Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1248
Peer-review started: August 8, 2021
First decision: November 11, 2021
Revised: November 18, 2021
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: June 27, 2022
Processing time: 319 Days and 7.7 Hours
Hepatitis C virus (HCV) is a leading cause of liver cirrhosis and hepatocellular carcinoma globally. Sofosbuvir/velpatasvir (SOF/VEL) is an effective pan-genotypic direct-acting antiviral combination for treatment of chronic HCV infection. While the addition of ribavirin (RBV) to SOF/VEL improved sustained virological response (SVR12) in genotype 3 (GT3) decompensated cirrhosis patients, the benefits of RBV in GT3 compensated cirrhosis patients receiving SOF/VEL remains unclear.
To evaluate the efficacy and safety of SOF/VEL, with or without RBV in GT3 compensated cirrhosis patients.
We searched four electronic databases (PubMed/Medline, Embase, Cochrane Library and Web of Science) from inception up to June 2021 using both free text and MeSH terms. There was no restriction on language, geography, publication dates and publication status (full text or abstracts). All GT3 compensated cirrhosis patients treated with 12 wk of SOF/VEL, with or without RBV, were included, regardless of age, gender or prior treatment experience. The primary outcome was sustained virological response 12-wk post-treatment (SVR12). The secondary outcome was treatment-related adverse events, as defined by symptomatic anemia requiring transfusion or a drop in hemoglobin beyond 2 g/dL. The pooled relative risk (RR), 95%CI and heterogeneity (I2) were estimated using Review Manager version 5.3.
From 1752 citations, a total of seven studies (2 randomized controlled trials, 5 cohort studies) with 1088 subjects were identified. The SVR12 was similar in GT3 compensated cirrhosis patients, regardless of the use of RBV, for both the intention-to-treat RR 1.03, 95%CI: 0.99-1.07; I2 = 0%) and the per-protocol analysis (RR: 1.03, 95%CI: 0.99-1.07; I2 = 48%). The overall pooled rate of treatment-related adverse events was 7.2%. Addition of RBV increased the pooled risk of treatment-related adverse events in GT3 compensated cirrhosis patients receiving SOF/VEL (RR: 4.20, 95%CI: 1.29-13.68; I2 = 0%). Subgroup analysis showed that RBV was associated with a higher SVR12 in GT3 compensated cirrhosis patients with baseline resistance-associated substitutions. However, addition of RBV did not significantly increase the SVR12 among treatment-experienced GT3 compensated cirrhosis patients.
Ribavirin was not associated with higher SVR12 in GT3 compensated cirrhosis patients receiving SOF/VEL. Our findings suggest a limited role for RBV as routine add-on therapy to SOF/VEL in GT3 compensated cirrhosis patients.
Core Tip: Ribavirin (RBV) as routine add-on therapy was not associated with higher sustained virological response in genotype 3 (GT3) compensated cirrhosis patients receiving sofosbuvir/velpatasvir (SOF/VEL), except in the subgroup of patients with baseline resistance-associated substitution mutation. As RBV is associated with a higher risk of treatment-related adverse event, RBV as routine add-on therapy to SOF/VEL should be reconsidered among compensated GT3 cirrhosis patients.
- Citation: Loo JH, Xu WXF, Low JT, Tay WX, Ang LS, Tam YC, Thurairajah PH, Kumar R, Wong YJ. Efficacy and safety of sofosbuvir/velpatasvir with or without ribavirin in hepatitis C genotype 3 compensated cirrhosis: A meta-analysis. World J Hepatol 2022; 14(6): 1248-1257
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1248.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1248
Hepatitis C virus (HCV) is an important cause of liver cirrhosis and hepatocellular carcinoma, affecting 71 million people globally[1]. Genotype 3 (GT3) is the second most common HCV genotype worldwide and is responsible for up to 30% of global HCV infections, especially in South and Central Asia[2,3]. GT3 HCV is associated with a higher incidence of liver steatosis[4], fibrosis progression[5] and liver cirrhosis[6]. GT3 HCV infection was also associated with poorer prognosis with an 80% increased risk of hepatocellular carcinoma[6] and 17% increased risk of all-cause mortality compared to other HCV genotypes[7].
The introduction of direct-acting antiviral (DAA) therapy has significantly improved the treatment success for HCV infection, thus providing a simplified approach for global HCV elimination. The improvement in treatment outcome was observed since the first generation of DAA, albeit to a lesser degree among GT3 HCV patients with cirrhosis or prior treatment experience[8,9]. Because of the poorer treatment response among GT3 HCV patients treated with DAA, GT3 HCV infection was considered the difficult-to-treat population. Currently, there are two approved pan-genotypic DAA regimens available, namely sofosbuvir and velpatasvir (SOF/VEL), as well as glecaprevir and pibrentasvir. While both regimens are highly efficacious with sustained virological response 12-wk post-treatment (SVR12) rates beyond 95% in most scenarios, only SOF/VEL is approved to treat decompensated HCV cirrhosis patients[10,11].
The potential of ribavirin (RBV) as add-on therapy to SOF/VEL to improve SVR12 in HCV patients remains an area of interest. Ribavirin, a guanosine nucleoside analog, has been used in HCV treatment regimens since the pre-DAA era. It is postulated that RBV interferes with viral replication by direct and indirect means. RBV directly inhibits viral mRNA polymerase by binding to the nucleotide binding site of the enzyme and indirectly, by inducing error prone mutagenesis and promoting T-helper-type-1-mediated immune responses[12]. The addition of RBV to a SOF/VEL regimen improves SVR rates where there is pre-existing baseline NS5A Y93H resistance-associated substitutions (RAS). The ASTRAL-3 study reported an SVR of 97% vs 84% in patients with or without baseline RAS[13]. Indeed, American Associated for the Study of Liver Disease (AASLD) guidelines recommend adding RBV for compensated GT3 cirrhosis with baseline RAS or decompensated HCV cirrhosis, regardless of genotype[14]. While the use of RBV significantly increases the SVR12 in decompensated cirrhosis receiving SOF/VEL[15], the benefit of RBV remains controversial among GT3 compensated cirrhosis patients. A Spanish randomized controlled trial had demonstrated a comparable SVR12 among GT3 compensated cirrhosis patients treated with SOF/VEL, regardless of the use of RBV[16].
In routine clinical practice, the application of pretreatment RAS testing for patients with GT3 compensated cirrhosis is often limited by their cost and availability. Moreover, such a strategy should be balanced with the need for closer monitoring for adverse events from RBV such as anemia[17]. In order to address these gaps, we performed a systematic review and meta-analysis to compare the efficacy and safety of RBV in GT3 compensated cirrhosis patients treated with SOF/VEL.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for data extraction and reporting[18]. All potential literature was identified from a comprehensive search of four electronic databases, namely PubMed/Medline, Embase, Cochrane and Web of Science, from initiation up to 1 June 2021, with the help of a medical librarian. There was no restriction on language, geography, publication dates and publication status (full text and abstract). The search keywords included a combination of “sofosbuvir”, “velpatasvir”, “ribavirin”, and "hepatitis C" using both the free text and MeSH terms as detailed in Supplementary Table 1. Additionally, a relevant search by Reference Citation Analysis (https://www.referencecitationanalysis.com) was conducted. All GT3 compensated cirrhosis patients treated with 12 wk of SOF/VEL, with or without RBV, were included, regardless of age, gender or prior treatment experience. References of all included studies were manually searched for additional studies. We also included grey literature from abstracts published in major conferences from 2015 to 2020.
In this meta-analysis, we included all studies that met the following inclusion criteria: (1) Studies that evaluated patients with hepatitis C GT3 compensated cirrhosis; (2) studies that evaluated the efficacy or safety of SOF/VEL, with or without RBV; and (3) reported SVR12, and/or treatment-related adverse events as study outcomes. We excluded case reports, case series, review articles, editorials, guidelines, and animal or pediatric studies. Two authors independently performed the initial screening of titles and abstracts during the primary search. The full texts of all relevant studies were extracted and reviewed. Any discrepancy in the article selection was resolved by consensus and discussion with a third coauthor.
The data from each study were independently extracted by two authors from the included studies using a predefined standardized form. The data extracted included study design, sample size, demographic of study participants, GT3 subtypes, coinfection with human immunodeficiency virus (HIV), baseline RAS, history of prior treatment, SVR12, and treatment-related adverse events. Treatment-related adverse events were defined as symptomatic anemia requiring transfusion or a drop in hemoglobin > 2 g/dL due to RBV. Corresponding authors were contacted in the event of any missing information.
We used Review Manager Software version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to perform our meta-analysis. The effect measures were presented as relative risk ratio (RR) and their respective 95%CI. The meta-analysis was analyzed using the random-effects model as the a priori model. P < 0.05 was considered to be statistically significant. The statistical heterogeneity was evaluated using Cochran’s Q test and I2 statistics[19]. We defined substantial heterogeneity across the study when P was < 0.10 in the Cochran Q test and I2 > 50%. Publication bias of the primary outcome was assessed based on funnel plot symmetry.
Prespecified subgroup analyses were performed based on study design [randomized controlled trial (RCT) vs non-RCT] and publication status (full text vs abstracts). Because non-RCT and abstracts are more susceptible to selection and recall bias, we also performed sensitivity analyses to estimate the effect size by the serial exclusion of individual studies and using a fixed-effect model to assess the reliability of our findings.
We used the Cochrane Risk of Bias 2.0 tool to assess randomized studies based on sequence generation, allocation concealment, performance bias, detection bias and reporting bias[20]. The Newcastle–Ottawa Scale was used to assess cohort studies based on selection, comparability and exposure[21]. Based on a total score ≥ 7, 4-6 or ≤ 3, each cohort study was classified as low, moderate or high risk of bias, respectively. Two authors independently assessed the risk of bias of all included studies. All discrepancy in risk of bias assessment was resolved by consensus with a third coauthor.
A total of 1752 citations were identified using our search strategy (Supplementary Figure 1). After removing duplicates and the title screen, we included a total of 69 studies for full-text review. Sixty-two studies were excluded for the following reasons: decompensated cirrhosis as study population (n = 6); intervention did not involve SOF/VEL and RBV (n = 42); and no comparison of outcomes by genotype (n = 14). Finally, seven studies fitted our inclusion criteria, as shown in the PRISMA flowchart (Supplementary Figure 1).
Seven studies, including 1,088 subjects (506 in the SOF/VEL with RBV group and 582 in the SOF/VEL without RBV group), were included in the final analysis. Five studies were published as full manuscripts[16,22-25], and two were published as abstracts[26,27]. The patient characteristics of all included studies are summarized in Table 1. The proportion of patients with GT3a and GT3b subtype was 99.5% and 0.5%, respectively[15]. The pooled rate of HIV coinfection was 13.0% (35/269)[16,23]. Overall, the proportion of subjects with baseline NS5A RASs mutation and prior treatment history was 6.4% (17/264) and 39.6% (127/321), respectively. The proportion of patients with baseline RAS mutation and prior treatment were comparable between the intervention and control groups[16,23]. Four studies had a low risk of bias (Supplementary Figure 2 and Supplementary Table 2). Three studies have a moderate risk of bias due to concerns over the severity of liver disease between intervention and control groups[24,26,27].
| Ref. | Study design | Sample size (n) | Age | Subtypes (3A/3B), (%) | Co-infection with HIV (%) | Baseline NS5A RAS (%) | Prior treatment (%) | SVR12 ITT (%) | SVR12 PP (%) | Treatment-related adverse event (%) |
| Pianko et al[22], 2015 | RCT | 52 | I: 54.0 (44-65); C: 56 (45-68) | NR | 0 | NR | I: 100.0; C: 100.0 | I: 96.2; C: 88.5 | I: 96.2; C: 88.5 | NR |
| Esteban et al[16], 2018 | RCT | 204 | I: 51 ± 7.6; C: 51 ± 7.3 | I: 100.0/0.0; C: 99.0/1.0 | I: 15.5; C: 13.9 | I: 21.8; C: 19.4 | I: 27.2; C: 26.7 | I: 96.1; C: 91.1 | I: 96.1; C: 92.0 | I: 4.9; C: 1.0 |
| von Felden et al[23], 2018 | Cohort study | 65 | NR | NR | I: 2.9; C: 13.3 | I: 11.4; C: 0.0 | I: 37.1; C: 23.3 | I: 100.0; C: 96.7 | NR | NR |
| Drysdale et al[26], 2019 | Cohort study | 414 | NR | NR | NR | NR | NR | NR | I: 98.0; C: 91.7 | NR |
| Pasulo et al[27], 2019 | Cohort study | 130 | NR | NR | NR | NR | NR | NR | I: 93.9; C: 98.4 | NR |
| Hlaing et al[24], 2019 | Cohort study | 60 | NR | NR | NR | NR | NR | NR | I: 100.0; C: 96.0 | I: 31.4; C: 8.0 |
| Wong et al[30], 2020 | Cohort study | 163 | NR | NR | NR | NR | NR | I: 97.8; C: 97.5 | I: 97.8; C: 97.5 | NR |
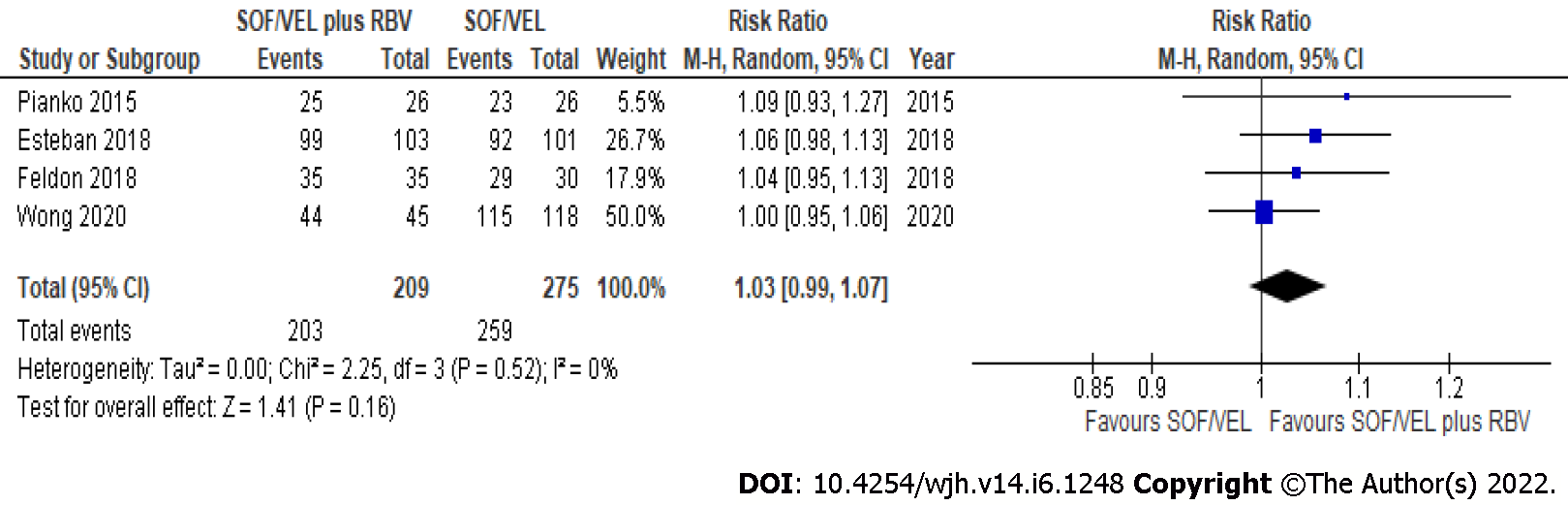
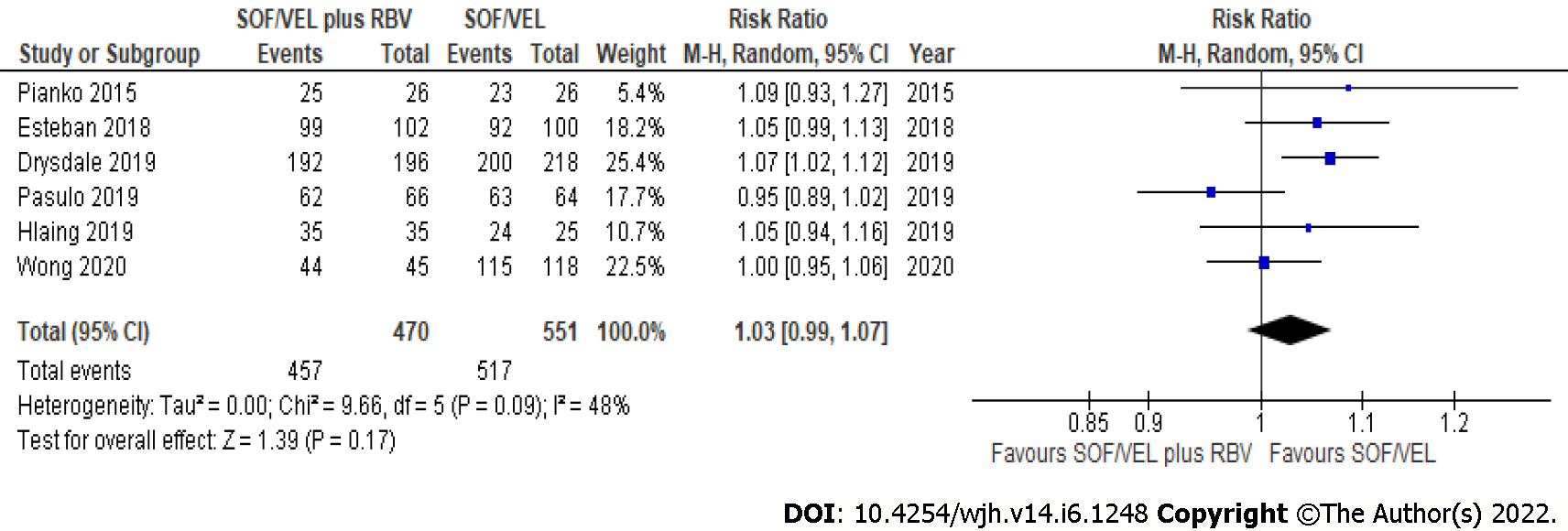
All seven studies (1088 subjects) reported SVR12 in GT3 compensated cirrhosis patients treated with SOF/VEL. The overall pooled rate of SVR12 based on intention-to-treat (ITT) and per-protocol (PP) analysis was 95.5% (462/484) and 95.4% (974/1021), respectively. The SVR12 was similar regardless of the use of RBV in GT3 compensated cirrhosis based on ITT (RR: 1.03, 95%CI: 0.99-1.07; I2 = 0%) (Figure 1) and PP (RR: 1.03, 95%CI: 0.99-1.07; I2 = 48%) analysis (Figure 2). The SVR12 remained comparable when subgroup analysis was performed based on study design, with less heterogeneity observed among RCTs (RR: 1.06, 95%CI: 1.00-1.13; I2 = 0%) (Table 2).
| Outcome | Subgroup | No. of studies | Effect size (RR with 95%CI) | I2 | |
| SVR12 (ITT analysis) | Overall | 4 | 1.03 (0.99-1.07) | 0 | |
| Study design | RCT | 2 | 1.06 (0.99-1.13) | 0 | |
| Non-RCT | 2 | 1.01 (0.97-1.06) | 0 | ||
| Effect estimates | Fixed model | 4 | 1.04 (1.00-1.08) | 0 | |
| Odd’s ratio | 4 | 2.32 (0.91-5.89) | 0 | ||
| SVR12 (PP analysis) | Overall | 6 | 1.03 (0.99-1.07) | 48 | |
| Study design | RCT | 2 | 1.06 (1.00-1.13) | 0 | |
| Non-RCT | 4 | 1.02 (0.97-1.07) | 65 | ||
| Publication type | Full-text | 4 | 1.00 (0.96-1.04) | 0 | |
| Abstract | 2 | 0.99 (0.88-1.10) | 86 | ||
| Effect estimates | Fixed model | 6 | 1.04 (1.01-1.07) | 48 | |
| Odd’s ratio | 6 | 2.36 (1.07-5.19) | 14 |
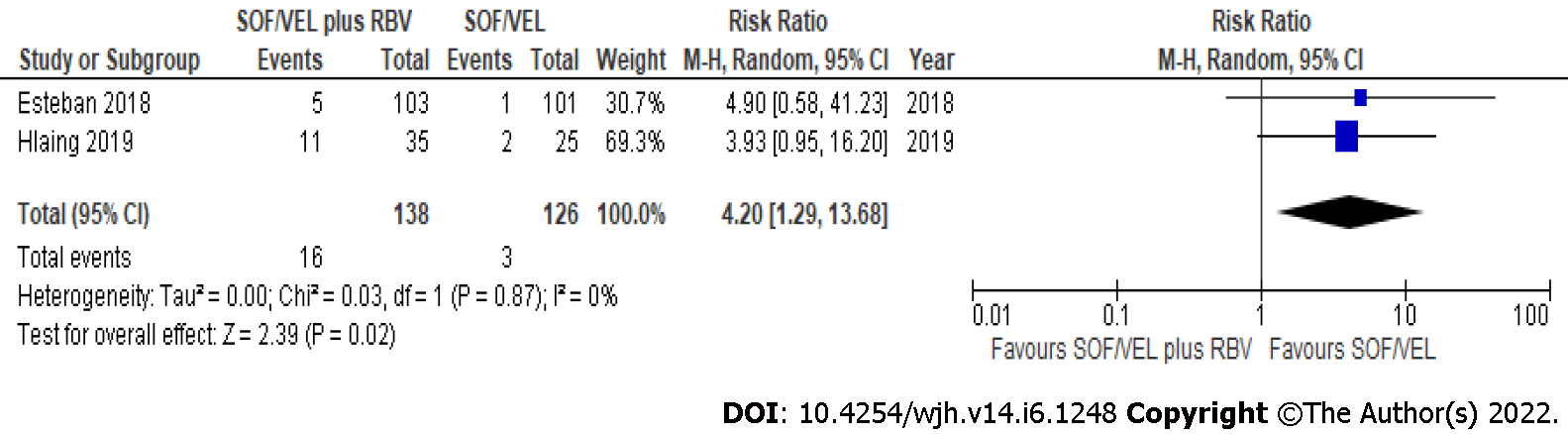
The overall pooled rate of treatment-related adverse events was 7.2% (95%CI: 4.4-11.0)[16,24]. Treatment with SOF/VEL plus RBV increases the pooled risk of treatment-related adverse events compared to SOV/VEL without RBV (RR: 4.20, 95%CI: 1.29-13.68; I2 = 0%) (Figure 3).
Treatment-experienced: The overall SVR12 among treatment-experienced GT3 compensated cirrhosis patients was 96.4%[16]. The use of RBV did not result in a higher SVR12 among treatment-experienced GT3 compensated cirrhosis patients (96% vs 96%).
Baseline RAS mutation: Baseline RAS testing was performed in 17.0% of subjects, from two studies[16,22]. Among those with baseline RAS mutation, the addition of RBV was associated with a higher SVR12 in patients treated with SOF/VEL (96% vs 87%, P = 0.12).
We performed sensitivity analysis to assess whether an individual study had a dominant effect on the overall pooled results. No individual study with a dominant effect was detected after serial exclusion of individual studies. Our findings remained consistent when analysis was performed using a fixed-effect model and OR as the effect measure (Table 2). Based on I2 analysis for heterogeneity, significant statistical heterogeneity was noted with the analysis for SVR12 for PP cohorts, which was reduced when only RCTs were considered. The funnel plot did not reveal significant publication bias for our primary outcome (Supplementary Figure 3).
GT3 HCV cirrhosis is considered the last frontier of HCV microelimination in the era of DAA use. Not only is GT3 the second most common genotype globally, affecting 45 million HCV patients worldwide[28], it has also been associated with significantly poorer outcomes, including higher risk of steatosis, faster progression to cirrhosis, and accelerated progression to hepatocellular carcinoma[29]. The benefit of RBV among GT3 compensated cirrhosis receiving SOF/VEL remains controversial. While the European Association for the Study of the Liver guidelines recommend routine RBV use, the AASLD guidelines recommend RBV only when baseline RAS mutation is present.
In this meta-analysis, we found that RBV has a limited role as a routine add-on therapy in GT3 compensated cirrhosis treated with SOF/VEL. The overall SVR12 was similar, regardless of the use of RBV. This finding remained robust when subgroup analysis was performed based on study design and prior treatment experience. In terms of safety, the addition of RBV increased the pooled risk of treatment-related adverse events, defined as symptomatic anemia requiring transfusion or a drop in hemoglobin > 2 g/dL. Five studies reported severe adverse events, defined as the need for hospitalization, intensive care unit, permanent disability, death and treatment cessation[16,22-25]. Overall, treatment-related severe adverse events were rare (0.8%) and were comparable regardless to the use of RBV. The most common minor adverse event was asthenia, followed by headache[16,24].
Our findings suggest that the routine use of RBV in GT3 compensated cirrhosis patients treated with SOF/VEL should be reconsidered. Similar findings were observed in real-world studies demonstrating high SVR12 of around 95% in GT3 compensated cirrhosis patients, regardless the use of RBV[25,30]. Given the limited benefit and higher risk of treatment-related adverse events with RBV use, 12 wk of SOF/VEL among GT3 compensated cirrhosis patients provides a simplified approach to safely omit the need for routine genotype and resistance testing, thus allowing rapid treatment upscale[31]. Meanwhile, retreatment using the combination of SOF, VEL and voxilaprevir has also been shown to be an efficacious strategy, both in clinical trials and real-world settings[32,33].
There were several strengths in our meta-analysis. First, we conducted a comprehensive search of four electronic databases, including grey literature, with the help of a medical librarian. All relevant data were extracted independently using a predefined template to compare both the efficacy and safety of RBV and SOF/VEL in GT3 compensated cirrhosis patients. All corresponding authors were contacted for any missing data through emails. All included studies were homogeneous in terms of patient characteristics, intervention, and outcome measures. Finally, our findings remained robust under various permutations of sensitivity analysis. To our knowledge, this is the first meta-analysis evaluating the safety and efficacy of adding RBV to SOF/VEL, specifically among GT3 compensated cirrhosis patients.
We acknowledge that there were limitations to this study. First, the number of subjects with baseline RAS mutations tested were small and only derived from two studies[16,22]. Although SVR12 was higher in the RBV group, it did not achieve statistical significance. Moreover, few papers reported the specific side effects during the treatment period, thus it was not possible to investigate the dose-dependent effect of RBV. We were unable to exclude indication bias among the nonrandomized trials. Although the decision to initiate RBV may be confounded by indication bias, our findings were consistent between RCTs and non-RCTs. Finally, more studies are needed to investigate the treatment outcome among GT3b patients because GT3b are under-represented from the existing literature[34].
Among GT3 compensated cirrhosis patients, adding RBV to 12 wk of SOF/VEL did not significantly increase SVR12. As RBV was associated with a higher risk of treatment-related adverse events, routine addition of RBV among GT3 compensated cirrhosis patients receiving SOF/VEL should be reconsidered.
With direct-acting antiviral therapy that is safe, effective and simple to use, future research should address linkage of care of hepatitis C virus (HCV) to achieve elimination.
As ribavirin (RBV) is associated with a higher risk of treatment-related adverse events, RBV as routine add-on therapy to sofosbuvir/velpatasvir (SOF/VEL) should be reconsidered among compensated genotype 3 (GT3) cirrhosis patients.
RBV as routine add-on therapy was not associated with higher sustained virological response at 12 wk post-treatment (SVR12) in GT3 compensated cirrhosis patients receiving SOF/VEL.
Systematic review and meta-analysis.
Our study aimed to evaluate the efficacy and safety of SOF/VEL, with or without RBV in GT3 compensated cirrhosis patients.
In routine clinical practice, the application of pretreatment resistance-associated substitution testing for patients with GT3 compensated cirrhosis is often limited by cost and availability. Moreover, such a strategy should be balanced with the need for closer monitoring for adverse events from RBV such as anemia. In order to address these gaps, we performed a systematic review and meta-analysis to compare the efficacy and safety of RBV in GT3 compensated cirrhosis patients treated with SOF/VEL.
SOF/VEL is an effective pan-genotypic direct-acting antiviral combination for the treatment of chronic HCV infection. While the addition of RBV to SOF/VEL improved SVR12 in GT3 decompensated cirrhosis patients, the benefits of RBV in GT3 compensated cirrhosis patients receiving SOF/VEL remains unclear.
The authors would like to thank Professor Rajender Reddy, Professor Naomi Khine Than Hliang and Dr Wilcon James for their kind assistance in our meta-analysis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferenci P, Austria; Yoshioka K, Japan A-Editor: Liu X, China S-Editor: Zhang H L-Editor: Kerr C P-Editor: Zhang H
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 2. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1145] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 3. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 4. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Müllhaupt B, Borovicka J, Heim M, Moradpour D, Cerny A, Malinverni R, Francioli P, Negro F; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 7. | McCombs J, Matsuda T, Tonnu-Mihara I, Saab S, Hines P, L'italien G, Juday T, Yuan Y. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med. 2014;174:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 – the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 10. | Younossi ZM, Stepanova M, Sulkowski M, Foster GR, Reau N, Mangia A, Patel K, Bräu N, Roberts SK, Afdhal N, Nader F, Henry L, Hunt S. Ribavirin-Free Regimen With Sofosbuvir and Velpatasvir Is Associated With High Efficacy and Improvement of Patient-Reported Outcomes in Patients With Genotypes 2 and 3 Chronic Hepatitis C: Results From Astral-2 and -3 Clinical Trials. Clin Infect Dis. 2016;63:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA; ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 12. | Te HS, Randall G, Jensen DM. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (N Y). 2007;3:218-225. [PubMed] |
| 13. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 14. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 528] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 15. | Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton M; ASTRAL-4 Investigators. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 16. | Esteban R, Pineda JA, Calleja JL, Casado M, Rodríguez M, Turnes J, Morano Amado LE, Morillas RM, Forns X, Pascasio Acevedo JM, Andrade RJ, Rivero A, Carrión JA, Lens S, Riveiro-Barciela M, McNabb B, Zhang G, Camus G, Stamm LM, Brainard DM, Subramanian GM, Buti M. Efficacy of Sofosbuvir and Velpatasvir, With and Without Ribavirin, in Patients With Hepatitis C Virus Genotype 3 Infection and Cirrhosis. Gastroenterology. 2018;155:1120-1127.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Feld JJ, Jacobson IM, Sulkowski MS, Poordad F, Tatsch F, Pawlotsky JM. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017;37:5-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40131] [Article Influence: 10032.8] [Reference Citation Analysis (2)] |
| 19. | Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane 2021. [DOI] [Full Text] |
| 20. | Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane 2021. [DOI] [Full Text] |
| 21. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12615] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 22. | Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, McNally J, Brainard DM, Han L, Doehle B, Mogalian E, McHutchison JG, Rabinovitz M, Towner WJ, Gane EJ, Stedman CA, Reddy KR, Roberts SK. Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | von Felden J, Vermehren J, Ingiliz P, Mauss S, Lutz T, Simon KG, Busch HW, Baumgarten A, Schewe K, Hueppe D, Boesecke C, Rockstroh JK, Daeumer M, Luebke N, Timm J, Schulze Zur Wiesch J, Sarrazin C, Christensen S. High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther. 2018;47:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Hlaing NKT, Nangia G, Tun KT, Lin S, Maung MZ, Myint KT, Kyaw AMM, Maung ST, Sein Win S, Bwa AH, Loza BL, Win KM, Reddy KR. High sustained virologic response in genotypes 3 and 6 with generic NS5A inhibitor and sofosbuvir regimens in chronic HCV in myanmar. J Viral Hepat. 2019;26:1186-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Wong YJ, Thurairajah PH, Kumar R, Tan J, Fock KM, Law NM, Li W, Kwek A, Tan YB, Koh J, Lee ZC, Kumar LS, Teo EK, Ang TL. Efficacy and safety of sofosbuvir/velpatasvir in a real-world chronic hepatitis C genotype 3 cohort. J Gastroenterol Hepatol. 2021;36:1300-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Drysdale K, Townley C, Mahomed F, Foster G. Effectiveness of therapy in 16,567 directly-acting antiviral treated people in England: High response rates in genotype 3 hepatitis C infection regardless of degree of fibrosis, but ribavirin improves response in cirrhosis. Int Liv Congress. 2019;. [DOI] [Full Text] |
| 27. | Pasulo L, Gambato M, Spinetti A. Treatment of 320 genotype 3 cirrhotic patients with 12 week Sofosbuvir/Velpatasvir with or without ribavirin: real life experience from Italy. Dig Liver Dis. 2019;51. |
| 28. | Chan A, Patel K, Naggie S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs. 2017;77:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC, Gordien E, Vicaut E, Baghad I, Beaugrand M. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Wilton J, Wong S, Yu A, Ramji A, Cook D, Butt ZA, Alvarez M, Binka M, Darvishian M, Jeong D, Bartlett SR, Pearce ME, Adu PA, Yoshida EM, Krajden M, Janjua NZ. Real-world Effectiveness of Sofosbuvir/Velpatasvir for Treatment of Chronic Hepatitis C in British Columbia, Canada: A Population-Based Cohort Study. Open Forum Infect Dis. 2020;7:ofaa055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Wong YJ, Thurairajah PH, Kumar R, Fock KM, Law NM, Chong SY, Manejero FG, Ang TL, Teo EK, Tan J. The impact of unrestricted access to direct-acting antiviral among incarcerated hepatitis C virus-infected patients. Clin Mol Hepatol. 2021;27:474-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, Borgia SM, Shafran SD, Workowski KA, Pearlman B, Hyland RH, Stamm LM, Svarovskaia E, Dvory-Sobol H, Zhu Y, Subramanian GM, Brainard DM, McHutchison JG, Bräu N, Berg T, Agarwal K, Bhandari BR, Davis M, Feld JJ, Dore GJ, Stedman CAM, Thompson AJ, Asselah T, Roberts SK, Foster GR. Efficacy of 8 Weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in Patients With Chronic HCV Infection: 2 Phase 3 Randomized Trials. Gastroenterology. 2017;153:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 33. | Papaluca T, Roberts SK, Strasser SI, Stuart KA, Farrell G, MacQuillan G, Dore GJ, Wade AJ, George J, Hazeldine S, O'Beirne J, Wigg A, Fisher L, McGarity B, Sawhney R, Sinclair M, Thomas J, Valiozis I, Weltman M, Wilson M, Woodward A, Ahlenstiel G, Haque M, Levy M, Prewett E, Sievert W, Sood S, Tse E, Valaydon Z, Bowden S, Douglas M, New K, O'Keefe J, Hellard M, Doyle J, Stoove M, Thompson AJ. Efficacy and Safety of Sofosbuvir/Velpatasvir/Voxilaprevir for Hepatitis C Virus (HCV) NS5A-Inhibitor Experienced Patients With Difficult to Cure Characteristics. Clin Infect Dis. 2021;73:e3288-e3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, Wu S, Xu M, Tang H, Cheng J, Le Manh H, Gao Y, Mou Z, Sobhonslidsuk A, Dou X, Thongsawat S, Nan Y, Tan CK, Ning Q, Tee HP, Mao Y, Stamm LM, Lu S, Dvory-Sobol H, Mo H, Brainard DM, Yang YF, Dao L, Wang GQ, Tanwandee T, Hu P, Tangkijvanich P, Zhang L, Gao ZL, Lin F, Le TTP, Shang J, Gong G, Li J, Su M, Duan Z, Mohamed R, Hou JL, Jia J. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |