published elsewhere[9-11]. Briefly, it consists of the following steps: (1) Skin incision; (2) Bladder catheter insertion; (3) Peritoneal catheter placement; (4) Pump pocket creation and catheter tunneling; (5) Catheter attachment to the pump; and (6) Closure of the surgical incisions[11].
As with any new surgical technology, there is an unavoidable learning curve before achieving an acceptable level of success. In Europe, implantation is usually performed surgically under general anesthesia and takes an average of 60 min[9]. In the United States and Canada, a less invasive method for implantation has been developed, using an interventional radiology technique. In the recently published North American multicenter MOSAIC study, most procedures (29 out of 30) were performed by interventional radiology, and 11 patients were implanted under conscious sedation or local anesthetic[12,13]. Briefly, the peritoneal catheter was inserted under ultrasound guidance into the right lower quadrant, and excess ascites was removed to prevent leakage and catheter migration. The bladder catheter was inserted above the pubis symphysis and correct placement was confirmed by aspiration of urine or dyed saline or contrast-enhanced fluoroscopy. A subcutaneous pocket was then created by an incision 5 cm in length at the midclavicular line, 5-6 cm below the costal border, mostly on the right quadrant (76% of patients). Both catheters were then tunneled to the pump pocket, connected to the pump, and fixed in place with sutures; the alfapump® was finally housed in the pocket before multilayer closure[13]. In this study, technical success was obtained in all patients. The median duration of hospitalization was 4 d (range: 2-69 d). After a 3-mo follow-up period, three serious adverse events were classified as “procedure-related” (one bleeding at the site of bladder catheter insertion, one fluid leakage at the implant site of the pump, and one bacterial peritonitis 26 d after implantation). At 3 mo, two pumps had been explanted for infectious complications (cellulitis and pump pocket infection). Four re-interventions were performed, mostly because of peritoneal catheter dysfunction (three cases). This minimally invasive approach remains infrequent in European centers but a series of three cases reported by a team from Birmingham provided encouraging results[14]. Whatever the method used for implantation, a Sequana Medical implant specialist must be present during the procedure, to check that the pump is working properly, and in the event of a dysfunction, to have a back-up alfapump® available. During the hospitalization, which lasts approximately 4 to 7 d in the absence of complications, the patient must receive appropriate therapeutic education and training in the use of the pump. In particular, the patient must be able to alert the physician immediately if symptoms occur, such as suture loosening, an inflammatory aspect at the surgical site, abdominal pain, reconstitution of abundant ascites, fever, or urinary symptoms. Notably, the presence of the alfapump® contraindicates the subsequent use of magnetic resonance imaging (risk of displacement of the pump and catheters, and damage to the system). Explantation of the pump may be necessary in some cases (death, LT, local complication, or pump dysfunction); this decision must be made on a case-by-case basis and in a multidisciplinary manner. The median life span of the device is around 2 years.
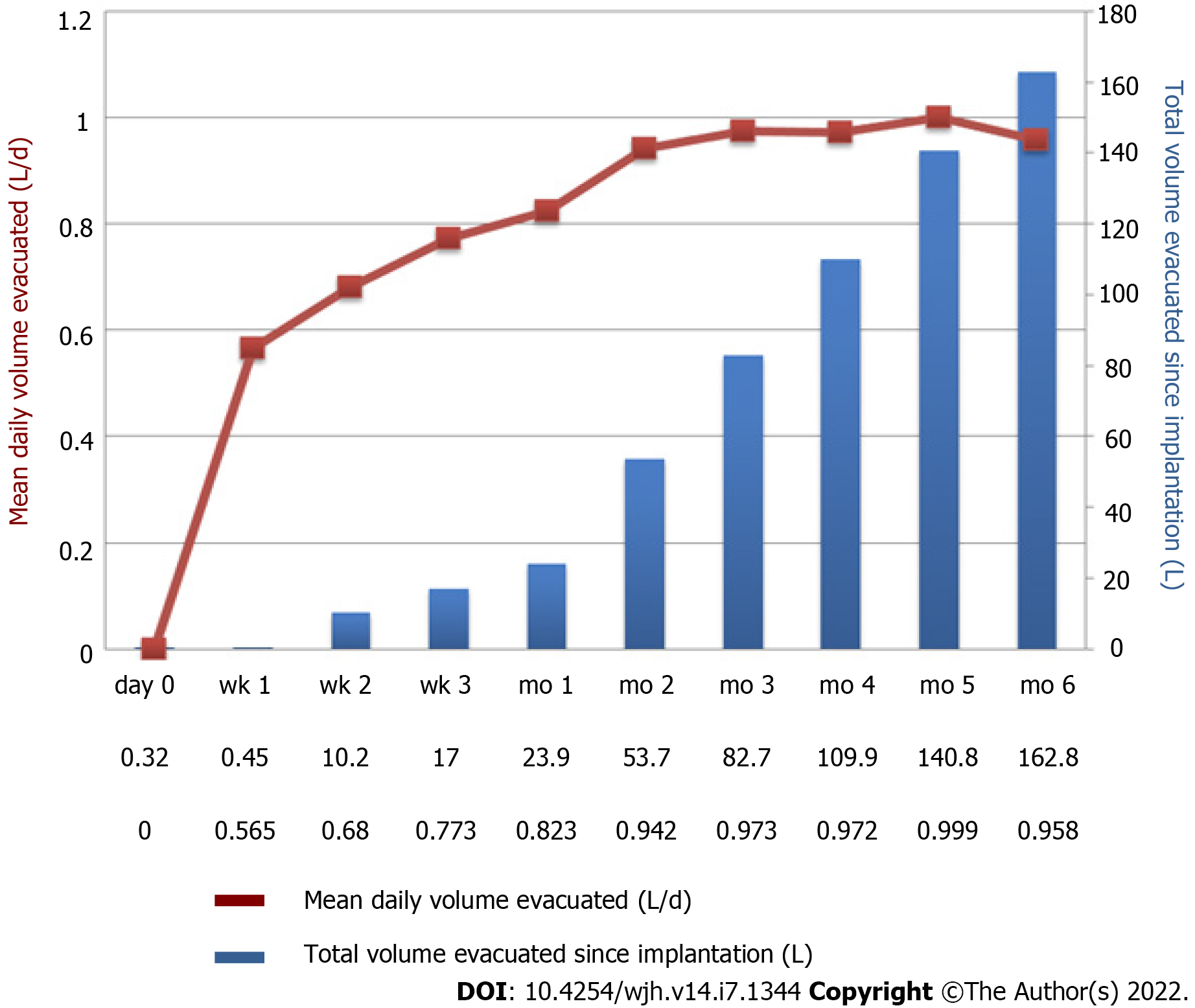
Most studies evaluating the efficacy of the alfapump® device included relatively small numbers of selected patients, generally not very old, with preserved liver function (Table 1). The international landmark PIONEER study performed in 40 patients showed a significant decrease in the number of monthly paracenteses in the “alfapump®” group compared to the “conventional treatment” group (0.2 vs 3.4; P < 0.01)[15]. More recently, a large prospective, multicenter, open-label, randomized, controlled study (RCT) was conducted in five European countries and aimed to evaluate the safety and efficacy of the alfapump® system in cirrhotic patients with RA in comparison with LVP[16]. This study included 60 patients (29 in the “alfapump®” group and 31 in the “SoC” (standard of care) group). Time to first LVP (primary endpoint) was significantly longer in the “alfapump®” group compared with “SoC” (hazard ratio: 0.13, P < 0.001). A total of 10/29 patients (37%) required LVP after pump implantation, mostly due to insufficient pumped volumes (4 patients) or device issues (5 patients). A recent meta-analysis of nine studies, including the European RCT[16] and eight observational studies[12,14,15,17-21], evaluated the efficacy of alfapump® in a total of 196 patients[22]. Despite significant heterogeneity between the studies (some of which were retrospective[17,21]), the proportion of patients receiving an alfapump® who no longer required paracentesis after pump implantation was 62%. This significant reduction in the need for paracentesis after pump implantation persisted over time (average follow-up time ranging from 6 to 24 mo)[22]. Interestingly, the reduced use of paracentesis is accompanied by an early and prolonged improvement in nutritional status[12,16]. In the study by Bureau et al[16], there was a significant improvement in brachial circumference, tricipital skinfold thickness, and hand grip strength in the first 3 mo after alfapump® placement compared to the control group.
| Ref. | Study design | Main exclusion criteria | N patients | Mean age (yr) | MELD score2 | Follow-up | Efficacy of the device | Mortality during follow-up, patients, n (%) | Liver transplantation after pump implantation (%) |
| Bellot et al[15], 2013 | Observational Prospective | Life expectancy < 6 mo Creatinine > 176 µmol/L in the 7 d prior to inclusion Bilirubin > 85 µmol/L Malignancy (including HCC) HE and/or GI bleeding related to portal hypertension in the 2 wk prior to inclusion | 40 | 59 | 12 | 6 mo | Number of paracentesis/mo/patient: 3.4 vs 0.24; P < 0.01 | 8 (25) | 5 (12) |
| Thomas et al[20], 2015 | Observational Prospective | Na | 10 | Na | 16 | Median: 165 d (maximum: 379 d) | Number of paracentesis/mo/patient: 3.4 ± 0.8 vs 0.4 ± 1.0 P < 0.0001 | 3 (30) | 1 (10) |
| Bureau et al[16], 2017 | RCT: alfapump (G1) vs iterative paracentesis (G2) | Creatinine > 176 µmol/L HCC outside Milan criteria Inability to use the device | G1: 27 G2: 31 | 61 | 12 | 6 mo | Median number of paracentesis on day 28 G1 vs G2: 0.3 vs 1.2; P < 0.001 | G1 vs G2: 22 vs 13, P = NS | 3 (11) |
| Stirnimann et al[18], 2017 | Observational Prospective | Inability to use the device | 56 | 62 | 13 | Median: 5.8 mo (maximum: 26 mo) | Number of paracentesis/mo/patient: 2.9 ± 1.8 vs 0.3 ± 0.3, P = NA | 23 (41) | 9 (16) |
| Solà et al[19], 2017 | Observational Prospective | Creatinine > 176 µmol/L Bilirubin > 85 µmol/L ≥ 2 urinary tract infections or SBP in the 6 mo prior to inclusion HCC outside Milan criteria | 10 | 59 | 11 | 12 mo | Number of paracentesis/3 mo/patient 7.5 vs 2.4; P = NA | 5 (50) | NA |
| Solbach et al[17], 2018 | Retrospective | Na | 21 | 56 | 15 | Na | Number of paracentesis/wk/patient: 2.3 ± 2.7 vs 0; P = NA | Median survival: 153 d | 4 (19) |
| Wong et al[20], 2020 | Observational Prospective | MELD score > 21 HE stage > II in the 15 d prior to inclusion > 2 systemic or local infections in the 6 mo prior to inclusion Bilirubin > 85 μmol/L Creatinine > 132 μmol/L GFR < 30 mL/min/1.73 m2 | 301 | 60 | 11 | 12 mo | Number of paracentesis/mo/patient: 2.4 ± 1.4 vs 0.2 ± 0.4; P < 0.05 | 4 (13.3) | 3 (10) |
| Will et al[24], 2020 | Retrospective TIPS vs alfapump | Na | 40 | 59 | 16 | Median: 4.7 mo (maximum: 24 mo) | Number of paracentesis: no more paracentesis at 6 mo for 43% of patients | 24 (60) | 11 (28) |
The effect of alfapump® on quality of life was specifically studied in the RCT by Bureau et al[16] and in the MOSAIC study[12,23], and it was shown that quality of life, assessed by the Chronic Liver Disease Questionnaire, was significantly improved in patients with “alfapump®” compared to patients who underwent iterative paracentesis, in particular due to a reduction in ascites-related symptoms[12,16,23]. This benefit may be of interest in patients not eligible for LT.
It is noteworthy that no prognostic impact of alfapump® has been demonstrated so far. In the European RCT, the overall survival at 6 mo was not different in the “alfapump®” group compared to the “iterative paracentesis” group (77% vs 87%, P = 0.35)[16]. In the series reported by Stirnimann et al[18], the median TFS of patients with alfapump® was only 9.8 mo, and the TFS rate was only 40% at 12 mo. The better TFS rate at 12 mo (57%) observed in the North American series could be explained, at least partially, by the lower severity of patients at inclusion. More insights should be provided by a European clinical trial that is currently recruiting subjects (NCT04326946), in which the primary endpoint is 6-mo post-implant survival.
A retrospective, single-centre, observational study compared the outcome of patients with RA treated with TIPS (n = 19) vs alfapump® (n = 40)[24]. As expected, patients receiving alfapump® had more impaired liver function (MELD-Na 16 vs 12; P = 0.04) and more frequently had encephalopathy (47% vs 16%; P = 0.02). Within the 6 mo following the procedure, the proportion of patients who did not require further paracentesis was 58% in the “TIPS” group vs 43% in the “alfapump®” group (P = NS). Two patients (10%) were transplanted in the “TIPS” group during the follow-up, vs 11 (27%) in the “alfapump®” group. In the subgroup of patients with a MELD-Na score below 15, 12-mo TFS was significantly higher in the “TIPS” group (65% vs 23% in the “alfapump®” group, P = 0.02), but the retrospective design of this study makes the results questionable. Two hypotheses can be proposed to explain the high mortality rate in patients from the “alfapump®” group who did not undergo LT. The first and major explanation is that, although alfapump® is an effective treatment to control ascites, it does not protect the patient against the other complications of persistent portal hypertension. The second hypothesis is related to the specific complications of the device, which are not rare (Tables 2 and 3) and may impact on prognosis per se or indirectly, if explantation of the pump is required.
| Ref. | Patients (n) | AKI occurrence during follow-up | Variation in creatinine levels before vs after implantation | Peritoneal infections (n episodes) | Urinary tract infections (n episodes) |
| Bellot et al[13], 2013 | 40 | 13 episodes, 11 patients | 106 vs 127 µmol/L at 3 mo (P = NA) 105 µmol/L at 6 mo (P = NA) | 12 | 3 |
| Thomas et al[20], 2015 | 10 | 3 episodes | 168 vs 221 µmol/L at 2 mo (P = NS) | NA | NA |
| Bureau et al[16], 20171 | 27 | After day 7: G1 vs G2: 17 vs 11 episodes; P = NS | G1 vs G2, at 3 mo: Increase of 18.1 vs 8.1 µmol/L (P = NS) | NA | NA |
| Stirnimann et al[18], 2017 | 56 | NA | Increase of 46 µmol/L at 3 mo (P = NA) | 5 | 1 |
| Solà et al, 2017 | 10 | 18 episodes, 14 after day 7 in 7 patients | 96 vs 105 µmol/L at 12 mo (P = NS) | 3 | 8 |
| Solbach et al[17], 2018 | 21 | 0 | 140 vs 168 µmol/L at 3 mo (P = NS) | 11 | 4 |
| Wong et al[20], 2020 | 30 | 11 episodes after day 7 in 9 patients | 93 vs 107 µmol/L at 12 mo (P = NA) | 1 | 3 |
| Ref. | Patients (n) | Peritoneal catheter dysfunction (n patients) | Bladder catheter dysfunction (n patients) | Pump dysfunction (n patients) | Pump pocket complication (n patients) | Explanted/replaced pumps |
| Bellot et al[15], 2013 | 40 | 5 | 9 | 2 | Infection: 2 Wound: 2 | 13/NA |
| Thomas et al[20], 2015 | 10 | 0 | Kinking: 1 | 1 | Infection: 1 Wound: 2 | 1/0 |
| Bureau et al[16], 2017 | 27 | 2 | 3 | 12 | 3 | 3/4 |
| Stirnimann et al[18], 2017 | 56 | Blockage: 13 Displacement: 2 Disconnection: 1 Twist: 2 | Blockage: 1 Migration: 1 | Clogging: 2 Humidity: 2 Communication: 4 Faulty sensor: 3 | Infection: 2 Wound: 2 | 17/11 |
| Solà et al[19], 2017 | 10 | Migration: 2 Blockage: 1 | 2 | Charging problem: 2 Transient blockage:2 | 1 | 2/1 |
| Karkhanis et al[14], 2017 | 3 | 0 | Migration: 1 | 1 | 2 | |
| Solbach et al[17], 2018 | 21 | Obstructions: 6 | Dislocations: 5 | 4 | 4 | 4/2 |
| Wong et al[20], 2020 | 30 | 13 | 1 | 3 | 4 | 10/9 |
| Will et al[24], 2020 | 40 | NA | Obstructions: 9 | NA | NA | 12/40 |
Assessing the safety of the device remains challenging since most of the reported series do not include a control group. The heterogeneity of inclusion and non-inclusion criteria across studies (Table 1) hinders the interpretation of the results.
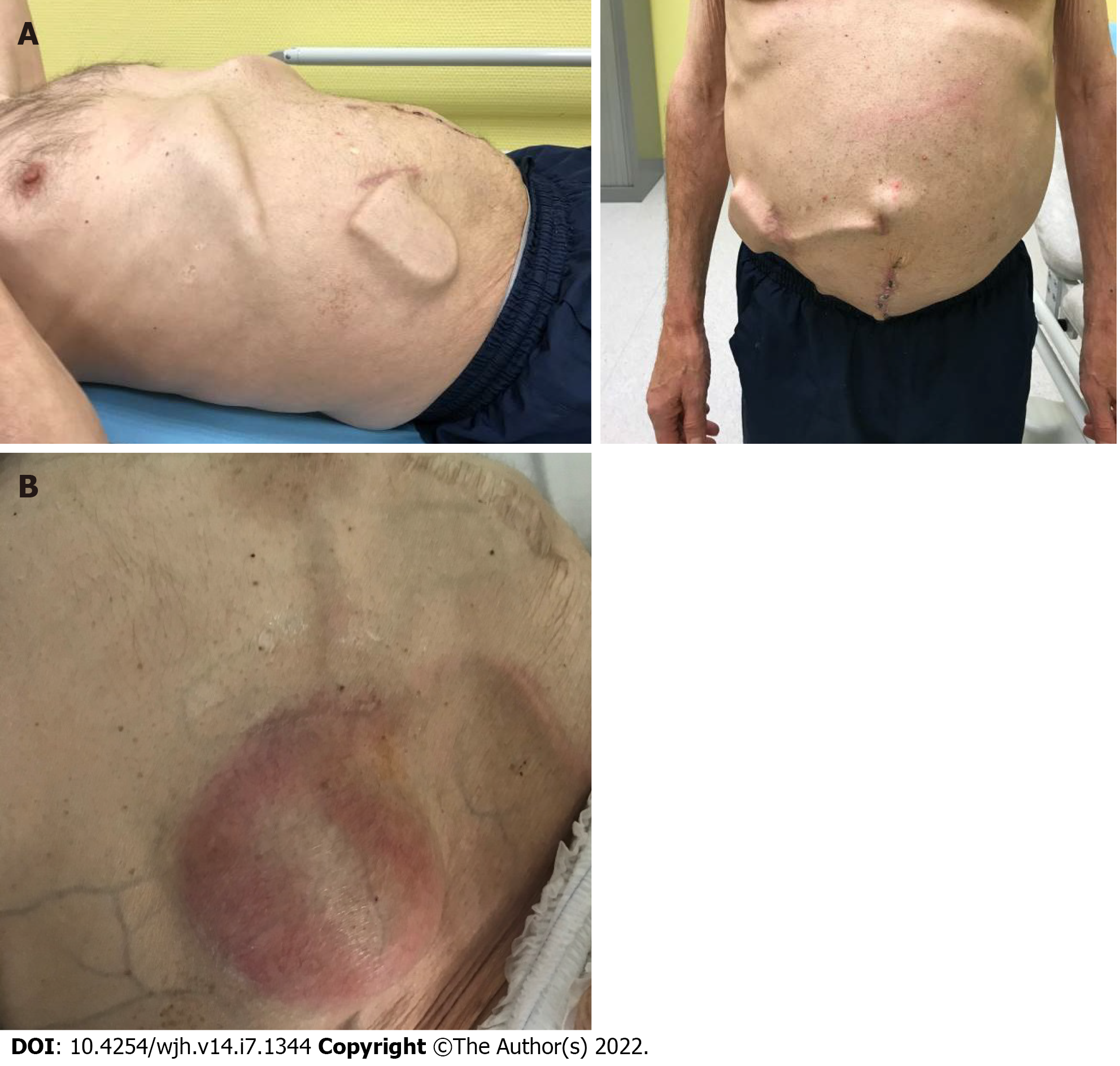
Complications directly related to the device are frequent. Among 100 patients with available data, Lepida et al reported a pooled estimate rate of overall pump-related adverse events of 0.77 (95%CI: 0.64-0.87) with low heterogeneity[22]. Some of these events may require re-intervention, or even pump removal, which is not an uncommon event during follow-up (Table 2). We note, amongst others, the following events: Dysfunction of the peritoneal catheter due to blockage (debris, fibrin clots, or peritoneal aspiration) or displacement, more rarely dysfunction of the bladder catheter (occlusion and disconnection), migration or dysfunction of the pump, and infection of the pump pocket (Figure 3).
Among the frequently reported adverse events of the pump, AKI may occur in up to 30% of patients during follow-up[22]. However, the heterogeneous definitions used for AKI and the widely varying timeframe between pump implantation and assessment of renal function must be taken into consideration in the interpretation of this finding. It should be noted that the existence of chronic renal failure (based on serum creatinine values > 133 to 176 µmol/L or glomerular filtration rate < 30 to 50 mL/min depending on the series) was an ineligibility criterion for alfapump® in most studies (Table 1). An association between alfapump® and renal function deterioration at 6 mo was suggested in a series of ten patients followed for 1 year[19], but these results were not confirmed in the MOSAIC cohort[12]. In the European RCT, almost half of the patients experienced AKI, which was observed during the first week after implantation in 41% of them, but 75% of patients recovered their previous renal function[16]. In the meta-analysis, the mean increase in serum creatinine after implantation was 23 µmol/L (95%CI: 10-35)[22]. Several distinct and interrelated mechanisms may contribute to the deterioration of renal function in the postoperative period, such as changes in intra-abdominal pressure, systemic inflammation, and hemodynamic changes. In the medium term, it has been suggested that the continuous removal of ascites could cause circulatory dysfunction[19], thus favoring a deterioration of renal function. However, data regarding the impact of alfapump® implantation on the hemodynamic parameters are limited and conflicting[12,16,19] and this hypothesis has not been confirmed so far[25]. The issue of long-term albumin administration to prevent post-paracentesis circulatory dysfunction in these patients is not clear-cut, due to a lack of published data, and is therefore left at the discretion of the clinician in charge of the patient[26]. The ANSWER study provides some evidence that the benefits of long-term albumin administration in decompensated cirrhosis could be due to improved circulatory function and reduced proinflammatory cytokines[27]. However, the dosage, duration, and frequency of administration remain open to debate. Consequently, expert recommendations[10] advise following current guidelines regarding the use of albumin infusion after implantation, i.e., whenever AKI occurs; experts also considered albumin infusion whenever total daily volume of ascites removed exceeds one liter[10,28].
The second common adverse effect of pump implantation is the occurrence of bacterial infection. In the meta-analysis by Lepida et al, the incidence rates of ascites fluid infection and urinary tract infection were 27% and 20%, respectively[22]. In the North American study, 15 bacterial infections occurred in 13 patients during the 12-mo follow-up, of whom 12 were considered to be related to the alfapump®[12]. Again, the absence of a control group limits the interpretation of these data. In the European RCT, the incidence of infectious events was similar in both the “alfapump®” and “standard treatment” groups[16]. Although the risk of developing multidrug-resistant infections related to long-term antibiotic prophylaxis remains a concern[7], patients receiving alfapump® have a particularly high risk of infection, and consequently long-term antibiotic prophylaxis should be maintained unless the patient's condition improves significantly (which occurs rarely). Norfloxacin at 400 mg/d remains the antibiotic of choice but, in the future, other molecules (such as rifaximin) with lower bacterial resistance and a better safety profile may be an alternative approach for long-term antibiotic prophylaxis[28]. Whatever the choice of antibiotic used for long-term prophylaxis, regular screening for multidrug-resistant organisms in these cirrhotic patients should be considered during antibiotic prophylaxis, in order to re-evaluate this strategy whenever multidrug-resistant Gram-negative bacteria or quinolone-resistant Gram-negative bacteria emerge[29]. However, two recent studies have provided more optimistic results regarding the long-term use of quinolones. The first observed that the incidence of infections caused by multidrug-resistant bacteria did not differ between the norfloxacin and placebo groups in patients with decompensated cirrhosis[30], while in the Global Study, no association was found between quinolone prophylaxis and multidrug-resistant bacterial infections, even when analysis was performed within different geographical areas[31].
According to data on the efficacy and safety of the alfapump® device, it appears that the selection of candidates for insertion of an alfapump® as well as their pre-therapeutic evaluation must be rigorous (Figure 4). Multidisciplinary evaluation involving surgeons, anesthetists, and hepatologists is recommended. In fact, relative contraindications are frequent in these frail patients with RA (for example, pre-existing kidney injury, severe malnutrition or sarcopenia, cognitive impairment due to hepatic encephalopathy, significant peripheral oedema, and bed confinement[10]) and the risk-benefit ratio should be carefully considered. When LT is not possible, alfapump® implantation may be a satisfactory solution to improve the patient's quality of life, provided that there are no severe comorbidities that could threaten the short-term prognosis or compromise the success of the implantation procedure and/or the use of the device.
In patients who are candidates for LT, but with a long estimated waiting time until transplantation (notably when there is no possibility of prioritizing LT), alfapump® implantation may be discussed whenever TIPS is contraindicated. Few reports are available about the use of alfapump® in patients awaiting LT. A recent single-centre retrospective study among 22 patients listed for LT in Switzerland aimed to demonstrate the feasibility of LT in patients with an alfapump®[32]. In this cohort, the median (range) MELD score at alfapump® implantation was 15 (8-25), and only 14/22 patients underwent LT within an average of 6 mo after the pump implantation. The pump was removed before LT and at the end of the LT procedure in three and eight patients, respectively, and left in place in three patients for a limited period of time. No technical issues were attributed to the alfapump® during the LT procedure. The authors reported that eight patients died before LT, seven while on the waiting list, and one after being delisted due to progressive liver disease. The causes of death among the patients on the waiting list were progressive liver disease in four (of whom one had a bacterial infection of unknown focus and another suffered from peritonitis), and multi-organ failure in three patients (who respectively developed pump pocket empyema, an abdominal wall phlegmon with communication into the abdominal cavity, and septic shock associated with probable infected abdominal focus). A last patient died after small bowel perforation not directly related to the pump catheter. The lack of a control group of patients listed for LT with RA and treated by iterative LVP, precludes any firm conclusions. However, while these results suggest that alfapump® does not technically compromise LT, they also emphasize the high risk of severe infection in these patients carrying intra-abdominal foreign material.
The alfapump® offers interesting perspectives that warrant further evaluation.
Frailty: Frailty is recognized as a determining factor in the overall prognosis of cirrhotic patients and contributes to mortality on the LT waiting list[33,34]. By enabling an improvement in nutritional status and a return to physical activity, we may speculate that the alfapump® device could limit sarcopenia and frailty, but data regarding this potential benefit are scarce and this point warrants specific evaluation in dedicated studies.
Percutaneous treatment of hepatocellular carcinoma: By reducing the quantity of ascites, alfapump® renders the percutaneous treatment of hepatocellular carcinoma possible. To date, this was reported in only one case report[35], but this therapeutic approach warrants further study.
Cure of hernia: A retrospective study of European multicenter data recently showed that patients who had concomitant umbilical or inguinal hernia repair and alfapump® placement had a shorter hospital stay, fewer complications, and better survival without paracentesis than patients undergoing emergency hernia surgery[36]. Hernia surgery concomitant with the implantation of the alfapump® enables the patient to undergo programmed surgery and to avoid the usual postoperative drainage, since the pump achieves ascites control. However, these data must be confirmed prospectively before this “concomitant” approach can be recommended. In the current state of knowledge, experts discourage concomitant repair of hernias[10].
Prevention of multidrug-resistant bacterial infections: Due to the decrease in hospitalizations for paracentesis, patients with alfapump® may be less exposed to nosocomial bacterial infections, which mainly involve multi-drug resistant bacteria. This may be of interest for patients who are candidates for LT. However, this potential benefit has not yet been evaluated in the long-term, and must be balanced against the risk of infections related to the procedure.
Cost-effectiveness: The overall cost of the procedure (implantation and patient follow-up), compared with that of standard treatment (iterative paracentesis), is a crucial point for the routine use of alfapump®. This cost in the first 6 mo after implantation is higher than that of the SoC treatment, mainly due to the cost of the device and the surgical intervention (about 30000 Euros), but tends to stabilize thereafter[16]. The ongoing French multicenter randomized medico-economic study (ARIAPUMP protocol, NCT03506893) comparing two management approaches for RA, namely, alfapump® implantation and iterative paracentesis, will make it possible to compare the costs of the long-term care for both these strategies, taking into account whether or not there is programmed LT. The radiological approach offers interesting perspectives in reducing the perioperative risk of morbidity in frail patients. Whether this mini-invasive technique can significantly reduce the duration of the post-procedure hospital stay, or the rate of local complications, has not yet been demonstrated, due to insufficient data and a lack of head-to-head studies.
Alfapump® is a device that has proven its effectiveness in reducing the need for iterative paracentesis and in improving the quality of life of cirrhotic patients with refractory ascites. It should be considered in particular for patients contraindicated for a TIPS, regardless of the patient’s eligibility for LT. To minimize the risk of complications after implantation, careful selection of these frail patients is essential. The concerns related to the cost of the device, the surgical procedure of implantation, as well as the potential complications that can occur are not fully resolved yet, but the implantation technique could evolve towards a "minimally invasive" approach, with a view to reducing the risks and improving the cost-effectiveness of the implantation. Patient information and active participation of the patient are two prerequisites for successful management. Additional studies, particularly real-world data from large heterogeneous populations with long-term follow-up, are required to clarify some unresolved issues, notably concerning the acceptable limits of liver and kidney function, age, forms of albumin compensation, or cost-effectiveness. There are currently several ongoing observational studies (NCT04326946, NCT03973866, and NCT03506893) that will hopefully provide a more complete picture of the advantages and disadvantages of this innovative device.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng KC, China; Elshimi E, Egypt; Isac S, Romania; Tsoulfas G, Greece S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 710] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 3. | Moreau R, Delègue P, Pessione F, Hillaire S, Durand F, Lebrec D, Valla DC. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 5. | De Gottardi A, Thévenot T, Spahr L, Morard I, Bresson-Hadni S, Torres F, Giostra E, Hadengue A. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7:906-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 7. | Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 9. | Stirnimann G, Banz V, Storni F, De Gottardi A. Automated low-flow ascites pump for the treatment of cirrhotic patients with refractory ascites. Therap Adv Gastroenterol. 2017;10:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 10. | Aagaard NK, Malago M, De Gottardi A, Thomas M, Sauter G, Engelmann C, Aranovich D, Cohen M, Thévenot T, Ehmann T, Capel J, Angeli P, Jalan R, Stirnimann G. Consensus care recommendations for alfapump® in cirrhotic patients with refractory or recurrent ascites. BMC Gastroenterol. 2022;22:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Dembinski J, Aranovich D, Banz V, Ehmann T, Klein I, Malago M, Richter N, Schnitzbauer AA, Staszewicz W, Tautenhahn HM, Capel J, Regimbeau JM. Surgical technique for placement of the automated low flow ascites pump (Alfapump). Langenbecks Arch Surg. 2020;405:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wong F, Bendel E, Sniderman K, Frederick T, Haskal ZJ, Sanyal A, Asrani SK, Capel J, Kamath PS. Improvement in Quality of Life and Decrease in Large-Volume Paracentesis Requirements With the Automated Low-Flow Ascites Pump. Liver Transpl. 2020;26:651-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Bendel EC, Sniderman K, Shaw C, Frederick RT, Wong F, Sanyal A, Asrani SK, Kamath PS, Capel J, Haskal ZJ. Feasibility and Procedural Safety of alfapump System Implantation by IR: Experience from the MOSAIC Study, a Multicenter, Open-Label Prospective Study in Cirrhotic Patients with Refractory Ascites. J Vasc Interv Radiol. 2020;31:1256-1262.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Karkhanis S, Jones R, Willis A, Mccarthy E, Zia Z, Mehrzad H, O'rourke J, Holt A, Tripathi D. Radiological insertion of automated low flow ascitic pump (alfapump®) system for management of medically refractory ascites. BJR Case Rep. 2017;3:20170025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Bellot P, Welker MW, Soriano G, von Schaewen M, Appenrodt B, Wiest R, Whittaker S, Tzonev R, Handshiev S, Verslype C, Moench C, Zeuzem S, Sauerbruch T, Guarner C, Schott E, Johnson N, Petrov A, Katzarov K, Nevens F, Zapater P, Such J. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol. 2013;58:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rosi S, MacDonald S, Malago M, Stepanova M, Younossi ZM, Trepte C, Watson R, Borisenko O, Sun S, Inhaber N, Jalan R. Alfapump® system vs. large volume paracentesis for refractory ascites: A multicenter randomized controlled study. J Hepatol. 2017;67:940-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Solbach P, Höner Zu Siederdissen C, Wellhöner F, Richter N, Heidrich B, Lenzen H, Kerstin P, Hueper K, Manns MP, Wedemeyer H, Jaeckel E. Automated low-flow ascites pump in a real-world setting: complications and outcomes. Eur J Gastroenterol Hepatol. 2018;30:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Stirnimann G, Berg T, Spahr L, Zeuzem S, McPherson S, Lammert F, Storni F, Banz V, Babatz J, Vargas V, Geier A, Stallmach A, Engelmann C, Trepte C, Capel J, De Gottardi A. Treatment of refractory ascites with an automated low-flow ascites pump in patients with cirrhosis. Aliment Pharmacol Ther. 2017;46:981-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Solà E, Sanchez-Cabús S, Rodriguez E, Elia C, Cela R, Moreira R, Pose E, Sánchez-Delgado J, Cañete N, Morales-Ruiz M, Campos F, Balust J, Guevara M, García-Valdecasas JC, Ginès P. Effects of alfapump™ system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transpl. 2017;23:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Thomas MN, Sauter GH, Gerbes AL, Stangl M, Schiergens TS, Angele M, Werner J, Guba M. Automated low flow pump system for the treatment of refractory ascites: a single-center experience. Langenbecks Arch Surg. 2015;400:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Nair S, Robinson E, Fiona S, Macdonald S, Mergental H, Talbot D, Tripathi D, Griffiths B, Jalan R, McPherson S. PWE-141 Alfapump for the treatment of refractory ascites: a “real world” experience from the uk. Gut. 2015;64:A274-5. |
| 22. | Lepida A, Marot A, Trépo E, Degré D, Moreno C, Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther. 2019;50:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Stepanova M, Nader F, Bureau C, Adebayo D, Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V, Simon-Talero M, Cordoba J, Angeli P, Rossi S, MacDonald S, Capel J, Jalan R, Younossi ZM. Patients with refractory ascites treated with alfapump® system have better health-related quality of life as compared to those treated with large volume paracentesis: the results of a multicenter randomized controlled study. Qual Life Res. 2018;27:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Will V, Rodrigues SG, Stirnimann G, Gottardi A, Bosch J, Berzigotti A. Transjugular intrahepatic portosystemic shunt and alfapump® system for refractory ascites in liver cirrhosis: Outcomes and complications. United European Gastroenterol J. 2020;8:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kumar K, Pillai VB. Improvement in Quality of Life and Decrease in Large-Volume Paracentesis Requirements With the Automated Low-Flow Ascites Pump. Liver Transpl. 2020;26:1539-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S, Svegliati-Baroni G, Fagiuoli S, Romanelli RG, Cozzolongo R, Di Marco V, Sangiovanni V, Morisco F, Toniutto P, Tortora A, De Marco R, Angelico M, Cacciola I, Elia G, Federico A, Massironi S, Guarisco R, Galioto A, Ballardini G, Rendina M, Nardelli S, Piano S, Elia C, Prestianni L, Cappa FM, Cesarini L, Simone L, Pasquale C, Cavallin M, Andrealli A, Fidone F, Ruggeri M, Roncadori A, Baldassarre M, Tufoni M, Zaccherini G, Bernardi M; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 28. | Shamsaddini A, Gillevet PM, Acharya C, Fagan A, Gavis E, Sikaroodi M, McGeorge S, Khoruts A, Albhaisi S, Fuchs M, Sterling RK, Bajaj JS. Impact of Antibiotic Resistance Genes in Gut Microbiome of Patients With Cirrhosis. Gastroenterology. 2021;161:508-521.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Mücke MM, Mayer A, Kessel J, Mücke VT, Bon D, Schwarzkopf K, Rüschenbaum S, Queck A, Göttig S, Vermehren A, Weiler N, Welker MW, Reinheimer C, Hogardt M, Vermehren J, Herrmann E, Kempf VAJ, Zeuzem S, Lange CM. Quinolone and Multidrug Resistance Predicts Failure of Antibiotic Prophylaxis of Spontaneous Bacterial Peritonitis. Clin Infect Dis. 2020;70:1916-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Moreau R, Elkrief L, Bureau C, Perarnau JM, Thévenot T, Saliba F, Louvet A, Nahon P, Lannes A, Anty R, Hillaire S, Pasquet B, Ozenne V, Rudler M, Ollivier-Hourmand I, Robic MA, d'Alteroche L, Di Martino V, Ripault MP, Pauwels A, Grangé JD, Carbonell N, Bronowicki JP, Payancé A, Rautou PE, Valla D, Gault N, Lebrec D; NORFLOCIR Trial Investigators. Effects of Long-term Norfloxacin Therapy in Patients With Advanced Cirrhosis. Gastroenterology. 2018;155:1816-1827.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 32. | Storni F, Stirnimann J, Banz V, De Gottardi A, Stirnimann G. Treatment of refractory ascites with an automated low flow ascites pump in patients awaiting liver transplantation. J Liver Transpl. 2021;4:100037. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 34. | Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 35. | Weil D, Christmann PY, Sailley N, Thévenot T. Letter: innovative use of the alfapump system to treat a small hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;47:695-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Nguyen-Khac E, Sarba R, Spahr L, Staszewicz W, DeGottardi A, Storni F, Elkrief L, Dokmak S, Valla D, Pricope D, Sabbagh C, Regimbeau JM. Combined treatment of refractory ascites with an alfapump® plus hernia repair in the same surgical session: A retrospective, multicentre, European pilot study in cirrhotic patients. J Visc Surg. 2021;158:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |