Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1182
Peer-review started: January 14, 2022
First decision: March 24, 2022
Revised: March 28, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 27, 2022
Processing time: 159 Days and 20.5 Hours
Oxidative damage of DNA and RNA has been associated with mortality of patients with different diseases. However, there is no published data on the potential use of DNA and RNA oxidative damage to predict the prognosis of patients with hepatocellular carcinoma (HCC) undergoing liver transplantation (LT).
To determine whether patients with increased DNA and RNA oxidative damage prior to LT for HCC have a poor LT prognosis.
Patients with HCC who underwent LT were included in this observational and retrospective study. Serum levels of all three oxidized guanine species (OGS) were measured prior to LT since guanine is the nucleobase that forms DNA and RNA most prone to oxidation. LT mortality at 1 year was the end-point study.
Surviving patients (n = 101) showed lower serum OGS levels (P = 0.01) and lower age of the liver donor (P = 0.03) than non-surviving patients (n = 13). An association between serum OGS levels prior to LT and 1-year LT (odds ratio = 2.079; 95% confidence interval = 1.356-3.189; P = 0.001) was found in the logistic regression analysis.
The main new finding was that high serum OGS concentration prior to LT was associated with the mortality 1 year after LT in HCC patients.
Core Tip: The potential use of DNA and RNA oxidative damage to predict prognosis of patients with hepatocellular carcinoma who underwent liver transplantation is unknown. In this retrospective study serum levels of the three oxidized guanine species before liver transplantation in 114 patients were measured. One-year survivor patients showed lower serum oxidized guanine specie levels than non-survivor patients (P = 0.01). These preliminary results could induce studies to clarify the potential role of oxidative damage in the prognosis of liver transplantation patients due to hepatocellular carcinoma and to explore the use of antioxidant agents to reduce oxidative stress in those patients.
- Citation: Lorente L, Rodriguez ST, Sanz P, González-Rivero AF, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Cerro P, Portero J, Barrera MA. DNA and RNA oxidative damage in hepatocellular carcinoma patients and mortality during the first year of liver transplantation. World J Hepatol 2022; 14(6): 1182-1189
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1182.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1182
Liver transplantation (LT) could be the treatment of choice in some patients with hepatocellular carcinoma (HCC)[1-4], which is the most common malignant liver tumor and is responsible for many deaths. LT may be an appropriate choice because it treats liver failure and removes the liver tumor[5-8].
The possible contribution of the oxidative state in chronic liver disease progression and in hepatocarcinogenesis development has been suggested[9-12]. RNA, DNA, lipids and proteins could be damaged by reactive oxygen species during oxidative stress. The five types of nucleobases present in RNA and DNA are adenine, guanine, cytosine, uracil and thymine; but only four types of those nucleobases constitute RNA and DNA. In both, RNA and DNA, guanine, adenine and cytosine are present. In addition, uracil is present in RNA and thymine in DNA. Guanine is the nucleobase most prone to oxidation since it has the lowest redox potential[13-16]. The three species of oxidized guanine species (OGS) are 8-hydroxyguanine from DNA or RNA, 8-hydroxyguanosine from RNA, and 8-hydroxy-2’-deoxyguanosine from DNA.
An association between DNA and RNA oxidative damage and mortality has been found in patients with other diseases such as sepsis[17]. Greater DNA oxidative damage (assessed by concentrations of 8-hydroxy-2’-deoxyguanosine in liver biopsy samples) has been found in patients with chronic hepatic disease with HCC than without it[18,19]. However, there is no published data about the potential use of DNA and RNA oxidative damage to predict the prognosis of patients with HCC and who underwent LT. Therefore, the aim in our study was to analyze the potential association between increased oxidative DNA and RNA damage before LT for HCC and poorer LT prognosis.
We included patients who underwent LT due to HCC between May 2001 to May 2017. LT were carried out in the Hospital Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain). This observational and retrospective study was performed after the approval by the Institutional Review Board. Patients were included after the written informed consent was obtained by the LT recipient or a family member. All LT donors were brain dead. Serum samples were obtained before LT and frozen at -80 ºC, and serum concentrations of 8-hydroxy-2’-deoxyguanosine were determined in those samples.
Sex, age, nodule size, degree of tumor differentiation, Child-Pugh score[20], infiltration, serum alpha-fetoprotein level, macrovascular invasion, multinodular tumor, portal hypertension (determined either by clinical data or by hepatic venous pressure gradient), microvascular invasion, model for end-stage liver disease score[21] by hepatic function, treatment before LT, LT technique and inside Milan criteria[22] before and after LT were registered. In addition, age of LT donor was registered. One-year LT survival was considered our end-point study.
Serum samples were taken about 2 h before LT. Afterwards samples were placed in a -80 ºC freezer. We had previously determined serum caspase-3 levels in some of these patients[23], and in this research we determined serum OGS levels. We used kits called DNA/RNA Oxidative Damage ELISA Kit® (by Cayman Chemical Corporation in Ann Arbor, United States) to determine serum OGS concentrations. The detection limit of these kits was 0.45 ng/mL. All determinations were carried out in the same Laboratory Department blinded to clinical data.
Categorical variables, presented as frequency (percentage), were compared using the χ2 test. Continuous variables, presented as median (percentiles 25 and 75), were compared using the test of Mann-Whitney. The ability of serum OGS concentrations prior to LT to predict 1-year LT mortality was analyzed using receiver operating characteristic curve. The Kaplan-Meier 1-year LT survival curves were constructed with a serum OGS concentration cut-off (3.3 ng/mL) selected on the basis of Youden’s J-index. The association between serum OGS levels and 1-year LT controlling for serum caspase-3 levels and age of liver donor was analyzed using the logistic regression analysis. MedCal 15.2.1 (Ostend, Belgium) and SPSS 17.0 (by SPSS Inc. in Chicago, IL, United States) were used to perform the statistical analyses.
We included 114 patients in the study, of which 101 remained alive 1 year after LT and 13 died during the first year after LT. Surviving LT patients in comparison to non-surviving patients showed lower serum OGS concentrations prior to LT (P = 0.01) and lower liver donor age (P = 0.03) (Table 1). No significant differences between surviving and non-surviving patients regarding sex, liver receptor age, nodule size, serum alpha-fetoprotein levels, degree of tumor differentiation, microvascular invasion, multinodular tumor, infiltration, macrovascular invasion, Child-Pugh score, model for end-stage liver disease score, portal hypertension, treatment prior to LT, LT technique and inside Milan criteria before and after LT were observed (Table 1). Significant differences were not found (P = 0.20) in serum OGS concentrations in regard to the cause of death: 8 (61.5%) sepsis, 3 (23.1%) multiple organ failure, 1 (7.7%) recurrence of hepatitis C virus infection and 1 (7.7%) recurrence of HCC.
| 1 yr survivor patients, n = 101 | 1 yr non-survivor patients, n = 13 | P value | |
| Serum OGS (ng/mL)–median (p 25-75) | 2.80 (2.20-4.00) | 4.00 (2.70-10.25) | 0.01 |
| Age of liver recipient (yr)-median (p 25-75) | 58 (52-62) | 57 (55-63) | 0.61 |
| Serum alpha-fetoprotein (ng/dL)-median (p 25-75) | 7.4 (4.0-21.6) | 8.4 (4.3-130.5) | 0.62 |
| Protein (g/dL)-median (p 25-75) | 6.70 (6.10-7.10) | 6.70 (5.58-7.63) | 0.90 |
| Leukocytes count–median × 103/mm3 (p 25-75) | 4.57 (3.48-6.01) | 4.52 (3.27-7.77) | 0.89 |
| Albumin (g/dL)-median (p 25-75) | 3.29 (2.89-3.99) | 3.47 (3.14-3.93) | 0.45 |
| Creatinine (mg/dL)-median (p 25-75) | 0.90 (0.78-1.10) | 1.02 (0.75-1.10) | 0.27 |
| BMI (kg/m2)-median (p 25-75) | 27.3 (24.3-29.7) | 28.7 (24.9-31.8) | 0.26 |
| Nodules size (cm)-median (p 25-75) | 2.9 (2.0-3.4) | 3.2 (1.8-4.9) | 0.40 |
| MELD score-median (p 25-75) | 15 (11-18) | 15 (13-17) | 0.77 |
| Age of liver donor (yr)-median (p 25-75) | 51 (35-62) | 62 (49-72) | 0.03 |
| Gender female, n (%) | 19 (18.8) | 0 | 0.12 |
| Child-Pugh score, n (%) | 0.06 | ||
| A | 46 (45.5) | 10 (76.9) | |
| B | 29 (28.7) | 3 (23.1) | |
| C | 26 (25.7) | 0 | |
| Infiltration, n (%) | 32 (31.7) | 3 (23.1) | 0.75 |
| Macrovascular invasion, n (%) | 4 (4.0) | 0 | 0.99 |
| Microvascular invasion, n (%) | 19 (18.8) | 2 (15.4) | 0.99 |
| Multinodular tumor, n (%) | 27 (26.7) | 4 (30.8) | 0.75 |
| Portal hypertension, n (%) | 64 (63.4) | 9 (69.2) | 0.77 |
| Treatment previously to LT, n (%) | 56 (55.4) | 8 (61.5) | 0.77 |
| PEI, n (%) | 26 (25.7) | 5 (38.5) | 0.33 |
| RFA, n (%) | 6 (5.9) | 0 | 0.99 |
| TACE, n (%) | 18 (17.8) | 3 (23.1) | 0.71 |
| Liver resection, n (%) | 3 (3.0) | 0 | 0.99 |
| Mixed treatment, n (%) | 3 (3.0) | 0 | 0.99 |
| Transplantation technique, n (%) | 0.99 | ||
| By-pass | 44 (43.6) | 6 (46.2) | |
| Piggy back | 57 (56.4) | 7 (53.8) | |
| Degree of tumor differentiation, n (%) | 0.11 | ||
| Well | 76 (75.2) | 11 (84.6) | |
| Moderate | 24 (23.8) | 1 (7.7) | |
| Poor | 1 (1.0) | 1 (7.7) | |
| Inside Milan criteria previously to LT, n (%) | 96 (95.0) | 12 (92.3) | 0.53 |
| Inside Milan criteria after LT, n (%) | 85 (84.2) | 10 (76.9) | 0.45 |
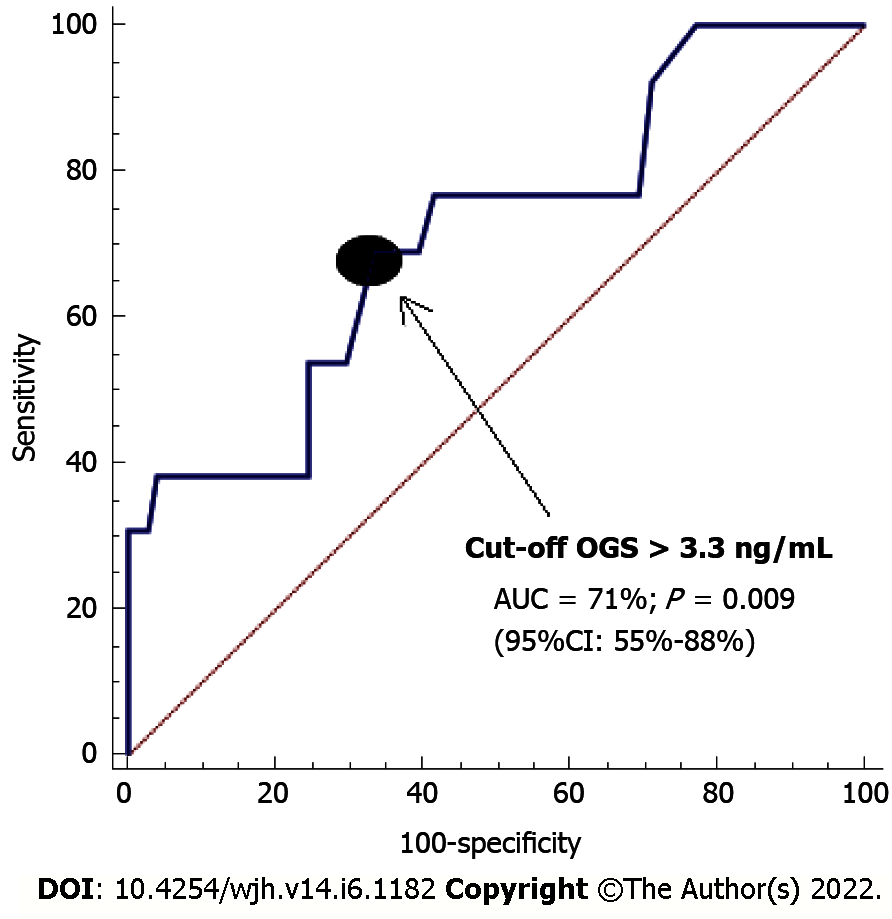
In logistic analysis, an association was found between serum OGS and 1-year LT mortality, controlling for serum caspase-3 and liver donor age [odds ratio = 2.079; 95% confidence interval (CI): 1.356-3.189; P = 0.001] (Table 2). On the receiver operating characteristic analysis, the area under the curve of pre-LT serum OGS concentrations for predicting 1-year LT mortality was found to be 71% (95%CI: 55%-88%; P = 0.009) (Figure 1).
| Odds ratio | 95%CI | P value | |
| Age of liver donor (age) | 1.087 | 1.019-1.160 | 0.01 |
| Serum oxidized guanine species levels (ng/mL) | 2.079 | 1.356-3.189 | 0.001 |
| Serum caspase-3 levels (ng/mL) | 4.178 | 1.709-10.211 | 0.002 |
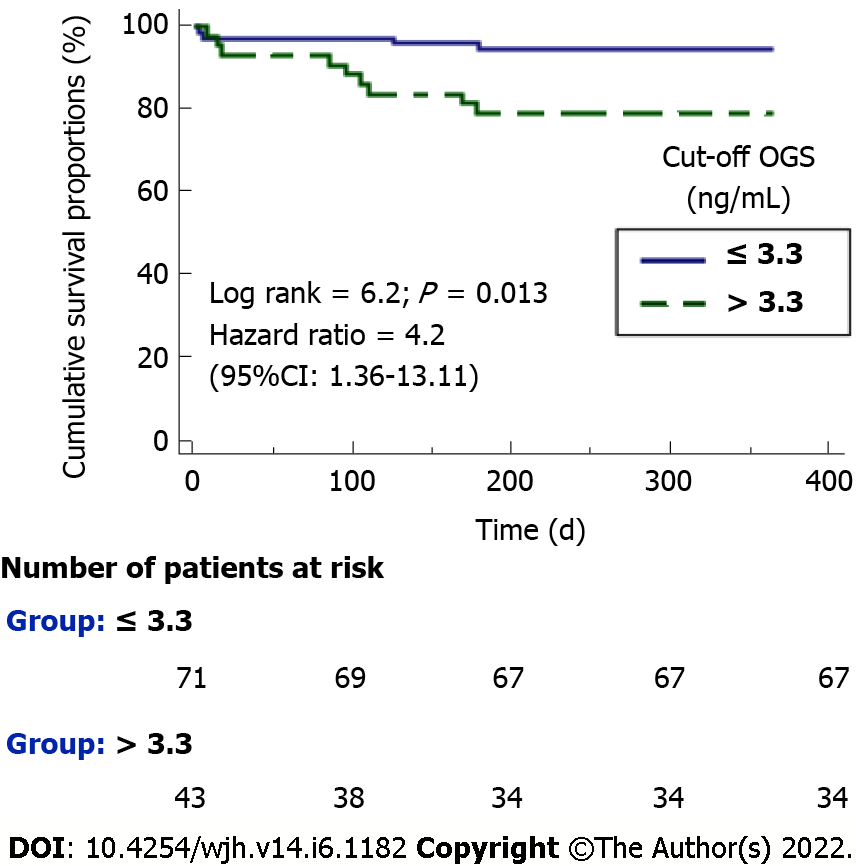
Serum OGS levels with a cut-off point of 3.3 ng/mL showed a sensitivity of 69% (39%-91%), specificity of 66% (56%-74%), positive likelihood ratio of 2.1 (1.3-3.2), negative likelihood ratio of 0.5 (0.2-1.1), positive predictive value of 21% (14%-29%) and negative predictive value of 94% (88%-98%) for 1-year LT mortality prediction. The Kaplan-Meier survival analysis showed a higher 1-year LT mortality risk in patients with serum OGS levels prior to LT above 3.3 ng/mL (hazard ratio = 4.2; 95%CI: 1.36-13.11; P = 0.01) (Figure 2).
To our knowledge, our study is the first reporting data about the determination of DNA and RNA oxidative damage to predict prognosis of patients with HCC who underwent LT. The main finding was that high serum OGS prior to LT was associated with the mortality 1 year after LT. Greater oxidative DNA damage (assessed by 8-hydroxy-2’-deoxyguanosine concentration in liver biopsy specimens) has been found in patients with chronic liver disease with HCC compared to those without[18,19]. However, the association between serum OGS concentration and LT mortality is a new finding of our study.
These higher serum OGS levels found in non-surviving LT patients are in line with those found in patients with other diseases, such as sepsis[17], and could be in relation with a higher oxidative status that could favor multiple organ dysfunction and death of patients.
There were some limitations of our study. First, we have not determined serum 8-hydroxy-2’-deoxyguanosine change after LT to explore which is a better serum marker for prognosis (before or after LT). Second, we have not determined serum 8-hydroxy-2’-deoxyguanosine in healthy controls or chronic liver patients without HCC. However, the objective of our study was to determine whether patients with increased oxidative DNA and RNA damage before undergoing LT for HCC have poorer LT prognosis. Third, we have not determined other markers of oxidative stress for nucleic acids, such as abasic sites or 8-nitroguanosine 3’,5’-cyclic monophosphate. Fourth, we have not determined 8-hydroxy-2’-deoxyguanosine in the liver to explore its correlation with serum levels. Fifth, the regression analysis did not allow the introduction of more variables due to the low number of deceased patients. However, one strength of our study was that the association between mortality and serum OGS has been also previously found in patients with other diseases such as sepsis[17].
The possible contribution of an oxidative state in chronic liver disease progression and in hepatocarcinogenesis development has been suggested. In addition, the potential use of antioxidant agents in patients with chronic liver diseases has also been suggested[9-12]. Therefore, these preliminary results could induce studies to clarify the potential role of oxidative damage in the prognosis of LT patients due to HCC and to explore the use of antioxidant agents to reduce oxidative stress in those patients.
The main new finding was that high serum OGS concentrations prior to LT were associated with mortality 1 year after LT in HCC patients.
Oxidative damage of DNA and RNA has been associated with mortality of patients with various diseases.
There is no published data on the potential use of DNA and RNA oxidative damage to predict the prognosis of patients with liver transplantation (LT) due to hepatocellular carcinoma (HCC).
The aim in our study was to analyze the potential association between increased oxidative DNA and RNA damage before LT due to HCC and poorer LT prognosis.
In this observational, retrospective study, patients with HCC who underwent LT were included. Serum levels of all three oxidized guanine species (OGS) were measured prior to LT because guanine is the nucleobase with a higher risk of oxidation. LT mortality at 1 year was the end point of the study.
Surviving patients (n = 101) showed lower serum OGS levels (P = 0.01) and lower age of liver donor (P = 0.03) than non-surviving patients (n = 13). An association between serum OGS prior to LT and 1-year LT (odds ratio = 2.079; 95% confidence interval: 1.356-3.189; P = 0.001) was found in the logistic regression analysis.
The main new finding was that high serum OGS concentration prior to LT was associated with 1-year LT mortality.
These preliminary results could induce studies to clarify the potential role of oxidative damage in the prognosis of LT patients due to HCC and to explore the use of antioxidant agents to reduce oxidative stress in those patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Son TQ, Viet Nam; Tanaka Y, Japan A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 3. | Verslype C, Rosmorduc O, Rougier P; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41-vii48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 4. | Cescon M, Bertuzzo VR, Ercolani G, Ravaioli M, Odaldi F, Pinna AD. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunological state on recurrence and prognosis. World J Gastroenterol. 2013;19:9174-9182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol. 2015;7:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Guerrero-Misas M, Rodríguez-Perálvarez M, De la Mata M. Strategies to improve outcome of patients with hepatocellular carcinoma receiving a liver transplantation. World J Hepatol. 2015;7:649-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: Surgeon's view on latest findings and future perspectives. World J Hepatol. 2015;7:1168-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Takaki A, Yamamoto K. Control of oxidative stress in hepatocellular carcinoma: Helpful or harmful? World J Hepatol. 2015;7:968-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (2)] |
| 10. | Choi J, Corder NL, Koduru B, Wang Y. Oxidative stress and hepatic Nox proteins in chronic hepatitis C and hepatocellular carcinoma. Free Radic Biol Med. 2014;72:267-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R, Accardo M, Agresti M, Caraglia M, Sperlongano P. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;9:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Hoshida Y. Molecular signatures and prognosis of hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2011;57:311-322. [PubMed] |
| 13. | Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018;14:669-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Markkanen E. Not breathing is not an option: How to deal with oxidative DNA damage. DNA Repair (Amst). 2017;59:82-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Kino K, Hirao-Suzuki M, Morikawa M, Sakaga A, Miyazawa H. Generation, repair and replication of guanine oxidation products. Genes Environ. 2017;39:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | AbdulSalam SF, Thowfeik FS, Merino EJ. Excessive Reactive Oxygen Species and Exotic DNA Lesions as an Exploitable Liability. Biochemistry. 2016;55:5341-5352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lorente L, Martín MM, González-Rivero AF, Pérez-Cejas A, Abreu-González P, Ortiz-López R, Ferreres J, Solé-Violán J, Labarta L, Díaz C, Palmero S, Jiménez A. Association between DNA and RNA oxidative damage and mortality in septic patients. J Crit Care. 2019;54:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Schwarz KB, Kew M, Klein A, Abrams RA, Sitzmann J, Jones L, Sharma S, Britton RS, Di Bisceglie AM, Groopman J. Increased hepatic oxidative DNA damage in patients with hepatocellular carcinoma. Dig Dis Sci. 2001;46:2173-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Tanaka H, Fujita N, Sugimoto R, Urawa N, Horiike S, Kobayashi Y, Iwasa M, Ma N, Kawanishi S, Watanabe S, Kaito M, Takei Y. Hepatic oxidative DNA damage is associated with increased risk for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer. 2008;98:580-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5733] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 21. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 22. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5305] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 23. | Lorente L, Rodriguez ST, Sanz P, González-Rivero AF, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Cerro P, Portero J, Barrera MA. High serum caspase-3 levels in hepatocellular carcinoma prior to liver transplantation and high mortality risk during the first year after liver transplantation. Expert Rev Mol Diagn. 2019;19:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |