Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1162
Peer-review started: February 4, 2022
First decision: April 17, 2022
Revised: April 24, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: June 27, 2022
Processing time: 138 Days and 22.8 Hours
Alcohol consumption increases the risk of hepatocellular carcinoma (HCC) in patients with pre-existing liver disease, including viral hepatitis. However, studies on the impact of alcohol consumption on the outcomes of HCC are limited. We hypothesized that alcohol had an additional effect with chronic viral hepatitis infection on treatment outcomes after transarterial chemoembolization (TACE) in patients with intermediate-stage HCC (Barcelona Clinical Liver Cancer [BCLC] -B).
To evaluate the additional effect of alcohol on treatment outcomes of TACE among HCC patients with viral hepatitis.
This study, conducted at Hatyai Hospital in Thailand, included HCC patients over 18 years of age with chronic viral hepatitis. Records of HCC patients with viral hepatitis classified as BCLC-B who underwent TACE as the first treatment modality between 2014 and 2019 were retrospectively reviewed. Patients with chronic viral hepatitis only were categorized under group A, and those with chronic viral hepatitis and concurrent alcohol consumption were categorized under group B. Both groups were compared, and the Cox proportional-hazards model was used to identify the survival-influencing variables.
Of the 69 patients, 53 were categorized in group A and 16 in group B. There were no statistically significant differences in tumor characteristics between the two patient groups. However, Group A had a statistically significantly higher proportion of complete response (24.5% vs 0%, P = 0.030) and a higher median survival rate (26.2 mo vs 8.4 mo; log-rank P = 0.012) compared to group B. Factors associated with decreased survival in the proportional-hazards model included alcohol consumption (hazards ratio [HR], 2.377; 95% confidence interval [CI], 1.109-5.095; P = 0.026), presence of portal hypertension (HR, 2.578; 95%CI, 1.320–5.037; P = 0.006), largest tumor size > 5 cm (HR, 3.558; 95%CI, 1.824-6.939; P < 0.001), and serum alpha-fetoprotein level > 100 ng/mL (HR, 2.536; 95%CI, 1.377-4.670; P = 0.003).
In HCC BCLC B patients with chronic viral hepatitis, alcohol consumption is an independent risk factor for increased mortality and decreases the rate of complete response and survival after TACE.
Core Tip: Regular alcohol consumption is associated with increased hepatocellular carcinoma (HCC) risk, particularly in patients with pre-existing chronic liver diseases, including viral hepatitis B and C infection. However, data on the impact of alcohol consumption on HCC outcomes after treatment with transarterial chemoembolization (TACE) remain limited. This study is the first to address the additional effect of alcohol on treatment outcomes of transarterial chemoembolization TACE among HCC patients with viral hepatitis.
- Citation: Rattanasupar A, Chang A, Prateepchaiboon T, Pungpipattrakul N, Akarapatima K, Songjamrat A, Pakdeejit S, Prachayakul V, Piratvisuth T. Impact of alcohol consumption on treatment outcome of hepatocellular carcinoma patients with viral hepatitis who underwent transarterial chemoembolization . World J Hepatol 2022; 14(6): 1162-1172
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1162.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1162
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide[1]. As the incidence of HCC is almost the same as the number of annual deaths caused by this malignancy, it is also the third leading cause of cancer-related mortality worldwide[2]. Most HCC patients are diagnosed late, subsequently leading to poor clinical outcomes and often making palliative treatment their only option[2,3]. For patients with intermediate-stage HCC (Barcelona Clinical Liver Cancer [BCLC] B), transarterial chemoembolization (TACE) with preserved liver function has been shown to improve survival[2,3].
Chronic viral hepatitis infection, in particular with hepatitis B virus (HBV) and hepatitis C virus (HCV), are important risk factors for HCC. In fact, HBV and HCV are estimated to be responsible for 50%-90% of HCC cases worldwide[4]. Alcohol use disorder is associated with intravenous injections and bloodborne infections; heavy alcohol consumption has been reported to be much higher among individuals screened for chronic viral hepatitis than the general population[5]. Due to the strong association of alcohol misuse with alcohol-associated liver diseases, liver cirrhosis, and cancer[6,7], alcohol has been categorized as a human carcinogen[8]. Alcohol consumption enhances or accelerates hepatocarcinogenesis in patients with other pre-existing chronic liver diseases, especially chronic viral hepatitis infection[9,10]. However, studies on the impact of alcohol consumption on HCC outcomes after treatment are limited.
The study’s objective was to verify the additional effect of alcohol on treatment outcomes of TACE among intermediate-stage HCC (BCLC B) patients with viral hepatitis.
This retrospective cohort study was conducted at Hatyai Hospital (a regional referral tertiary center in southern Thailand). The study protocol was approved by the Institutional Review Board of Hatyai Hospital (protocol number HYH EC 105-64-01) and conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived because patient information was de-identified before analysis.
The inclusion criterion was HCC patients > 18 years of age with chronic viral hepatitis classified as BCLC B who underwent TACE as the first treatment modality between January 2014 and December 2019. The exclusion criteria were as follows: (1) Received any curative treatment for HCC; (2) infiltrative tumor or extrahepatic metastasis; (3) renal, cerebral, or cardiopulmonary dysfunction; (4) presence of other concurrent malignancy; and (5) insufficient data for analysis.
A retrospective review of the medical records of each patient was performed manually by two independent investigators (with at least five years of experience in the field of hepatology), and a third investigator (senior consultant who had an experience of more than ten years) was consulted to resolve disagreements or discrepancies. For each patient, data were extracted from the demographic and clinical variables (including age, sex, body mass index, comorbidities, clinical presentation, and laboratory results), tumor characteristics (including the number of tumors and the size and stage of the tumors) at the time of diagnosis. Data on clinical outcomes included the number of total sessions of TACE and responses after all treatments were completed.
After discussion by a multidisciplinary team, TACE treatment was offered to patients and conducted after a consensus was reached between doctors and patients. Written informed consent was obtained from all the patients before the procedure. Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was performed to evaluate tumor status prior to TACE. Conventional TACE was performed by experienced interventional radiologists. A single intravenous dose of antibiotic prophylaxis with third-generation cephalosporin was routinely administered, except in patients who were prescribed antibiotics for other indications. After assessment of feeding vessels to the segment where the tumor was located, a mixture of a cytotoxic drug (such as doxorubicin or mitomycin C) and iodized oil (Lipiodol; Guerbet, Milan, Italy) was injected, followed by embolization using gelatin sponge particles under fluoroscopic monitoring. We routinely assessed the treatment response at 4-6 wk after the procedure using dynamic contrast-enhanced CT or MRI.
HCC was diagnosed based on the American Association for the Study of Liver Disease (AASLD) criteria as previously described, and the BCLC system was used for tumor staging[2]. We stratified patients into two groups, namely “group A” consisting of patients with chronic viral hepatitis only and “group B” consisting of patients with concurrent chronic viral hepatitis and alcohol consumption. Viral hepatitis was defined as infection with either HBV or HCV as confirmed by a history of positive serological results (hepatitis B virus surface antigen and hepatitis C virus antibody) accompanied by the presence of HBV DNA or HCV RNA. Alcohol consumption was defined as daily alcohol consumption of at least 40 g[11]. The diagnosis of cirrhosis was based on clinical features, imaging, and histology. The presence of portal hypertension was confirmed if the patients had any of the following: (1) Ascites; (2) esophageal or gastric varices; and (3) splenomegaly accompanied by a platelet count < 100000/mm³[2]. Hepatic function was assessed using the Child–Turcotte–Pugh score[12] and the model of end-stage liver disease[13]. The patient’s performance status was classified according to the Eastern Cooperative Oncology Group Performance Status scale[14].
Complete response (CR) after treatment was defined as the disappearance of any intra-tumor enhancement in all target lesions, as demonstrated by dynamic enhanced cross-sectional imaging based on the modified Response Evaluation Criteria in Solid Tumors[15]. Overall survival (OS) was calculated from the date of diagnosis of HCC until either death (using data from the Thailand civil registrations) or the last follow-up date. The censored survival time was January 1, 2021.
Categorical variables were expressed using descriptive statistics and assessed for statistically significant differences using Pearson’s chi-square or Fisher's exact test. For continuous variables, data were presented as mean ± standard deviation (SD) or median and interquartile range (IQR) and tested for statistically significant differences using the Student's t-test and Wilcoxon rank-sum test. Survival analysis was performed using the Kaplan–Meier method, and the log-rank test was used to analyze statistical differences between the two groups. The Cox proportional hazards model was used to identify variables influencing survival. After univariate analysis, sex, age, and other variables with probabilities (P values) < 0.2 were included in the multivariate analyses. All data analyses were performed using the statistical program Stata Version 15.1 (StataCorp LLC, College Station, TX, United States). Statistical significance was set at P < 0.05.
A total of 69 patients met the inclusion criteria and were enrolled in the study. The average age was 55.5 ± 9.9 years, and 51 (73.9%) were men. Of these patients, 53 were classified into group A (chronic viral hepatitis only) and 16 into group B (concurrent chronic viral hepatitis and alcohol consumption). Comparisons of demographic data are shown in Table 1. The proportion of female patients in group A was higher than that in group B (34.0% vs 0%, P = 0.007). When compared between the two groups, serum albumin level in group A was significantly higher (mean ± SD = 3.6 ± 0.7 g/dL vs 3.2 ± 0.4 g/dL, P = 0.017), while serum aspartate aminotransferase (AST) level in group B was significantly higher (median [IQR] = 63.0 [42.0 to 116.0] mg/dL vs 96.5 [73.5 to 155.0] mg/dL). The proportion of patients with chronic hepatitis B tended to be higher in group A compared to group B (64.2% vs 37.5%, P = 0.058), while the proportion of patients with chronic hepatitis C tended to be higher in group B compared to group A (37.7% vs 62.5%, P = 0.080).
| Variables | Group A (n = 53), % | Group (n = 16), % | P value |
| Female sex | 18 (34.0) | 0 (0) | 0.007 |
| Age (yr): mean ± SD | 56.1 ± 10.5 | 53.6 ± 7.5 | 0.365 |
| Body mass index (kg/m2): mean ± SD | 23.2 ± 4.3 | 22.0 ± 3.1 | 0.298 |
| Underlying disease | |||
| Diabetic mellitus | 10 (18.9) | 2 (12.5) | 0.718 |
| Hypertension | 9 (17.0) | 2 (12.5) | 1.000 |
| Dyslipidemia | 2 (3.8) | 0 (0) | 1.000 |
| Hepatitis B virus infection | 34 (64.2) | 6 (37.5) | 0.058 |
| Hepatitis C virus infection | 20 (37.7) | 10 (62.5) | 0.080 |
| Hepatitis B and C virus coinfection | 1 (1.9) | 0 (0) | 1.000 |
| Cirrhosis | 53 (100) | 16 (100) | N/A |
| Child–Turcotte–Pugh classification | 0.109 | ||
| A | 35 (66.0) | 7 (43.8) | |
| B | 18 (34.0) | 9 (56.2) | |
| Presence of portal hypertension | 36 (67.9) | 10 (62.5) | 0.687 |
| Laboratory data | |||
| Hemoglobin (g/dL): mean ± SD | 12.3 ± 1.9 | 12.2 ± 1.9 | 0.883 |
| Platelet median (×103/mL): Median (IQR) | 119 (78 to 208) | 116 (64 to 175) | 0.803 |
| Serum creatinine (mg/dL): Median (IQR) | 0.9 (0.7 to 1.0) | 0.8 (0.7 to 0.9) | 0.257 |
| Serum Albumin (g/dL): mean ± SD | 3.6 ± 0.7 | 3.2 ± 0.4 | 0.017 |
| Total bilirubin (mg/dL): Median (IQR) | 1.0 (0.6 to 2.0) | 1.7 (0.9 to 2.1) | 0.155 |
| Aspartate aminotransferase (mg/dL), median (IQR) | 63.0 (42.0 to 116.0) | 96.5(73.5 to 155.0) | 0.013 |
| Alanine aminotransferase (mg/dL), median (IQR) | 41.0 (23.0 to 76.0) | 52.5 (45.0 to 85.0) | 0.151 |
| International normalized ratio: mean ± SD | 1.2 ± 0.2 | 1.2 ± 0.4 | 0.654 |
| Hepatitis B viral load (IU/mL): Median (IQR) | 1450 (Undetectable to 165000) | 32650 (13700 to 966000) | 0.706 |
| Alpha-fetoprotein (ng/mL): Median (IQR) | 20.5 (9.3 to 499.8) | 176.45 (13.3 to 992.2) | 0.207 |
| MELD: mean ± SD | 9 (7 to 12) | 11 (8 to 12) | 0.307 |
| ECOG score | 1.000 | ||
| 0 | 42 (79.2) | 13 (81.2) | |
| 1 | 11 (20.8) | 3 (18.8) |
There were no significant differences in tumor characteristics between these two patient groups (Table 2). The median number of TACE sessions was not significantly different between the two groups; the proportion of patients who achieved CR after treatment was statistically significantly higher in group A than in group B (24.5% vs 0%, P = 0.030).
| Variables | Group A (n = 53), % | Group (n = 16), % | P value |
| Multinodular (> 1 lesion) | 44 (83.0) | 14 (87.5) | 1.000 |
| Largest tumor size (cm): Median (IQR) | 5.3 (3.7 to 9.0) | 4.3 (2.6 to 9.0) | 0.399 |
| Largest tumor sized > 5 cm | 27 (50.9) | 9 (56.2) | 0.710 |
| Number of TACE sessions: median (IQR) | 2 (1 to 3) | 2 (1 to 3) | 0.301 |
| Achieved complete respond | 13 (24.5) | 0 (0) | 0.030 |
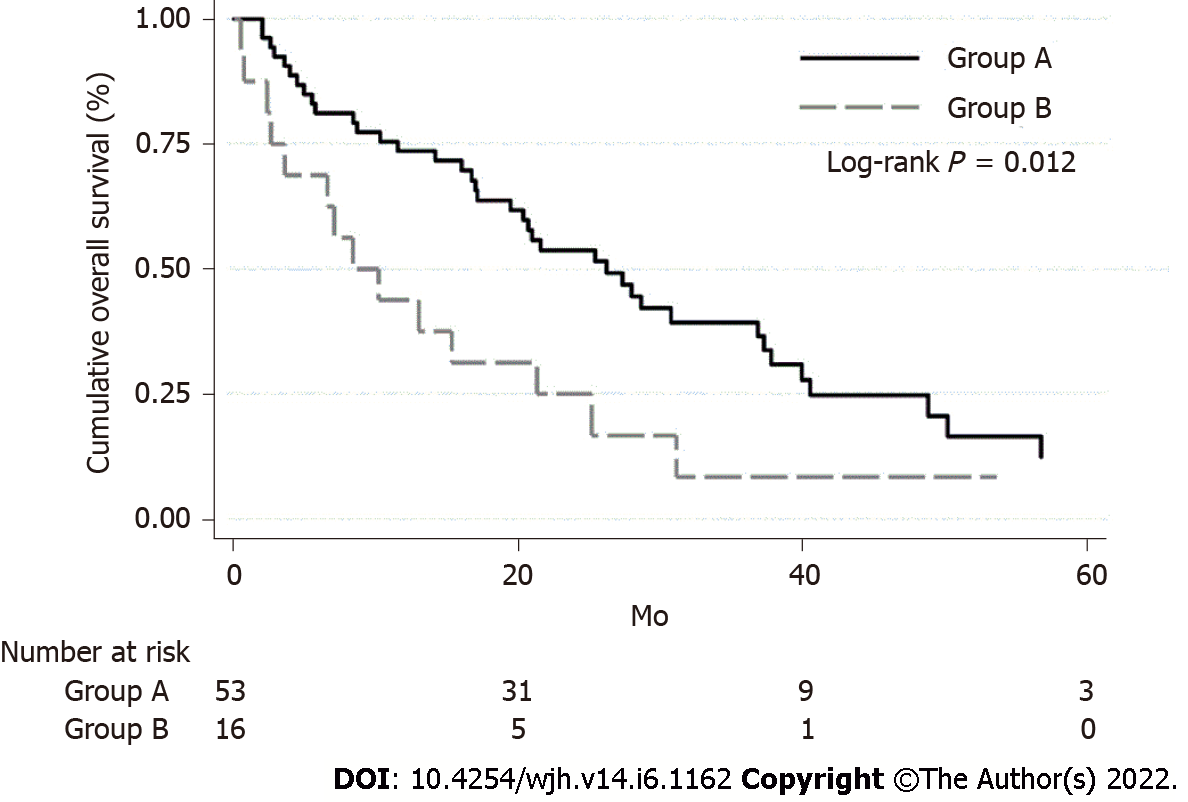
Based on the Kaplan–Meier method, the survival rate of patients in group A was significantly higher than in group B (median OS was 26.2 mo in group A and 8.4 mo in group B; log-rank P = 0.012) (Figure 1).
To identify the factors of OS after TACE in HCC patients with viral hepatitis, the Cox proportional-hazards model was used. In the multivariate analysis, factors associated with a decreased OS included alcohol consumption (hazards ratio [HR], 2.377; 95% confidence interval [CI], 1.109-5.095; P = 0.026), presence of portal hypertension (HR, 2.578; 95%CI, 1.320-5.037; P = 0.006), largest tumor size > 5 cm (HR, 3.558; 95%CI, 1.824-6.939; P < 0.001), and serum alpha-fetoprotein level > 100 ng/mL (HR, 2.536; 95%CI, 1.377-4.670; P = 0.003) (Table 3).
| Factor | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | HR | 95%CI | P value | |
| Female sex | 0.722 | 0.384-1.358 | 0.312 | 1.103 | 0.516-2.359 | 0.800 |
| Age, every 1-year increase | 0.977 | 0.947-1.008 | 0.148 | 1.000 | 0.968-1.034 | 0.979 |
| Body mass index < 18.5 | 0.937 | 0.439-2.002 | 0.867 | |||
| Hepatitis B infection | 0.841 | 0.487-1.453 | 0.535 | |||
| Hepatitis C infection | 1.270 | 0.737-2.191 | 0.389 | |||
| Alcohol consumption | 2.185 | 1.172-4.074 | 0.014 | 2.377 | 1.109-5.095 | 0.026 |
| Serum albumin > 3.5 g/dL | 0.717 | 0.414-1.240 | 0.234 | |||
| Alpha-fetoprotein > 100 ng/mL | 2.174 | 1.249-3.783 | 0.006 | 2.536 | 1.377-4.670 | 0.003 |
| Child–Turcotte–Pugh classification | 0.115 | 0.793 | ||||
| A | 1 | (reference) | 1 | (reference) | ||
| B | 1.558 | 0.898-2.704 | 1.114 | 0.498-2.492 | ||
| MELD score > 10 | 1.133 | 0.652-1.968 | 0.657 | |||
| Presence of portal hypertension | 1.743 | 0.952-3.191 | 0.072 | 2.578 | 1.320-5.037 | 0.006 |
| ECOG | 0.270 | |||||
| 0 | 1 | (reference) | ||||
| 1 | 1.436 | 0.755-2.731 | ||||
| Multinodular (> 1 lesion) | 1.141 | 0.512-2.543 | 0.747 | |||
| Largest tumor sized > 5 cm | 2.203 | 1.242-3.906 | 0.007 | 3.558 | 1.824-6.939 | < 0.001 |
This retrospective cohort study was based on a series of patients with intermediate-stage HCC (BCLC B) who underwent TACE and reflects “real-life” outcome data from a Government Hospital in a middle-income country. The principal findings of this study were as follows: First, HCC BCLC B patients with chronic viral hepatitis concurrent with alcohol consumption showed a decreased rate of CR and survival after TACE than those who had chronic viral hepatitis alone; and second, after adjusting for confounding factors, alcohol consumption was observed as an independent risk factor of increased mortality after TACE in individuals with chronic viral hepatitis.
It has been well documented that regular alcohol consumption is associated with increased HCC risk, with a significant dose-dependent response relationship between the amount of alcohol intake and the risk of HCC[16,17]. Recent meta-analysis demonstrated that consumption of even a small amount of alcohol is related to cancer risk[18]. The risk of HCC in alcohol consumption may differ depending on the severity of baseline liver status[19]. For patients with pre-existing chronic liver diseases, including HBV and HCV, alcohol consumption has a synergistic effect on the development of HCC, although the risk threshold remains uncertain[1,20,21]. However, the data on the impact of alcohol consumption on HCC outcomes after treatment remains limited.
To the best of our knowledge, this was the first study evaluating the impact of alcohol consumption on treatment outcomes among patients with intermediate-stage HCC after TACE in individuals with chronic viral hepatitis. According to the tumor characteristics, there were no significant differences in the number and size of tumors between the two groups. Patients with chronic viral hepatitis concurrent with alcohol consumption developed a lower rate of CR and had decreased survival rate after TACE than those who had chronic viral hepatitis alone. These results underscore that alcohol consumption provides worse outcomes after TACE when concomitant with chronic viral hepatitis. There are many possible reasons to explain this finding.
First, patients with alcohol-related HCC are linked to poor general conditions, including performance status and hepatic reserve[22]. This is consistent with the results of our study demonstrating liver status in patients with viral hepatitis infection and alcohol consumption which was poorer in both synthesis (lower albumin level) and evidence of inflammation (higher AST level) than that in patients with viral hepatitis only. The impaired clinical status could be caused by the direct effect of ethanol on the liver, alcohol-associated malnutrition, or brain cognitive dysfunction occurring in chronic alcohol abuse[23,24]. Poorer general conditions at the time of HCC detection were associated with a higher rate of non-HCC-related complications than viral-related-HCC, which resulted in shorter survival[22,25-28]. In addition, continuing alcohol abuse precludes providing treatment options for best supportive care as a result of worsening survival[29]. In Thailand, most patients with HCC who abused alcohol still had concurrent alcohol consumption, leading to ongoing liver function deceleration and limited treatment options[22,28,29]. This could explain why patients with chronic viral hepatitis and alcohol abuse had a lower rate of CR and shorter OS than patients with chronic viral hepatitis alone in this study.
Second, alcohol can accelerate the progression of liver disease in patients with chronic viral hepatitis (B or C) by several mechanisms. Alcohol increases intestinal permeability to various substances, especially bacteria-derived liposaccharides from the gut to the liver, stimulating Kupffer cell activity and promoting inflammatory cascade resulting in progression of fibrosis[30,31]. Acetaldehyde, which is derived from the metabolism of ethanol, is a carcinogen and a highly toxic substance that plays a major role in the necroinflammation of hepatocytes[1]. Besides the direct biological impact of alcohol, the association between alcohol consumption and chronic viral hepatitis infection has been identified. Chronic alcohol consumption led to increased replication of viral hepatitis virus (both HBV and HCV)[32,33] and altered immune response, which is associated with promoting hepatocyte injury resulting in hepatic deterioration[34,35]. Heavy alcohol drinking was associated with rapid progression of fibrosis and development of cirrhosis in patients with HBV infection[36]. Among HCV patients, excessive alcohol consumption was strongly associated with decompensated cirrhosis[37]. In addition, HBV infection compromises the host function of antioxidant defense, which promotes alcoholic liver injury[38]. For these reasons, alcohol consumption and chronic viral hepatitis can synergize the lifetime risk of liver disease progression and ultimately increase the risk of death, as seen in our study[37,39].
Third, alcohol may alter the biological pattern of HCC in patients with viral hepatitis. Kubo et al[40] demonstrated that the proportion of well-differentiated HCC was lower among those with massive alcohol consumption than those without alcohol use. Undifferentiated HCC is more aggressive and metastatic[41]. Okada et al[42] also reported that patients with excessive alcohol consumption had a short tumor-free and overall survival after treatment.
Fourth, alcohol consumption is linked with different types of liver disease. Alcohol abuse, especially heavy alcohol consumption, cause changes in lipid metabolism resulting in aggravation of non-alcoholic liver disease (NASH), which affects treatment outcomes. NASH-related HCC is associated with poorer OS than HCC in patients with cirrhosis from other etiologies[43].
In addition to the impact of alcohol on treatment outcome, our study revealed the other factors that affect the risk of mortality, including the presence of portal hypertension, serum AFP > 100 ng/mL and larger tumor size. Consistent with the findings of a previous study, Scheiner et al[44] demonstrated that portal hypertension was a significant poor prognostic factor in HCC patients undergoing TACE. After TACE, transient hepatic hypoxia enhanced the upregulation of vascular endothelial growth factor, which plays a significant role in cirrhosis progression and dysfunction[44,45]. Tumor burden is another factor that affects the prognosis of HCC. A larger tumor size provides higher tumor volume resulting in a worse prognosis, which is consistent with the results of our study. A previous study reported that the elevation of serum AFP levels correlated with the tumor size in HCC[46]. In our study, patients with serum AFP levels of more than 100 ng/mL showed an increase in the risk of death with an odds ratio of 2.5. This supports that AFP is not only a diagnostic tool but also a prognostic tool of HCC.
Our study has several limitations. First, this was a single center study conducted in a tertiary care center in a developing country in Southeast Asia. According to a previous study by our group, the rate of adherence to the international guidelines of HCC treatment in developing Asian countries was decreased because of the regional culture in which the aggressive treatment options were not preferred extensively in patients with non-curative malignancies[47]. Second, this study was retrospective in nature. All variables were obtained from a review of medical records, which may have caused misclassification bias and missing data. Minimization of these errors was attempted using two independent reviewers, and a third reviewer made the final decision in case discrepancies were found. Third, some information that might affect survival (e.g., data on non-alcoholic fatty liver disease, viral status, and alcohol abstinence) was unavailable. Finally, the study population size was relatively small, and the number of patients among the two groups was disproportionate (53 in group A and 16 in group B). A future prospective study with a larger sample size and appropriately balanced heterogenous participants to eliminate bias and confirm the findings of this study is needed.
In HCC BCLC B, patients with chronic viral hepatitis concurrent with alcohol consumption had a decreased CR rate and survival post-TACE than those who had viral hepatitis infection only. Alcohol consumption was observed as an independent risk factor of increased mortality after TACE in individuals with viral hepatitis. The burden of alcohol is high globally and is avoidable, although difficult to prevent. The results of this study remind us that alcohol consumption will continue to be important, and strategies modified for these factors to limit their impact at the individual and population levels need to be continued.
Alcohol consumption increases the risk of hepatocellular carcinoma (HCC) in patients with pre-existing liver disease, including viral hepatitis. However, the impact of alcohol consumption on the outcomes of HCC remained questionable.
We hypothesized that alcohol had an additional effect with chronic viral hepatitis infection on treatment outcomes after transarterial chemoembolization (TACE) in patients with intermediate-stage HCC (Barcelona Clinical Liver Cancer [BCLC] -B).
We aims to evaluate the additional effect of alcohol on treatment outcomes of TACE among HCC patients with viral hepatitis.
We conducted a retrospective review the records of 69 HCC patients with viral hepatitis classified as BCLC B who underwent TACE as the first-line treatment between 2014 and 2019 at Hatyai Hospital. Patients with chronic viral hepatitis only were categorized under group A and those with chronic viral hepatitis and concurrent alcohol consumption were categorized under group B. Both groups were compared, and the Cox proportional hazards model was used to identify variables influencing survival.
We find that patients who had chronic viral hepatitis alone had a statistically significantly higher proportion of complete response (24.5% vs 0%, P = 0.030) and a higher median survival rate (26.2 mo vs 8.4 mo; log-rank P = 0.012) than those with chronic viral hepatitis concurrent with alcohol consumption. Alcohol consumption was an independent factor associated with decreased survival in the proportional hazards model included (hazards ratio [HR], 2.377; 95% confidence interval [CI], 1.109-5.095; P = 0.026).
In HCC BCLC B patients with chronic viral hepatitis, alcohol consumption is an independent risk factor for increased mortality and decreases the rate of complete response and survival after TACE.
This research underscore that alcohol consumption leads to worse outcomes after TACE in intermediate stage HCC patients with chronic viral hepatitis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Gastroenterological Association of Thailand.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nath L, India; Xu X, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Iida-Ueno A, Enomoto M, Tamori A, Kawada N. Hepatitis B virus infection and alcohol consumption. World J Gastroenterol. 2017;23:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3247] [Article Influence: 463.9] [Reference Citation Analysis (1)] |
| 3. | European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236]. J Hepatol. 2019;70:817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Schottenfeld D, Beebe-Dimmer JL, Buffler PA, Omenn GS. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health. 2013;34:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Rosman AS, Waraich A, Galvin K, Casiano J, Paronetto F, Lieber CS. Alcoholism is associated with hepatitis C but not hepatitis B in an urban population. Am J Gastroenterol. 1996;91:498-505. [PubMed] |
| 6. | Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, Neuman MG, Rehm J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 7. | Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, Roerecke M. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 439] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Chuang SC, Lee YC, Wu GJ, Straif K, Hashibe M. Alcohol consumption and liver cancer risk: a meta-analysis. Cancer Causes Control. 2015;26:1205-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C, Porru S, Nardi G. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 10. | Matsushita H, Takaki A. Alcohol and hepatocellular carcinoma. BMJ Open Gastroenterol. 2019;6:e000260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 171] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 13. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3679] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 14. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 15. | Sato Y, Watanabe H, Sone M, Onaya H, Sakamoto N, Osuga K, Takahashi M, Arai Y; Japan Interventional Radiology in Oncology Study Group-JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Minami T, Tateishi R, Fujiwara N, Nakagomi R, Nakatsuka T, Sato M, Uchino K, Enooku K, Nakagawa H, Fujinaga H, Izumiya M, Hanajiri K, Asaoka Y, Kondo Y, Tanaka Y, Otsuka M, Ohki T, Arai M, Tanaka A, Yasuda K, Miura H, Ogata I, Kamoshida T, Inoue K, Koike Y, Akamatsu M, Mitsui H, Fujie H, Ogura K, Yoshida H, Wada T, Kurai K, Maekawa H, Obi S, Teratani T, Masaki N, Nagashima K, Ishikawa T, Kato N, Moriya K, Yotsuyanagi H, Koike K. Impact of Obesity and Heavy Alcohol Consumption on Hepatocellular Carcinoma Development after HCV Eradication with Antivirals. Liver Cancer. 2021;10:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Chao X, Wang S, Hlobik M, Ballabio A, Ni HM, Ding WX. Loss of Hepatic Transcription Factor EB Attenuates Alcohol-Associated Liver Carcinogenesis. Am J Pathol. 2022;192:87-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Park H, Shin SK, Joo I, Song DS, Jang JW, Park JW. Systematic Review with Meta-Analysis: Low-Level Alcohol Consumption and the Risk of Liver Cancer. Gut Liver. 2020;14:792-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Hagström H, Thiele M, Sharma R, Simon TG, Roelstraete B, Söderling J, Ludvigsson JF. Risk of Cancer in Biopsy-Proven Alcohol-Related Liver Disease: A Population-Based Cohort Study of 3410 Persons. Clin Gastroenterol Hepatol. 2022;20:918-929.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Vandenbulcke H, Moreno C, Colle I, Knebel JF, Francque S, Sersté T, George C, de Galocsy C, Laleman W, Delwaide J, Orlent H, Lasser L, Trépo E, Van Vlierberghe H, Michielsen P, van Gossum M, de Vos M, Marot A, Doerig C, Henrion J, Deltenre P. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J Hepatol. 2016;65:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Ganne-Carrié N, Nahon P, Chaffaut C, N'Kontchou G, Layese R, Audureau E, Chevret S; CIRRAL group; ANRS CO12 CirVir group. Impact of cirrhosis aetiology on incidence and prognosis of hepatocellular carcinoma diagnosed during surveillance. JHEP Rep. 2021;3:100285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | McClain CJ, Rios CD, Condon S, Marsano LS. Malnutrition and Alcohol-Associated Hepatitis. Clin Liver Dis. 2021;25:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Nunes PT, Kipp BT, Reitz NL, Savage LM. Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction. Int Rev Neurobiol. 2019;148:101-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Bucci L, Garuti F, Camelli V, Lenzi B, Farinati F, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Maida M, Felder M, Morisco F, Gasbarrini A, Gemini S, Foschi FG, Missale G, Masotto A, Affronti A, Bernardi M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group; Italian Liver Cancer ITA LI CA Group. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment Pharmacol Ther. 2016;43:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Costentin CE, Mourad A, Lahmek P, Causse X, Pariente A, Hagège H, Dobrin AS, Becker C, Marks B, Bader R, Condat B, Héluwaert F, Seitz JF, Lesgourgues B, Denis J, Deuffic-Burban S, Rosa I, Decaens T; CHANGH Study Group. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: Results of a prospective, nationwide study. Cancer. 2018;124:1964-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stål P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Schütte K, Bornschein J, Kahl S, Seidensticker R, Arend J, Ricke J, Malfertheiner P. Delayed Diagnosis of HCC with Chronic Alcoholic Liver Disease. Liver Cancer. 2012;1:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Costentin CE, Sogni P, Falissard B, Barbare JC, Bendersky N, Farges O, Goutte N. Geographical Disparities of Outcomes of Hepatocellular Carcinoma in France: The Heavier Burden of Alcohol Compared to Hepatitis C. Dig Dis Sci. 2020;65:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 32. | Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O'Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Larkin J, Clayton MM, Liu J, Feitelson MA. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology. 2001;34:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Pianko S, Patella S, Ostapowicz G, Desmond P, Sievert W. Fas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infection. J Viral Hepat. 2001;8:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, Tani S, Goto M. An epidemiologic study of effects of alcohol in the liver in hepatitis B surface antigen carriers. Am J Epidemiol. 1988;128:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M, Vogel A, Capron F, Chedid A, Bedossa P; PANFIBROSIS Group. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Schwarzinger M, Baillot S, Yazdanpanah Y, Rehm J, Mallet V. Contribution of alcohol use disorders on the burden of chronic hepatitis C in France, 2008-2013: A nationwide retrospective cohort study. J Hepatol. 2017;67:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035-6043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 39. | Lee M, Kowdley KV. Alcohol's effect on other chronic liver diseases. Clin Liver Dis. 2012;16:827-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Kubo S, Kinoshita H, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Kuroki T. High malignancy of hepatocellular carcinoma in alcoholic patients with hepatitis C virus. Surgery. 1997;121:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, Shimamoto F, Asahara T. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Okada S, Ishii H, Nose H, Okusaka T, Kyogoku A, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Effect of heavy alcohol intake on long-term results after curative resection of hepatitis C virus-related hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:867-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 618] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 44. | Qu K, Yan Z, Wu Y, Chen Y, Qu P, Xu X, Yuan P, Huang X, Xing J, Zhang H, Liu C, Zhang J. Transarterial chemoembolization aggravated peritumoral fibrosis via hypoxia-inducible factor-1α dependent pathway in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Scheiner B, Ulbrich G, Mandorfer M, Reiberger T, Müller C, Waneck F, Trauner M, Kölblinger C, Ferlitsch A, Sieghart W, Peck-Radosavljevic M, Pinter M. Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma. United European Gastroenterol J. 2019;7:850-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Abbasi A, Bhutto AR, Butt N, Munir SM. Corelation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62:33-36. [PubMed] |
| 47. | Chang A, Utarabhand R, Khaimook A, Songjamrat A, Pakdeejit S, Rattanasupa A. Adherence to AASLD Recommendation Guideline for Treatment Hepatocellular Carcinoma: Single Center Analysis of the Regional Hospital of Thailand. J Med Assoc Thai. 2018;101:1708-1715. [DOI] [Full Text] |