Published online Jul 27, 2022. doi: 10.4254/wjh.v14.i7.1291
Peer-review started: January 13, 2022
First decision: April 16, 2022
Revised: April 27, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: July 27, 2022
Processing time: 194 Days and 22.8 Hours
The diabetogenic potential of liver cirrhosis (LC) has been known for a long time, and the name "hepatogenous diabetes" (HD) was coined in 1906 to define the condition. Diabetes mellitus (DM) that develops as a consequence of LC is referred to as HD. In patients with LC, the prevalence rates of HD have been reported to vary from 21% to 57%. The pathophysiological basis of HD seems to involve insulin resistance (IR) and pancreatic β-cell dysfunction. The neuro
Core Tip: Hepatogenous diabetes appears to be the most prevalent form of diabetes in patients with liver cirrhosis. It is linked to the pathophysiological alterations and severity of cirrhosis. However, it is still an underappreciated problem and is not recognized as a distinct entity by scientific organizations. This article discusses the current state of knowledge about hepatogenous diabetes, including evidence of its existence and clinical implications.
- Citation: Kumar R, García-Compeán D, Maji T. Hepatogenous diabetes: Knowledge, evidence, and skepticism. World J Hepatol 2022; 14(7): 1291-1306
- URL: https://www.wjgnet.com/1948-5182/full/v14/i7/1291.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i7.1291
The maintenance of glucose homeostasis necessitates a coordinated response of insulin secretion, hepatic and peripheral glucose uptake, and suppression of hepatic glucose synthesis. The same is achieved via a complex control process involving several tissues and inter-organ crosstalk, including the liver, pancreas, muscles, and adipose tissues, as well as a number of circulating factors[1]. The liver plays a key role in glucose homeostasis by regulating multiple glucose metabolism pathways such as glycolysis, glycogenolysis, gluconeogenesis, and glycogenesis[2-4]. Therefore, hepatic dysfunction is likely to have an impact on glucose metabolism. In fact, the association between liver cirrhosis (LC) and diabetes mellitus (DM) has been known for a long time[5,6]. The prevalence of diabetes in patients with LC ranges from 20% to 70%, which is significantly higher than the 6.28% prevalence of type 2 DM (T2DM) in the general population[7,8]. The wide range of reported prevalence rates appears to be due to heterogeneity in the studied population, stage of liver disease, and evaluation method(s).
In 1906, Naunyn first coined the term “hepatogenous diabetes” (HD) to describe DM caused by LC[9]. In the subsequent years, the association between DM and LC was studied more thoroughly, with hyperinsulinemia, insulin resistance (IR), and pancreatic-β-cell dysfunctions being commonly reported[10-14]. Although cirrhosis due alcohol, non-alcoholic fatty liver disease (NAFLD), hepatitis C viruses (HCV), and hemochromatosis has been deemed a diabetogenic condition, multiple studies have shown that diabetogenic potential of cirrhosis cuts across etiologies. Emerging evidences suggest that in patients with LC, a complex interplay between the liver, pancreas, skeletal muscles, gut, and adipose tissues is involved in the pathogenesis of impaired glucose tolerance (IGT) and HD[7,9,15,16]. However, despite plethora of evidence, HD is still not regarded as a distinct disease or a recognized complication of LC[7,17,18]. Such skepticism among scientific bodies appears to be paradoxical. The global acceptance of this term is important in order to spur more research in this area.
DM that develops as a result of LC is referred to as HD[19]. For HD to be diagnosed, DM must have occurred after the onset of cirrhosis. In practice, however, distinguishing HD from T2DM can be challenging, especially in early cirrhosis, because both DM and cirrhosis have a long, indolent, and clinically silent course, making it difficult to determine which condition appeared first. Furthermore, the association between diabetes and LC is bidirectional, as patients with T2DM can develop NAFLD which may progress to cirrhosis[20]. Certain etiological agents of LC such as ethanol, NAFLD, HCV, and hemochromatosis, have a direct diabetogenic effect which can lead to DM even before onset of cirrhosis, posing a classification dilemma. Therefore, there is an unmet need to develop a consensus-based criteria for defining HD in LC patients in order to ensure consistency in future clinical research.
There are a number of soft indicators that can help distinguish HD from T2DM[7,17-19]. Unlike T2DM, HD can occur in patients with LC who don't have metabolic risk factors including a high body mass index, hyperlipidemia, or a family history of diabetes. HD patients frequently have normal fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) but abnormal oral glucose tolerance tests (OGTTs), whereas T2DM patients often have high FBG. In addition, the degree of hyperinsulinemia and IR is substantially higher in HD than in T2DM patients (Table 1).
| Following characteristics favour a diagnosis of HD |
| Occurrence after the onset of liver cirrhosis |
| Low prevalence of metabolic risk factors1 or a family history of DM |
| Normal fasting glycemia but abnormal oral glucose tolerance test |
| Low prevalence of microvascular complications, such as diabetic retinopathy |
| Associated with higher levels of hyperinsulinemia, insulin resistance, and an increased risk of hypoglycemia due to high glycemic variability |
| Higher association with the severity of liver cirrhosis and liver related complications |
| Remission after a liver transplantation |
The prevalence of all types of DM (T2DM + HD) in patients with LC has been reported to range from 20% to 70%[7]. Overall, the prevalence of DM varies depending on the etiology of LC. In a recent systematic review of 58 studies (n = 9705), the overall prevalence of DM in adult patients with LC was 31%. Patients with NAFLD-cirrhosis had the highest prevalence of diabetes (56%), followed by cryptogenic cirrhosis (51%), while patients with HCV and HBV cirrhosis had 32.2% and 22.2%, respectively[21]. Due to multiple shared risk factors, the prevalence of DM is higher in metabolic cirrhosis than in viral cirrhosis. However, because HD in its true sense refers to diabetes induced by liver dysfunction per se, the etiology of cirrhosis may have little bearing on its occurrence. Many studies, however, have not reported the differential prevalence of T2DM and HD. In studies where prevalence of HD was specifically looked at using OGTT, the rates ranged from 21% to 57% (Table 2). Wang et al[22] and Ramachandran et al[23] reported HD prevalence rates of 15.9% and 29.2%, respectively, based on clinical history alone, i.e., onset of DM after diagnosis of LC. The relatively lower prevalence rates of HD in their studies signify the relevance of performing an OGTT. To detect DM in LC patients, an OGTT is required because FBG and HbA1c levels may be erroneously low[24,25]. LC patients who have normal FBG and HbA1c values but an abnormal OGTT are likely to have HD. Because of the pathophysiological differences as well as clinical and therapeutic ramifications, HD must be distinguished from T2DM.
| Ref. | Patients (n) | Diagnostic method | HD, n (%) | IGT, n (%) |
| Holstein et al[31] | 35 | OGTT | 20 (57) | 13 (37) |
| Tietge et al[114] | 100 | OGTT | 35 (35)1 | 38 (38) |
| Nishida et al[25] | 46 | OGTT | 21 (38)1 | 13 (23) |
| García-Compeán et al[30] | 130 | OGTT | 28 (21.5) | 36 (38.5) |
| Jeon et al[29] | 195 | OGTT | 108 (55.4) | 169 (86.7) |
| Ramachandran et al[23] | 202 | Clinical history2 | 59 (29.2) | NS |
| Wang et al[22] | 207 | Clinical history2 | 33 (15.97) | NS |
| Vasepalli et al[28] | 121 | OGTT | 52 (42.9) | 58 (47.9) |
The severity of liver disease appears to influence the prevalence of DM in LC[26,27]. In a prospective study on compensated LC patients with a normal glucose tolerance (n = 100) at baseline, a diabetic response to OGTT was noted in 4.4% and 21.2% after a 1-year and 4-year follow-up, respectively. The incidence of DM was even higher (35.3% at 2 years) among patients whose Child-Pugh class worsened during follow-up. Notably, the incidence of diabetes was unaffected by gender, etiology, or a family history of diabetes, suggesting that diabetes was likely to be hepatogenous[26]. In another study, DM was present in 20.5%, 56%, and 61% in Child Pugh class A, B, and C, respectively[27]. The presence of HD was significantly related to a higher model for end stage liver disease (MELD) scores (> 15), large varices, and hepatocellular carcinoma (HCC) in a study[28]. HD was significantly associated with a high Child-Pugh's scores [odds ratio (OR) = 1.43] and hepatic venous pressure gradients (HVPG) (OR = 1.15) in a study by Jeon et al[29]. García-Compean et al[19] found that renal impairment and family history of DM were only two factors significantly differed between T2DM and HD[30]. Holstein et al[31] reported a very high prevalence of HD (57%) in a study cohort in which 56% of LC patients belonged to Child-Pugh class B or C. Thus, the available evidence suggests that the severity of LC, rather than the etiology, influences the development of HD[26-29]. In summary, HD seems to constitute a significant proportion of DM in patients with LC. The worsening diabetogenic potential of LC in parallel with the severity of liver disease suggests a detrimental impact of liver failure on glucose metabolism.
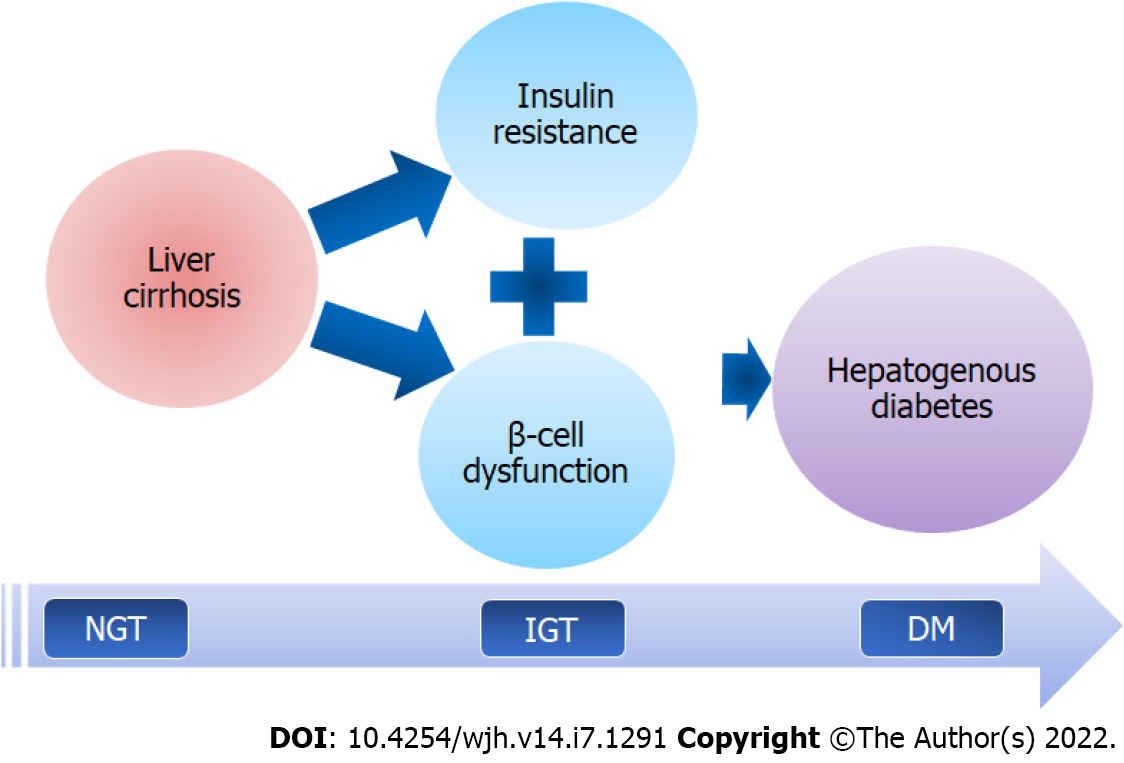
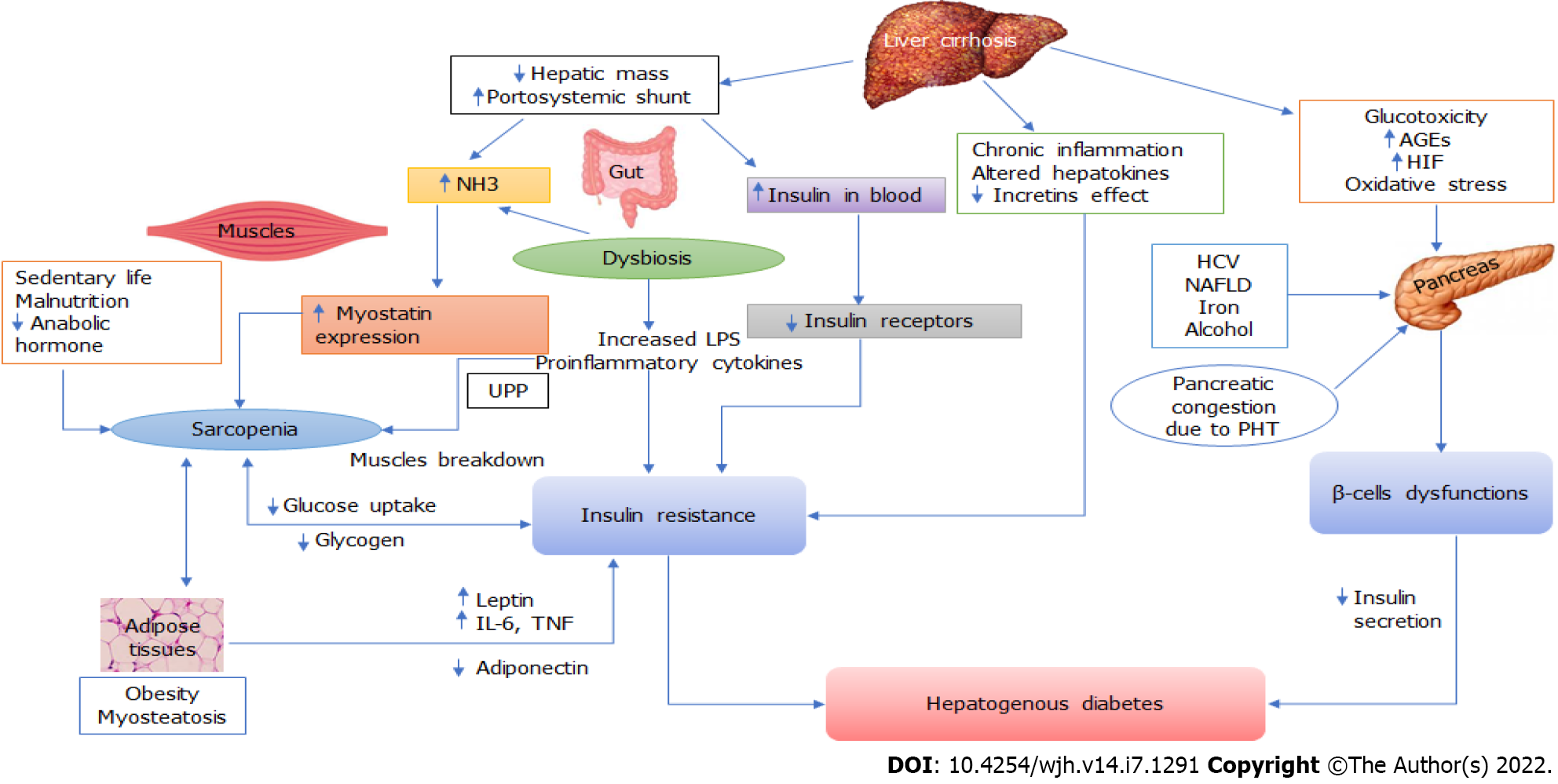
The pathophysiology of HD is complex and poorly understood. This appears to be caused by two major factors: IR and pancreatic β-cell dysfunction (Figure 1). The development of IR is triggered by neurohormonal changes, endotoxemia, and chronic inflammation of LC. The toxic effects eventually reach the pancreatic islets, causing β-cell dysfunction, which leads to the development of HD. Recently, roles of hepatokines, adipokines, gut dysbiosis, hyperammonemia, sarcopenia and myosteatosis have emerged in the pathogenesis of metabolic disturbances in LC, including IR and glucose intolerance (Figure 2). Thus, the identification of mechanisms that connect multi-organ dysfunction might unravel a novel understanding of HD pathophysiology.
Several studies have confirmed hyperinsulinemia and IR in LC patients[10-12,32]. Hyperinsulinemia appears to be caused primarily by two abnormalities: decreased hepatic extraction and portosystemic shunting of insulin. Hyperglucagonemia, due to insufficient hepatic metabolism, may also contribute to hyperinsulinemia[33]. An augmented insulin secretion due to pancreatic islet hypertrophy may contribute to hyperinsulinemia before the development of significant β-cell dysfunction[34]. Persistent hyperinsulinemia leads to IR as insulin receptors are downregulated over target cell membranes[35]. Insulin sensitivity has been reported to be normalized when hyperinsulinemia is reduced[36]. Many studies have found a link between clinically significant portal hypertension and elevated IR[37,38], which could be due to the existence of a portosystemic shunt. In LC patients, hyperinsulinemia deteriorates after the placement of a trans jugular intrahepatic portosystemic shunt (TIPS)[39]. In contrast, balloon-occluded retrograde transvenous obliteration (BRTO) of portosystemic shunts has been shown to ameliorate hyperinsulinemia in portal hypertensive patients[40].
Clamp studies of whole-body glucose utilization have shown that IR in patients with LC is due to reduction in nonoxidative glucose disposal, which includes glucose conversion to glycogen or fat, as well as anaerobic glycolysis[41,42]. Since extrahepatic glucose metabolism accounts for majority (approximately 85%) of total body glucose metabolism under glucose clamp condition, reduction in nonoxidative glucose disposal leads to significant IR at peripheral tissues[43,44]. Many other studies have consistently demonstrated that diminished insulin-dependent glucose transport into skeletal muscle and a reduction in glycogen synthesis are mainly responsible for the reduction in peripheral glucose turnover in patients with LC[45,46]. On the other hand, there appears to be no significant hepatic IR in LC[47]. Thus, skeletal muscle is the primary site of IR in patients with LC.
Despite reports of pancreatic islet cell proliferation in patients with LC, insulin-positive islet area was found to be considerably reduced[34,48,49]. In comparison to the control and T2DM groups, Sakata et al[49] found that patients with LC have lower insulin expression and higher expression of the pancreatic transcription factor PDX-1 in their islets. Studies on animal models of LC and portal hypertension have also found a decreased insulin secretion from the pancreatic islets despite hyperinsulinemia[13,14]. In a recent study, pancreas in LC patients showed congestive changes on dynamic contrast enhanced ultrasound and histopathology. In addition, decreased insulin secretion was found to be associated with pancreatic congestive changes. Despite the islets' expansion, the fraction of insulin-positive region per islet decreased, and this was negatively correlated with thickness of pancreatic vein due to portal hypertension[14]. These data indicate that even when glucose tolerance is impaired, pancreatic hyposecretion can occur in LC patients. The inability of the pancreatic β-cell to compensate for worsening IR appears signal the switch from IGT to HD. An improved β-cell function is also required for the regression of diabetes after liver transplantation (LT)[50].
Chronic hyperglycemia can produce toxic damage to the pancreatic islets, resulting in β-cells' dysfunction[51-53]. The accumulation of advanced glycation end products (AGEs), which are normally eliminated by the liver, accelerates this process by causing oxidative stress in β-cells. The systemic low-grade hypoxia generated by advanced LC contributes to the further deterioration of β-cells function[54]. Increased expression of hypoxia-inducible factors (HIF, mainly HIF-1α) has been reported in many liver diseases, including NAFLD and alcoholic liver disease[55]. HIF-1α is known to regulate cellular glucose uptake, glycolytic enzyme activity, and insulin sensitivity[56,57]. Apart from directly affecting glucose metabolism, activation of HIF-1 in patients with LC can elicit an inflammatory response in β-cells, contributing to the development of overt DM[58].
In patients with LC, a number of disease-specific mechanisms of β-cell dysfunction may be operating. Chronic alcohol use and hemochromatosis produce glucokinase downregulation and increased oxidative stress, resulting in increased β-cells apoptosis and decreased glucose-induced insulin production, respectively[59,60]. The combination of chronic hyperglycemia and high free fatty acid levels in NAFLD causes glucolipotoxicity, leading to pancreatic β-cells injury[61]. Chronic HCV infection, on the other hand, causes pancreatic islets injury by a combination of autoimmune-mediated and direct cytopathic processes[62-64].
Incretins serve an important function in maintaining glucose homeostasis. Enteroendocrine cells produce two naturally occurring incretins, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide, which regulate glycemic control by boosting insulin secretion and lowering glucagon secretion during postprandial period. Dipeptidyl peptidase 4 (DPP-4) is a membrane-associated peptidase that has a wide range of organ distribution and exhibits pleiotropic effects through its peptidase activity. DPP4 inactivates GLP-1, which leads to the development of IGT and DM[65]. Cirrhotic patients had higher serum DPP-4 activity and hepatic DPP-4 expression, which reduces incretin effects[66]. Thus, decreased incretin effects could play a role in the development of HD.
The gut microbiota is involved in the host immunity, metabolism, and intestinal endocrine function[67]. Patients with LC frequently have changes in the composition and function of the gut microbiota with associated damage to the gut barrier, bacterial translocation, and systemic inflammation[68,69]. The translocation of gut-derived endotoxins (notably lipopolysaccharide, LPS), which activates of toll-like receptors, is involved in the pathogenesis of IR[70]. Metabolic endotoxemia mediated by LPS/CD14 system dysregulates inflammatory tone, leading to diabetes and adiposity[70]. Dysbiosis of the gut has been associated with obesity, metabolic diseases, and DM[71]. Gut dysbiosis also contributes to hyperammonemia in LC patients which has some role in the development of peripheral IR[72]. The human gut microbiota produces a variety of compounds, including branched-chain amino acids, whose circulation levels are linked to the risk of IR and DM[73]. The gut microbiome of CLD patients with sarcopenia was found to be pro-diabetogenic in a recent study, with a high abundance of gram-negative bacteria containing LPS on the one hand and a low Firmicutes/Bacteroidetes ratio on the other[74]. Therefore, gut dysbiosis could play an important role in the pathogenesis of HD in advanced LC.
Hyperammonemia is a frequent abnormality in LC due to impaired hepatic detoxification to urea and bypass via portosystemic shunt. Hyperammonemia has been associated with IR for a long time[75]. Hyperammonemia in LC is linked to enhanced myostatin expression in the skeletal muscle[76]. Because myostatin is a negative regulator of muscle protein synthesis, a greater serum ammonia level can promote a rise in myostatin in skeletal muscle, leading to sarcopenia progression and its adverse consequences[77,78]. Hyperammonemia is also associated with myosteatosis which affects glucose transport and glycogen synthesis by downregulating muscle insulin receptors[79,80]. Thus, myostatin appears to have a role in cirrhosis-induced peripheral IR, as it causes sarcopenia and myosteatosis.
Skeletal muscle is responsible for the majority of postprandial glucose consumption, making it an essential insulin target organ for glucose uptake and utilization[81]. As a result, skeletal muscle loss might result in substantial IR[81,82]. Sarcopenia, or the loss of skeletal muscle mass, quality, and strength, is common in LC patients and is linked to IR and DM[83,84]. Sarcopenia in LC is caused by an imbalance in muscle protein turnover, which is influenced by a number of metabolic variables such as hyperammonemia, amino acid deficiency, hormone imbalance, gut dysbiosis, IR, and chronic inflammation[81-84]. Sarcopenia and DM appear to have a bidirectional link. On the one hand, sarcopenia is common among DM patients; on the other hand, sarcopenia has been linked to an increased risk of DM[85]. Sarcopenia is frequently accompanied by myosteatosis, mitochondrial dysfunction, macrophage infiltration, and inflammatory cytokine release, all of which contribute to IR as well as lower glucose uptake and utilization[83,84]. Previous studies have shown that skeletal muscles secrete a variety of cytokines, such as IL-6 and irisin, that regulate insulin sensitivity and promote metabolism[86,87]. Thus, impairment of muscle secretory function due to sarcopenia may contribute to the development of DM in LC patients. Sarcopenic obesity, which affects up to 35% of patients waiting for a liver transplant, has a higher influence on metabolic profile than either condition alone[88].
Hepatokines and adipokines, proteins that regulate systemic metabolism and energy homeostasis, are secreted by the liver and adipose tissues, respectively[89-91]. Crosstalk between hepatokines, adipokines, and myokines influences inflammation and fat metabolism in adipose and skeletal muscle, which can contribute to IR[90,92,93]. Additionally, some hepatokines influence insulin secretion by the pancreas, which can independently affect peripheral tissue glucose uptake and metabolism. Hepatokines are known to contribute to the pathogenesis of metabolic syndrome, NAFLD, and 2DM[90,92]. Furthermore, several hepatokines control pancreatic insulin secretion, which can affect glucose uptake and metabolism in peripheral tissues independently. Many hepatokines, including fetuin A, fetuin B, retinol-binding protein 4, and selenoprotein P, have been linked to the induction of metabolic dysfunction[93]. Therefore, their significance in metabolic abnormalities of LC is worth investigating. Resistin is an adipokine that reduces insulin sensitivity in adipocytes, skeletal muscles, and hepatocytes. Serum resistin level has been found to be significantly elevated in patients with LC, which may contribute to IR[94].
The presence of DM (HD+T2DM) in patients with LC is associated with numerous complications and poor outcomes (Table 3). Because most studies have not stratified DM into HD and T2DM, the individual impact of HD cannot be ascertained[16,18,95,96]. However, because HD is a direct complication of liver cirrhosis, it is likely to have a greater negative impact on prognosis of liver cirrhosis than T2DM.
| Ref. | Design | n | Main outcomes/remarks |
| Bianchi et al[112] | Retro-prospective | 354 | 5 yr survival: 41% with DM and 56% without DM (P = 0.005) |
| Holstein et al[31] | Prospective cohort | 52 | 51% of HD patients died within median of 5.7 yr after diagnosis of DM. Remark: No data on non-diabetic control |
| Moreau et al[136] | Prospective cohort | 75 | Survival in patients with and without DM: 18% and 58%, respectively |
| Sigal et al[97] | Cross-sectional | 65 | Incidence and severity of HE was higher in diabetics and DM was an independent risk factor for HE (P = 0.0008). Remark: study involved only HCV cirrhosis |
| Nishida et al[25] | Prospective cohort | 56 | 5 yr survival was 94%, 68% and 56%, with NGT, IGT and DM, respectively |
| Tietge et al[114] | Case-control study | 100 | Pre-transplant IGT or DM was risk factor for post-LT DM. Remark: Only 31 patients were prospectively evaluated |
| Jeon et al[29] | Prospective cohort | 195 | HD correlated significantly with HVPG and VH. Post-prandial hyperglycemia correlation with risk of VH in 6 mo |
| García-Compeán et al[113] | Prospective cohort | 100 | 5 yr cumulated survival was lower in IGT patients than NGT (31.7% vs 71.6%, P = 0.02) |
| Elkrief et al[106] | Retrospective cohort | 348 | DM was independently associated with ascites, infections, HE, HCC and mortality. Remarks: Only HCV cirrhosis studied |
| Yang et al[104] | Prospective cohort | 146 | DM was among independent predictors of VH (OR = 4.90) |
| Jepsen et al[98] | Database analysis | 863 | Diabetic patients had a higher episode of first-time overt HE and HE progression beyond grade 2 than non-diabetics. Remarks: Original trials used vaptan which could be a confounder |
| Khafaga et al[137] | Prospective case-control | 60 | Proportion of VH (46.4% vs 10%), HE (36% vs 10%) and mortality (16.6% vs 6.7%) was higher among diabetics compared to non-diabetic LC |
| Qi et al[105] | Retrospective | 145 | In-hospital mortality was 20.6% in diabetics and 4.3% in nondiabetics (P = 0.003) |
| Hoehn et al[116] | Retrospective | 12442 | Diabetic recipients had longer hospitalization (10 vs 9 d) and higher peri-transplant mortality (5% vs 4%) |
| Yang et al[110] | Retrospective cohort | 739 | DM increased the risk of HCC in non-HCV cirrhosis (HR = 2.1) |
| Routhu et al[100] | Retrospective cohort | 895 | DM was an independent predictor of HE |
| Ramachandran et al[23] | Prospective cohort | 222 | HD patients had higher incidence of gall stones (27% vs 13%) and urinary infection (28% vs 7%), compared to those without DM |
| Tergast et al[108] | Prospective | 475 | DM patients had an increased risk for SBP (HR = 1.51), especially when HbA1c values ≥ 6.4% |
| Wang et al[22] | Retrospective | 207 | Rebleeding rate following variceal endotherapy was higher (approximately 5 times) in diabetics, including HD, than non-diabetics at 1, 3, and 6 mo |
| Rosenblatt et al[109] | Retrospective (National database) | 906559 | Uncontrolled DM was associated with an increased risk of bacterial infection (OR = 1.33) and death (OR = 1.62) |
| Labenz et al[138] | Prospective cohort s | 240 | DM was independently associated with covert HE. The risk of HE and overt HE was more pronounced when HbA1c ≥ 6.5% |
Many complications of LC, including hepatic encephalopathy (HE), variceal hemorrhage (VH), sepsis, and hepatocellular carcinoma (HCC) have been associated with DM. DM has been associated with an increased incidence and severity of HE in patients with LC. In a study, the proportion of patients with severe HE was found to be higher in diabetic than in nondiabetic patients (60% vs 20%, P = 0.007)[97]. Jepsen et al[98] reported that diabetic LC patients had a higher incidence of first-time overt HE in a year (26% vs 15.8%) as well as greater risk of HE progression > grade 2 (64% vs 42%), compared to non-diabetic LC. DM is an independent predictor of HE after TIPS in LC patients[99,100]. Possible mechanisms by which DM can promote HE include induction of intestinal glutaminase, intestinal bacterial overgrowth, hyperammonemia, sepsis, and development of a chronic inflammatory state[101-103].
Chronic hyperglycemia may induce splanchnic hyperemia in LC patients leading to an increased portal pressure and risk of VH[22,29,104]. In a prospective study (n = 194), HD (55.4%) was significantly associated with increased portal pressure and risk of VH[29]. Yang et al[104] also reported DM as an independent predictor of VH in LC patients (OR = 2.99). Wang et al[22] have reported an increased risk of rebleeding following endoscopic variceal ligation in HD patients (44% vs 13.9% in 6 mo). DM also increases the mortality risk following upper gastrointestinal bleeding in LC patients (OR = 5.7)[105].
DM increases the risk of bacterial infections in patients with LC[106]. In a study on hospitalized LC patients (n = 178), the prevalence of bacterial infections was higher among diabetics than non-diabetic (85% vs 48%, P < 0.0001)[107]. DM increases the risk of spontaneous bacterial peritonitis in LC (HR = 1.51)[108]. Furthermore, uncontrolled DM in LC has greater risk of bacterial infection, suggesting that glycemic control could be a modifiable target[108,109]. DM is also a risk factor for HCC in LC patients. In a cohort study, DM increased the risk of HCC in patients with non-HCV cirrhosis (HR = 2.1) but not in HCV-cirrhosis, who already have a very high risk of developing HCC[110]. Takahashi et al[111] have reported that 2-hours post-glucose-challenge hyperglycemia was significant factor for HCC development in HCV-RNA–positive patients (HR = 6.9).
The survival rate in patients with LC is significantly reduced in presence of DM[25,106,112,113]. Bianchi et al[112] reported that 5-year survival of LC patients with or without DM was 41% and 56%, respectively, P = 0.005). In a prospective study on 100 compensated LC patients, 5-year cumulated survival rates were lower (31.7% vs 71.6%) in those with abnormal OGTT normal OGTT (P = 0.02)[113]. In an another prospective study, the cumulative 5-year survival was 94.7% in LC with normal glucose tolerance compared to 56.6% in those with DM on OGTT[25]. In a recent French study, DM had a greater impact on survival in early stages of LC patients (MELD score < 10), suggesting that the severity of liver disease can mask the deleterious effect of DM[106]. In a longitudinal study, Holstein et al[31] also found that all deaths in HD patients were due to complications related to LC rather than diabetes-related complications. This could be because of advanced liver failure in HD patients which shortens the time for diabetes complications to emerge.
The pre-transplant DM also has adverse impact on outcomes of liver transplantation. Tietge et al[114] demonstrated that pre-liver transplant IGT or DM are the major risk factors for post-transplant diabetes. In a meta-analysis of 20 studies (n = 4580), impaired glucose metabolism was among the risk factors for new onset DM after LT[115]. Post-LT DM is associated with increased risk of mortality and multiple morbid outcomessuch as cardiovascular disease, infection, biliary complications, renal impairment, and graft rejection[116-118].
Ever since Naunyn first coined the term "hepatogenous diabetes" in 1906, there has been a lot of research on this subject, especially in the1970s and 1980s, but the momentum faded little bit and the term HD began to lose its recognition and appeal. To date, the most scientific bodies, such as the American Diabetes Association (ADA) and the American Association for the Study of Liver Disease (AASLD), do not recognize HD as a distinct entity. As a result, HD is underestimated by most medical fraternity belonging to gastroenterology and endocrinology departments. There are no consensus-based diagnostic criteria or therapeutic guidelines for HD. Such skepticism does not appear to be justified. A strong link between LC and diabetes, several evidences of impaired glucose metabolism in LC patients, and a number of characteristics that distinguish HD from T2DM, all point to HD being a separate disease (Table 1). In addition, a number of factors have recently been identified as playing a role in the pathogenesis of impaired glucose metabolism in LC, including sarcopenia, sarcopenic obesity, gut dysbiosis, hyperammonemia, and hepato-adipokines. We believe that the time has arrived for scientific bodies to acknowledge HD as a distinct entity. This will pave the road and create doors for a large number of researchers to work on this topic in greater depth.
There are no standardized guidelines for managing diabetes in LC patients. Currently, T2DM and HD are being treated in a similar manner[7,20,50,96]. In general, insulin is recommended for LC at all stages, while many oral hypoglycemic agents (OHA) are being used in LC up to Child-Pugh class B. A number of pathophysiological changes caused by LC, such as changes in the hepatic blood flow, fluid balance, hypoalbuminemia, and gut dysbiosis, might impact the bioavailability, distribution, and metabolism of antidiabetic medicines, posing a risk to patients. As a result, the majority of current OHA are considered unsafe for LC in Child-Pugh class-C, the stage which has the highest frequency of HD. Because the pathophysiology of T2DM and HD differs, the therapeutic approach should differ accordingly. However, because the pathophysiology of T2DM and HD differs, the therapeutic approach may need to be adjusted. Several pathophysiological changes produced by cirrhosis, such as degree of hepatic dysfunction, large portosystemic shunt, sarcopenia, gut dysbiosis, and hyperammonemia, all of which have an indirect impact on HD, could influence treatment choices, including drug selection (Table 4).
| Condition | Antidiabetic drug with pros and cons | Preferences |
| Obesity | Metformin, SGLT2i, and GLP-1 agonists promote weight loss; DPP-4 inhibitors are weight neutral; Sulfonylureas, Pioglitazone, and Insulin promote weight gain | Should be preferred; May be considered; Consider alternative |
| Sarcopenia | Metformin and TZD appears to have favorable effect on muscles mass; SGLT2 inhibitors, SUs (especially glibenclamide and glinides) may increase the risk of sarcopenia | Should be preferred; Consider alternative |
| Hyperammonemia/Recurrent HE | Metformin and AGIs cause reduction of blood ammonia levels and risk of HE | May be preferred |
| Renal impairment | Insulin and linagliptin appear to be safe; SGLT-2 inhibitors may be considered with dose modification. It has added diuretic advantage; Metformin increases the risk of lactic acidosis | Should be preferred; May be considered; Should be avoided |
| Hypoglycemia | Insulin in SU have high risk of hypoglycaemia; Metformin, PZD, DPP4i and SGLT2 inhibitors have low risk of hypoglycaemia | Should be avoided; May be considered |
| LC with dysplastic liver lesion/high serum AFP | Metformin decreases the risk of HCC; DPP4 inhibitors and pioglitazone inhibit HCC development in experimental model; Insulin increases risk of HCC | Should be preferred; May be consider; Should be avoided |
A moderate caloric restriction may be recommended for HD patients, particularly those who are overweight or obese. However, because of sarcopenia and sarcopenic obesity, it is important to maintain a sufficient protein intake to avoid muscle loss. Physical exercise may aid in the preservation and restoration of muscle function and mass while also improving IR. Physical exercise has also been shown to improve the HVPG and nutritional status in LC patients[119,120]. Because HD is a direct complication of LC and is associated with severity of cirrhosis, improving hepatic dysfunction and portal hypertension should be one of the important goals of HD treatment. Etiology-specific therapy (for HCV, hepatitis B, autoimmune hepatitis, etc.) and non-selective β-blocker to control portal hypertension may play a role in preventing, delaying, or attuning HD in LC patients. In a recent prospective study of 96 acute-on-chronic liver failure patients, 51 (53.1%) of whom had new-onset diabetes, most likely HD, the glycemic indices improved in one-third of patients following improvement of their liver function without taking anti-hyperglycemic medication[121].
Among the OHAs that can be considered for HD patients are metformin, glucagon-like peptide-1 (GLP-1) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, thiazolidinediones (TZD), alpha-glucosidase inhibitors (AGI) and sodium glucose co-transporter-2 (SGLT2) inhibitors[[7,20,50,96]. Glycemic targets for HD patients should be set based on postprandial glucose levels rather than HbA1c or FBG. Metformin can be an important therapeutic agent for HD, because it is free of hepatic metabolism, plasma protein binding, and hypoglycemia risk, as well as having other benefits like cardio protection and a lower risk of HCC and HE[122-124]. However, metformin should be avoided if there is concurrent renal impairment with an eGFR of less than 45 mL/min per 1.73 m2 due to the significant risk of lactic acidosis[50]. Upregulation of DPP-4 expression in LC patients contributes to the development IR[66]. Therefore, incretin-based antidiabetic agents, like GLP-1 receptor agonists (Liraglutide) and DPP-4 inhibitors can be an important agent for HD. They are generally safe in LC patients, increase muscle mass, and pose little risk of hypoglycemia or weight gain[125,126]. Recently, a group of investigators from Taiwan have raised safety concerns about use of metformin and DPP4 inhibitors in LC[127,128]. From analysis of Taiwan's National Health Insurance Research Database, investigators found that metformin use (> 1000 mg/d) in patients with compensated LC patients was associated with higher risks of mortality and decompensation[127]. Similarly, DPP-4 inhibitor was found to be associated with higher risks of hepatic decompensation and failure in another study[128]. These results should be viewed with caution, as the findings need to be validated in prospective studies. In a recent study, sulfonylureas (SU) was found to be associated with lower risks of all-cause mortality and major cardiovascular events in LC patients with diabetes[129]. However, SU should be better avoided in HD patients because of a high risk of hypoglycemia. HD patients are already at high risk of hypoglycemia due to poor glycogen storage and reduced gluconeogenesis capacity. Due to hypoglycemia, stringent glycemic control should not be attempted in HD patients.
In obese HD patients, metformin, SGLT2i, and GLP-1 agonists can be preferred because they tend to promote weight loss. When sarcopenia is severe, metformin, GLP-1 agonist (Liraglutide), and DPP-4 inhibitors are preferable[123]. SUs and SGLT2 inhibitors may increase the risk of sarcopenia[130,131]. Metformin or AGI, both of which have a positive effect on blood ammonia levels and the risk of HE, should be considered in hyperammonemic HD patients. Metformin effect is mediated partially by inhibition of glutaminase activity in enterocytes, while AGI (acarbose) stimulates the gut peristalsis and proliferation of the saccarolytic bacteria[132,133]. If there is a large portosystemic shunt in such patients, shunt occlusion using BRTO may be considered. Alteration of gut dysbiosis using probiotics is another option that requires investigation.
Insulin therapy is considered to be the safest and most effective for patients with LC, and it is currently the sole option available for LC patients of Child-Pugh class C. However, there are many concerns about the use of insulin in HD patients who have a higher degree of hyperinsulinemia and IR than LC patients with T2DM. The insulin requirements in such patients might vary greatly, making it difficult to maintain glycemic control without increasing the risk of hypoglycemia. Insulin use has also been associated with HCC in LC patients[133]. Hence, it should be avoided in patients who are at high risk of developing HCC, such as those with dysplastic liver nodules and elevated serum alpha fetoprotein levels. In a recent study, insulin use in LC patients with diabetes was found to be associated with increased risks of hypoglycemia, cardiovascular events, liver-related complications, and mortality compared to insulin nonusers[134]. Given these considerations, insulin cannot be regarded an optimal anti-diabetic treatment for LC patients, and the search for a better alternative should be prioritized.
Finally, HD should be reversible after LT because it is caused by LC. There have been reports of HD reversibility with LT, however this does not occur in all patients[135]. In one study, DM regressed in 63.9% of patients after LT, while DM never regressed in 36% of patients after two years of follow-up. The reversibility of HD appears to be determined by the level of pre-LT pancreatic ß-cell injury and its improvement after LT. Grancini et al[50] found that improved β-cell function plays a major role in favoring diabetes regression following LT, in the presence of a sustained improvement of IR. With progression of LC, progressive accumulation of toxic materials (AGEs, HIF, etc.) may lead to severe non-repairable ß-cells injury, making the chances of HD reversibility less likely. The diabetogenic potential of immunosuppressive therapies could also be one of the reasons behind non-reversibility of diabetes following LT.
In conclusion, the evidence suggests that patients with LC can have two forms of diabetes: T2DM and HD, with HD appearing to be the predominant type. HD is a direct complication of LC since it is strongly linked to the pathophysiological alterations and severity of LC. However, HD is still an underappreciated problem that isn't even recognized as a separate entity by scientific organizations. To maintain consistency in clinical research, future directions will first require recognition of HD as a distinct entity, followed by the creation of a consensus definition for HD. Understanding the complex pathophysiology of LC leading to HD, including changes in the liver-multiorgan cross-talk, will also be critical for providing evidence-based management recommendations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tamori A, Japan; Wang CR, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020;472:1273-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 2. | Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 504] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 3. | Roden M, Bernroider E. Hepatic glucose metabolism in humans--its role in health and disease. Best Pract Res Clin Endocrinol Metab. 2003;17:365-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C. Liver glucose metabolism in humans. Biosci Rep. 2016;36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | del Olmo JA, Serra MA, Rodrigo JM. Liver cirrhosis and diabetes mellitus. J Hepatol. 1996;24:645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 6. | Muting D, Wohlgemuth D, Dorsett R. Liver cirrhosis and diabetes mellitus. Geriatrics. 1969;24:91-99. [PubMed] |
| 7. | Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 8. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1650] [Article Influence: 412.5] [Reference Citation Analysis (2)] |
| 9. | Perseghin G, Mazzaferro V, Sereni LP, Regalia E, Benedini S, Bazzigaluppi E, Pulvirenti A, Leão AA, Calori G, Romito R, Baratti D, Luzi L. Contribution of reduced insulin sensitivity and secretion to the pathogenesis of hepatogenous diabetes: effect of liver transplantation. Hepatology. 2000;31:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 10. | Johnson DG, Alberti KG, Faber OK, Binder C. Hyperinsulinism of hepatic cirrhosis: Diminished degradation or hypersecretion? Lancet. 1977;1:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Letiexhe MR, Scheen AJ, Gérard PL, Bastens BH, Pirotte J, Belaiche J, Lefèbvre PJ. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab. 1993;77:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinaemia in Child's disease grade B liver cirrhosis investigated in free living conditions. Gut. 2002;51:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 13. | Gomis R, Fernández-Alvarez J, Pizcueta P, Fernández M, Casamitjana R, Bosch J, Rodés J. Impaired function of pancreatic islets from rats with portal hypertension resulting from cirrhosis and partial portal vein ligation. Hepatology. 1994;19:1257-1261. [PubMed] |
| 14. | Kuroda T, Hirooka M, Koizumi M, Ochi H, Hisano Y, Bando K, Matsuura B, Kumagi T, Hiasa Y. Pancreatic congestion in liver cirrhosis correlates with impaired insulin secretion. J Gastroenterol. 2015;50:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 15. | Armandi A, Rosso C, Caviglia GP, Bugianesi E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 16. | Nath P, Anand AC. Hepatogenous Diabetes: A Primer. J Clin Exp Hepatol. 2021;11:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 17. | García-Compeán D, González-González JA, Lavalle-González FJ, González-Moreno EI, Villarreal-Pérez JZ, Maldonado-Garza HJ. Hepatogenous diabetes: Is it a neglected condition in chronic liver disease? World J Gastroenterol. 2016;22:2869-2874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kumar R. Hepatogenous Diabetes: An Underestimated Problem of Liver Cirrhosis. Indian J Endocrinol Metab. 2018;22:552-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8:13-20. [PubMed] |
| 20. | Kumar R, Priyadarshi RN, Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J Clin Transl Hepatol. 2020;8:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Lee WG, Wells CI, McCall JL, Murphy R, Plank LD. Prevalence of diabetes in liver cirrhosis: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 22. | Wang X, Mei X, Kong D. Effects of diabetes on the rebleeding rate following endoscopic treatment in patients with liver cirrhosis. Exp Ther Med. 2020;20:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 23. | Ramachandran TM, Rajneesh AHR, Zacharia GS, Adarsh RP. Cirrhosis of Liver and Diabetes Mellitus: The Diabolic Duo? J Clin Diagn Res. 2017;11:OC01-OC05. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 24. | Imano E, Nishida T, Shibata M, Kanda T. Significance of oral glucose tolerance test for the diagnosis of diabetes mellitus in patients with liver cirrhosis. Intern Med. 1999;38:918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, Suzuki M, Kanda T, Kawano S, Hiramatsu N, Hayashi N, Hori M. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 26. | Gentile S, Loguercio C, Marmo R, Carbone L, Del Vecchio Blanco C. Incidence of altered glucose tolerance in liver cirrhosis. Diabetes Res Clin Pract. 1993;22:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Grancini V, Trombetta M, Lunati ME, Zimbalatti D, Boselli ML, Gatti S, Donato MF, Resi V, D'Ambrosio R, Aghemo A, Pugliese G, Bonadonna RC, Orsi E. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: Role of severity of liver disease. J Hepatol. 2015;63:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Vasepalli P, Noor MT, Thakur BS. Hepatogenous Diabetes - A Report from Central India. J Clin Exp Hepatol. 2022;12:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Jeon HK, Kim MY, Baik SK, Park HJ, Choi H, Park SY, Kim BR, Hong JH, Jo KW, Shin SY, Kim JM, Kim JW, Kim HS, Kwon SO, Kim YJ, Cha SH, Kim DJ, Suk KT, Cheon GJ, Kim YD, Choi DH, Lee SJ. Hepatogenous diabetes in cirrhosis is related to portal pressure and variceal hemorrhage. Dig Dis Sci. 2013;58:3335-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, Vázquez-Elizondo G, Villarreal-Pérez JZ, Maldonado-Garza HJ. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann Hepatol. 2012;11:240-248. [PubMed] |
| 31. | Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Bosch J, Gomis R, Kravetz D, Casamitjana R, Terés J, Rivera F, Rodés J. Role of spontaneous portal-systemic shunting in hyperinsulinism of cirrhosis. Am J Physiol. 1984;247:G206-G212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Sherwin R, Joshi P, Hendler R, Felig P, Conn HO. Hyperglucagonemia in Laennec's cirrhosis. The role of portal-systemic shunting. N Engl J Med. 1974;290:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 156] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Saitoh M. [Studies on histopathology of pancreas in portal hypertension]. Nihon Shokakibyo Gakkai Zasshi. 1984;81:1444-1452. [PubMed] |
| 35. | Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31 Suppl 2:S262-S268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 554] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 36. | Petrides AS, Stanley T, Matthews DE, Vogt C, Bush AJ, Lambeth H. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology. 1998;28:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Erice E, Llop E, Berzigotti A, Abraldes JG, Conget I, Seijo S, Reverter E, Albillos A, Bosch J, García-Pagán JC. Insulin resistance in patients with cirrhosis and portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1458-G1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Zardi EM, Di Matteo FM, Pacella CM, Sanyal AJ. Invasive and non-invasive techniques for detecting portal hypertension and predicting variceal bleeding in cirrhosis: a review. Ann Med. 2014;46:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Deschênes M, Somberg KA. Effect of transjugular intrahepatic portosystemic shunt (TIPS) on glycemic control in cirrhotic patients with diabetes mellitus. Am J Gastroenterol. 1998;93:483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Ishikawa T, Shiratsuki S, Matsuda T, Iwamoto T, Takami T, Uchida K, Terai S, Yamasaki T, Sakaida I. Occlusion of portosystemic shunts improves hyperinsulinemia due to insulin resistance in cirrhotic patients with portal hypertension. J Gastroenterol. 2014;49:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 41. | Petrides AS, Groop LC, Riely CA, DeFronzo RA. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest. 1991;88:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Müller MJ, Fenk A, Lautz HU, Selberg O, Canzler H, Balks HJ, von zur Mühlen A, Schmidt E, Schmidt FW. Energy expenditure and substrate metabolism in ethanol-induced liver cirrhosis. Am J Physiol. 1991;260:E338-E344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Petrides AS, DeFronzo RA. Glucose metabolism in cirrhosis: a review with some perspectives for the future. Diabetes Metab Rev. 1989;5:691-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Meyer-Alber A, Hartmann H, Stümpel F, Creutzfeldt W. Mechanism of insulin resistance in CCl4-induced cirrhosis of rats. Gastroenterology. 1992;102:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Selberg O, Burchert W, vd Hoff J, Meyer GJ, Hundeshagen H, Radoch E, Balks HJ, Müller MJ. Insulin resistance in liver cirrhosis. Positron-emission tomography scan analysis of skeletal muscle glucose metabolism. J Clin Invest. 1993;91:1897-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Kruszynska Y, Williams N, Perry M, Home P. The relationship between insulin sensitivity and skeletal muscle enzyme activities in hepatic cirrhosis. Hepatology. 1988;8:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Proietto J, Alford FP, Dudley FJ. The mechanism of the carbohydrate intolerance of cirrhosis. J Clin Endocrinol Metab. 1980;51:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Takei K, Suda K. [Study of mechanisms of pancreatic fibrosis and structural changes in liver cirrhotic patients]. Nihon Shokakibyo Gakkai Zasshi. 1997;94:92-100. [PubMed] |
| 49. | Sakata M, Kawahara A, Kawaguchi T, Akiba J, Taira T, Taniguchi E, Abe M, Koga H, Kage M, Sata M. Decreased expression of insulin and increased expression of pancreatic transcription factor PDX-1 in islets in patients with liver cirrhosis: a comparative investigation using human autopsy specimens. J Gastroenterol. 2013;48:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Grancini V, Trombetta M, Lunati ME, Boselli ML, Gatti S, Donato MF, Palmieri E, Resi V, Pugliese G, Bonadonna RC, Orsi E. Central role of the β-cell in driving regression of diabetes after liver transplantation in cirrhotic patients. J Hepatol. 2019;70:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 51. | Kruszynska YT, Goulas S, Wollen N, McIntyre N. Insulin secretory capacity and the regulation of glucagon secretion in diabetic and non-diabetic alcoholic cirrhotic patients. J Hepatol. 1998;28:280-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Picardi A, D'Avola D, Gentilucci UV, Galati G, Fiori E, Spataro S, Afeltra A. Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab Res Rev. 2006;22:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (4)] |
| 53. | Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 185] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Moreau R, Lee SS, Soupison T, Roche-Sicot J, Sicot C. Abnormal tissue oxygenation in patients with cirrhosis and liver failure. J Hepatol. 1988;7:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 56. | Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 317] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 57. | Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 58. | Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O'Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 59. | Kim JY, Song EH, Lee HJ, Oh YK, Park YS, Park JW, Kim BJ, Kim DJ, Lee I, Song J, Kim WH. Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem. 2010;285:37251-37262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305-5312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Weir GC. Glucolipotoxicity, β-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes. 2020;69:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 62. | Antonelli A, Ferrari SM, Ruffilli I, Fallahi P. Cytokines and HCV-related autoimmune disorders. Immunol Res. 2014;60:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Bose SK, Ray R. Hepatitis C virus infection and insulin resistance. World J Diabetes. 2014;5:52-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 64. | Chen J, Wang F, Zhou Y, Jiang J, Ksimu S, Zhang X, Li JZ, Niu J, Wang Q. Chronic hepatitis C virus infection impairs insulin secretion by regulation of p38δ MAPK-dependent exocytosis in pancreatic β-cells. Clin Sci (Lond). 2020;134:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2842] [Article Influence: 149.6] [Reference Citation Analysis (1)] |
| 66. | Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013;19:2298-2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 129] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 67. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2321] [Article Influence: 257.9] [Reference Citation Analysis (0)] |
| 68. | Bajaj JS. Altered Microbiota in Cirrhosis and Its Relationship to the Development of Infection. Clin Liver Dis (Hoboken). 2019;14:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Muñoz L, Borrero MJ, Úbeda M, Conde E, Del Campo R, Rodríguez-Serrano M, Lario M, Sánchez-Díaz AM, Pastor O, Díaz D, García-Bermejo L, Monserrat J, Álvarez-Mon M, Albillos A. Intestinal Immune Dysregulation Driven by Dysbiosis Promotes Barrier Disruption and Bacterial Translocation in Rats With Cirrhosis. Hepatology. 2019;70:925-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 70. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4562] [Article Influence: 253.4] [Reference Citation Analysis (1)] |
| 71. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2012] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 72. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 73. | Gojda J, Cahova M. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 74. | Yamamoto K, Ishizu Y, Honda T, Ito T, Imai N, Nakamura M, Kawashima H, Kitaura Y, Ishigami M, Fujishiro M. Patients with low muscle mass have characteristic microbiome with low potential for amino acid synthesis in chronic liver disease. Sci Rep. 2022;12:3674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 75. | Schlienger JL, Imler M. Effect of hyperammonemia on insulin-mediated glucose uptake in rats. Metabolism. 1978;27:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 76. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 77. | Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Takata R, Iijima H, Nishiguchi S. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle. 2017;8:915-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 78. | García PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 79. | Thandassery RB, Montano-Loza AJ. Role of Nutrition and Muscle in Cirrhosis. Curr Treat Options Gastroenterol. 2016;14:257-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 80. | Correa-de-Araujo R, Addison O, Miljkovic I, Goodpaster BH, Bergman BC, Clark RV, Elena JW, Esser KA, Ferrucci L, Harris-Love MO, Kritchevsky SB, Lorbergs A, Shepherd JA, Shulman GI, Rosen CJ. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front Physiol. 2020;11:963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (2)] |
| 81. | Merz KE, Thurmond DC. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr Physiol. 2020;10:785-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 82. | Zhang H, Lin S, Gao T, Zhong F, Cai J, Sun Y, Ma A. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 83. | Marasco G, Dajti E, Ravaioli F, Brocchi S, Rossini B, Alemanni LV, Peta G, Bartalena L, Golfieri R, Festi D, Colecchia A, Renzulli M. Clinical impact of sarcopenia assessment in patients with liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2021;15:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 85. | Son JW, Lee SS, Kim SR, Yoo SJ, Cha BY, Son HY, Cho NH. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES. Diabetologia. 2017;60:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 86. | Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 1897] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 87. | Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 430] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 88. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 89. | Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40:1367-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (2)] |
| 90. | de Oliveira Dos Santos AR, de Oliveira Zanuso B, Miola VFB, Barbalho SM, Santos Bueno PC, Flato UAP, Detregiachi CRP, Buchaim DV, Buchaim RL, Tofano RJ, Mendes CG, Tofano VAC, Dos Santos Haber JF. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 91. | Kucukoglu O, Sowa JP, Mazzolini GD, Syn WK, Canbay A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J Hepatol. 2021;74:442-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (2)] |
| 92. | Shi J, Fan J, Su Q, Yang Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front Endocrinol (Lausanne). 2019;10:703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 93. | Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (2)] |
| 94. | Yagmur E, Trautwein C, Gressner AM, Tacke F. Resistin serum levels are associated with insulin resistance, disease severity, clinical complications, and prognosis in patients with chronic liver diseases. Am J Gastroenterol. 2006;101:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Coman LI, Coman OA, Bădărău IA, Păunescu H, Ciocîrlan M. Association between Liver Cirrhosis and Diabetes Mellitus: A Review on Hepatic Outcomes. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 97. | Sigal SH, Stanca CM, Kontorinis N, Bodian C, Ryan E. Diabetes mellitus is associated with hepatic encephalopathy in patients with HCV cirrhosis. Am J Gastroenterol. 2006;101:1490-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Jepsen P, Watson H, Andersen PK, Vilstrup H. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J Hepatol. 2015;63:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 99. | Yin X, Zhang F, Xiao J, Wang Y, He Q, Zhu H, Leng X, Zou X, Zhang M, Zhuge Y. Diabetes mellitus increases the risk of hepatic encephalopathy after a transjugular intrahepatic portosystemic shunt in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Routhu M, Safka V, Routhu SK, Fejfar T, Jirkovsky V, Krajina A, Cermakova E, Hosak L, Hulek P. Observational cohort study of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS). Ann Hepatol. 2017;16:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Ampuero J, Ranchal I, Nuñez D, Díaz-Herrero Mdel M, Maraver M, del Campo JA, Rojas Á, Camacho I, Figueruela B, Bautista JD, Romero-Gómez M. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. 2012;7:e49279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 102. | Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 103. | Basu S, Zethelius B, Helmersson J, Berne C, Larsson A, Arnlöv J. Cytokine-mediated inflammation is independently associated with insulin sensitivity measured by the euglycemic insulin clamp in a community-based cohort of elderly men. Int J Clin Exp Med. 2011;4:164-168. [PubMed] |
| 104. | Yang CH, Chiu YC, Chen CH, Tsai MC, Chuah SK, Lee CH, Hu TH, Hung CH. Diabetes mellitus is associated with gastroesophageal variceal bleeding in cirrhotic patients. Kaohsiung J Med Sci. 2014;30:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Qi X, Peng Y, Li H, Dai J, Guo X. Diabetes is associated with an increased risk of in-hospital mortality in liver cirrhosis with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2015;27:476-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R, Durand F, Marcellin P, Rautou PE, Valla D. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 107. | Diaz J, Monge E, Roman R, Ulloa V. Diabetes as a risk factor for infections in cirrhosis. Am J Gastroenterol. 2008;103:248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Tergast TL, Laser H, Gerbel S, Manns MP, Cornberg M, Maasoumy B. Association Between Type 2 Diabetes Mellitus, HbA1c and the Risk for Spontaneous Bacterial Peritonitis in Patients with Decompensated Liver Cirrhosis and Ascites. Clin Transl Gastroenterol. 2018;9:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Rosenblatt R, Atteberry P, Tafesh Z, Ravikumar A, Crawford CV, Lucero C, Jesudian AB, Brown RS Jr, Kumar S, Fortune BE. Uncontrolled diabetes mellitus increases risk of infection in patients with advanced cirrhosis. Dig Liver Dis. 2021;53:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol. 2016;111:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 111. | Takahashi H, Mizuta T, Eguchi Y, Kawaguchi Y, Kuwashiro T, Oeda S, Isoda H, Oza N, Iwane S, Izumi K, Anzai K, Ozaki I, Fujimoto K. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, González-González JA, Muñoz-Espinosa LE, Villarreal-Pérez JZ, Maldonado-Garza HJ. Subclinical abnormal glucose tolerance is a predictor of death in liver cirrhosis. World J Gastroenterol. 2014;20:7011-7018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Tietge UJ, Selberg O, Kreter A, Bahr MJ, Pirlich M, Burchert W, Müller MJ, Manns MP, Böker KH. Alterations in glucose metabolism associated with liver cirrhosis persist in the clinically stable long-term course after liver transplantation. Liver Transpl. 2004;10:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Li DW, Lu TF, Hua XW, Dai HJ, Cui XL, Zhang JJ, Xia Q. Risk factors for new onset diabetes mellitus after liver transplantation: A meta-analysis. World J Gastroenterol. 2015;21:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 116. | Hoehn RS, Singhal A, Wima K, Sutton JM, Paterno F, Steve Woodle E, Hohmann S, Abbott DE, Shah SA. Effect of pretransplant diabetes on short-term outcomes after liver transplantation: a national cohort study. Liver Int. 2015;35:1902-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, Wang T, Liang J, He S, Gao J, Zhou J, Yu M, Fan J, Gao X. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Morbitzer KA, Taber DJ, Pilch NA, Meadows HB, Fleming JN, Bratton CF, McGillicuddy JW, Baliga PK, Chavin KD. The impact of diabetes mellitus and glycemic control on clinical outcomes following liver transplant for hepatitis C. Clin Transplant. 2014;28:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, Torre A, Duarte-Rojo A. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol. 2016;7:e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 120. | Brustia R, Savier E, Scatton O. Physical exercise in cirrhotic patients: Towards prehabilitation on waiting list for liver transplantation. A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 121. | Hu H, Hu X, Tian C, Zhu Y, Liu Y, Cheng Q, Yang F, Liu J, Li Y, Lin S. Diabetes is associated with poor short-term prognosis in patients with hepatitis B virus-related acute-on-chronic liver failure. Hepatol Int. 2021;15:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (3)] |
| 122. | Wu CN, Tien KJ. The Impact of Antidiabetic Agents on Sarcopenia in Type 2 Diabetes: A Literature Review. J Diabetes Res. 2020;2020:9368583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC, Beaugrand M. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 124. | Kaplan DE, Serper M, John BV, Tessiatore KM, Lerer R, Mehta R, Fox R, Aytaman A, Baytarian M, Hunt K, Albrecht J, Taddei TH; Veterans Outcomes and Cost Associated with Liver disease Study Group. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients With Diabetes and Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:2148-2160.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 125. | Hong Y, Lee JH, Jeong KW, Choi CS, Jun HS. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J Cachexia Sarcopenia Muscle. 2019;10:903-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 126. | Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 127. | Yen FS, Huang YH, Hou MC, Hwu CM, Lo YR, Shin SJ, Hsu CC. Metformin use and cirrhotic decompensation in patients with type 2 diabetes and liver cirrhosis. Br J Clin Pharmacol. 2022;88:311-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 128. | Yen FS, Wei JC, Yip HT, Hwu CM, Hou MC, Hsu CC. Dipeptidyl peptidase-4 inhibitors may accelerate cirrhosis decompensation in patients with diabetes and liver cirrhosis: a nationwide population-based cohort study in Taiwan. Hepatol Int. 2021;15:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Yen FS, Lai JN, Wei JC, Chiu LT, Hwu CM, Hou MC, Hsu CC. Sulfonylureas may be useful for glycemic management in patients with diabetes and liver cirrhosis. PLoS One. 2020;15:e0243783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Cetrone M, Mele A, Tricarico D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr Diabetes Rev. 2014;10:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 131. | Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. 2019;10:193-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Gentile S, Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, Magliano PL, Gravina AG, Torella R. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Donadon V, Balbi M, Zanette G. Hyperinsulinemia and risk for hepatocellular carcinoma in patients with chronic liver diseases and Type 2 diabetes mellitus. Expert Rev Gastroenterol Hepatol. 2009;3:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Yen FS, Lai JN, Wei JC, Chiu LT, Hsu CC, Hou MC, Hwu CM. Is insulin the preferred treatment in persons with type 2 diabetes and liver cirrhosis? BMC Gastroenterol. 2021;21:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 135. | Shetty A, Wilson S, Kuo P, Laurin JL, Howell CD, Johnson L, Allen EM. Liver transplantation improves cirrhosis-associated impaired oral glucose tolerance. Transplantation. 2000;69:2451-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 136. | Moreau R, Delègue P, Pessione F, Hillaire S, Durand F, Lebrec D, Valla DC. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 137. | Khafaga S, Khalil K, Mohamed A, Miada M, Mahmoud S, Mohammad M. Acute Variceal Bleeding in Patients with Liver Cirrhosis with and without Diabetes. Liver Res Open. 1:14-20. [DOI] [Full Text] |
| 138. | Labenz C, Nagel M, Kremer WM, Hilscher M, Schilling CA, Toenges G, Kuchen R, Schattenberg JM, Galle PR, Wörns MA. Association between diabetes mellitus and hepatic encephalopathy in patients with cirrhosis. Aliment Pharmacol Ther. 2020;52:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |