Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1319
Peer-review started: January 31, 2022
First decision: March 25, 2022
Revised: April 11, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: July 27, 2022
Processing time: 177 Days and 0.3 Hours
Gut microbiota plays an essential role in host homeostasis. It is involved in several physiological processes such as nutrients digestion and absorption, maintenance of intestinal epithelial barrier integrity and immune system self-tolerance. Especially the gut microbiota is assumed to play a crucial role in many gastro
Core Tip: The gut-liver axis plays an important role in the pathogenesis of liver diseases, including hepatocellular carcinoma (HCC). Growing evidence has supported the role of the gut microbiota in the development of HCC and as a prognostic and predictive factor. Thus, manipulation of the gut microbiota might represent a novel way to treat or prevent HCC.
- Citation: Spanu D, Pretta A, Lai E, Persano M, Donisi C, Mariani S, Dubois M, Migliari M, Saba G, Ziranu P, Pusceddu V, Puzzoni M, Astara G, Scartozzi M. Hepatocellular carcinoma and microbiota: Implications for clinical management and treatment. World J Hepatol 2022; 14(7): 1319-1332
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1319.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1319
Hepatocellular carcinoma (HCC) is an aggressive malignancy and almost exclusively develops in patients with chronic liver disease and cirrhosis. While viral hepatitis, especially hepatitis B virus (HBV) infection and hepatitis C virus (HCV) infection represent one ofe the most important cause of cirrhosis and HCC in low-income countries and Asia, alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD) are the main cause for developing cirrhosis and HCC in high income countries. The pathogenesis of HCC is multi factorial, driven by a circle of liver injury, inflammation, and regeneration that typically spans decades. Next to predisposing factors, as already mentioned, increasing evidence points towards a key role of the bacterial microbiome and bacterial metabolites in the development of chronic liver disease (CLD).
The human gut is one of the most complex structures in the body and is colonized by trillions of microorganisms including bacteria, fungi, viruses and protists. Among them, bacteria are the main inhabitants[1]. In recent years there has been increasing attention to the possible relationship between the gut microbiota and the process of carcinogenesis. Increasing data have suggested that the gut microbiota is related to a variety of cancers, particularly of the gastrointestinal tract, and consequently it has been hypothesized that this link may lead to the development of targeted therapies against the gut microbiome[2,3].
Among the various types of cancer, hepatocellular carcinoma is also included. As is known, the liver does not contain a microbiome, but is closely connected to the gut via the portal venous system, constituting the intestine – microbiota – liver axis[4].
The balance of the intestinal microbiota is essential for a physiological and correct functioning of the metabolism and immunity and, even more importantly, of the intestinal barrier[5]. In fact, homeostasis between the host and the microbiota is maintained precisely by the multilayered intestinal barrier. A disruption in this balance can lead to a malfunction of the intestinal barrier, resulting in chronic inflammation and dysbiosis. Although the mechanisms by which the microbiota is related to cancer are not yet fully understood, the above two are key factors in the carcinogenesis process[6] and several studies have observed significant alterations in the composition of gut microbiota in patients with chronic liver disease especially with a reduction in beneficial bacteria and an increase in pathogenic bacteria[7]. For this reason, numerous studies have investigated the potential use of therapies targeting the microbiota, such as prebiotics, probiotics and fecal microbiota transplantation (FMT). In particular, their potential role in the treatment of different liver diseases (such as hepatic encephalopathy, steatohepatitis and cirrhosis) and in different types of cancer (such as gastrointestinal cancers, breast cancer and melanoma) has been studied[8].
Indeed, in recent years, researchers have focused their attention on the possible prognostic and predictive role of gut microbiota composition, in particular in the response to therapy with immune checkpoint inhibitors (ICIs). It seems that a variation in the composition of the gut microbiota can influence the efficacy of treatments, in particular of immunotherapy, and the presence of specific gut microbes increases this efficacy[9].
Microbiome role in the development and growth of neoplastic lesions has assumed ever greater interest in recent studies. Human microbiota, defined as the population of microorganisms that colonize the body, has in fact been shown to play a crucial role both in physiological and pathological mechanisms. In addition to bacteria, the gut microbiota also contains eukaryotes as fungi, and some types of viruses.
Bacterial gut microbiota promotes disease development and progression not only locally, such as inflammatory bowel disease (IBD)[10,11], but also in distant locations such as the brain, heart, hematopoietic system and liver[12-16].
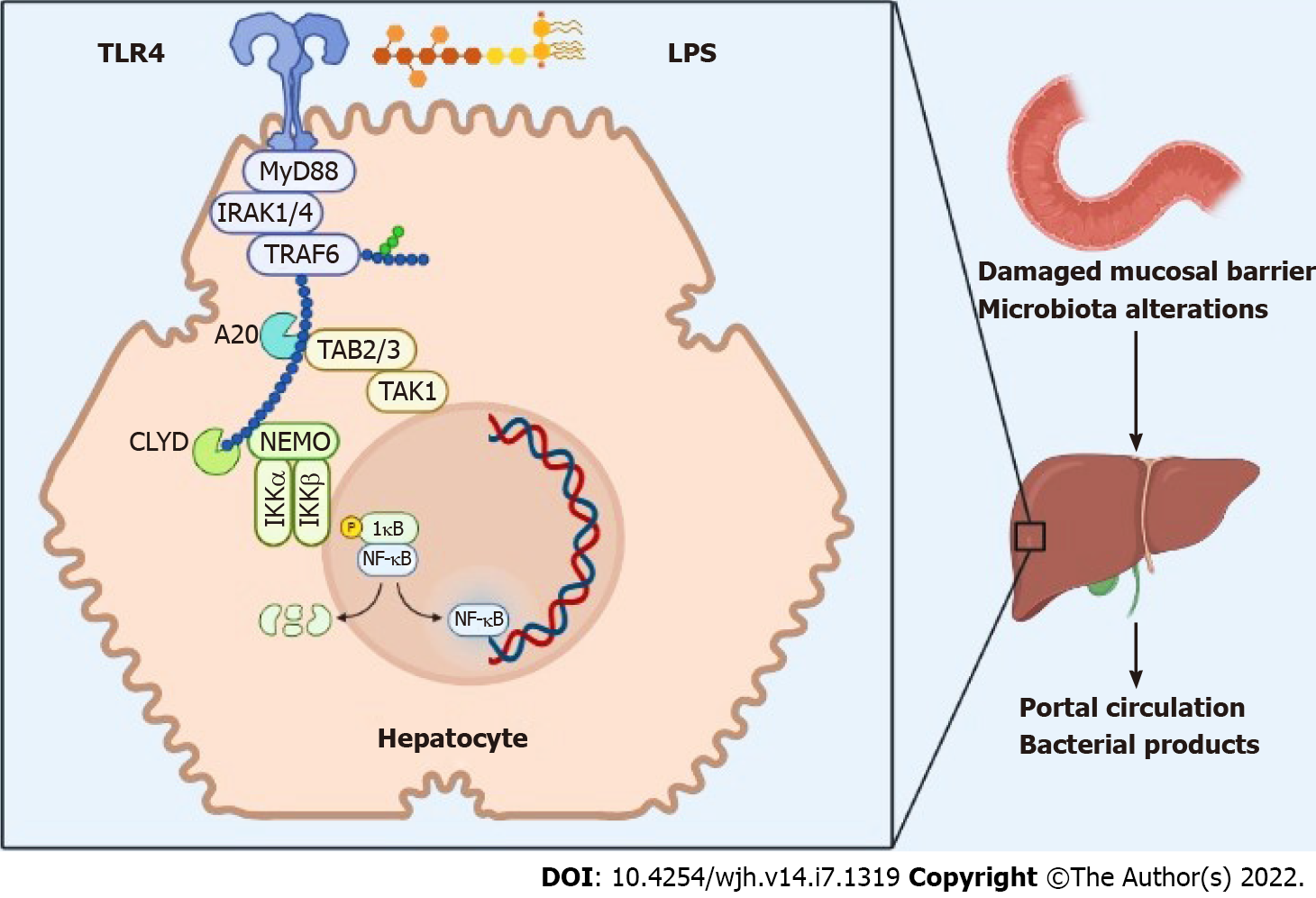
Given its direct anatomical connection through the portal vein, the liver is closely connected to the intestine. This is called “intestine-microbiota-liver axis”[6,17]. In fact, the liver receives blood rich in nutrients absorbed by the intestine, but it is also the first "filter" organ for the intestinal microbiota, of the MAMP (microbe-associated molecular pattern), toxins and bacterial metabolites. These products can subsequently trigger inflammatory responses via pattern recognition receptors.
Damage to the intestinal barrier, associated with alterations of the intestinal microbiota in CLD contribute to the onset of chronic inflammation. The inflammation, in turn, progresses, leading to tissue reworking with fibrosis. The processes of rehashing on an inflammatory basis increase the risk of developing HCC as the last step of the entire pathological process[7,18-20].
Bile acids represent another factor that acts in this complex system. In fact, these have the function of regulating the intestinal epithelial barrier, the proliferation of epithelial cells of the mucosa via the farnesoid X-activated receptor - dependent and the epidermal growth factor receptor - dependent pathways and controlling the growth and adhesion of intestinal bacteria[21,22].
At the hepatic level, bacterial products activate toll-like receptors (TLRs), in particular TLR-4, which, in turn, activates the NF-kB pathway, which determines the constitutive initiation of a mitogenic signal that is associated with an inhibition of programmed cell death. Chronic damage exposes the liver to a prolonged action of several TLR ligands and other bacterial substances, which represent inflammatory mediators that promote the development of chronic liver disease as well as laying the foundations for the subsequent development of hepatocellular cancer[20] (Figure 1).
Most cases of HCC develop on a basis of fibrosis and cirrhosis, which is the most important risk factor for the development of liver cancer. However, the presence of underlying liver diseases of various etiologies may contribute to the increased specific risk for developing HCC on a cirrhotic basis[23] (Table 1).
| Microbiota balance | |||
| Ponziani et al[38] | Cirrhosis + HCC | ↑ Bacteroides; ↑ Ruminococcus; ↑ Enterobacteriaceae | ↓ Bifidobacterium; ↓ Akkermansia |
| Ren et al[41] | HBV cirrhosis + HCC | ↑ Actinobacteria | ↓ Verrucomicrobia |
| Liu et al[97] | NBNC cirrhosis + HCC | ↑ Escherichia; ↑ Enterococcus | ↓ Faecalibacterium;↓ Ruminococcus;↓ Ruminoclostridium |
| Huang et al[39] | HBV cirrhosis + HCC | ↑ Bacteroides; ↑ Lachnospiracea incertae sedis; ↑ Clostridium XIVa | |
Although the percentage of NAFLD patients who develop HCC is small, the high incidence carries a high risk of developing hepatocellular carcinoma. Several studies in animal models have shown that the microbiome of obese patients has the ability to extract more nutrients, and in mice deprived of bacterial flora there was a reduction in body weight despite an increased caloric intake[24,25].
In dysbiotic mice fed a high-fat diet, choline is converted to methylamine, which involves a reduction in circulating plasma levels of phosphatidilcholine. Subsequently, low phosphatidylcholine levels lead to impaired secretion of VLDL, reducing hepatic lipid export, inducing hepatic steatosis[26,27].
The contribution of gut microbiota to non alcoholic steatosis hepatitis, a progressive form of NAFLD, is not as well documented as its role in earlier disease stages. An high-fat diet (HFD) increases intestinal permeability in mice with a noticeable increase in LPS serum levels[28,29].
About half of the cases of cirrhosis are caused by alcohol consumption. Indeed, serum LPS levels are increased in patients who have made chronic use of alcohol. Ethanol and its metabolite acetaldehyde have the ability to interrupt the intercellular junctions in the intestine, allowing bacteria and their products to pass into the bloodstream[30].
The microbiota-TLR4 axis plays an important role in this process. In fact, in several studies conducted on animal models, TLR4 deprived mice, subjected to intestinal disinfection, have shown a reduction in inflammation and oxidative stress[31-33].
Fibrosis represents a risk factor for the development of HCC. Literature data highlight an important contribution of the microbiota-TLR4 axis to liver fibrosis[34].
Studies in knockout mice have shown a key role for TLR4 and other mediators in the TLR4 signaling pathway, such as CD14 and lipopolysaccharide binding protein, in experimental models of hepatic fibrosis.
Conversely, other studies have shown a protective role of bacterial flora against the development of liver inflammation and fibrosis[35,36].
Evidence supports the role of the commensal microbiota as a hepatoprotective, although an alteration in its internal balance can lead to the prevalence of harmful species that can cause liver damage.
There is currently little data on the role of the microbiota in chronic viral hepatitis at the moment. Current data suggests that dysbiosis in patients with viral hepatitis cirrhosis is similar to that in patients with cirrhosis from other causes[37]. Further studies will be needed to investigate the close interconnections between microbiome composition and tumor development and growth.
Given the important role of the gut microbiota in liver carcinogenesis, growing attention towards using microbiome patterns as a predictive, prognostic or diagnostic biomarker of HCC is observed. The gut microbiome dysbiosis has been evaluated in NAFLD- and HBV/HCV-related HCC patients to find new clinical features and outcomes biomarkers in this setting[38,39]. In 2019, Ponziani et al[38] found that cirrhotic patients with NAFLD and HCC lack protective bacteria and have an enhanced intestinal inflammation with an increased level of IL8, IL13, CCL3, CCL4, CCL5, and faecal calprotectin concentration. HCC was associated with increased abundance of Bacteroidetes together with a reduction of Verrucomicrobiaceae, Bifidobacteriaceae, Akkermansia, Bifidobacterium.
In a more recent study, HCC tumour burden was associated with the presence of specific gut microbes, distinguished by the enrichment of Bacteroides, Lachnospiracea incertae sedis, and Clostridium XIVa. Patients with these three genera mounted a weaker host liver anti-tumour inflammatory response[39]. Primary bile acids increased CXCL16 expression, which regulates NK cell accumulation, whereas secondary bile acids showed the opposite effect. Feeding secondary bile acids or colonization of bile acid-metabolizing bacteria reversed both NK cell accumulation and inhibition of liver tumour growth in mice with altered gut commensal bacteria. Removing gram-positive bacteria by antibiotic treatment with vancomycin, which contains the bacteria mediating primary-to-secondary bile acid conversion, induced hepatic NK cell accumulation and decreased liver tumour growth in mice. These data suggest that Clostridium XIVa influences bile acid-controlled NK cell accumulation. In normal liver tissue from human HCC patients, primary bile acid cheno-deoxycholic acid levels correlated with CXCL16 expression, whereas an inverse correlation was observed with secondary bile acid glycolithocholate[40]. Higher bile acid levels (≥ 16 μmol/L) indicated worse clinical outcomes among HBV-related HCC patients with enrichment of Bacteroides, Lachnospiracea incertae sedis, and Clostridium XIVa. These results show that correlation between gut microbiota and serum bile acids in tumour immune microenvironment could potentially influence tumour burden and clinical outcomes in HBV-related HCC[40].
In 2019, Ren et al[41] evaluated the potential role of microbiome as a non-invasive biomarker for HCC. The authors collected 486 faecal samples from East, Central, and Northwest China. Using 16S rRNA Miseq sequencing, 3 groups were identified: early HCC, cirrhosis, and healthy controls. Actinobacteria, 13 genera including Gemmiger and Parabacteroides were increased in HCC compared with cirrhosis. Additionally, butyrate-producing genera was decreased, and lipopolysaccharide-producing was increased in HCC in comparison to healthy controls. Interestingly, 30 microbial markers were identified through a fivefold cross-validation on a random forest model between 75 early HCC and 105 non-HCC samples. This was the first study characterizing the gut microbiome in early HCC as a non-invasive tool to diagnosed early stage of HCC.
In literature, three studies analysed the potential predictive and prognostic role of gut microbiota in HCC.
Zheng et al[42] analyzed the characteristics and changes in the gut microbiota during treatment with anti-PD-1 immunotherapy drugs in eight patients with HCC. Responders (R) had, during the entire treatment, a higher richness of taxa and a greater number of genes than the no-responder (NR). Before the start of treatment, Bacteroidetes was the most abundant phylum, followed by Firmicutes and Proteobacteria in both R and NR[41]. As treatment progressed, the microbial composition at the phylum level in R remained relatively stable, while proteobacteria often increased in NR, with a prevalence of Escherichia coli. Furthermore, the methanogenesis pathway was found to be correlated with R. obeum and Lactobacillus species, and furthermore the generated methane would appear to improve oxidative stress damage and suppress host inflammatory response. Other pathways with potential benefits include sulfate reduction and carbon fixing functions that were correlated with R. obeum, carotenoid biosynthesis correlated with B. cellulosilyticus and A. colihominis, and unsaturated fatty acid metabolism associated with C. comes[42].
Li et al[9] collected microbiome samples from 65 patients with metastatic HCC being treated with ICI therapy. The analyzes showed that patients with a high presence of Faecalibacterium had a significantly prolonged PFS compared to those with low presence (P = 0.006). In contrast, patients with a high presence of Bacteroidales had a reduced PFS compared with those with low presence (P = 0.002).
Chung et al[43] studied the effects of the gut microbiota in eight adult patients with HCC treated with nivolumab (anti PD-1). Reported data showed that responder patients had a significantly lower Firmicutes/Batteroidetes ratio (10% vs 66.7%, P <0.05) and a higher Prevotella/Batteroides ratio (22.99 vs 2.312, P = 0.024 ) than non-responders. These results indicate that the F/B ratio and P/B ratio could serve as predictive markers of non-response to nivolumab therapy. Akkermansia species was also found in two responders, indicating that this could also be a useful prognostic marker of response to nivolumab therapy in patients with advanced HCC.
In the last decade, several research efforts have been made to identify potential prognostic and predictive biomarkers of response with target therapy[44-46]. In this perspective, interest in interaction between the gut microbiome and HCC targeted therapy is increasing, even if no data are available up to date. It has been shown that among anthocyanins, delphinidin possesses strong antitumor activity through various mechanisms such as downregulation of matrix metalloproteinase (MMP)[47], inhibition of angiogenesis and tumour cell migration[48], growth suppression of ERα-positive cancer both in vitro and in vivo[49] and apoptosis promotion[50,51].
These mechanisms are, in turn, the target of targeted therapies. Therefore, a possible implementation of the targeted therapies activity can be profoundly influenced by the composition of the intestinal microbiome. Furthermore, particular microbes could become a future therapeutic target to potentially improve the effectiveness of cancer treatment. However, further studies are needed to better understand the predictive and prognostic role of the gut microbiome in HCC and to investigate the potential benefits of microbiome modulation.
Gut microbiota dysfunction is known to lead to dysfunction of local, locoregional, and systemic immune systems, causing the dissolution of epithelial barriers, and as a consequence, transfers biofilm microbes and their components into mesenteric lymph nodes and peripheral circulation. Furthermore, dysbiosis may induce a neutrophils gathering into the intestinal epithelium that modifies the profiles of inflammatory cytokine and chemokine, stimulates the T helper 17 and effector T-cells, resulting in a negative feedback control of the microbiota[52]. Aging, antibiotics, xenobiotics, smoking, hormones, and diet can be responsible for dysbiosis which is a risk factors for cancer onset and, on the other hand, influences the therapeutic outcomes, as for chemotherapy or immunotherapy, interfering directly or indirectly with therapeutic mechanisms[53].
ICIs are promising anticancer agents and according to several recent studies, the gut micro-biome may play a critical role in regulating immunotherapy responses. Thus, the microbiome can influence the host response to ICIs (PD-1/ PD-L1 blockade, or CTLA-4 inhibition)[52,53] (Table 2).
| Treatment | Responder | Non responder | |
| Zheng et al[42] | Anti-PD1 | ↑ Akkermansia muciniphila; ↑ Ruminococcaceae | ↑Proteobacteria |
| Li et al[9] | Anti-PD1 | ↑ Faecalibacterium | ↑ Bacteroidales |
| Chung et al[43] | Anti-PD1 | Dialister pneumosintes; Escherichia coli; Lactobacillus reteri; Streptococcus mutans; Enterococcus faecium; Streptococcus gordonii; Veillonella atypica; Granulicatella sp.; Trchuris trichiura; ↑ Firmicutes/Bacteroidetes ratio; ↓ Prevotella/Bacteroides ratio | Citrobacter freundii; Azospirillum sp.Enterococcus durans; ↑ Akkermansia species |
CD152, well known as CTLA-4, a T-cell surface receptor activated by two ligands (CD80 or CD86) expressed on antigen-presenting cells, induces an inhibitory signal in the early activated T-cell[54,55]. The other immune checkpoint repressing T-cell response is PD-1 (programmed death cell 1) sided is on the surface of activated T-cells, whereas its ligand PD-L1 is expressed on tumor cells surfaces and antigen-presenting cells (macrophages and dendritic cells). Their bond induces T-cell inactivation[56].
Various studies have recently examinated the role of gut microbiota in melanoma patients treated with ICIs showing a strict correlation between gut microbiota and response to immunotherapy and how differences in microbiome composition could influence treatment efficacy[56-60].
Notably, Akkermansia muciniphila was associated with a better response to ICIs. In metastatic melanoma patients with a benefit from ICIs treatment, the so-called responders, several bacteria phyla were detected in abundance: Faecalibacterium, Ruminococcaceae, and Clostridiales. Nevertheless, non-responder patients had a higher amount of Bacteroidales.
According to several studies, both preclinical and clinical, A. muciniphila, Alistipes indistinc-tus, Bacteroides, B. cepacia, D. formicigenerans, Parabacteroides merdae/distasonis, C. aerofa-ciens, Eubacterium spp., Veillonella parvula, Klebsiella pneumoni-ae, Bifidobacterium spp., Lactobacillus spp., Streptococcus parasanguinis, Blautia spp., E. hi-rae, E. faecium, H. filiformis, Faecalibacterium prausnitzii, Gemmiger formicilis, and Ruminococ-caceae family seems to improve the effectiveness of ICIs facilitating antitumor immunity.
Gut microbiota enhanced with B. thetaiotaomicron, Roseburia intestinalis, Anaerotruncus coli-hominis, Blautia obeum, and some combination of antibiotics were associated with a compromised ICIs' efficacy[53].
Changes in the expression of cytokines and immune cells due to alterations in the gut microbiota induced distinct therapeutic responses[61].
As for HCC, ICIs are approved for clinical practice usage[62-64]. Up to now, programmed cell death (PD) ligand-1 (PD-L1) expression, tumor mutation burden, and microenvironmental immune cells have been associated with ICIs effectiveness. A previous study conducted by Shen et al[65] investigated the relationship between the gut microbiome, analyzed through 16S rRNA and shotgun whole-genome sequencing on stool samples collected at baseline and after eight weeks treatment, and the effectiveness of immunotherapy in patients with advanced HCC. The study enlisted thirty-six patients (31 males and 5 females). There was no difference in the baseline gut microbiome between responders and non-responders. Also, the composition of gut microbiota showed no difference induced by ICIs. The study failed to demonstrate an association between gut microbiota and ICI efficacy, maybe due to the size of the sample examined, the different treatment, and the host factors influencing and affecting the gut microbiota, along with a non-standardized method to collect and process the stool samples leading to poor reproducibility. Also, dysbiosis is common in patients with chronic liver diseases. Therefore, it may affect the response to ICIs treatment as the study results, too.
On the contrary, a study by Chung et al[43] enrolled eight patients with HCC who received nivol
A retrospective study by Li et al[9] focused on the composition of gut microbiota in patients with HCC treated with ICIs. In particular, patients were gathered on the basis of the abundance of Bacteroidales in nonresponders and Faecalibacterium in responder patients. As a result, an abundance of Faecalibaterium correlated with an increased PFS, while a great amount of Bacteroidales is as-sociated with low PFS. However, the small size of the sample and the retrospective nature of this study limited the validity of the results observed.
Another study by Zheng et al[42] enlisted eight patients with HCC, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and a Child-Pugh Class A receiving camrelizumab, an anti-PD-1 agent, after progression to sorafenib. Stool samples were collected and analyzed at baseline and week 3 and 12, respectively. In the responders patients the species that composed the gut microbiota were: Bacteroidetes, Firmicutes and Proteobacteria. In non-responder Proteobacteria were predominant. Oral intake of Bifidobacterium may promote antitumor growth induced by ICIs. A. Muciniphila and Ruminococcacaeae were observed in the intestinal microbiota and have a role in immunomodulatory functions. Oral A. Mucinophila could revive the effectiveness of ICIs.
A clinical trial was recently opened at the National Cancer Institute that aims to combine vancomycin-based antibiotic treatment with immune checkpoint inhibitors (NCT03785210). This study may attempt to answer the question of whether the combination of immunotherapy with microbiota selection could have a beneficial effect in HCC patients[67].
Summing up, evidence with low statistical power is available up to now. Further studies are war-ranted for the time being. Also, a standardized method to collect and analyze fecal samples may help obtain reproducibility.
Curative options for advanced HCC are very limited, and driving the need to develop new therapeutics. Greater understanding about bacterial function, its impact on the host, its contribution to the loss of barrier function and the gut–liver immune system will lay the foundations for novel therapeutic approaches to the treatment of chronic liver disease that will attenuate progression to cirrhosis and HCC. To improve their efficacy, these therapies should focus on preventing the progression from chronic liver disease to cirrhosis and from compensated to decompensated cirrhosis. Bacteriotherapy could restore microbiome composition, reduce intestinal permeability (and thus reduce endotoxemia) and attenuate the chronic inflammatory environment in the liver; in this way, there is the potential that progression of disease and tumour development could be delayed or halted. Ideally, these therapeutic approaches would be more effective if they targeted earlier stages of disease and aimed to reduce chronic inflammation and cirrhosis, rather than directly reduce tumour mass[68].
Potential routes to target intestinal microbiota community include diet, probiotics, prebiotics, antibiotics[69-73] and FMT.
FMT aims to replenish the gut with a “physiological” microbiome taken from the stool of healthy subjects. Fecal donors are carefully selected[74], with exclusion criteria such as a low or high body mass index (new-onset obesity has been reported in a transplant recipient of fecal microbiota isolated from an overweight donor[75]), high risk behavior for infectious diseases, gastro-intestinal disease, recent microbiota-altering treatment (antibiotics, immunosuppressive medication, antineoplastic agents), presence of specific medical issues such as auto-immune, atopic or neurologic disorders, cancer, or chronic pain syndrome. The method for microbiota isolation is simple: fecal matter is collected from selected donors, suspended (usually in saline solution) and mixed in a blender; the resulting liquefied stool is filtered through a strainer to remove fibers, and thus ready for transplant[8]. Currently, FMT has been approved as a clinical method for treating recurrent Clostridium difficile infection by 2013 guidelines[76] and its clinical effectiveness has reached approximately 90%[77]. Fecal microbiota can be delivered via endoscopy (e.g., colonoscopy or nasojejunal), enema or colonic transendoscopic enteral tubing[78-80]. Oral capsules has been developed showing efficacy comparable to delivery by colonoscopy regardless of whether fresh, frozen or lyophilised stools were used[81-83]. However, frequency of doses and optimal overall duration is still unclear as study parameters were not directly comparable across different studies.
There are several clinical studies regarding the use of probiotics as a novel and effective approach to treat or prevent chronic liver disease and HCC. Probiotic VSL#3, a combination of Bifidobacteria, Lactobacilli and Streptococcus thermophilus, could short inpatient time for patients with liver cirrhosis and hepatic encephalopathy[84]. A randomized controlled multicenter study investigates the role of probiotics in patients with alcoholic hepatitis (AH)[85]. The 117 patients were prospectively randomized to receive the 7 d of cultured Lactobacillus subtilis/Streptococcus faecium (1500 mg/d) or placebo (probiotics 60 and placebo 57). In the probiotics group albumin and TNFα showed significant difference. In addition, 7 d of oral supplementation with cultured L. subtilis/S. faecium was associated with restoration of bowel flora and improvement of microbial lipopolysaccharide in patients with AH. Another study in mice aimed to evaluate the role of FMT in reducing HFD-induced steatohepatitis in mice. The analysis was conducted by examining the microbiota structure of the rodents, the butyrate present in the caecal content, the intrahepatic lipids and the liver pathological conditions 8 weeks after FMT. The results documented a reduction in the degree of steatohepatitis after FMT, as indicated by the finding of a decrease in intrahepatic lipids, NAS score and intrahepatic pro-inflammatory cytokines (INF-γ and IL-17), with an increase in Foxp3, IL -4 and IL-22 [86]. Another study investigate the role of FMT in patients with untreated sever alcoholic hepatitis (SAH). Philips et al[87] discovered that Indices of liver disease severity improved significantly within the first week after FMT compared to HC and even survival was better in patients treated with FMT (87.5% vs 33.3%, P = 0.018). Philips et al[88] reported a case of a young male patient with corticosteroid nonresponsive severe alcoholic hepatitis in 2017. FMT led to rapid amelioration of appetite and hyperbilirubinemia. Notably, FMT was performed in 18 patients with persistent positive HBeAg[89]. FMT was effective for these patients via inducing HBeAg clearance, suggesting that regulating intestinal microbiota might be beneficial to chronic hepatitis B treatment. A Phase I clinical trial demonstrated that FMT restored antibiotic-induced microbial dysbiosis in patients with advanced liver cirrhosis[90]. Even more, the effect of FMT on hepatic encephalopathy has been confirmed in both animal models and human beings. FMT alleviated cognitive function and prevented hepatic necrosis in animal models, thereby triggering improvement of hepatic encephalopathy[91]. Kao et al[92] reported a significant improvement in serum ammonia and quality of life in a patient with hepatic encephalopathy after performing FMT. Bajaj et al[93] conducted a randomized clinical trial, which suggested that FMT has the potential to improve cognition and reduce hospitalizations in hepatic encephalopathy patients.
Recently, Baruch et al[94] reported the first-in-human clinical trials where they discovered how treatment with FMT was associated with favorable changes in immune cell infiltrates and gene expression profiles in both the gut lamina propria and the tumor microenvironment. These early findings have implications for modulating the gut microbiota in cancer treatment.
These results encourage further studies on the possible beneficial impact of gut microbiota “resetting” by FMT in HCC.
Despite the abundance of studies in the literature, the understanding of the mechanisms of the alteration of the gut microbiota in HCC remains incomplete and inadequate. Most clinical studies have limitations due to the fact that they are single center studies with small population samples, which compromises the applicability of the results. Furthermore, it is often complex to analyze the various etiologies of the hepatic disease, the stage of cirrhosis, the diet, the use of antibiotics for other causes, the consumption of alcohol; all these elements represent confounding factors that can determine important variations in the intestinal microbiota. Therefore these factors should be considered in the design of future studies, which would involve multiple centers, on a large scale.
The characteristics of dysbiosis change among patients with hepatocellular carcinoma depending on the different etiologies. Despite this, some studies have shown that the different etiology of HCC is not related to a condition of intestinal microbial dysbiosis[95], while other studies are conflicting. Chen et al[96] evaluated the differences in the gut microbiota in consideration of the etiology of liver disease. they found that the discriminant between HBV-related cirrhosis and primary biliary cirrhosis was indicated by two operative taxonomic units (OTU), OTU-23 (Neisseria) and OTU-36 (Gemella). Furthermore, the level of bacterial diversity and composition varied differently between patients with non-HBV and non-HCV HCC (NBNC-HCC) and patients with HBV-HCC[97], and between patients with HBV-HCC and NAFLD-HCC[41,98]. Therefore, these data suggest that in designing future studies, the underlying etiology of liver disease should also be taken into account for identifying patterns of dysbiosis in HCC.
Currently, although several studies on the association between intestinal microbiota and HCC are emerging, data analyzing the causal relationship are still very limited[99]. From a methodological point of view, traditional sequencing technology and 16S rRNA sequencing (the most established genetic marker used for bacterial identification and classification), does not take into account rare eukaryotic cells and the absence of which could lead to loss of important information. Furthermore, it is necessary to consider that the use of low biomass samples, such as blood, can lead to contamination in the microbiome analysis[100].
Finally, despite extensive preclinical evidence, clinical trials focusing on the prevention and treatment of HCC by modulating the microbiota are still lacking. The greatest difficulties are found, in this sense, in the applicability of in vitro or in vivo studies to the human context.
To optimize the therapeutic response it could be useful to host protective intestinal bacteria depending on the type of treatment proposed. To confirm this, despite the current lack of clinical studies, the data obtained up to now with animal models have given interesting results in increasing the efficacy of treatments by modulating the bacterial flora. Some studies suggest that to increase the efficacy of the treatments it is necessary to keep intact the commensal microbiota which would mediate the cellular functions of the myeloid-derived cells present in the tumor microenvironment[101]. The results obtained from the implantation of fecal material of patients with melanoma in germ-free mice, led to an improvement in tumor control, increased the response of T lymphocytes and obtained a greater efficacy of treatment with the anti programmed cell death protein 1 (PD-1)[59]. Among these, Bifidobacterium spp. has been identified. as a component of the microbiota that improved the efficacy of treatment with anti programmed death-ligand 1 (PD-L1)[58], while several species of Bacteroides appear to have an implication in the antitumor effect of antigen-blocking anti-cytotoxic T cells (CTLA ) -4[57].
As regards hepatocarcinoma, a recent study has documented that the intestinal microbiota can increase the effectiveness of treatment with anti PD-1, increasing the sensitivity to immunotherapy[42,102]. Given the important results obtained from immunotherapy treatment in patients with advanced HCC[103], it is important to explore the data relating to the microbiota and the bacterial species interested in contributing to the beneficial effects of the immunotherapy. Furthermore, in patients with NAFLD-HCC, given the immunosuppressive phenotype exerted in peripheral blood mononuclear cells from a bacterial extract of these patients, the possible modulation of the microbiota could help in overcoming any resistance to immunotherapy in HCC[98].
In recent years, enormous progress has been made in the characterization of the gut microbiota and its association with different etiologies and severity of cancer diseases; various hypotheses have also been advanced in chronic liver disease and in the development of HCC and studies have been launched to characterize it. Furthermore, the work in rodent models and the greater understanding of the etiology of bacterial pathogens affecting liver disease has established the contribution of the gut microbiome in the progression of liver disease and its potential role as predictive and prognostic biomarkers.
Considering that patients with HCC and other CLDs are prone to alterations in the intestinal microbiota, it is tempting to assume that dysbiosis affects the efficacy of anticancer treatments including ICI in some categories of patients and that consequently the modulation of components of the microbiota can be managed to increase the activity of available treatments. Despite the growing interest on the part of researchers on the subject, for now it remains to be clarified whether the recent data obtained through animal models on the interaction of the immune response and the microbiota in some types of tumors, can also be applied to patients with hepatocarcinoma. Therefore, the orientation of research towards the intestinal microbiota, in particular the use of probiotics or the transplantation technique of the fecal microbiota, will be able to better direct towards new paradigms and personalized treatments with the aim of improving the effectiveness of the treatments available for HCC.
We acknowledge all the authors whose publications are referred in our article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rizzo A, Italy; Rostami-Nejad M, Iran; Vij M, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3709] [Cited by in RCA: 3183] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 2. | Garrett WS. Cancer and the microbiota. Science. 2015;348:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 958] [Article Influence: 95.8] [Reference Citation Analysis (1)] |
| 3. | Hamm AK, Weir TL. Editorial on "Cancer and the microbiota" published in Science. Ann Transl Med. 2015;3:175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Weng MT, Chiu YT, Wei PY, Chiang CW, Fang HL, Wei SC. Microbiota and gastrointestinal cancer. J Formos Med Assoc. 2019;118 Suppl 1:S32-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1814] [Article Influence: 181.4] [Reference Citation Analysis (57)] |
| 6. | Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 405] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 7. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 747] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 8. | Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer. 2019;145:2021-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Li L, Ye J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: A Chinese population-based study. Medicine (Baltimore). 2020;99:e21788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DP, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 473] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 11. | Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1069] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 12. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2783] [Cited by in RCA: 3137] [Article Influence: 261.4] [Reference Citation Analysis (0)] |
| 13. | Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1000] [Cited by in RCA: 1086] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 14. | Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 2393] [Article Influence: 199.4] [Reference Citation Analysis (0)] |
| 15. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4022] [Article Influence: 287.3] [Reference Citation Analysis (0)] |
| 16. | Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1368] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 17. | Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1223] [Article Influence: 101.9] [Reference Citation Analysis (2)] |
| 18. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1025] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 19. | Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1641] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 21. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 22. | Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Rattan P, Minacapelli CD, Rustgi V. The Microbiome and Hepatocellular Carcinoma. Liver Transpl. 2020;26:1316-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8726] [Article Influence: 459.3] [Reference Citation Analysis (1)] |
| 25. | Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6397] [Cited by in RCA: 5654] [Article Influence: 353.4] [Reference Citation Analysis (1)] |
| 26. | Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511-12516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 802] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 27. | Jiang XC, Li Z, Liu R, Yang XP, Pan M, Lagrost L, Fisher EA, Williams KJ. Phospholipid transfer protein deficiency impairs apolipoprotein-B secretion from hepatocytes by stimulating a proteolytic pathway through a relative deficiency of vitamin E and an increase in intracellular oxidants. J Biol Chem. 2005;280:18336-18340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4542] [Article Influence: 252.3] [Reference Citation Analysis (1)] |
| 29. | Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 395] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 33. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 504] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 34. | Affo S, Yu LX, Schwabe RF. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu Rev Pathol. 2017;12:153-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 503] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 35. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1553] [Article Influence: 86.3] [Reference Citation Analysis (1)] |
| 36. | Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, McKim SE, Parsons C, Rippe RA, Wheeler MD. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318-G1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Bajaj JS, Sterling RK, Betrapally NS, Nixon DE, Fuchs M, Daita K, Heuman DM, Sikaroodi M, Hylemon PB, White MB, Ganapathy D, Gillevet PM. HCV eradication does not impact gut dysbiosis or systemic inflammation in cirrhotic patients. Aliment Pharmacol Ther. 2016;44:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (1)] |
| 39. | Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, Lu H, Yin S, Ji J, Zhou L, Zheng S. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 40. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1023] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 41. | Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 42. | Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, Li P, Qin N, Fang W. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 364] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 43. | Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB, Joo YE, Hwang JE, Bae WK, Chung IJ, Shin MG, Shin JH. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27:7340-7349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 44. | Rapposelli IG, Shimose S, Kumada T, Okamura S, Hiraoka A, Di Costanzo GG, Marra F, Tamburini E, Forgione A, Foschi FG, Silletta M, Lonardi S, Masi G, Scartozzi M, Nakano M, Shibata H, Kawata K, Pellino A, Vivaldi C, Lai E, Takata A, Tajiri K, Toyoda H, Tortora R, Campani C, Viola MG, Piscaglia F, Conti F, Fulgenzi CAM, Frassineti GL, Rizzato MD, Salani F, Astara G, Torimura T, Atsukawa M, Tada T, Burgio V, Rimini M, Cascinu S, Casadei-Gardini A. Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open. 2021;6:100190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Faloppi L, Puzzoni M, Casadei Gardini A, Silvestris N, Masi G, Marisi G, Vivaldi C, Gadaleta CD, Ziranu P, Bianconi M, Loretelli C, Demurtas L, Lai E, Giampieri R, Galizia E, Ulivi P, Battelli N, Falcone A, Cascinu S, Scartozzi M. Angiogenesis Genotyping and Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Receiving Sorafenib: The ALICE-2 Study. Target Oncol. 2020;15:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Casadei-Gardini A, Rovesti G, Dadduzio V, Vivaldi C, Lai E, Lonardi S, Fornaro L, Pretta A, Zagonel V, Bernardini L, Astara G, D'Amico FE, Masi G, Rimini M, Scartozzi M, Cascinu S. Impact of Aspirin on clinical outcome in advanced HCC patients receiving sorafenib and regorafenib. HPB (Oxford). 2021;23:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Ho ML, Chen PN, Chu SC, Kuo DY, Kuo WH, Chen JY, Hsieh YS. Peonidin 3-glucoside inhibits lung cancer metastasis by downregulation of proteinases activities and MAPK pathway. Nutr Cancer. 2010;62:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Lee SJ, Hong S, Yoo SH, Kim GW. Cyanidin-3-O-sambubioside from Acanthopanax sessiliflorus fruit inhibits metastasis by downregulating MMP-9 in breast cancer cells MDA-MB-231. Planta Med. 2013;79:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Yoshimaru T, Komatsu M, Tashiro E, Imoto M, Osada H, Miyoshi Y, Honda J, Sasa M, Katagiri T. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Sci Rep. 2014;4:7355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Lewandowska U, Szewczyk K, Owczarek K, Hrabec Z, Podsędek A, Sosnowska D, Hrabec E. Procyanidins from evening primrose (Oenothera paradoxa) defatted seeds inhibit invasiveness of breast cancer cells and modulate the expression of selected genes involved in angiogenesis, metastasis, and apoptosis. Nutr Cancer. 2013;65:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, Li HB. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 450] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 52. | Rezasoltani S, Yadegar A, Asadzadeh Aghdaei H, Reza Zali M. Modulatory effects of gut microbiome in cancer immunotherapy: A novel paradigm for blockade of immune checkpoint inhibitors. Cancer Med. 2021;10:1141-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 654] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 54. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1693] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 55. | Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 56. | Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1367] [Cited by in RCA: 1233] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 57. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2520] [Article Influence: 252.0] [Reference Citation Analysis (0)] |
| 58. | Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1979] [Cited by in RCA: 2806] [Article Influence: 280.6] [Reference Citation Analysis (1)] |
| 59. | Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 2104] [Article Influence: 300.6] [Reference Citation Analysis (1)] |
| 60. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3264] [Article Influence: 466.3] [Reference Citation Analysis (0)] |
| 61. | Kim E, Ahn H, Park H. A review on the role of gut microbiota in immune checkpoint blockade therapy for cancer. Mamm Genome. 2021;32:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, Impera V, Dubois M, Persano M, Migliari M, Pretta A, Liscia N, Astara G, Scartozzi M. Immune Checkpoint Inhibitors in the Treatment of HCC. Front Oncol. 2020;10:601240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 63. | Lai E, Astara G, Ziranu P, Pretta A, Migliari M, Dubois M, Donisi C, Mariani S, Liscia N, Impera V, Persano M, Tolu S, Balconi F, Pinna G, Spanu D, Pireddu A, Saba G, Camera S, Musio F, Puzzoni M, Pusceddu V, Madeddu C, Casadei Gardini A, Scartozzi M. Introducing immunotherapy for advanced hepatocellular carcinoma patients: Too early or too fast? Crit Rev Oncol Hematol. 2021;157:103167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Dubois M, Persano M, Balconi F, Dubois M, Donisi C, Impera V, Liscia N, Mariani S, Migliari M, Musio F, Pinna G, Pireddu A, Pretta A, Saba G, Spanu D, Tolu S, Demurtas L, Puzzoni M, Ziranu P, Lai E, Astara G, Scartozzi M. Combining Immunotherapy with Anti-angiogenic Therapy: The New Standard of Care for Hepatocellular Cancer? J Clin Oncol Ther. 2021;3:117. [DOI] [Full Text] |
| 65. | Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC, Lei CH, Yeh CP, Hsu C, Hsu CH, Lin ZZ, Shao YY, Lu LC, Liu TH, Chen CH, Wu MS, Huang YH, Cheng AL. An Exploratory Study for the Association of Gut Microbiome with Efficacy of Immune Checkpoint Inhibitor in Patients with Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:809-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, Zhang X, Gong JF, Li J, Lu M, Wang X, Zhou J, Lu Z, Zhang Q, Tzeng DTW, Bi D, Tan Y, Shen L. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol Res. 2020;8:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 67. | Temraz S, Nassar F, Kreidieh F, Mukherji D, Shamseddine A, Nasr R. Hepatocellular Carcinoma Immunotherapy and the Potential Influence of Gut Microbiome. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Moreno-Gonzalez M, Beraza N. The Role of the Microbiome in Liver Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Khan MT, van Dijl JM, Harmsen HJ. Antioxidants keep the potentially probiotic but highly oxygen-sensitive human gut bacterium Faecalibacterium prausnitzii alive at ambient air. PLoS One. 2014;9:e96097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1304] [Article Influence: 144.9] [Reference Citation Analysis (0)] |
| 71. | Chassard C, de Wouters T, Lacroix C. Probiotics tailored to the infant: a window of opportunity. Curr Opin Biotechnol. 2014;26:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | de Vos WM. Fame and future of faecal transplantations--developing next-generation therapies with synthetic microbiomes. Microb Biotechnol. 2013;6:316-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: 'RePOOPulating' the gut. Microbiome. 2013;1:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 523] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 74. | Donor Recruitment for Fecal Microbiota Transplantation | Inflammatory Bowel Diseases |Oxford Academic [Internet]. [cited 2018 Feb 19]. Available from: https://academic.oup.com/ibdjournal/article/21/7/1600/4604260. |
| 75. | Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 76. | Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-98; quiz 499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1185] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 77. | Konturek PC, Haziri D, Brzozowski T, Hess T, Heyman S, Kwiecien S, Konturek SJ, Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol. 2015;66:483-491. [PubMed] |
| 78. | Peng Z, Xiang J, He Z, Zhang T, Xu L, Cui B, Li P, Huang G, Ji G, Nie Y, Wu K, Fan D, Zhang F. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc Int Open. 2016;4:E610-E613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 79. | Ding X, Li Q, Li P, Zhang T, Cui B, Ji G, Lu X, Zhang F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019;42:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 80. | Wang JW, Wang YK, Zhang F, Su YC, Wang JY, Wu DC, Hsu WH. Initial experience of fecal microbiota transplantation in gastrointestinal disease: A case series. Kaohsiung J Med Sci. 2019;35:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, Chang HJ, Coward S, Goodman KJ, Xu H, Madsen K, Mason A, Wong GK, Jovel J, Patterson J, Louie T. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2017;318:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 82. | Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 83. | Staley C, Hamilton MJ, Vaughn BP, Graiziger CT, Newman KM, Kabage AJ, Sadowsky MJ, Khoruts A. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. Am J Gastroenterol. 2017;112:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 84. | Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327-37.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 85. | Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, Cheon GJ, Choi DH, Ham YL, Shin DH, Kim EJ. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 87. | Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, Sharma MK, Maiwall R, Jindal A, Choudhary A, Hussain MS, Sharma S, Sarin SK. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 88. | Philips CA, Phadke N, Ganesan K, Augustine P. Healthy donor faecal transplant for corticosteroid non-responsive severe alcoholic hepatitis. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 90. | Bajaj JS, Kakiyama G, Savidge T, Takei H, Kassam ZA, Fagan A, Gavis EA, Pandak WM, Nittono H, Hylemon PB, Boonma P, Haag A, Heuman DM, Fuchs M, John B, Sikaroodi M, Gillevet PM. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology. 2018;68:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 91. | Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol. 2017;23:6983-6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 92. | Kao D, Roach B, Park H, Hotte N, Madsen K, Bain V, Tandon P. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology. 2016;63:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 93. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 451] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 94. | Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 972] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 95. | Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, Zuo X, Pan H, Zheng J, Wang F. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232-4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 96. | Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6:34055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 97. | Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 98. | Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 99. | Schmidt TSB, Raes J, Bork P. The Human Gut Microbiome: From Association to Modulation. Cell. 2018;172:1198-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 100. | Hornung BVH, Zwittink RD, Ducarmon QR, Kuijper EJ. Response to: 'Circulating microbiome in blood of different circulatory compartments' by Schierwagen et al. Gut. 2020;69:789-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1660] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 102. | Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, Matsumoto S, Inoue K, Muto M. Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw Open. 2020;3:e202895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 103. | Zhong C, Li Y, Yang J, Jin S, Chen G, Li D, Fan X, Lin H. Immunotherapy for Hepatocellular Carcinoma: Current Limits and Prospects. Front Oncol. 2021;11:589680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |