Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1111
Peer-review started: January 26, 2022
First decision: April 8, 2022
Revised: April 18, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: June 27, 2022
Processing time: 148 Days and 4.3 Hours
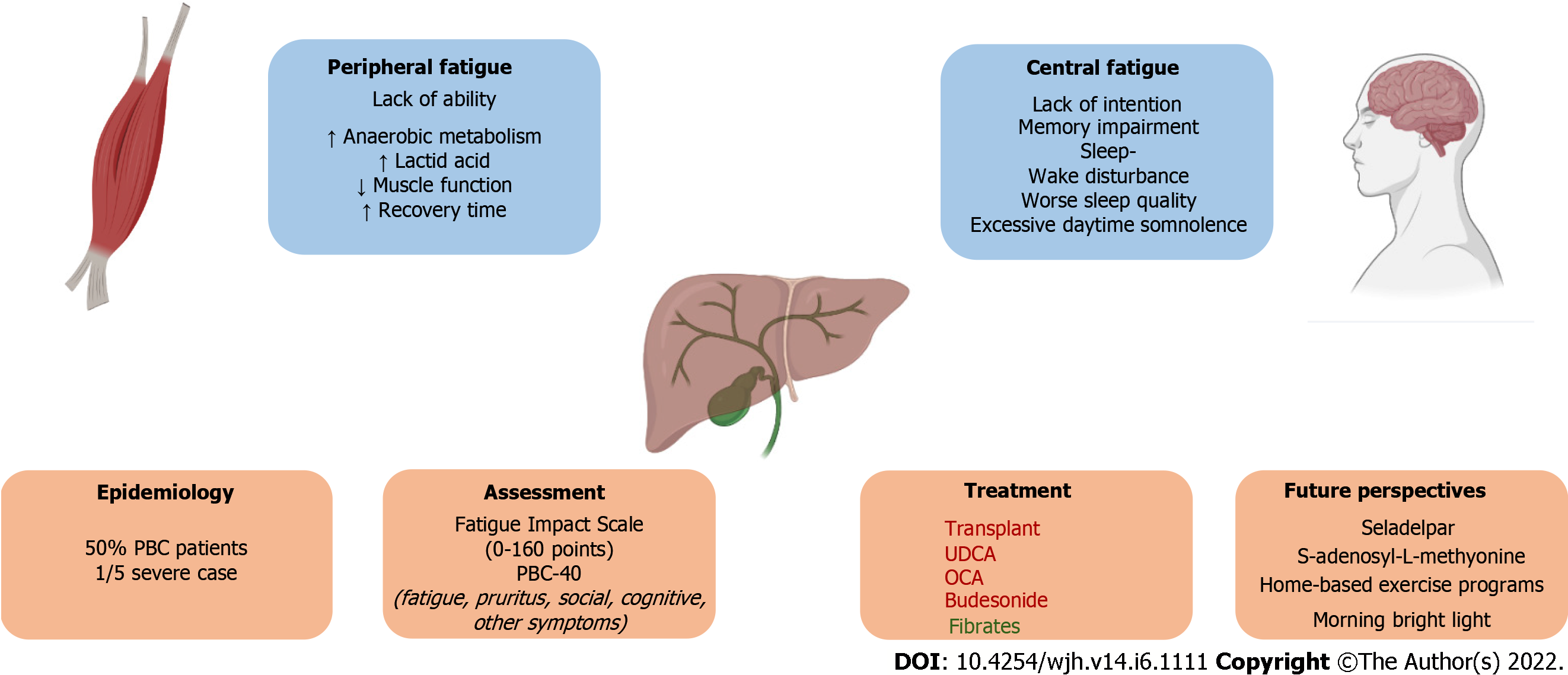
Fatigue is considered one of the most frequent and debilitating symptoms in primary biliary cholangitis (PBC), affecting over 50% of PBC patients. One in five patients with PBC suffer from severe fatigue, which significantly impairs quality of life. Fatigue is made up of a central and a peripheral component, whose pathophysiology is still greatly unresolved. Central fatigue is characterised by a lack of self-motivation and can manifest both in physical and mental activities (lack of intention). Peripheral fatigue includes neuromuscular dysfunction and muscle weakness (lack of ability). Peripheral fatigue could be explained by an excessive deviation from aerobic to anaerobic metabolism leading to excessive lactic acid accumulation and therefore accelerated decline in muscle function and prolonged recovery time. As opposed to itching, and with the exception of end-stage liver disease, fatigue is not related to disease progression. The objective of this review is to outline current understanding regarding the pathophysiology of fatigue, the role of comorbidities and contributing factors, the main tools for fatigue assessment, the failed therapeutic options, and future treatment perspectives for this disabling symptom. Since fatigue is an extremely common and debilitating symptom and there is still no licensed therapy for fatigue in PBC patients, further research is warranted to understand its causative mechanisms and to find an effective treatment.
Core Tip: Fatigue is considered one of the most frequent and debilitating symptoms in primary biliary cholangitis, affecting over 50% of patients. The objective of this review is to outline current understanding regarding the pathophysiology of fatigue, the role of comorbidities and contributing factors, the main tools for fatigue assessment, the failed therapeutic options, and future treatment perspectives for this disabling symptom. Since fatigue is an extremely common and debilitating symptom and there is still no licensed therapy for fatigue in PBC patients, further research is warranted to understand its causative mechanisms and to find effective treatment.
- Citation: Lynch EN, Campani C, Innocenti T, Dragoni G, Biagini MR, Forte P, Galli A. Understanding fatigue in primary biliary cholangitis: From pathophysiology to treatment perspectives. World J Hepatol 2022; 14(6): 1111-1119
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1111.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1111
Fatigue is considered one the most frequent and debilitating symptoms in primary biliary cholangitis (PBC), affecting over 50% of patients with PBC[1]. As opposed to itching, and with the exception of end-stage liver disease, fatigue is not related to disease progression[1,2]. One in five patients with PBC suffer from severe fatigue, which significantly impairs quality of life[3]. The severity of chronic fatigue symptoms in PBC predicts liver-related mortality and liver transplantation outcome[4]. Latitude and sun exposure might influence PBC phenotype, including fatigue status[5].
The objective of this review is to outline current understanding regarding the pathophysiology of fatigue, the role of comorbidities and contributing factors, the main tools for fatigue assessment, the available treatments, and future therapeutic options for this disabling symptom.
Fatigue is defined as an overwhelming sense of tiredness, lack of energy, and a feeling of exhaustion[6]. Its pathophysiology in PBC is still unresolved. It can be considered as made up of two different entities: Peripheral and central fatigue. Peripheral fatigue includes neuromuscular dysfunction and muscle weakness (lack of ability)[7]. Central fatigue is characterised by a lack of self-motivation and can manifest both in physical and mental activities (lack of intention)[7].
Anti-mitochondrial autoantibodies (AMA), which specifically target pyruvate dehydrogenase complex (PDC), are a hallmark of PBC[8]. In these patients, anti-PDC antibodies are mainly directed against the inner lipoyl domain of the PDC-E2 component, which has an alpha-lipoic acid covalently bound to a specific lysine residue, that is an absolute requirement for its enzymatic activity. The PDC-E2 comp
In fatigued PBC patients, there seems to be an excessive deviation from aerobic to anaerobic metabolism leading to excessive lactic acid accumulation and therefore accelerated decline in muscle function and prolonged recovery time. Various studies support this conclusion: Fatigued PBC patients perform worse than non-fatigued patients on hand grip test with no association with liver disease severity[10]; when bioenergetics of muscle function was assessed using 31P magnetic resonance spectroscopy in PBC patients, non-PBC patients with chronic fatigue syndrome, patients with primary sclerosing cholangitis, and controls, only patients with PBC showed increased post-exercise muscle acidosis and prolonged adenosine diphosphate and phosphocreatine recovery time suggesting mitochondrial dysfunction[11]. pH recovery appeared to be related to fatigue severity[11]. How AMA can induce PDC depletion or dysfunction in muscles of patients with PBC remains uncertain.
It should also be noted that the reduction of AMA through B-cell depletion with rituximab did not have any effect on fatigue, suggesting the existence of other fatigue-inducing pathophysiologic mechanisms than antibody-mediated damage[12]. In addition, peripheral fatigue measured by twitch interpolation did not differ between PBC patients and controls, although patients with PBC were not differentially assessed based on fatigue symptoms[13]. Twitch interpolation can supposedly distinguish central from peripheral fatigue as it allows to assess whether all motor units have been recruited by the central nervous system or not[13]. In centrally fatigued patients, central activation is low, and a smaller number of motor units are stimulated[13].
Patients with PBC often report cognitive symptoms, such as memory impairment, and higher rates of sleep-wake disturbance with delayed sleep timing, worse sleep quality, and excessive daytime somnolence[14], seemingly unrelated to liver disease severity[13,15]. Evidence supporting the central origin of fatigue in PBC patients is mostly made up of small-scale studies and its pathophysiology is unknown. Treatment for excessive daytime somnolence with modafinil was ineffective[16]. Mosher et al[17] studied the resting-state functional connectivity (rsFC) of deep grey matter brain structures (putamen, thalamus, amygdala, and hippocampus) using resting-state functional magnetic resonance imaging in 20 non-cirrhotic PBC patients compared with 21 matched controls. PBC patients exhibited significant alterations in rsFC levels as compared to controls. Fatigue, itch, and verbal working memory performance were associated with alterations of deep grey matter rsFC, possibly reflecting chronic immune-mediated signalling from the liver to the brain in PBC patients[17]. In a study by McDonald et al[13], twitch interpolation and paired-pulse trans-cranial magnetic stimulation were used to study central nervous system function in PBC patients and its relationship to fatigue symptoms. PBC patients had significantly lower levels of central activation[13]. Interestingly, no differences were found between transplanted and non-transplanted patients. However, a volitional contribution could justify the results and could not be excluded; central activation might be reduced as a protective mechanism to avoid exhaustion (due to peripheral fatigue?).
Altered central neurotransmission has been a leading hypothesis to explain the development of fatigue in PBC patients, involving both serotonergic and noradrenaline pathways. Unfortunately, no specific treatment to stimulate the serotonin pathway (ondansetron, fluvoxamine, or fluoxetine) has brought positive results[18,20].
Large-scale clinical studies are warranted that assess whether fatigue in PBC patients is predominantly central or peripheral, or both, in order to concentrate future research in the right direction.
There are many conditions and therapies which can cause fatigue or deteriorate existing weariness; in fatigued PCB patients, a complete assessment should be performed, and any detected condition should be addressed. Among these conditions, we can find autoimmune diseases such as hypothyroidism, anaemia, type II diabetes, nocturnal pruritus, autonomic dysfunction, dehydration, restless leg syndrome, and concurrent medications such as anti-hypertensive therapy. Depressive symptoms in PBC patients seem to be the consequence rather than the cause of fatigue, as the prevalence of a depressive disorder in patients with PBC does not seem to be higher than that in the general population[21].
A complete list of additive factors to fatigue burden is presented in Table 1.
| Conditions | Drugs |
| Addison disease; Anaemia; Autonomic dysfunction; Cancer; Chronic Lyme disease; Dehydration; Depression; Diabetes; Heart failure; Hypothyroidism; Infectious/inflammatory state; Myasthenia gravis; Multiple sclerosis; Obstructive sleep apnoea; Parkinson’s disease; Pregnancy; Renal failure; Restless legs syndrome; Tuberculosis | Antibiotics; Antidepressants; Anti-hypertensive therapy; Muscle relaxants; Opioids; Sedative-hypnotics |
Although fatigue is a ubiquitous symptom in medical practice, one single questionnaire might not fit the purpose of measuring fatigue in a specific group of patients. Each assessment tool should be validated not to compromise the quality of research. In a systematic review by Kim et al[22], the authors found that, between 1990 and 2019, patient reported outcomes in PBC had been mostly assessed with unlabelled, nonspecific versions of numeric ratings or Likert scales and that fatigue has been measured with over ten different instruments although ideally, the use of questionnaires should be standardised to allow comparison.
In a recent systematic review, Machado et al[6] evaluated existing fatigue scales commonly used to assess fatigue in patients with various medical conditions. Eleven fatigue scales were identified and analysed: Five were unidimensional [Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Brief Fatigue Inventory, Fatigue Severity Scale (FSS), Numerical Rating Scale-Fatigue, and Visual Analog Scale-Fatigue (VAS-F)] and six multidimensional [Fatigue Impact Scale (FIS), Checklist Individual Strength (CIS), Chalder Fatigue Scale (CFS), Multidimensional Assessment of Fatigue, Multidimensional Fatigue Inventory Scale, and Piper Fatigue Scale][6]. Unidimensional scales can be useful to assess severity or as screening tools, whereas multidimensional scores are more informative and can evaluate affective, cognitive, somatic, and behavioural manifestations of fatigue.
FACIT-F and FSS can be used as screening tools as they present a cut-off point to differentiate patients with fatigue vs non-fatigue. Eight of the previously reported scales (FACIT, FSS, BFI, CIS, MAF, MFI, FIS, and CFS) are able to detect disease progression or response to treatment[6].
Of all the above-mentioned fatigue-specific scales, the Fatigue Impact Scale is the only one which has been validated in PBC[23,24]. It was initially validated in 1994 by Fisk et al[25] in patients with multiple sclerosis and mild hypertension and was then found to be highly acceptable and consistent also in patients with PBC. It takes approximately 5 to 8 minutes to be completed and has a coefficient of reproducibility of 13% of the mean (vs 33% for the VAS-F)[23]. FIS measures the impact of fatigue on 40 aspects of daily life over the previous month. Patients are required to grade from zero to four how impaired has each aspect been to give a maximum score of 160 (severely fatigued). FIS assesses the impact of fatigue on psycho-social, cognitive, and physical activities[23].
However, the PBC-40 is the only instrument which can claim to be truly representative of PBC-related fatigue as it was originally developed for PBC patients. Jacoby et al [5] developed the PBC-40 in 2005 to assess PBC patients’ quality of life. It investigates the impact of the disease in six domains: Fatigue, pruritus, social, cognitive, and other symptoms. The patients rate 40 items on a five-point scale, with a higher score indicating a worse quality of life. It has also been adapted in shorter versions. As it includes a fatigue subscale with proven content validity, it is the ideal instrument for studies on PBC-related fatigue. None of the proposed questionnaires specifically differentiate between peripheral and central fatigue.
Fatigue can also be assessed by measuring objective (e.g., brain imaging, serological, and physical performance measures), as well as patient reported outcomes, but no consensus has been reached with regard to objective or combined assessments in PBC[7]. In the literature, there are a certain number of objective methods to distinguish central from peripheral fatigue. Peripheral fatigue, or impairment of muscle excitation, is most commonly evaluated with electromyography[26]. Serum lactate and IL-6 have been identified as the most accurate and valid biomarkers to measure muscle fatigue, although they are influenced by workload conditions and timing of testing with respect to exercise[27]. Other non-invasive methods have been employed for the detection of peripheral fatigue (e.g., acoustic myography)[28]. On the other hand, central fatigue can be assessed either with percutaneous nerve stimulation[29] or transcranial magnetic stimulation during maximal contractions[30]. If the stimulation evokes an extra-force, it means that not all muscle units have been recruited, suggesting that central fatigue is present[30].
Patients need to be advised and supported to develop coping strategies while retaining ownership of the problem. Pacing strategies (using available energy to its best advantage) and timing strategies (fatigue is worse later in the day typically so arranging key tasks for earlier in the day can make them less demanding) are recommended[3]. Awareness and understanding from carers should be promoted[3].
Ursodeoxycholic acid (UDCA) at a daily dosage of 13-15 mg/kg is the first line treatment for PBC. Although UDCA slows liver disease progression, increases transplant-free survival, and reduces mortality, it does not improve fatigue[1,31]. In China, a phase IV trial (NCT03345589) is being conducted to compare the efficacy of an intermediate dosage of UDCA of 18–22 mg/kg/day and the standard dose over 6 mo in achieving biochemical remission. Unfortunately, since Angulo et al found no symptom improvement with an UDCA dosage increase to 23-25/mg/kg/day, the same is to be expected with the intermediate dosage[32].
Obeticholic acid (OCA) is a semi-synthetic hydrophobic bile acid analogue which can be used in patients who experience an inadequate response or are intolerant to UDCA. It is administered at an initial dose of 5 mg which can be titrated to 10 mg according to tolerability at 6 mo[1]. Fatigue is not responsive to OCA therapy[33]. OCA is associated with a dose dependent exacerbation in pruritus which can impair sleep and worsen fatigue[33].
Fibrates are a readily available but unlicensed treatment option for patients with PBC[34]. In the BEZURSO trial, a multicentre, double-blind, placebo-controlled, phase III clinical trial, 100 patients with inadequate response to ursodeoxycholic acid were randomly assigned to receive benzafibrate at a daily dose of 400 mg or placebo. After a 24 mo follow-up, 15% of patients in the benzafibrate group vs 9% in the placebo group reported an improvement in fatigue so benzafibrate could be the first therapeutic drug for PBC which has an effect on fatigue[35]. However, in this trial, no validated metrics were used to assess fatigue, as it was only categorised as absent, intermittent, or continuous. Further studies are required to confirm these results.
Budesonide is a synthetic corticosteroid with a high first-pass metabolism in the liver, which was found to improve liver histology and biochemistry in PBC patients with interface hepatitis on biopsy[36]. A recent phase-III, double-blind, randomised trial comparing budesonide combined with UDCA and UDCA only did not detect any improvement in liver histology, nor was fatigue alleviated[37].
Modafinil is an approved treatment for daytime somnolence due to narcolepsy, sleep apnoea, and fatigue related to shift work sleep disorder. A randomised, double-blind, placebo-controlled study was conducted to assess the efficacy of modafinil for the treatment of fatigue in PBC did not show a significant improvement vs placebo[16], despite positive results from an uncontrolled study. The use of modafinil should therefore be limited to patients with formally diagnosed sleep disorders.
As previously mentioned, rituximab, which could have influenced fatigue severity by reducing circulating anti-PDC antibodies, did not significantly reduce fatigue in a single-centre randomised controlled trial with 57 participants[38]. The anaerobic threshold improved, possibly due to an effect on muscle bioenergetics dysfunction, but this did not lead to reduced fatigue symptoms. Interestingly, in two small sample studies on the use of plasmapheresis in PBC, in one study all patients who suffered from fatigue (4/5) reported reduced symptoms after treatment[39] and in the second study on 13 patients, the reduction of the PBC-40 fatigue domain score was statistically significant (30 vs 38, P = 0.004)[40].
Ondansetron (a 5HT1 A receptor antagonist) did not determine an improvement in fatigue in a crossover study[20], nor did the use of selective serotonin reuptake inhibitors (fluoxetine and fluvoxamine) show any effect on fatigue in randomised controlled trials[18,19].
The empirical use of antioxidant therapy (vitamins A, C and E, selenium, methionine, and ubiquinone) had no effect on fatigue scores in a randomised, placebo-controlled crossover trial[41].
In the last few decades, there has been a decrease in the need for liver transplantation (LT) in PBC, most probably due to the introduction of UDCA as standard therapy[42]. According to current guidelines, liver transplant for fatigue in PBC is not appropriate as fatigue persists after transplantation in most patients[1]. Montali et al[5] conducted a prospective study to assess the impact of LT on fatigue. Although fatigue scores were significantly lower after LT, nearly half of LT recipients reported ongoing fatigue (44% of the total cohort and 47% of patients with low Model for End Stage Liver Disease score). These results have been confirmed in later studies[43].
Seladelpar is a selective peroxisome proliferator activated receptor delta agonist which has recently been assessed in an open-label, uncontrolled phase 2 study in PBC patients[44]. After 1 year of treatment, PBC-40 fatigue scores improved in 55%-64% of patients. Patients also reported a decrease in itch and sleep disturbance. These results need to be confirmed in a placebo-controlled and randomised trial.
S-adenosyl-L-methionine, added to UDCA, can improve cholestasis in non-cirrhotic PBC patients, probably due to its hepatoprotective effects[45]. In an open label clinical trial on 24 PBC patients, there was a significant improvement in fatigue, assessed with the PBC-40 questionnaire[45].
Although causative mechanisms of fatigue in PBC are still unknown, therapeutic approaches have been sought to alleviate this debilitating symptom.
Since fatigue in patients with PBC could be caused by muscle bioenergetic abnormalities, as previously mentioned, Freer et al[46] have performed a phase 1, single-arm, open-label clinical trial evaluating a novel exercise programme in patients with PBC with moderate-severe fatigue. Thirty-one patients concluded the 12-week home-based exercise programme which consisted of individualised resistance, aerobic exercises, and telephone health calls, although the results have not yet been published[46]. Peripheral muscle excessive acidosis and delayed pH recovery which characterise PBC patients can be improved with repeated single exercise episodes[47]. This programme is of great interest as patients with PBC tend to lead a sedentary lifestyle due to fear of exacerbating fatigue, but muscle fatigability is increased when physical activity is reduced.
PBC is associated with poor sleep quality and delayed sleep-wake profile which can worsen the burden of fatigue. For this reason, Turco et al[15] conducted a pilot study to assess the efficacy of a short course of morning bright light treatment on sleep-wake patterns of fifteen PBC patients, six healthy individuals, and seven cirrhotic patients[15]. In patients with PBC, 15 d of light therapy resulted in subjective sleep quality improvement and a reduction in daytime sleepiness. In addition, sleep onset and get-up time were significantly advanced. Unfortunately, fatigue was not formally assessed, although daytime dysfunction due to somnolence was reported as improved[15].
Failed therapeutic options and future therapeutic perspectives for fatigue in PBC are summarised in Table 2.
| Treatment for fatigue in PBC | Ref. |
| Failed therapeutic options | |
| Ursodeoxycholic acid | Angulo et al[32], 1999 |
| Obeticholic acid | Hirschfield et al[33], 2015 |
| Budesonide | Hirschfield et al[37], 2021 |
| Fluoxetine | Talwalkar et al[18], 2006 |
| Fluvoxamine | ter Borg et al[19], 2004 |
| Ondansetron | Theal et al[20], 2005 |
| Rituximab | Khanna et al[14], 2019 |
| Modafinil | Silveira et al[16], 2017 |
| Methotrexate | Combes et al[48], 2005 |
| Oral antioxidant supplementation | Prince et al[23], 2003 |
| Lifestyle changes | |
| Morning bright light treatment | Turco et al[15], 2018 |
| Home-based exercise programme | Freer et al[46], 2021 |
| Possible future therapeutic options | |
| Fibrates | Corpechot et al[35], 2018 |
| Plasmapheresis | Wunsch et al[40], 2021 |
| S-adenosyl-L-methionine | Wunsch et al[45], 2018 |
| Seladelpar | Kremer et al[44], 2022 |
The key concepts presented in this review are illustrated in Figure 1.
The pathophysiology of fatigue in patients with PBC is still unresolved and as yet, there is no licensed therapy for fatigue in PBC patients. Since fatigue is an extremely common and debilitating symptom, further research is warranted to understand its causative mechanisms and to find effective treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; Associazione Italiana per lo Studio del Fegato; European Association for the Study of the Liver.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Manesis EK, Greece; Oltra E, Spain A-Editor: Lin FY, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | EAftSotLEa, Liver EAftSot. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [DOI] [Full Text] |
| 2. | Al-Harthy N, Kumagi T, Coltescu C, Hirschfield GM. The specificity of fatigue in primary biliary cirrhosis: evaluation of a large clinic practice. Hepatology. 2010;52:562-570. [PubMed] [DOI] [Full Text] |
| 3. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, CollierJ, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [DOI] [Full Text] |
| 4. | Shahini E, Ahmed F. Chronic fatigue should not be overlooked in primary biliary cholangitis. J Hepatol. 2021;75:744-745. [PubMed] [DOI] [Full Text] |
| 5. | Montali L, Gragnano A, Miglioretti M, Frigerio A, Vecchio L, Gerussi A, Cristoferi L, Ronca V, D'Amato D, O'Donnell SE, Mancuso C, Lucà M, Yagi M, Reig A, Jopson L, Pilar S, Jones D, Pares A, Mells G, Tanaka A, Carbone M, Invernizzi P. Quality of life in patients with primary biliary cholangitis: A cross-geographical comparison. J Transl Autoimmun. 2021;4:100081. [PubMed] [DOI] [Full Text] |
| 6. | Machado MO, Kang NC, Tai F, Sambhi RDS, Berk M, Carvalho AF, Chada LP, Merola JF, Piguet V, Alavi A. Measuring fatigue: a meta-review. Int J Dermatol. 2021;60:1053-1069. [PubMed] [DOI] [Full Text] |
| 7. | Gerber LH, Weinstein AA, Mehta R, Younossi ZM. Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol. 2019;25:3669-3683. [PubMed] [DOI] [Full Text] |
| 8. | Nilsson I, Palmer J, Apostolou E, Gottfries CG, Rizwan M, Dahle C, Rosén A. Metabolic Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Not Due to Anti-mitochondrial Antibodies. Front Med (Lausanne). 2020;7:108. [DOI] [Full Text] |
| 9. | Yeaman SJ, Kirby JA, Jones DE. Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev. 2000;174:238-249. [PubMed] [DOI] [Full Text] |
| 10. | Goldblatt J, James OF, Jones DE. Grip strength and subjective fatigue in patients with primary biliary cirrhosis. JAMA. 2001;285:2196-2197. [PubMed] [DOI] [Full Text] |
| 11. | Hollingsworth KG, Newton JL, Taylor R, McDonald C, Palmer JM, Blamire AM, Jones DE. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol. 2008;6:1041-1048. [PubMed] [DOI] [Full Text] |
| 12. | Aguilar MT, Chascsa DM. Update on Emerging Treatment Options for Primary Biliary Cholangitis. Hepat Med. 2020;12:69-77. [PubMed] [DOI] [Full Text] |
| 13. | McDonald C, Newton J, Lai HM, Baker SN, Jones DE. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. J Hepatol. 2010;53:1095-1100. [PubMed] [DOI] [Full Text] |
| 14. | Khanna A, Leighton J, Lee Wong L, Jones DE. Symptoms of PBC - Pathophysiology and management. Best Pract Res Clin Gastroenterol. 2018;34-35:41-47. [PubMed] [DOI] [Full Text] |
| 15. | Turco M, Cazzagon N, Franceschet I, Formentin C, Frighetto G, Giordani F, Cellini N, Mazzotta G, Costa R, Middleton B, Skene DJ, Floreani A, Montagnese S. Morning Bright Light Treatment for Sleep-Wake Disturbances in Primary Biliary Cholangitis: A Pilot Study. Front Physiol. 2018;9:1530. [PubMed] [DOI] [Full Text] |
| 16. | Silveira MG, Gossard AA, Stahler AC, Jorgensen RA, Petz JL, Ali AH, Lindor KD. A Randomized, Placebo-Controlled Clinical Trial of Efficacy and Safety: Modafinil in the Treatment of Fatigue in Patients With Primary Biliary Cirrhosis. Am J Ther. 2017;24:e167-e176. [DOI] [Full Text] |
| 17. | Mosher VAL, Swain MG, Pang JXQ. Primary Biliary Cholangitis Alters Functional Connections of the Brain's Deep Gray Matter. Clin Transl Gastroenterol. 2017;8:e107. [DOI] [Full Text] |
| 18. | Talwalkar JA, Donlinger JJ, Gossard AA, Keach JC, Jorgensen RA, Petz JC, Lindor KD. Fluoxetine for the treatment of fatigue in primary biliary cirrhosis: a randomized, double-blind controlled trial. Dig Dis Sci. 2006;51:1985-1991. [PubMed] [DOI] [Full Text] |
| 19. | ter Borg PC, van Os E, van den Broek WW, Hansen BE, van Buuren HR. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634]. BMC Gastroenterol. 2004;4:13. [PubMed] [DOI] [Full Text] |
| 20. | Theal JJ, Toosi MN, Girlan L, Heslegrave RJ, Huet PM, Burak KW, Swain M, Tomlinson GA, Heathcote EJ. A randomized, controlled crossover trial of ondansetron in patients with primary biliary cirrhosis and fatigue. Hepatology. 2005;41:1305-1312. [PubMed] [DOI] [Full Text] |
| 21. | van Os E, van den Broek WW, Mulder PG, ter Borg PC, Bruijn JA, van Buuren HR. Depression in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 2007;46:1099-1103. [PubMed] [DOI] [Full Text] |
| 22. | Kim HP, Lieber SR, Rogers ME. A Systematic Review of Patient-Reported Outcomes in Primary Biliary Cholangitis and Primary Sclerosing Cholangitis. Hepatol Commun. 2020;4:1502-1515. [DOI] [Full Text] |
| 23. | Prince MI, James OF, Holland NP, Jones DE. Validation of a fatigue impact score in primary biliary cirrhosis: towards a standard for clinical and trial use. J Hepatol. 2000;32:368-373. [PubMed] [DOI] [Full Text] |
| 24. | Huet PM, Deslauriers J, Tran A, Faucher C, Charbonneau J. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95:760-767. [PubMed] [DOI] [Full Text] |
| 25. | Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18 Suppl 1:S79-S83. [PubMed] [DOI] [Full Text] |
| 26. | Cifrek M, Medved V, Tonković S, Ostojić S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech (Bristol, Avon). 2009;24:327-340. [PubMed] [DOI] [Full Text] |
| 27. | Finsterer J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012;13:218. [DOI] [Full Text] |
| 28. | Laurent A, Plamondon R, Begon M. Central and Peripheral Shoulder Fatigue Pre-screening Using the Sigma-Lognormal Model: A Proof of Concept. Front Hum Neurosci. 2020;14:171. [PubMed] [DOI] [Full Text] |
| 29. | Rozand V, Grosprêtre S, Stapley PJ, Lepers R. Assessment of Neuromuscular Function Using Percutaneous Electrical Nerve Stimulation. J Vis Exp. 2015;. [PubMed] [DOI] [Full Text] |
| 30. | Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725-1789. [PubMed] [DOI] [Full Text] |
| 31. | Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD, Hirschfield GM, Parés A, Floreani A, Mayo MJ, Invernizzi P, Battezzati PM, Nevens F, Ponsioen CY, Mason AL, Kowdley KV, Lammers WJ, Hansen BE, van der Meer AJ. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357-365. [DOI] [Full Text] |
| 32. | Angulo P, Dickson ER, Therneau TM. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol. 1999;30:830-835. [DOI] [Full Text] |
| 33. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-61.e8. [PubMed] [DOI] [Full Text] |
| 34. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [PubMed] [DOI] [Full Text] |
| 35. | Corpechot C, Chazouillères O, Rousseau A. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171-2181. [DOI] [Full Text] |
| 36. | Mazzetti M, Marconi G, Mancinelli M, Benedetti A, Marzioni M, Maroni L. The Management of Cholestatic Liver Diseases: Current Therapies and Emerging New Possibilities. J Clin Med. 2021;10. [PubMed] [DOI] [Full Text] |
| 37. | Hirschfield GM, Beuers U, Kupcinskas L. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J Hepatol. 2021;74:321-329. [DOI] [Full Text] |
| 38. | Khanna A, Jopson L, Howel D. Rituximab Is Ineffective for Treatment of Fatigue in Primary Biliary Cholangitis: A Phase 2 Randomized Controlled Trial. Hepatology. 2019;70:1646-1657. [DOI] [Full Text] |
| 39. | Cohen LB, Ambinder EP, Wolke AM, Field SP, Schaffner F. Role of plasmapheresis in primary biliary cirrhosis. Gut. 1985;26:291-294. [PubMed] [DOI] [Full Text] |
| 40. | Wunsch E, Kruk B, Snarski E, Basak G, Krawczyk M, Milkiewicz P. Plasmapheresis in the treatment of chronic fatigue in patients with primary biliary cholangitis. Pol Arch Intern Med. 2021;131:205-207. [PubMed] [DOI] [Full Text] |
| 41. | Prince MI, Mitchison HC, Ashley D, Burke DA, Edwards N, Bramble MG, James OF, Jones DE. Oral antioxidant supplementation for fatigue associated with primary biliary cirrhosis: results of a multicentre, randomized, placebo-controlled, cross-over trial. Aliment Pharmacol Ther. 2003;17:137-143. [PubMed] [DOI] [Full Text] |
| 42. | Kuiper EM, Hansen BE, Metselaar HJ, de Man RA, Haagsma EB, van Hoek B, van Buuren HR. Trends in liver transplantation for primary biliary cirrhosis in the Netherlands 1988-2008. BMC Gastroenterol. 2010;10:144. [PubMed] [DOI] [Full Text] |
| 43. | Krawczyk M, Koźma M, Szymańska A, Leszko K, Przedniczek M, Mucha K, Foroncewicz B, Pączek L, Moszczuk B, Milkiewicz P, Raszeja-Wyszomirska J. Effects of liver transplantation on health-related quality of life in patients with primary biliary cholangitis. Clin Transplant. 2018;32:e13434. [PubMed] [DOI] [Full Text] |
| 44. | Kremer AE, Mayo MJ, Hirschfield G, Levy C, Bowlus CL, Jones DE, Steinberg A, McWherter CA, Choi YJ. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022;42:112-123. [PubMed] [DOI] [Full Text] |
| 45. | Wunsch E, Raszeja-Wyszomirska J, Barbier O, Milkiewicz M, Krawczyk M, Milkiewicz P. Effect of S-adenosyl-L-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J Gastrointestin Liver Dis. 2018;27:273-279. [PubMed] [DOI] [Full Text] |
| 46. | Freer A, Williams F, Durman S, Hayden J, Trivedi PJ, Armstrong MJ. Home-based exercise in patients with refractory fatigue associated with primary biliary cholangitis: a protocol for the EXerCise Intervention in cholesTatic LivEr Disease (EXCITED) feasibility trial. BMJ Open Gastroenterol. 2021;8. [PubMed] [DOI] [Full Text] |
| 47. | Hollingsworth KG, Newton JL, Robinson L, Taylor R, Blamire AM, Jones DE. Loss of capacity to recover from acidosis in repeat exercise is strongly associated with fatigue in primary biliary cirrhosis. J Hepatol. 2010;53:155-161. [PubMed] [DOI] [Full Text] |
| 48. | Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, McCashland TM, Zetterman RK, Peters MG, Di Bisceglie AM, Benner KG, Kowdley KV, Carithers RL Jr, Rosoff L Jr, Garcia-Tsao G, Boyer JL, Boyer TD, Martinez EJ, Bass NM, Lake JR, Barnes DS, Bonacini M, Lindsay KL, Mills AS, Markin RS, Rubin R, West AB, Wheeler DE, Contos MJ, Hofmann AF. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology. 2005;42:1184-1193. [PubMed] [DOI] [Full Text] |