Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1053
Peer-review started: January 17, 2022
First decision: March 8, 2022
Revised: April 1, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 27, 2022
Processing time: 157 Days and 12.8 Hours
Hepatitis C virus (HCV) is a common cause of liver disease and is associated with various extrahepatic manifestations (EHMs). This mini-review outlines the currently available treatments for HCV infection and their prognostic effect on hepatic manifestations and EHMs. Direct-acting antiviral (DAA) regimens are considered pan-genotypic as they achieve a sustained virological response (SVR) > 85% after 12 wk through all the major HCV genotypes, with high percentages of SVR even in advanced fibrosis and cirrhosis. The risk factors for DAA failure include old males, cirrhosis, and the presence of resistance-associated substitutions (RAS) in the region targeted by the received DAAs. The effectiveness of DAA regimens is reduced in HCV genotype 3 with baseline RAS like A30K, Y93H, and P53del. Moreover, the European Association for the Study of the Liver recommended the identification of baseline RAS for HCV genotype 1a. The higher rate of hepatocellular carcinoma (HCC) after DAA therapy may be related to the fact that DAA regimens are offered to patients with advanced liver fibrosis and cirrhosis, where interferon was contraindicated to those patients. The change in the growth of pre-existing subclinical, undetectable HCC upon DAA treatment might be also a cause. Furthermore, after DAA therapy, the T cell-dependent immune response is much weaker upon HCV clearance, and the down-regulation of TNF-α or the elevated neutrophil to lymphocyte ratio might increase the risk of HCC. DAAs can result in reactivation of hepatitis B virus (HBV) in HCV co-infected patients. DAAs are effective in treating HCV-associated mixed cryog
Core Tip: Direct-acting antivirals (DAAs) are achieving an over 85% sustained virological response in treating hepatitis C virus (HCV) infection. The risk factors for DAAs failure include old males, cirrhosis, and the presence of resistance-associated substitutions mainly in genotypes 1a and 3. The higher rate of hepatocellular carcinoma after DAA therapy may be due to offering DAA regimens to patients with advanced liver fibrosis and cirrhosis, where using interferon was contraindicated. The change in the growth of pre-existing subclinical, undetectable hepatocellular carcinoma upon DAA treatment might be a cause. DAAs are effective in treating HCV-associated mixed cryoglobulinemia, thrombocytopenia, rheumatological, renal, and cardiovascular diseases.
- Citation: Salama II, Raslan HM, Abdel-Latif GA, Salama SI, Sami SM, Shaaban FA, Abdelmohsen AM, Fouad WA. Impact of direct-acting antiviral regimens on hepatic and extrahepatic manifestations of hepatitis C virus infection. World J Hepatol 2022; 14(6): 1053-1073
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1053.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1053
The worldwide prevalence of chronic hepatitis C virus (HCV) infection is estimated to be 58 million people, and 1.5 million individuals get new HCV infection annually. The World Health Organization stated that, about 290 thousand patients died from hepatitis C-related complications in 2019[1]. In 2016, the World Health Assembly adopted the Global Health Sector Strategy on viral hepatitis. This strategy is directed towards eliminating both viral hepatitis B and C infections. To achieve the target objective, this will require the diagnosis of 90% of the infected patients, followed by treatment of 80% of the diagnosed individuals[2]. HCV leads to acute and chronic hepatitis, progressing to lifelong liver cirrhosis and cancer, and is associated with several extrahepatic manifestations (EHMs)[1]. The aim of antiviral treatment is HCV eradication, thus preventing disease progression and reducing the EHMs. This mini-review outlines the currently available treatments for HCV infection and their prognostic effect on hepatic manifestations and EHMs.
HCV possesses a single-stranded RNA genome that encodes a polyprotein, which is processed into ten proteins: E1, E2, core, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B[3]. The structural proteins E1, E2, and core are components of the virion and the nonstructural proteins NS3/4A, NS5A, and NS5B are involved in viral genome replication[4,5]. To enter the host cell, HCV requires a cascade of synchronized and sequentially ordered events where the virus binds to many receptors. HCV particles circulate, as lipoviroparticles (LVPs), in association with low-density lipoprotein (LDL) and very-LDL (VLDL) components, including apolipoproteins (such as Apo-B, Apo-AI, Apo-CI, and Apo-E)[6]. HCV core, E1, E2, and P7 are essential for cell-free and cell-to-cell viral transmission[7]. HCV recognition is initiated by Toll-like receptor 3 and retinoic acid-inducible gene I[8].
HCV infects hepatocytes through cell-free and cell-to-cell viral transmission (Table 1). LVPs circulate in the sinusoidal blood, and through sinusoidal endothelial fenestration, they become in contact with receptors on the basolateral membrane of hepatocytes[9]. The virus envelope glycoproteins and virus-associated lipoprotein components (particularly apoE) of LVPs attach to hepatocyte basolateral membranes through interaction with highly sulfated proteoglycans, particularly syndecans, LDL receptor (LDLr), and scavenger receptor class B type I (SR-BI) on the cell surface[10]. SR-B1, as both an entry factor and an attachment factor, has been shown to bind viral envelope proteins[11]. Knockdown of individual gene of LDLr or SR-B1 had a moderate impact on HCV infection. While, knockdown of genes of both receptors resulted in a much more pronounced effect[12].
| Diagnostic tool | Early fibrosis stages (METAVIR less than F2) | Fibrosis 2 | Fibrosis 3 | Compensated cirrhosis | Decompensated cirrhosis | Ref. |
| HCV antibody | Positive | Positive | Positive | Positive | Positive | AASLD and IDSA[30] |
| Quantitative HCV RNA (viral load) | Positive | Positive | Positive | Positive | Positive | |
| Platelet count < 150000/mm3) | Normal | Normal | Normal | < 150000/mm3 | < 150000/mm3 | |
| Total and direct bilirubin, ALT & AST | Normal/elevated | Normal/elevated | Normal/elevated | Elevated | Elevated | |
| Child- Pugh | -------- | -------- | -------- | Class A (scores 5-6) | Class B (scores 7-9); Class C (scores 10-15) | |
| FIB-4 Score | < 1.45 | ≥ 1.45 but < 2.67 | ≥ 2.67 but < 3.25 | ≥ 3.25 | > 3.25 | Filozof et al[31] |
| Fibroscan by transient elastography | 5.3 kPa | 7.4 kPa | 9.1 kPa | 13.2 kPa | 13.2 kPa | Platon et al[32] |
| Fibro test | < 0.48 | 0.48 - 0.58 | > 0.58 but < 0.74 | > 0.74 | > 0.74 | Laboratory Corporation of America[33] |
| Enhanced liver fibrosis test | < 7.7 | 7.7 | 9.8 | 11.3 | 11.3 | Lichtinghagen et al[34] |
| Aspartate aminotransferase to platelet ratio index | < 0.77 | 0.77 | 0.77 | ≥ 0.83 | ≥ 0.83 | Lin et al[35] |
| Liver nodularity and/or splenomegaly | Negative | Negative | Negative | Positive | Positive | AASLD and IDSA[30] |
| Prior liver biopsy | F0: No fibrosis; F1: Portal fibrosis without septa | F2: Portal Fibrosis with few septa | F3: Numerous septa without cirrhosis | F4: Cirrhosis | F4: Cirrhosis |
Attachment to SR-BI helps bind of LVPs to cluster of differentiation 81 (CD81), claudin-1 (CLDN1), and occludin (OCLN)[13]. Interaction of HCV with CD81 causes activation of epidermal growth factor receptor signaling and facilitates CD81 diffusion and formation of the HCV-CD81-CLDN1 complex[14]. This complex then interacts with OCLN, which mediates the clathrin-dependent internalization through interacting with GTPase dynamin[15]. Other entry factors have been demonstrated, such as CD36 which interacts directly with HCV E1 protein[16]. In addition, TIM-1/human hepatitis A virus cellular receptor 1/CD365 has been identified as a contributing factor to LVP attachment through interaction with phosphatidylserine exposed on the HCV envelope[17]. This interaction may enhance viral attachment and subsequent interaction with the main entry factors[18]. HCV uses cortactin (an actin-binding protein at the cell periphery) for its assembly to promote viral proliferation and controls cortactin phosphorylation to facilitate cell invasion. Cortactin may be involved in hepatic cell migration, so it may be a potential target to interfere with the HCV cellular pathogenesis[19].
SR-B1 has also a prominent role in cell-to-cell transmission. This type of transmission assists immune evasion and persistence. Cell-to-cell transmission may be the main route of HCV dissemination in chronically infected patients[20]. LIM and SH3 protein 1 (LASP-1) is a specific adhesion protein that plays an important role in the regulation of cell migration, proliferation, and protein-protein interactions. LASP-1 is an HCV NS5A-interacting partner. Both LASP-1 and NS5A are localized in the cytoplasm of HCV infected cells. RNA and protein levels of LASP-1 were increased in these cells, indicating that LASP-1 may be involved in HCV-induced liver pathogenesis[21].
HCV can also infect and replicate in other cell types, such as peripheral blood mononuclear cells (PBMCs) and bone marrow cells through cell-to-cell transmission[22]. HCV infects PBMCs and other cells through the interaction with CD81 molecules on the cell surface[23], allowing replication of HCV in the extrahepatic tissues, which is facilitated by the expression of miR-122[24]. B lymphocytes, particularly CD27+ memory B cells, can resist apoptosis and may serve as an HCV reservoir[25]. Infection of PBMCs with HCV leads to dysregulation of the signaling pathway mediators such as STAT-1 and IRF-1 and alterations in cytokine and chemokine production, including IL-1, IL-6, IL-8, and IL-10. Persistent HCV RNA and its antigens, combined with chronic immune activation, lead to exhaustion of PBMCs that become defective and more prone to programmed cell death[26]. Liu et al[27] prepared cell culture-derived infectious HCV particles (HCVcc) using Huh7 cells transfected with HCV RNA. They found that HCV entry into macrophages depends mainly on its phagocytic activity and does not depend on its cell receptors. Knockdown of CD81 had a minimal effect on the entry of HCVcc into macrophages. Exosomes have been demonstrated to contain HCV-RNA. However, the mechanism responsible for the transmission of HCV genomic RNA through exosomes is still not clarified[28].
Before starting direct-acting antiviral (DAA) therapy, liver disease severity should be assessed to detect clinically unapparent advanced fibrosis (METAVIR score F3) or cirrhosis (METAVIR score F4). In patients with cirrhosis, portal hypertension and esophageal varices should also be assessed[29]. These are important steps, as the choice of DAA regimens, prognosis, and hepatocellular carcinoma (HCC) surveillance every 6 months depend on the stage of fibrosis. Table 1 summarizes some of the current available HCV diagnostic and staging tests according to AASLD and IDSA[30], Filozof et al[31], and other studies[32-35]. Liver stiffness measurement (LSM) using transient elastography can assess the degree of liver fibrosis and portal hypertension. Aspartate aminotransferase to platelet ratio index and fibrosis-4 (FIB-4) are simple, inexpensive, and reliable panels of fibrosis biomarkers that can be used. However, these panels may be less sensitive among African patients. Both LSM and biomarkers are expected to be efficient in distinguishing cirrhosis vs no fibrosis, with the lower ability for intermediate degrees of fibrosis. The combination of blood biomarkers or the combination of LSM and a blood test may improve accuracy[36].
LSM importance after sustained virological response (SVR) remains uncertain. Several studies have reported the significant regression of LSM after treatment of HCV infection with DAAs[37]. However, it is still debatable whether the decrease of LSM and post-DAA HCV eradication are due to the suppression of viral necro-inflammatory activity or regression of liver fibrosis[38]. It is recommended that assessing the fibrosis stage after therapy using non-invasive tools should not be endorsed as they are unreliable in this setting[29].
Until 2011, pegylated interferon alpha (PEG-IFNα) with ribavirin (RBV) was the standard therapy for HCV infection, with an about 50% SVR[39]. The European Association for the Study of Liver Diseases (EASL)[29] recommended that the endpoint of therapy is undetectable HCV RNA either in serum or plasma by an assay with a lower limit of detection ≤ 15 IU/mL, 12 wk (SVR12) or 24 wk (SVR24) after the end of treatment[29]. In low-resource areas, as an alternative to HCV RNA, HCV antigen (HCV Ag) testing might be useful for diagnosis of active HCV infection and at the end of treatment[40].
The identification of HCV encoded proteins and their function allowed the development of highly effective DAA regimens against the NS3 protease, NS5A, and the NS5B polymerase[41]. The maximum effectiveness of therapy is obtained when the patients are treated at early stage before advanced liver fibrosis or cirrhosis[42,43]. According to the mechanism of action, DAAs can be classified into four different groups: NS3/4A protease inhibitors [Glecaprevir (GLE), Voxilaprevir (VOX), Grazoprevir, Paritaprevir (PTV), and Simeprevir (SIM)], NS5A protein inhibitors [Daclatasvir (DCV), Velpatasvir (VEL), Ledipasvir (LDV), Ombitasvir (OBV), Pibrentasvir (PIB), and Elbasvir], NS5B polymerase inhibitor-nucleoside analogue [Sofosbuvir (SOF)], and NS5B polymerase inhibitor-non-nucleoside analogue [Dasabuvir (DSV)]. These drugs are considered pan-genotypic as they achieve a SVR > 85% through all the major HCV genotypes[44]. All DAAs are effective for genotype 1 and 4 and SOF for genotype 2. While for genotype 3, SOF, DCV, and LDV are effective. For genotypes 5 and 6, a combination of two regimens (VEL/SOF and asunaprevir (ASV)/DCV/beclabuvir) is indicated. A review for 28 randomized clinical trials, enrolling more than 7000 HCV naïve patients, revealed that DAA regimens for 12 wk significantly increased SVR12 and SVR24 compared to placebo and HCV cure was achieved in about 90.5% of patients. DAAs were well tolerated with no increase in serious adverse effects[39].
DAAs are recommended for both naïve patients as well as those who failed to achieve SVR after prior treatment. In addition, treatment is recommended for patients with advanced fibrosis or cirrhosis, including decompensated cirrhosis. Guidelines for the global standard treatment established SOF + VEL or GLE/PIB as the first recommended drug regimen for naïve patients, irrespective to HCV genotype or the presence of compensated liver cirrhosis[29,45]. Moreover, lifelong monitoring for HCC is recommended for patients with advanced fibrosis and cirrhosis, even with SVR, as DAAs decrease, but does not eliminate the risk of HCC[29,46]. In a multicenter cohort study involving 868 HCV patients with liver cirrhosis treated with DAA regimens, SVR was attained at 90% in Child-Pugh A patients and 81% in Child-Pugh B/C patients. Within a median period of 28 months follow-up, 14% of patients with Child-Pugh A and 64% of those with Child-Pugh B/C developed disease progression[47]. The use of protease inhibitors is contraindicated in patients with decompensated cirrhosis or with prior episodes of decompensation. These inhibitors carry a substantially higher drug exposure and risk of toxicity due to their hepatic metabolization[48]. Thus, the fixed-dose combination of SOF and VEL is the treatment of choice for patients with decompensated (Child-Pugh B or C) cirrhosis or with compensated (Child-Pugh A) cirrhosis with prior episodes of decompensation[29]. Tables 2 summarizes the current recommended DAA regimens for treating HCV infection according to AASL/ADSA 2021[30].
| Treatment | No cirrhosis | Compensated cirrhosis | Decompensated cirrhosis | ||
| Naïve HCV infected patient | Previously treated patients | Naïve HCV infected patients | Previously treated patients | ||
| Sofosbuvir (400 mg)/Velpatasvir (100 mg) | 12 wk | Sofosbuvir (400 mg)/Velpatasvir (100 mg)/Voxilapevir (100 mg), 12 wk, for all genotypes. ALTERNATIVE: Glecaprevir (300 mg)/Pibrentasvir (120 mg), but not recommended for genotype 3 with Sofosbuvir/NS5A inhibitor | For genotypes 1, 2, 4, 5, and 6 & genotype 3 with NS5A-RAS Y93H negative, 12 wk, but not recommended for genotype 3 with NS5A-RAS Y93H positivity | Sofosbuvir (400 mg)/Velpatasvir (100 mg)/Voxilapevir (100 mg), 12 wk, for genotypes 1, 2, 4, 5, and 6; for genotype 3, 12 wk in addition to weight-based Ribavirin. ALTERNATIVE: Glecaprevir (300 mg)/Pibrentasvir (120 mg), but not recommended for genotype 3 with Sofosbuvir/NS5A inhibitor | Patients with HCV infection who have decompensated cirrhosis, i.e., Child-Pugh class B or class C, should be referred to a medical practitioner with expertise in that condition, ideally in a liver transplant center |
| Glecaprevir (300 mg)/Pibrentasvir (120 mg) | 8 wk | 16 wk in addition to Sofosbuvir (400 mg) + weight-based Ribavirin ALTERNATIVE: 12 wk of Sofosbuvir (400 mg)/Velpatasvir (100 mg)/Voxilapevir (100 mg) | 8 wk | 16 wk in addition to Sofosbuvir (400 mg) + weight-based Ribavirin. ALTERNATIVE: 12 wk of Sofosbuvir (400 mg)/Velpatasvir (100 mg)/Voxilapevir (100 mg) in addition to weight-based Ribavirin | |
| Elbasvir (50 mg)/Grazoprevir (100 mg) | 12 wk for genotype 1b | 12 wk Sofosbuvir (400 mg)/Velpatasvir (100 mg)/Voxilapevir (100 mg). However, Glecaprevir/Pibrentasvir for 16 wk is not recommended as an alternative for this group of patients | 12 wk for genotype 1B | NA | |
Risk factors for DAA failure include males with advanced liver fibrosis/cirrhosis, the presence of resistance-associated substitutions (RAS) in the region targeted by the received DAAs, and inadequacy of treatment. RAS linked to the NS5A gene are present at higher levels and persist for longer duration than those linked to the NS3/4 gene[49]. The naturally occurring RAS do not affect treatment efficacy, as they are present in a minority of circulating HCV virions. RAS resulting from treatment are present in the majority of the circulating HCV quasispecies, which decrease the efficacy of re-treatment with the same DAA class[50]. For DAA regimens involving LDV/SOF and DCV/SOF, the identification of baseline RASs for HCV genotype, such as 1a, is recommended to decide the treatment duration or if RBV addition is needed[51]. Eventually, the adverse impact of baseline RAS could be decreased by increasing duration of treatment or optimizing DAA regimens. However, a considerable percent of treatment failures is triggered by RAS acquired during therapy[49]. This may be related to the relatively low barrier to resistance of the NS5A region and the high genetic barrier of SOF. Moreover, the third-generation NS3 inhibitors are expected to have intermediate genetic barrier in HCV genotypes 1a and 1b and very high in non-1 genotypes[52]. A meta-analysis on 6500 HCV infected patients, reported reduced effectiveness of GLE/PIB in HCV genotype 3 with baseline RAS like A30K, Y93H, and P53del. Testing RAS for genotype 3 HCV infection is mandatory to improve the prognosis of treatment outcome and selection of therapy[53].
Baseline RAS were only identified in the NS5A region in Iranian patients with HCV genotypes 1a and 3a with no RAS in the NS5B region[54]. Among 539 Italian HCV genotype 3 patients (417 DAA-naïve and 135 DAA-failed), Sanger sequencing of NS3/NS5A/NS5B at baseline samples showed a higher prevalence of NS5A RAS in DAA-failed (5/13, 38.5%) vs DAA-naïve (61/393, 15.5%, P = 0.04) patients. The presence of baseline Y93H and/or A30K was associated with SVR rate of 72.2% vs 95.7% among patients without NS5A RAS (P = 0.002). Chen et al[55] and Pisaturo et al[52] reported at least one RAS among over 85% out of the studied 220 HCV naïve patients with DAA-based treatment. However, according to the recommendation of international guidelines, massive testing for RAS detection before starting DAAs treatment is not needed, with some exceptions[29,45]. RAS testing before treatment is recommended for HCV genotype 3 infected patients with liver cirrhosis, as those without a baseline Y93H RAS in NS5A are eligible for SOF/VEL therapy. While, those with baseline Y93H RAS could be treated with SOF/VEL/VOX or SOF/VEL plus RBV[45]. However, according to EASL guidelines, the same therapeutic regimen should be used to all compensated cirrhotic patients regardless of viral genotype[29]. Currently, the US Food and Drug Administration (FDA) and European Medical Agency (EMA) approved two types of DAA regimens, SOF/VEL/VOX and GLE/PIB, to treat patients with previous experience of DAAs failure[36,46-48]. The effectiveness of the regimen SOF/VEL/VOX plus or minus RBV for 12 wk among patients with DAA failure revealed that SVR at 12 wk ranged from 91% to 100%. Most patients tolerated retreatment well[58].
The impact of DAAs on the development of HCC is controversial. Meanwhile, it should be noted that in all studies, the risk of HCC remained even after successful HCV treatment. A meta-analysis study revealed that, the incidence rate for a new HCC was 3.3% (95% confidence interval: 1.2-9%) per year after DAA treatment[57]. Some studies reported that, the risk of de novo HCC after DAA therapy was reduced, while other studies noted a much higher HCC risk mainly within the first year after DAA therapy than later[58,59]. A retrospective cohort study was carried out on 243 consecutive HCV patients who received PEG-IFN/RBV and were followed for a median of 9.3 years, and 263 HCV patients who received DAA treatment and were followed for a median of 4.1 years. It revealed that a considerably increased hazard was associated with DAA treatment[60]. A French study conducted on 1270 HCV patients revealed that, the differences of the occurrence of HCC after IFN and DAA regimens could be explained by the higher prevalence of Child-Pugh class B, portal hypertension, and diabetes among DAA-treated patients vs IFN-induced SVR patients. A time-dependent Cox model weighted by inverse probability of treatment was used to overcome selection bias. This model shows that DAAs were not significantly associated with an increase in the risk of HCC occurrence (P = 0.73), nor with a more aggressive pattern of presentation[61].
The higher rate of HCC after DAA therapy may be because they are the drugs of choice for treating old patients and those with liver cirrhosis and end-stage liver disease as IFN was not indicated to treat such patients[62]. The possible clarification of the elevated incidence of HCC after the start of DAA therapy, might be the change in the growth of pre-existing subclinical and undetectable HCC upon DAA treatment[60]. A high HCC risk after DAA treatment was also reported, especially in individuals with uncharacterized liver nodules[63]. Owusu Sekyere et al[64] detected a reduced HCC specific tumor response upon DAA-induced HCV clearance. In HCV patients who subsequently developed HCC, the T cell-dependent immune response was much weaker, indicating their important role in inhibiting tumor growth. DAA therapy for HCV was associated with a weakening of the strength of HCC-specific CD8+ but not CD4+ T cell responses in cirrhotic patients in vitro. Moreover, a mechanism like cellular behavior after eradication of HCV by DAA therapy may increase the HCC growths as detected in early test models[59]. Recently, Lu et al[65] concluded that the down-regulation of tumor necrosis factor α (TNF-α) after successful DAA therapy increases the risk of HCC and the inhibition of TNF-α might attenuate the host immune surveillance against tumor cells. These findings might provide a clue for the pathogenesis of HCC and a strategy for HCC surveillance based on risk stratification. In Egypt, HCC was found to be significantly aggressive in HCV patients treated with DAAs, especially among those with an elevated neutrophil to lymphocyte ratio (P= 0.012)[66]. It is recommended to screen for HCV every 6 months for cases with cirrhosis and every 12 months for those without cirrhosis after DAA therapy[45].
DAAs appear safe for patients with a history of treated HCC and are not associated with an increased risk for cancer recurrence except for cases with vascular invasion, where aggressive HCC recurrence was reported[67]. For HCC patient candidates for a liver transplant, decisions regarding the timing of DAA treatment depend on organ availability and region wait times and should be individualized[68]. Moreover, Fouad et al[69] suggested that anti-HCV therapy in HCC patients should be postponed until further research for safety and effectiveness is carried out.
Hepatitis B virus (HBV) and HCV are the major causes of liver disease worldwide. The administration of compulsory HBV vaccination is effective in providing long-term protection against infection, even with low seroprotection rate, proved by the presence of high anamnestic response rate after being given a HBV challenging dose[70,71]. However, poly-transfused vaccinated individuals, either with or without HCV infection, are at risk of HBV infection. In these patients, HBV-DNA was detected even among HBsAg negative patients (occult HBV infection)[72,73]. The co-infection with both viruses increases the rates of cirrhosis and HCC[74,75]. When both HBV and HCV are present in the same cell, reciprocal inhibition of one viral genome by the other virus takes place and leads to the dominance of one virus over the other. The dominant virus replicates more actively and inhibits the replication of the non-dominant virus. However, co-dominance may occur if there is nearly equal replication of both HBV and HCV[76]. In 2018, EASL recommended that hepatitis C patients should be tested for HB surface (HBs) antigen, HB core antibody (anti-HBc), and HBs antibody (anti-HBs) prior to starting DAA-based treatment. In HBs antigen positive patients, concurrent HBV nucleoside/nucleotide analogue therapy is indicated. For anti-HBc positive patients with negative HBsAg, serum alanine transaminase (ALT) levels should be monitored and both HBs antigen and HBV DNA should be tested, if ALT levels rise or do not return to normal during or after anti-HCV therapy. In anti-HBs and anti-HBc antibodies positive patients, monitoring of serum ALT levels is indicated[51].
Co-infected patients may experience HBV reactivation after the cure of their HCV by PEG-IFN or DAA-based therapy and anti-HBV therapy should be started if clinically indicated[77]. The US FDA warned of the higher risk and earlier onset of HBV reactivation with DAA treatment[78]. This can be expected since HCV DAAs have no direct or immunomodulatory effect on the replication of HBV. Close monitoring of HBV infection status is settled by all guidelines with the implementation of IFN-free regimens. A retrospective study revealed that only 9 out of 62290 patients treated with DAAs had HBV reactivation. Eight patients were known to be HBsAg positive, and one patient was known to be isolated anti-HBc-positive. Seventeen other patients had a small increase in HBV DNA levels that did not qualify as HBV reactivation[79].
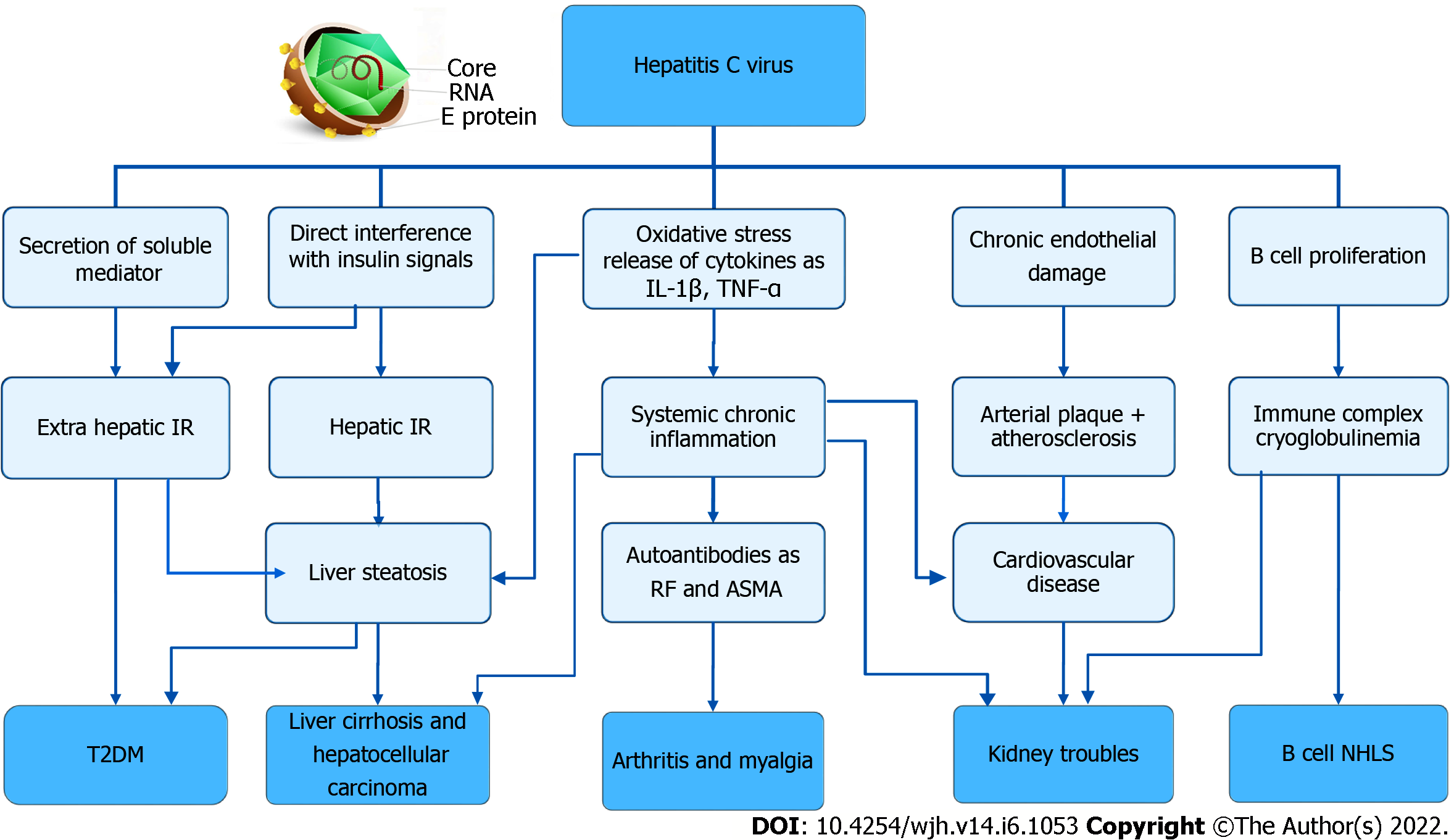
HCV can cause extrahepatic diseases that lead to an increase in the overall mortality. Figure 1 shows the pathophysiology of HCV infection in hepatic and extrahepatic diseases. The following extrahepatic diseases are related to HCV infection:
Chronic HCV infection is a common cause of mixed cryoglobulinemic vasculitis (MCV). In about 40%-60% of patients with chronic HCV infection, circulating mixed cryoglobulins are detected. However, overt cryoglobulinemia vasculitis (CV) is observed in only 5%-10% of patients[80,81]. As shown in Figure 1, the pathogenesis of MCV involves viral-induced activation of B cell clones which generate pathogenic IgM with rheumatoid factor (RF) activity. Monoclonal IgM and polyclonal IgG bind together and recognize hepatitis C nucleocapsid and core antigens. The resulting circulating immune complexes deposit in vascular beds of small-to-medium vessels, enhancing complement activation, leukocyte recruitment, and vasculitis[82]. The clinical manifestations of the disease are variable, ranging from mild symptoms such as purpura, arthralgia, and fatigue to more serious life-threatening complications resulting from neurologic and renal involvement[83].
Treatment of HCV-MCV is challenging. The main goal is SVR in order to down-regulate the B-cell arm of autoimmunity that is triggered by the virus. DAA regimens are now the drug of choice for HCV-associated MCV. The combination of PEG-IFNα with RBV has been abandoned for their side-effects, including the immune-stimulatory effects[84]. With DAA therapy, the rate of SVR after 12 months of therapy was the same for HCV patients with and without mixed cryoglobulinemia (MC). However, MCV may persist or reappear in some patients after SVR[85]. Moreover, new onset cases of cryoglobulinemic glomerulonephritis were also reported[86]. After DAA therapy for HCV associated MC, 64% to 96% of the patients improved clinically; however, the immunological response (defined by marked reduction or disappearance of circulating cryoglobulins and normalization of the levels of RF and C4) was only from 48% to 89%[87].
Artemova et al[88] reported complete disappearance of cryoglobulinemia among 48% of HCV-CV patients and a decrease in cryoglobulins among 17% of them. Response rates of HCV-CV after DAA treatment vary according to the organ involvement. A higher response rate (75%-100%) was attained for cutaneous and musculoskeletal presentations, while lower response rates (30%-70%) were attained in peripheral nerve and renal involvement[89]. The lag in immunologic and/or clinical response behind the viral clearance may be due to delay in the clearance of cryoglobulins from the circulation after successful antiviral therapy or to the persistence of the RF-producing memory B-cell clones for at least 24 wk[90]. Occult HCV infection is another possible explanation especially in case of cryoglobulinemic glomerulonephritis[91]. Abdelhamid et al[92] suggested that some forms of alteration in the immune system can be responsible for the persistent HCV-related immune disease after viral clearance. The recommended drug for patients with persistent or recurrent MCV after SVR is Rituximab, which is a B-cell depleting monoclonal antibody. In rapidly progressing or fulminant cases or severe exacerbation of vasculitis causing life-threatening complications, plasmapheresis is added to remove the circulating cryoglobulins[93].
Thrombocytopenia is a common complication in chronic HCV infection, causing an increased risk of bleeding[94,95]. The prevalence and severity of thrombocytopenia increase with the progression of liver disease and the development of hepatocellular damage and hepatic fibrosis[96]. Its prevalence is 6% in chronic liver disease patients, while it is 24% in chronic HCV infected patients and increases to 78% in cirrhotic patients[94-97]. The pathophysiology of thrombocytopenia in chronic HCV is multifactorial and largely related to the severity of hepatic infection. It includes splenomegaly and the related hypersplenism causing platelet sequestration, auto-immunogenicity, impaired production of thrombopoietin due to advanced fibrosis, possible direct effect of HCV as direct bone marrow suppression, and therapeutic adverse effects[94-96].
In the IFN era, starting or maintaining IFN therapy was a great challenge in the treatment of chronic HCV patients with thrombocytopenia[96]. IFN causes a further decrease in the platelet count in up to 13% of patients[98]. On the other hand, DAA treatment achieve an over 95% SVR at 24 wk among HCV infected thrombocytopenic patients with advanced fibrosis and cirrhosis. Moreover, the platelet count showed statistically significant improvement[99,100]. Chen et al[97] found that 99.6% of chronic HCV infected patients with thrombocytopenia receiving DAA treatment achieved a SVR and thrombocytopenia improved significantly in 41.7% of them. Another study reported a highly effective and safe DAA regimen, with improvement of platelet count in 73% of thrombocytopenic patients, especially in mild to moderate stages of hepatic fibrosis[95].
HCV infection is associated with several glomerulopathies including membranoproliferative glomerulonephritis (MPGN). It is associated with MCV in 80%-95% of the cases. Other HCV glomerular diseases include membranous nephropathy, proliferative glomerulonephritis, focal segmental glomerulosclerosis, fibrillary glomerulonephritis, IgA nephropathy, immunotactoid glomerulopathy, and renal thrombotic microangiopathy. HCV infection also increases the risk of chronic kidney disease (CKD). The association between chronic HCV infection and CKD is more significant with high HCV viral load and HCV genotype 2[84,101,102].
In MPGN associated with MCV, the developed immune complex deposits in the mesangium, capillaries, and urinary space of glomeruli, which can be manifested as nephrotic and nephritic syndromes[91,103,104]. Furthermore, HCV can cause kidney damage through direct cytopathic effect by viral invasion of the renal parenchyma (mesangial, endothelial, and tubular cells of the kidney) and through nephrotoxicity of drugs used for its treatment. Additionally, non-immunological pathways as oxidative stress or pro-inflammatory cytokines help the development of renal disease by vascular injury as shown in Figure 1. In addition, HCV infected patients may have an increased risk of insulin resistance, which develops during the inflammation process and exacerbates renal damage[105-108]. In very few cases, viral NS3 was found in the glomerular deposits, capillary walls, and the mesangium[84].
Previously, HCV kidney manifestations were treated with PEG-IFN plus RBV; however, these drugs presented low efficacy, low SVR (< 50%), and severe side effects as acute renal failure, graft failure, and hemolytic anemia[109,110]. DAA therapy improves glomerular filtration rate, decreases proteinuria and hematuria, and shortens treatment duration to only 8-12 wk without significant side effects[111-113]. Delays in initiation of DAA therapy could have deleterious effects[114-116]. In patients with an estimated glomerular filtration rate (eGFR) > 30 ml/min/1.73 m2, the use of SOF with SIM with or without ribavirin decreased proteinuria and improved eGFR[117,118]. There are different approved regimens for patients having an eGFR < 30 ml/min/1.73 m2 or those on dialysis: (1) OBV + PTV + Ritonavir (RITV) + DSV; (2) RBV + Elbasvir + Grazoprevir; and (3) GLE + PIB. The side effects were only mild general symptoms like fatigue, insomnia, dizziness, and headache[119-121]. Furthermore, DCV and ASV are important options, especially for patients with renal impairment since both DCV and ASV have minimal renal excretion[122].
HCV infected patients have impaired glucose metabolism with hyperinsulinemia due to decreased insulin catabolism or insulin resistance (IR). Up to 60%-80% of HCV cases have glucose intolerance, 20% of them develop type 2 diabetes (T2DM), and up to 41%-70% of them have IR. T2DM which develops as a complication of HCV infection is known as hepatogenous diabetes[123-125].
HCV can induce IR through direct and indirect ways as shown in Figure 1. The viral core protein can directly interfere with intracellular insulin signaling by inhibiting the expression of insulin receptor substrate (IRS)-1 and IRS-2. HCV replicates in the pancreatic β-cells, causing impairment of their function involved in glucose metabolism. In addition, HCV infection can indirectly induce IR due to oxidative stress, liver steatosis, release of inflammatory cytokines such as TNF-α, interleukin (IL)-1, IL-6, and leptin, phosphorylation of the insulin-1 receptor substrate and protein kinase B, and up-regulation of gluconeogenic genes such as glucose 6 phosphatase and phosphoenolpyruvate carboxy kinase[84,126,127].
SVR with PEG-IFN and RBV is associated with decreased IR after 24 wk of therapy[128,129]. DAAs improve the IR by 90%. Treatment with DAA regimens has ameliorated hyperglycemia, recovered pancreatic beta-cell function, and reduced cytokine production[130-132]. Furthermore, the eradication of HCV by DAAs such as SOF-based regimen led to the improvement in hemoglobin A1c percentage[133,134].
The rheumatologic and musculoskeletal manifestations are the most common EHMs, affecting 40-80% of HCV infected patients. MC is one of the causes of HCV associated rheumatologic manifestations (RM)[135]. Cryoglobulins were found to be deposited in small vessels of joints[136]. These manifestations are numerous and diverse, including fatigue, arthritis or arthralgia, myalgia, polyarthralgia, fibromyalgia, poly/dermato-myositis, sicca syndrome, and non-inflammatory musculoskeletal pain. The articular involvement is usually bilateral, symmetrical, and non-deforming, and it usually targets small joints such as the metacarpophalangeal joints, the proximal interphalangeal joints, wrists, and fingers. The knee, ankles, and back may be also affected[137,138]. RF is usually positive in these cases but anti-cyclic citrullinated peptide antibodies are negative and can be used to differentiate HCV arthropathy from early rheumatoid arthritis[139].
Sicca syndrome has been reported in 20 to 30% of patients with HCV infection. This may be due to the presence of the virus in the human salivary glands, where it can replicate. It is characterized by high RF titers, higher-frequency cryoglobulins, low antinuclear antibodies (ANA), hypocomplementemia, and a lower frequency of anti-Ro/SSA and anti-La/SSB autoantibodies[84]. Myalgia is a common finding in HCV infected patients, and it occurs in about 15% of cases. The mechanism of RM is possibly related to direct action of the virus as it was detected in muscle fibers. Figure 1 shows that these RM are mostly mediated by immunological mechanisms rather than being related to the infection of extra-hepatic tissues. HCV envelope E2 protein binds with CD81 expressed on the membrane of B-cells, forming a complex that decreases the threshold for activation of B-cells and also causes reduction of its apoptosis. These lead to aberrant activation of B-lymphocytes as well as their prolonged survival, therefore increasing the production of antibodies (including the auto-antibodies) and systemic inflammation[140]. Tissue damages, either directly by viruses or as a result of immune aggressions against infected cells, result in the release of a large number of tissue antigens. Additionally, it has been previously postulated that similarities between HCV antigens and host antigens are partly responsible for the development of ANA and anti-smooth muscle antibodies (ASMA)[141]. RF was detected in 70% of patients, followed by ANA (20 to 40%), anticardiolipin antibodies (15%), antithyroid antibodies (12%), and ASMA (7%)[140].
IFN-based regimens for HCV infection lead to exacerbation of rheumatic diseases and worsening of preexisting autoimmune disorders or even developing a new one[142]. DAAs reduce the viral load and therefore decrease the production of antibodies. The eradication of HCV with DAAs supports improving the articular manifestations[141]. SOF and DCV with or without ribavirin combination therapy are an effective and safe treatment with minimal side effects for eradication of HCV infection and amelioration of HCV related RM[143,144].
Chronic HCV infection has a significant, direct or indirect impact on the increased risk of cardiovascular diseases (CVD)[84]. HCV infection is associated with a 27% increase in risk of CVD and cerebrovascular atherosclerotic diseases including stroke events compared with uninfected controls[145,146]. The risk of death from cerebrovascular causes has been correlated with HCV RNA levels[147]. A meta-analysis of nine case-control studies showed a two-fold higher risk of carotid plaques in HCV infected individuals compared with uninfected controls[148]. Moreover, a higher prevalence of anti-HCV antibodies was detected in patients with cardiomyopathies and myocarditis than in the general population. The negative strand of HCV-RNA was detected in cardiac tissue, suggesting replication of the virus. These two findings indicate a direct association between HCV and cardiac injury, with CVD and heart failure seen in patients with HCV infection[145,149]. The effect of HCV infection on the risk of cardiovascular events was greater among older patients with hypertension or diabetes[146].
HCV infection leads to the development of atherosclerosis through different mechanisms as shown in Figure 1. A direct involvement of HCV in the induction of atherosclerosis, as the virus lives and replicates in thrombotic tissue, causes a chronic inflammatory reaction that participates in thrombus growth and instability[150]. Endothelial cells express HCV entry receptors which support viral replication. Moreover, HCV causes endothelial dysfunction through promoting migration and proliferation of smooth muscle cells from the tunica media to the intimal surface. HCV alters endothelial permeability, causes cell apoptosis and so, produces endothelial dysfunction[151]. Indirect mechanisms of atherosclerosis have been proposed, such as chronic low-grade systemic inflammation and activation of T helper cells with the release of pro-atherogenic cytokines and chemokines (e.g., IL-1, IL-6, and TNF)[152,153]. These in turn induce soluble vascular adhesion molecule 1 at the endothelial level, which has been found to be associated with endothelial dysfunction as well as the risk of CVD. HCV also interferes with glucose and lipid metabolism, leading to IR, diabetes, and liver steatosis, which are known factors that induce atherosclerosis[151]. A high TNF-α/ adiponectin ratio was found in HCV infected patients that is related to the development of IR and atherosclerosis[154].
Clearance of HCV by DAAs is associated with an improvement in atherosclerosis and metabolic and immunological conditions that promote the development of CVD[152]. Several studies reported the association between the achievement of SVR by DAAs and a significant reduction of the risk of acute coronary syndrome, CVD, and heart failure[84,155,156]. In pre-diabetic patients, Sasso et al[157] conducted a prospective multicenter study on prediabetic HCV positive cohort. They concluded that HCV eradication by DAAs allows a significant reduction of major CVD in the pre-diabetic population, regardless of the severity of liver disease and CV risk factors (age and hypercholesterolemia). This positive effect is mainly due to an improvement of serum markers of endothelial dysfunction and glucose metabolism[158,159].
A positive association was present between HCV and B-cell non-Hodgkin lymphoma (NHL) and it was detected among 5-15% of HCV patients. NHL includes marginal zone lymphoma, diffuse large B-cell lymphoma, lymphoplasmacytic lymphoma, follicular lymphoma, Burkitt’s lymphoma, non-Hodgkin T-cell lymphoma, and primary cutaneous T-cell lymphoma[160-162].
There are several HCV mechanisms determining neoplastic lymphoproliferative diseases. Bcell receptors are continuously stimulated by HCV viral antigens, leading to consecutive Bcell proliferation. HCV replication inside Bcells produces HCVderived viral proteins that induce genetic damage in the Bcells. The HCV envelope protein E3 binds to CD81 on the surface of Blymphocytes and forms a complex with CD19 and CD21, which in turn stimulates intracellular proliferative signals. When HCV enters B-cells, it causes oxidative stress that might result in mutations and defective DNA repair. Moreover, HCV infections are associated with increased frequencies of BCL6 and p53 gene mutations in Bcells. Accordingly, HCVrelated lymphomagenesis may be attributed to either chronic viral antigen stimulation or genetic mutations that lead to the clonal expansion and malignant transformation of Bcells[163-165]. In addition, MC is considered as a B-cell benign lymphoproliferative disorder frequently induced by HCV infection[166]. Figure 1 shows the pathophysiology of HCV infection in the occurrence of NHL.
DAA regimens in combination or after the completion of immunochemotherapy should be recommended. DAA treatment has been reported to reduce the frequency of the malignant B-cells in peripheral blood of patients affected by HCV-related lymphoproliferative disorders[167-169]. Moreover, DAAs might have a lower anti-lymphoma activity than IFN[84,170].
Up to 50% of patients with chronic HCV infection has neuropsychiatric symptoms. Among the reported psychiatric symptoms in chronic HCV patients are brain fog, depression, anxiety, weakness, and fatigue. These alterations lead to impaired quality of life[171]. The term HCV-associated neurocognitive disorder is used to refer to fundamental cognitive deficits unrelated to the severity of liver disease, viral load, and genotype and therefore distinct from the potentially reversible complications seen in patients with minimal hepatic encephalopathy (MHE)[172].
Chronic HCV patients exhibit prevalent involvement of the frontal lobe, which is responsible for alterations of executive functions[173] and the posterior regions of the cerebral cortex, particularly the occipital and parietal lobes[174]. Therefore, they exhibit difficulties in problem solving, monitoring one’s own behavior, self-control, cognitive flexibility, working memory, volition, sustained attention, and logical reasoning, in addition to verbal learning and verbal recall[175,176]. On the other hand, cognitive domains related to posterior brain regions, primarily involved in visuospatial, visual perceptual abilities, and constructive practice regions are mainly altered in patients with MHE[172]. Moreover, T2DM as an EHM in HCV infected patients may be associated with cognitive impairments[177]. Conversely, some studies did not confirm the association between chronic HCV infection and neurologic disorders[178]. Direct neuroinvasion changes in metabolic pathways and cerebral and systemic inflammation have been proposed as pathogenetic mechanisms[179]. Central fatigue and depression may share the same neurobiological causal pathways triggered by HCV infection[180]. Lower levels of dopamine were found in the ascending reticular activating and limbic systems among fatigued patients with HCV infection possibly from cytokine-induced reduction in tetrahydrobiopterin, which is an enzyme involved in the dopamine synthesis[181,182]. Impaired serotonin transmission implicated in depression has also been correlated with increased fatigue[183].
The prevalence of depression in HCV patients ranges from 20 to 50%, compared to a 10% prevalence in the general population[184]. In the US National Health and Nutrition Examination Survey, a cross-sectional study involving 10231 patients suffering from various liver diseases, only HCV was found to be independently associated with depression. The presence and severity of depression were independent of cirrhotic status, viral load, degree of hepatic inflammation, and use of IFN[185]. They suggested the presence of another exclusive HCV-mediated mechanism contributing to depression. Depression among people with HCV infection may be partially attributed to social and occupational limitations that may precede the infection, often causing viral acquisition, e.g., through intravenous drug use. Awareness of infection with subsequent poor acceptance and social stigma contributes to depressive symptoms independent of socioeconomic status or educational level[186]. Associated HCV complications such as cirrhosis, ascites, and encephalopathy, and comorbidities such as IR, RM, and CVD lead to limitation in physical function, and thus create higher physical load leading to increased depression[187].
The use of IFN was poorly tolerated as it was associated with neuropsychiatric disorders and impaired health related quality of life (HRQOL) in up to 70% of patients[172]. These disorders induced depression during and at the end of IFN treatment[188]. On the other hand, DAA regimens showed no significant psychiatric side effects and patients experience an improved HRQOL while on treatment, regardless of the stage of liver disease[189-192]. Viral clearance attained by DAAs improves fatigue, physical function, mental health, cognitive functions, and quality of life[193-196]. Several studies reported a significant reduction in the choline/creatine and myo-inositol/creatine ratios as well as an increase in cognitive functions in HCV infected patients who reached SVR compared to untreated patients or patients who did not achieve SVR[173,195]. It is suggested that the persistence of some cognitive symptoms at the end of therapy with DAAs can be attributed to compartmentalization of virus in the central nervous system that may represent a potential source of its reactivation[182,197].
Mazzaro et al[84] recommended starting DAAs as early as possible in the natural history of HCV infection. Early therapeutic approach not only will cure many of the EHMs that are still in a reversible stage, but it also can prevent those that develop due to delayed treatment.
HCV is a common cause of liver disease and is associated with a variety of EHMs. Among these manifestations are the rheumatologic diseases, T2DM, IR, several glomerulopathies, cardiovascular diseases, neuropsychiatric, and cognitive disorders.
DAA regimens are considered pan-genotypic as they achieve a SVR of > 85% at 12 wk through all the major HCV genotypes as well as a very high percentage of SVR even in advanced fibrosis and cirrhosis. DAAs improved the symptoms of EHMs and reduced the risk of complications. The risk factors for DAA failure include advanced liver fibrosis/cirrhosis and the presence of RAS in the region targeted by the received DAAs. The effectiveness of GLE/PIB is reduced in HCV genotype 3 with baseline RAS like A30K, Y93H, and P53del. Baseline RAS testing is recommended for HCV genotype 3 infected patients with liver cirrhosis, as those without a baseline Y93H RAS in NS5A are eligible for SOF/VEL therapy. While, those with baseline Y93H RAS could be treated with SOF/VEL/VOX or SOF/VEL plus RBV. Moreover, EASL recommended the identification of baseline RAS for HCV genotype 1a. No RAS was reported in the NS5B region. The higher rate of HCC after DAA therapy may be explained by the fact that DAA regimens are offered to patients with advanced liver fibrosis and compensated or decompensated cirrhosis, where IFN was contraindicated. In addition, the change in the growth of pre-existing subclinical undetectable HCC upon DAA treatment might be a cause. Furthermore, after DAA therapy, the T cell-dependent immune response is much weaker upon HCV clearance, and the down-regulation of TNF-α or the elevated neutrophil to lymphocyte ratio might increase the risk of HCC. DAAs appear to be safe for patients with a history of treated HCC except for cases with vascular invasion. DAAs can result in reactivation of HBV in HCV co-infected patients.
Concerning EHMs, DAAs are now the drug of choice for HCV-associated MCV, and they can achieve clinical and immunological responses for cutaneous and musculoskeletal manifestations, and peripheral nerve and renal involvement. DAAs have rapid and high effectiveness in thrombocytopenia. They also improve IR by 90%, increased glomerular filtration rate, and decrease proteinuria, hematuria, articular manifestations, and lymphoproliferative disorders. Moreover, HCV clearance by DAAs allows a significant improvement in atherosclerosis and metabolic and immunological conditions with a reduction of major cardiovascular events, regardless of the severity of liver disease. DAA treatment also improves physical function, fatigue, and HRQOL greatly during and at the end of treatment as well as at SVR. Viral clearance attained by DAAs improves also cognitive functions in patients with subtle cognitive defects independent of their liver condition. Early therapeutic approach with DAAs not only will cure many of the EHMs that are still in a reversible stage, but it can also prevent those develop due to delayed treatment.
The authors would like to acknowledge El-Atroush ES and Kamal OA for designing and drawing the figure presented in the minireview.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fateh A, Iran; Mukhopadhyay A, India A-Editor: Poddighe D, Kazakhstan S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | World Health Organization (WHO). Hepatitis C. [cited 20 March 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c on 29.2.2022. |
| 2. | WHO 2021 World Health Organization (WHO). Interim guidance for country validation of viral hepatitis elimination. [cited 20 March 2022]. Available from: https://www.who.int/publications/i/item/9789240028395 on 11.3.2022. |
| 3. | Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. 2020;71:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Sillanpää M, Melén K, Porkka P, Fagerlund R, Nevalainen K, Lappalainen M, Julkunen I. Hepatitis C virus core, NS3, NS4B and NS5A are the major immunogenic proteins in humoral immunity in chronic HCV infection. Virol J. 2009;6:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Farhang Zangneh H, Wong WWL, Sander B, Bell CM, Mumtaz K, Kowgier M, van der Meer AJ, Cleary SP, Janssen HLA, Chan KKW, Feld JJ. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2019;17:1840-1849.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Catanese MT, Uryu K, Kopp M, Edwards TJ, Andrus L, Rice WJ, Silvestry M, Kuhn RJ, Rice CM. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci U S A. 2013;110:9505-9510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Zhao F, Zhao T, Deng L, Lv D, Zhang X, Pan X, Xu J, Long G. Visualizing the Essential Role of Complete Virion Assembly Machinery in Efficient Hepatitis C Virus Cell-to-Cell Transmission by a Viral Infection-Activated Split-Intein-Mediated Reporter System. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Antonelli A, Ferri C, Galeazzi M, Giannitti C, Manno D, Mieli-Vergani G, Menegatti E, Olivieri I, Puoti M, Palazzi C, Roccatello D, Vergani D, Sarzi-Puttini P, Atzeni F. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26:S39-S47. [PubMed] |
| 9. | Lozach PY, Amara A, Bartosch B, Virelizier JL, Arenzana-Seisdedos F, Cosset FL, Altmeyer R. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279:32035-32045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Lefèvre M, Felmlee DJ, Parnot M, Baumert TF, Schuster C. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated apolipoprotein E. PLoS One. 2014;9:e95550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017-5025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 891] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 12. | Yamamoto S, Fukuhara T, Ono C, Uemura K, Kawachi Y, Shiokawa M, Mori H, Wada M, Shima R, Okamoto T, Hiraga N, Suzuki R, Chayama K, Wakita T, Matsuura Y. Lipoprotein Receptors Redundantly Participate in Entry of Hepatitis C Virus. PLoS Pathog. 2016;12:e1005610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Tawar RG, Schuster C, Baumert TF. HCV Receptors and Virus Entry. Springer. 2016;81-103. [DOI] [Full Text] |
| 14. | Zona L, Lupberger J, Sidahmed-Adrar N, Thumann C, Harris HJ, Barnes A, Florentin J, Tawar RG, Xiao F, Turek M, Durand SC, Duong FH, Heim MH, Cosset FL, Hirsch I, Samuel D, Brino L, Zeisel MB, Le Naour F, McKeating JA, Baumert TF. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe. 2013;13:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Sourisseau M, Michta ML, Zony C, Israelow B, Hopcraft SE, Narbus CM, Parra Martín A, Evans MJ. Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies. PLoS Pathog. 2013;9:e1003244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Cheng JJ, Li JR, Huang MH, Ma LL, Wu ZY, Jiang CC, Li WJ, Li YH, Han YX, Li H, Chen JH, Wang YX, Song DQ, Peng ZG, Jiang JD. CD36 is a co-receptor for hepatitis C virus E1 protein attachment. Sci Rep. 2016;6:21808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Wang J, Qiao L, Hou Z, Luo G. TIM-1 Promotes Hepatitis C Virus Cell Attachment and Infection. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kachko A, Costafreda MI, Zubkova I, Jacques J, Takeda K, Wells F, Kaplan G, Major ME. Determinants in the Ig Variable Domain of Human HAVCR1 (TIM-1) Are Required To Enhance Hepatitis C Virus Entry. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Nguyen LP, Nguyen TTT, Nguyen HC, Pham HT, Han KM, Choi DH, Park EM, Kang SM, Tark D, Lim YS, Hwang SB. Cortactin Interacts with Hepatitis C Virus Core and NS5A Proteins: Implications for Virion Assembly. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol. 2011;85:596-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | Choi JW, Kim JW, Nguyen LP, Nguyen HC, Park EM, Choi DH, Han KM, Kang SM, Tark D, Lim YS, Hwang SB. Nonstructural NS5A Protein Regulates LIM and SH3 Domain Protein 1 to Promote Hepatitis C Virus Propagation. Mol Cells. 2020;43:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 421] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 23. | Rosso C, Caviglia GP, Ciruolo M, Ciancio A, Younes R, Olivero A, Giordanino C, Troshina G, Abate ML, Rizzetto M, Pellicano R, Saracco GM, Bugianesi E, Smedile A. Clinical outcomes in chronic hepatitis C long-term responders to pre-direct antiviral agents: a single-center retrospective study. Minerva Med. 2019;110:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Fukuhara T, Kambara H, Shiokawa M, Ono C, Katoh H, Morita E, Okuzaki D, Maehara Y, Koike K, Matsuura Y. Expression of microRNA miR-122 facilitates an efficient replication in nonhepatic cells upon infection with hepatitis C virus. J Virol. 2012;86:7918-7933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Ito M, Masumi A, Mochida K, Kukihara H, Moriishi K, Matsuura Y, Yamaguchi K, Mizuochi T. Peripheral B cells may serve as a reservoir for persistent hepatitis C virus infection. J Innate Immun. 2010;2:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Alhetheel AF. Impact of Hepatitis C Virus Infection of Peripheral Blood Mononuclear Cells on the Immune System. Frontiers in Virology. 2022;1:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Wang W, Zou Z, Hu Z, Fan Q, Xiong J. Hepatitis C Virus Entry into Macrophages/Monocytes Mainly Depends on the Phagocytosis of Macrophages. Dig Dis Sci. 2019;64:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Colpitts CC, Tsai PL, Zeisel MB. Hepatitis C Virus Entry: An Intriguingly Complex and Highly Regulated Process. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 30. | AASLD and IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C, 2020. [cited 20 March 2022]. Available from: http://www.hcvguidelines.org on 11.12.2021. |
| 31. | Filozof CM, Jones S, Goldstein BJ. Liver Fibrosis as Assessed by the FIB-4 Index in Patients with Type 2 Diabetes (T2DM). Diabetes. 2018;67:1570-P. [DOI] [Full Text] |
| 32. | Lupsor Platon M, Stefanescu H, Feier D, Maniu A, Badea R. Performance of unidimensional transient elastography in staging chronic hepatitis C. Results from a cohort of 1,202 biopsied patients from one single center. J Gastrointestin Liver Dis. 2013;22:157-166. [PubMed] |
| 33. | Laboratory Corporation of America. Noninvasive assessment of liver fibrosis and necroinflammatory activity for patients with HCV. [cited 20 January 2022]. Available from: https://www.labcorp.com/tests/related-documents/L9465 on 11.3.2022. |
| 34. | Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 35. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 791] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 36. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 37. | Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27-38.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 38. | Piedade J, Pereira G, Guimarães L, Duarte J, Victor L, Baldin C, Inacio C, Santos R, Chaves Ú, Nunes EP, Grinsztejn B, Veloso VG, Fernandes F, Perazzo H. Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes. Sci Rep. 2021;11:11681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Pecoraro V, Banzi R, Cariani E, Chester J, Villa E, D'Amico R, Bertele' V, Trenti T. New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials. J Clin Exp Hepatol. 2019;9:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Flores GL, Mota JC, da Silva Andrade LT, Lopes RS, Bastos FI, Villar LM. Performance of HCV Antigen Testing for the Diagnosis and Monitoring of Antiviral Treatment: A Systematic Review and Meta-Analysis. Biomed Res Int. 2022;2022:7348755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Bukh J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65:S2-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Kondili LA, Gaeta GB, Brunetto MR, Di Leo A, Iannone A, Santantonio TA, Giammario A, Raimondo G, Filomia R, Coppola C, Amoruso DC, Blanc P, Del Pin B, Chemello L, Cavalletto L, Morisco F, Donnarumma L, Rumi MG, Gasbarrini A, Siciliano M, Massari M, Corsini R, Coco B, Madonia S, Cannizzaro M, Zignego AL, Monti M, Russo FP, Zanetto A, Persico M, Masarone M, Villa E, Bernabucci V, Taliani G, Biliotti E, Chessa L, Pasetto MC, Andreone P, Margotti M, Brancaccio G, Ieluzzi D, Borgia G, Zappulo E, Calvaruso V, Petta S, Falzano L, Quaranta MG, Weimer LE, Rosato S, Vella S, Giannini EG. Incidence of DAA failure and the clinical impact of retreatment in real-life patients treated in the advanced stage of liver disease: Interim evaluations from the PITER network. PLoS One. 2017;12:e0185728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Quaranta MG, Ferrigno L, Monti M, Filomia R, Biliotti E, Iannone A, Migliorino G, Coco B, Morisco F, Vinci M, D'Ambrosio R, Chemello L, Massari M, Ieluzzi D, Russo FP, Blanc P, Verucchi G, Puoti M, Rumi MG, Barbaro F, Santantonio TA, Federico A, Chessa L, Gentile I, Zuin M, Parruti G, Morsica G, Kondili LA; PITER Collaborating Group. Advanced liver disease outcomes after hepatitis C eradication by human immunodeficiency virus infection in PITER cohort. Hepatol Int. 2020;14:362-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Virus Infection. [cited 20 January 2022]. Available from: https://www.who.int/publications/i/item/9789241550345 on 1/6/2022. |
| 45. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 46. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 47. | Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, de Knegt RJ, Hansen BE, Janssen HLA, de Man RA, Feld JJ, van der Meer AJ. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 48. | Wedemeyer H, Craxí A, Zuckerman E, Dieterich D, Flisiak R, Roberts SK, Pangerl A, Zhang Z, Martinez M, Bao Y, Calleja JL. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir±dasabuvir±ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: A meta-analysis. J Viral Hepat. 2017;24:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Dietz J, Susser S, Vermehren J, Peiffer KH, Grammatikos G, Berger A, Ferenci P, Buti M, Müllhaupt B, Hunyady B, Hinrichsen H, Mauss S, Petersen J, Buggisch P, Felten G, Hüppe D, Knecht G, Lutz T, Schott E, Berg C, Spengler U, von Hahn T, Berg T, Zeuzem S, Sarrazin C; European HCV Resistance Study Group. Patterns of Resistance-Associated Substitutions in Patients With Chronic HCV Infection Following Treatment With Direct-Acting Antivirals. Gastroenterology. 2018;154:976-988.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 50. | Parigi TL, Torres MCP, Aghemo A. Upcoming direct acting antivirals for hepatitis C patients with a prior treatment failure. Clin Mol Hepatol. 2019;25:360-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461-511. |
| 52. | Pisaturo M, Starace M, Minichini C, De Pascalis S, Macera M, Occhiello L, Messina V, Sangiovanni V, Claar E, Precone D, Stornaiuolo G, Stanzione M, Gentile I, Brancaccio G, Martini S, Masiello A, Megna AS, Coppola C, Federico A, Sagnelli E, Persico M, Lanza AG, Marrone A, Gaeta GB, Coppola N. Patients with HCV genotype-1 who have failed a direct-acting antiviral regimen: virological characteristics and efficacy of retreatment. Antivir Ther. 2019;24:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Zhang Y, Jiang X, Zhao Y, Xu Y. Effect of baseline resistance-associated substitutions on the efficiency of glecaprevir/pibrentasvir in chronic hepatitis C subjects: A meta-analysis. J Viral Hepat. 2021;28:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Rahimi P, Sharafi H, Bahramali G, SajadianFard F, Asadi NS, Alavian SM, Iranpur Mobarakeh V, Moravej SZ. Prevalence of Naturally-Occurring NS5A and NS5B Resistance-Associated Substitutions in Iranian Patients With Chronic Hepatitis C Infection. Front Microbiol. 2020;11:617375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Chen Q, Perales C, Soria ME, García-Cehic D, Gregori J, Rodríguez-Frías F, Buti M, Crespo J, Calleja JL, Tabernero D, Vila M, Lázaro F, Rando-Segura A, Nieto-Aponte L, Llorens-Revull M, Cortese MF, Fernandez-Alonso I, Castellote J, Niubó J, Imaz A, Xiol X, Castells L, Riveiro-Barciela M, Llaneras J, Navarro J, Vargas-Blasco V, Augustin S, Conde I, Rubín Á, Prieto M, Torras X, Margall N, Forns X, Mariño Z, Lens S, Bonacci M, Pérez-Del-Pulgar S, Londoño MC, García-Buey ML, Sanz-Cameno P, Morillas R, Martró E, Saludes V, Masnou-Ridaura H, Salmerón J, Quíles R, Carrión JA, Forné M, Rosinach M, Fernández I, García-Samaniego J, Madejón A, Castillo-Grau P, López-Núñez C, Ferri MJ, Durández R, Sáez-Royuela F, Diago M, Gimeno C, Medina R, Buenestado J, Bernet A, Turnes J, Trigo-Daporta M, Hernández-Guerra M, Delgado-Blanco M, Cañizares A, Arenas JI, Gomez-Alonso MJ, Rodríguez M, Deig E, Olivé G, Río OD, Cabezas J, Quiñones I, Roget M, Montoliu S, García-Costa J, Force L, Blanch S, Miralbés M, López-de-Goicoechea MJ, García-Flores A, Saumoy M, Casanovas T, Baliellas C, Gilabert P, Martin-Cardona A, Roca R, Barenys M, Villaverde J, Salord S, Camps B, Silvan di Yacovo M, Ocaña I, Sauleda S, Bes M, Carbonell J, Vargas-Accarino E, Ruzo SP, Guerrero-Murillo M, Von Massow G, Costafreda MI, López RM, González-Moreno L, Real Y, Acero-Fernández D, Viroles S, Pamplona X, Cairó M, Ocete MD, Macías-Sánchez JF, Estébanez A, Quer JC, Mena-de-Cea Á, Otero A, Castro-Iglesias Á, Suárez F, Vázquez Á, Vieito D, López-Calvo S, Vázquez-Rodríguez P, Martínez-Cerezo FJ, Rodríguez R, Macenlle R, Cachero A, Mereish G, Mora-Moruny C, Fábregas S, Sacristán B, Albillos A, Sánchez-Ruano JJ, Baluja-Pino R, Fernández-Fernández J, González-Portela C, García-Martin C, Sánchez-Antolín G, Andrade RJ, Simón MA, Pascasio JM, Romero-Gómez M, Antonio Del-Campo J, Domingo E, Esteban R, Esteban JI, Quer J. Deep-sequencing reveals broad subtype-specific HCV resistance mutations associated with treatment failure. Antiviral Res. 2020;174:104694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Belperio PS, Shahoumian TA, Loomis TP, Backus LI. Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir in 573 direct-acting antiviral experienced hepatitis C patients. J Viral Hepat. 2019;26:980-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Shiha G, Mousa N, Soliman R, Nnh Mikhail N, Adel Elbasiony M, Khattab M. Incidence of HCC in chronic hepatitis C patients with advanced hepatic fibrosis who achieved SVR following DAAs: A prospective study. J Viral Hepat. 2020;27:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Tampaki M, Savvanis S, Koskinas J. Impact of direct-acting antiviral agents on the development of hepatocellular carcinoma: evidence and pathophysiological issues. Ann Gastroenterol. 2018;31:670-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Karbeyaz F, Kissling S, Jaklin PJ, Bachofner J, Brunner B, Müllhaupt B. Rates of Hepatocellular Carcinoma After Start of Treatment for Chronic Hepatitis C Remain High with Direct Acting Antivirals: Analysis from a Swiss Liver. Journal of Hepatocellular Carcinoma. 2021;8565-8574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Roudot-Thoraval F, Audureau E; ANRS CO12 CirVir Group. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology. 2018;155:1436-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 62. | Celsa C, Stornello C, Giuffrida P, Giacchetto CM, Grova M, Rancatore G, Pitrone C, Di Marco V, Cammà C, Cabibbo G. Direct-acting antiviral agents and risk of Hepatocellular carcinoma: Critical appraisal of the evidence. Ann Hepatol. 2022;27 Suppl 1:100568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Owusu Sekyere S, Schlevogt B, Mettke F, Kabbani M, Deterding K, Wirth TC, Vogel A, Manns MP, Falk CS, Cornberg M, Wedemeyer H. HCC Immune Surveillance and Antiviral Therapy of Hepatitis C Virus Infection. Liver Cancer. 2019;8:41-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Lu MY, Yeh ML, Huang CI, Wang SC, Tsai YS, Tsai PC, Ko YM, Lin CC, Chen KY, Wei YJ, Hsu PY, Hsu CT, Jang TY, Liu TW, Liang PC, Hsieh MY, Lin ZY, Chen SC, Huang CF, Huang JF, Dai CY, Chuang WL, Yu ML. Dynamics of cytokines predicts risk of hepatocellular carcinoma among chronic hepatitis C patients after viral eradication. World J Gastroenterol. 2022;28:140-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Khalid J, Umar M, Ur-Rehman T, Ali M, Khan GM. Tumor aggression among hepatitis-C related hepatocellular carcinoma patients: an observational study regarding the impact of anti-HCV therapy. Infect Agent Cancer. 2020;15:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Rich NE, Singal AG. Direct-Acting Antiviral Therapy and Hepatocellular Carcinoma. Clin Liver Dis (Hoboken). 2021;17:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Renzulli M, Buonfiglioli F, Conti F, Brocchi S, Serio I, Foschi FG, Caraceni P, Mazzella G, Verucchi G, Golfieri R, Andreone P, Brillanti S. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. Eur Radiol. 2018;28:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Fouad M, El Kassas M, Ahmed E, El Sheemy R. Tumor characteristics of hepatocellular carcinoma after direct-acting antiviral treatment for hepatitis C: Comparative analysis with antiviral therapy-naive patients. World J Hepatol. 2021;13:1743-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Sami SM, Salama II, Abdel-Latif GA, El Etreby LA, Metwally AI, Abd El Haliem NF. Hepatitis B Seroprotection and the Response to a Challenging Dose among Vaccinated Children in Red Sea Governorate. Open Access Maced J Med Sci. 2016;4:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Salama II, Sami SM, Said ZN, Salama SI, Rabah TM, Abdel-Latif GA, Elmosalami DM, Saleh RM, Abdel Mohsin AM, Metwally AM, Hassanin AI, Emam HM, Hemida SA, Elserougy SM, Shaaban FA, Fouad WA, Mohsen A, El-Sayed MH. Early and long term anamnestic response to HBV booster dose among fully vaccinated Egyptian children during infancy. Vaccine. 2018;36:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | El-Sayed MH, Said ZNA, Abo-Elmagd EK, Ebeid FSE, Salama II. High Risk of HBV Infection Among Vaccinated Polytransfused Children With Malignancy. J Pediatr Hematol Oncol. 2021;43:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Said ZN, El-Sayed MH, El-Bishbishi IA, El-Fouhil DF, Abdel-Rheem SE, El-Abedin MZ, Salama II. High prevalence of occult hepatitis B in hepatitis C-infected Egyptian children with haematological disorders and malignancies. Liver Int. 2009;29:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Pol S, Haour G, Fontaine H, Dorival C, Petrov-Sanchez V, Bourliere M, Capeau J, Carrieri P, Larrey D, Larsen C, Marcellin P, Pawlostky JM, Nahon P, Zoulim F, Cacoub P, de Ledinghen V, Mathurin P, Negro F, Pageaux GP, Yazdanpanah Y, Wittkop L, Zarski JP, Carrat F; French Anrs Co22 Hepather Cohort. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther. 2017;46:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 75. | Liu CJ, Tseng TC, Yang WT, Su TH, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. Profile and value of FIB-4 in patients with dual chronic hepatitis C and B. J Gastroenterol Hepatol. 2019;34:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Sagnelli E, Sagnelli C, Macera M, Pisaturo M, Coppola N. An update on the treatment options for HBV/HCV coinfection. Expert Opin Pharmacother. 2017;18:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 77. | Shih YF, Liu CJ. Hepatitis C Virus and Hepatitis B Virus Co-Infection. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 79. | Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017;66:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 80. | Wang CR, Tsai HW. Human hepatitis viruses-associated cutaneous and systemic vasculitis. World J Gastroenterol. 2021;27:19-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 81. | Comarmond C, Cacoub P, Saadoun D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Therap Adv Gastroenterol. 2020;13:1756284820942617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76:818-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Cacoub P, Comarmond C, Domont F, Savey L, Saadoun D. Cryoglobulinemia Vasculitis. Am J Med. 2015;128:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 84. | Mazzaro C, Quartuccio L, Adinolfi LE, Roccatello D, Pozzato G, Nevola R, Tonizzo M, Gitto S, Andreone P, Gattei V. A Review on Extrahepatic Manifestations of Chronic Hepatitis C Virus Infection and the Impact of Direct-Acting Antiviral Therapy. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 85. | Sollima S, Milazzo L, Peri AM, Torre A, Antinori S, Galli M. Persistent mixed cryoglobulinaemia vasculitis despite hepatitis C virus eradication after interferon-free antiviral therapy. Rheumatology (Oxford). 2016;55:2084-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Ghosn M, Palmer MB, Najem CE, Haddad D, Merkel PA, Hogan JJ. New-onset hepatitis C virus-associated glomerulonephritis following sustained virologic response with direct-acting antiviral therapy . Clin Nephrol. 2017;87 (2017):261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Lauletta G, Russi S, Pavone F, Vacca A, Dammacco F. Direct-acting antiviral agents in the therapy of hepatitis C virus-related mixed cryoglobulinaemia: a single-centre experience. Arthritis Res Ther. 2017;19:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 88. | Artemova M, Abdurakhmanov D, Ignatova T, Mukhin N. Persistent hepatitis C virus-associated cryoglobulinemic vasculitis following virus eradication after direct-acting antiviral therapy. Hepatology. 2017;65:1770-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, de Saint Martin L, Comarmond C, Bouyer AS, Musset L, Poynard T, Resche Rigon M, Cacoub P. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology. 2017;153:49-52.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 90. | Del Padre M, Todi L, Mitrevski M, Marrapodi R, Colantuono S, Fiorilli M, Casato M, Visentini M. Reversion of anergy signatures in clonal CD21low B cells of mixed cryoglobulinemia after clearance of HCV viremia. Blood. 2017;130:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Sikorska-Wiśniewska M, Sikorska K, Wróblewska A, Liberek T, Perkowska-Ptasińska A, Dębska-Ślizień A. Recurrence of Cryoglobulinemia Secondary to Hepatitis C in a Patient with HCV RNA (-) Negative in the Serum. Case Rep Nephrol Dial. 2021;11:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Abdelhamid WAR, Shendi A, Zahran M, Elbary EA, Fadda S. Hepatitis C-related membranoproliferative glomerulonephritis in the era of direct antiviral agents. J Bras Nefrol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Zignego AL, Pawlotsky JM, Bondin M, Cacoub P. Expert opinion on managing chronic HCV in patients with mixed cryoglobulinaemia vasculitis. Antivir Ther. 2018;23:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Chen YC, Ko PH, Lee CC, Tseng CW, Tseng KC. Baseline thrombopoietin level is associated with platelet count improvement in thrombocytopenic chronic hepatitis C patients after successful direct-acting antiviral agent therapy. BMC Gastroenterol. 2021;21:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Saif-Al-Islam M, Abdelaal UM, Younis MA, Alghany Algahlan HA, Khalaf S. Effect of Direct-Acting Antiviral Therapy on Thrombocytopenic Patients with Hepatitis C Virus-Related Chronic Liver Disease. Gastroenterol Res Pract. 2021;2021:8811203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Dahal S, Upadhyay S, Banjade R, Dhakal P, Khanal N, Bhatt VR. Thrombocytopenia in Patients with Chronic Hepatitis C Virus Infection. Mediterr J Hematol Infect Dis. 2017;9:e2017019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Chen YC, Tseng CW, Tseng KC. Rapid platelet count improvement in chronic hepatitis C patients with thrombocytopenia receiving direct-acting antiviral agents. Medicine (Baltimore). 2020;99:e20156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Hermos JA, Quach L, Gagnon DR, Weber HC, Altincatal A, Cho K, Lawler EV, Grotzinger KM. Incident severe thrombocytopenia in veterans treated with pegylated interferon plus ribavirin for chronic hepatitis C infection. Pharmacoepidemiol Drug Saf. 2014;23:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 100. | Soliman Z, El Kassas M, Elsharkawy A, Elbadry M, Hamada Y, ElHusseiny R, M El-Nahaas S, Fouad R, Esmat G, Abdel Alem S. Improvement of platelet in thrombocytopenic HCV patients after treatment with direct-acting antiviral agents and its relation to outcome. Platelets. 2021;32:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Henson JB, Sise ME. The association of hepatitis C infection with the onset of CKD and progression into ESRD. Semin Dial. 2019;32:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Guo S, Kapp ME, Beltran DM, Cardona CY, Caster DJ, Reichel RR, et al. Spectrum of Kidney Diseases in Patients With Hepatitis C Virus Infection. A 10-Year Study. Am J Clin Pathol. 2021;156:399-408. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Iovănescu VF, Constantinescu AF, Streba CT, Zaharie SI, Vere CC, Mandache E, Penescu MN, MoŢa E. Clinical and pathological considerations on renal diseases in patients with chronic viral hepatitis. Rom J Morphol Embryol. 2016;57:401-406. [PubMed] |
| 104. | Ratiu IA, Peride I, Fratila O, Adriana B, Bako GC, Ratiu C, et al. Efficacy of Direct Acting Antivirals and the Role of Corticosteroids in Rapidly Progressive Cryoglobulinemic Glomerulonephritis Associated to Hepatitis C Related Liver Cirrhosis. Case Report. Annals of Case Reports. 2020;14:1-5. [DOI] [Full Text] |
| 105. | Barsoum RS, William EA, Khalil SS. Hepatitis C and kidney disease: A narrative review. Journal of Advanced Research. 2017;8:113-130. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Lai T-S, Lee M-H, Yang H-I, You S-L, Lu S-N, Wang L-Y, et al. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease. Kidney International. 2017;92:703-709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Fabrizi F, Donato FM, Messa P. Association Between Hepatitis C Virus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ann Hepatol. 2018;17:364-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 108. | Fabrizi F, Cerutti R, Dixit V, Messa P. The impact of antiviral therapy for HCV on kidney disease: a systematic review and meta-analysis. Nefrologia (Engl Ed). 2020;40:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Suda G, Ogawa K, Kimura M, Nakai M, Sho T, Morikawa K, et al. Novel Treatment of Hepatitis C Virus Infection for Patients with Renal Impairment. J Clin Transl Hepatol. 2016;4: 320-327. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Kao CC, Lin YS, Chu HC, Fang TC, Wu MS, Kang YN. Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. J Clin Med. 2018;7:1-14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Rutledge SM, Chung RT, Sise ME. Treatment of hepatitis C virus infection in patients with mixed cryoglobulinemic syndrome and cryoglobulinemic glomerulonephritis. Hemodial Int. 2018;22 Suppl 1:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Salvadori M, Tsalouchos A. Hepatitis C Virus Infection and Renal Disorders. REVIEW ARTICLE. Journal of Renal and Hepatic Disorders. 2019;3:1-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Arruda RM, Batista AD, Filgueira NA, Moura IF, Sette LH, Lopes EP. Remission of long-term hepatic and renal disease induced by HCV after direct-acting antivirals therapy. Braz J Nephrol. 2021;43:117-120. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Elmowafy AY, El Maghrabi HM, Zahab MA, Elwasif SM, Bakr MA. Sofosbuvir and Daclatsvir in Treatment of Hepatitis C Virus-related Membranoproliferative Glomerulonephritis With Cryoglobulinemia in a Patient With Hepatitis C Genotype 4. Iran J Kidney Dis. 2018;12:382-384. [PubMed] |
| 115. | Bohorquez H, Velez JCQ, Lusco M, Scheuermann J, Cohen AJ. Hepatitis C-associated focal proliferative glomerulonephritis in an aviremic recipient of a hepatitis C-positive antibody donor liver. Am J Transplant. 2021;21:2895-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Muro K, Toda N, Yamamoto S, Yanagita M. The Successful Treatment of a Case of HCV-associated Cryoglobulinemic Glomerulonephritis with Rituximab, Direct-acting Antiviral Agents, Plasmapheresis and Long-term Steroid Despite Serologically Persistent Cryoglobulinemia. Intern Med. 2021;60:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Lens S, Rodriguez-Tajes S, Llovet L-P, Maduell F, Londoño M-C. Treating Hepatitis C in Patients with Renal Failure. Digestive Diseases. 2017;339-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Palombo SB, Wendel EC, Kidd LR, Yazdi F, Naljayan MV. MPGN and mixed cryoglobulinemia in a patient with hepatitis C - new treatment implications and renal outcomes. Clin Nephrol Case Stud. 2017;5:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Isnard Bagnis C, Cacoub P. Hepatitis C Therapy in Renal Patients: Who, How, When? Infect Dis Ther. 2016;5:313-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Schrezenmeier E, Wu K, Halleck F, Liefeldt L, Brakemeier S, Bachmann F, Kron S, Budde K, Duerr M. Successful Recovery of Acute Renal Transplant Failure in Recurrent Hepatitis C Virus-Associated Membranoproliferative Glomerulonephritis. Am J Transplant. 2017;17:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 121. | Angeletti A, Cantarelli C, Cravedi P. HCV-Associated Nephropathies in the Era of Direct Acting Antiviral Agents. Front Med (Lausanne). 2019;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Shimada M, Nakamura N, Endo T, Yamabe H, Nakamura M, Murakami R, Narita I, Tomita H. Daclatasvir/asunaprevir based direct-acting antiviral therapy ameliorate hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis: a case report. BMC Nephrol. 2017;18:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Ezzat WM, Elhosary YA, Abdulla NA, Raslan HM, Saleh OM, Ibrahim MH, et al. Insulin resistance and early virological response in chronic HCV infection. Journal of Genetic Engineering and Biotechnology. 2013;11:69-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 124. | Adinolfi LE, Petta S, Fracanzani AL, Nevola R, Coppola C, Narciso V, Rinaldi L, Calvaruso V, Pafundi PC, Lombardi R, Staiano L, Di Marco V, Solano A, Marrone A, Saturnino M, Rini F, Guerrera B, Troina G, Giordano M, Craxì A, Sasso FC. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes Metab. 2020;22:2408-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 125. | Urganci N, Kalyoncub D, Geylani-Gulec S. Insulin resistance in children with chronic hepatitis C and its association with response to IFN-alpha and ribavirin. Revista de Gastroenterología de México. 2021;86: 140-144. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Hum J, Jou JH. The link between hepatitis C virus and diabetes mellitus: Improvement in insulin resistance after eradication of hepatitis C virus. Clin Liver Dis (Hoboken). 2018;11:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Jeong D, Karim ME, Wong S, Wilton J, Butt ZA, Binka M, Adu PA, Bartlett S, Pearce M, Clementi E, Yu A, Alvarez M, Samji H, Velásquez García HA, Abdia Y, Krajden M, Janjua NZ. Impact of HCV infection and ethnicity on incident type 2 diabetes: findings from a large population-based cohort in British Columbia. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Huang JF, Huang CF, Yeh ML, Dai CY, Hsieh MH, Yang JF, Huang CI, Lin YH, Liang PC, Lin ZY, Chen SC, Yu ML, Chuang WL. The outcomes of glucose abnormalities in chronic hepatitis C patients receiving interferon-free direct antiviral agents. Kaohsiung J Med Sci. 2017;33:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Tsai MC, Kao KL, Huang HC, Chen W, Fang CK, Sung FC, Wu SI, Stewart R. Incidence of Type 2 Diabetes in Patients With Chronic Hepatitis C Receiving Interferon-Based Therapy. Diabetes Care. 2020;43:e63-e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Ribaldone DG, Sacco M, Saracco GM. The Effect of Viral Clearance Achieved by Direct-Acting Antiviral Agents on Hepatitis C Virus Positive Patients with Type 2 Diabetes Mellitus: A Word of Caution after the Initial Enthusiasm. Journal of Clinical Medicine. 2020;9:1-18. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 131. | Mahmoud B, Moneim AA, Mabrouk D. The impact of HCV eradication on hyperglycemia, insulin resistance, cytokine production, and insulin receptor substrate-1 and 2 expression in patients with HCV infection. Clin Exp Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Yosef T, Ibrahim WA, El Ghandour A, Attia S, El Nakeep S. Efect of diferent direct-acting antiviral regimens for treatment of nondiabetic hepatitis C virus-infected Egyptian patients on insulin resistance and sensitivity. Egypt J Intern Med. 2021;33:1-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 133. | Zignego AL, Ramos-Casals M, Ferri C, Saadoun D, Arcaini L, Roccatello D, Antonelli A, Desbois AC, Comarmond C, Gragnani L, Casato M, Lamprecht P, Mangia A, Tzioufas AG, Younossi ZM, Cacoub P; ISG-EHCV. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun Rev. 2017;16:523-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 134. | Andres J, Barros M, Arutunian M, Zhao H. Treatment of Hepatitis C Virus and Long-Term Effect on Glycemic Control. J Manag Care Spec Pharm. 2020;26:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Treppo E, Quartuccio L, Ragab G, De Vita S. Rheumatologic manifestations of Hepatitis C Virus. Hepatitis C Infection: A Systemic Disease. Review. Minerva Medica. 2021;112:201-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Shahin AA, Zayed HS, Said M, Amer SA. Efficacy and safety of sofosbuvir-based, interferon-free therapy : The Management of rheumatologic extrahepatic manifestations associated with chronic hepatitis C virus infection. Z Rheumatol. 2018;77:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Flores-Chávez A, Carrion JA, Forns X, Ramos-Casals M. Extrahepatic manifestations associated with chronic Hepatitis C Virus infection. Rev Esp Sanid Penit. 2017;19:87-97. [RCA] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 138. | Kchir H, Kaffel D, Cherif D, Hamdi W, Maamouri N. Rheumatologic manifestations during chronic viral hepatitis C. Tunis Med. 2019;97:1251-1257. [PubMed] |
| 139. | Ezzat WM, Raslan HM, Aly AA, Emara NA, El Menyawi MM, Edrees A. Anti-cyclic citrullinated peptide antibodies as a discriminating marker between rheumatoid arthritis and chronic hepatitis C-related polyarthropathy. Rheumatol Int. 2011;31:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Priora M, Borrelli R, Parisi S, Ditto MC, Realmuto C, Laganà A, et al. Autoantibodies and Rheumatologic Manifestations in Hepatitis C Virus Infection. Biology. 2021;10:1-12. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Priora M, Realmuto C, Parisi S, Ditto MC, Borrelli R, Peroni CL, Laganà A, Fusaro E. Rheumatologic manifestations of hepatitis C in the era of direct-acting antiviral agents. Minerva Gastroenterol Dietol. 2020;66:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 142. | Mohanty A, Salameh S, Butt AA. Impact of Direct Acting Antiviral Agent Therapy upon Extrahepatic Manifestations of Hepatitis C Virus Infection. Curr HIV/AIDS Rep. 2019;16:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | de Oliveira IMX, da Silva RSU. Rheumatological Manifestations Associated with Viral Hepatitis B or C. Journal of the Brazilian Society of Tropical Medicine. 2019;52: 1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | Alian SM, Wahba MO, Gomaa AF, Khalil SS. The efficacy and safety of direct-acting antiviral drugs in the management of hepatitis C virus-related arthritis. Egyptian Rheumatology and Rehabilitation. 2020;47:1-8. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 145. | Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 146. | Cacoub P. Hepatitis C Virus Infection, a New Modifiable Cardiovascular Risk Factor. Gastroenterology. 2019;156:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 147. | Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 148. | Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, Cammà C. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology. 2016;150:145-155.e4; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 149. | Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 150. | Nevola R, Acierno C, Pafundi PC, Adinolfi LE. Chronic hepatitis C infection induces cardiovascular disease and type 2 diabetes: mechanisms and management. Minerva Med. 2021;112:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 152. | Adinolfi LE, Rinaldi L, Nevola R. Chronic hepatitis C, atherosclerosis and cardiovascular disease: What impact of direct-acting antiviral treatments? World J Gastroenterol. 2018;24:4617-4621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 153. | Zampino R, Marrone A, Rinaldi L, Guerrera B, Nevola R, Boemio A, Iuliano N, Giordano M, Passariello N, Sasso FC, Albano E, Adinolfi LE. Endotoxinemia contributes to steatosis, insulin resistance and atherosclerosis in chronic hepatitis C: the role of pro-inflammatory cytokines and oxidative stress. Infection. 2018;46:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 155. | Onoue K, Saito Y. Hepatitis C Virus and Cardiovascular Disease. Circ J. 2018;82:1503-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 156. | Su X, Zhao X, Deng JL, Li SN, Du X, Dong JZ, Ma CS. Antiviral treatment for hepatitis C is associated with a reduced risk of atherosclerotic cardiovascular outcomes: A systematic review and meta-analysis. J Viral Hepat. 2021;28:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 157. | Sasso FC, Pafundi PC, Caturano A, Galiero R, Vetrano E, Nevola R, Petta S, Fracanzani AL, Coppola C, Di Marco V, Solano A, Lombardi R, Giordano M, Craxi A, Perrella A, Sardu C, Marfella R, Salvatore T, Adinolfi LE, Rinaldi L. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr Metab Cardiovasc Dis. 2021;31:2345-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 158. | Adinolfi LE, Petta S, Fracanzani AL, Coppola C, Narciso V, Nevola R, Rinaldi L, Calvaruso V, Staiano L, Di Marco V, Marrone A, Pafundi PC, Solano A, Lombardi R, Sasso FC, Saturnino M, Rini F, Guerrera B, Troina G, Giordano M, Craxì A. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis. 2020;296:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 159. | Muñoz-Hernández R, Ampuero J, Millán R, Gil-Gómez A, Rojas Á, Macher HC. Hepatitis C Virus Clearance by Direct-Acting Antivirals Agents Improves Endothelial Dysfunction and Subclinical Atherosclerosis: HEPCAR Study. Clin Transl Gastroenterol. 2020;11:e00203. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 160. | Sakai H, Miwa T, Ikoma Y, Hanai T, Nakamura N, Imai K, Kitagawa J, Shirakami Y, Kanemura N, Suetsugu A, Takai K, Shiraki M, Shimizu M. Development of diffuse large B-cell lymphoma after sofosbuvir-ledipasvir treatment for chronic hepatitis C: A case report and literature review. Mol Clin Oncol. 2020;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | Mert D, Merdin A, Ceken S, Dal MS, Ertek M, Altuntas F. Evaluation of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus seroprevalence in patients with diffuse large B cell lymphoma and Hodgkin's lymphoma. J Cancer Res Ther. 2021;17:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 162. | Samonakis DN, Psyllaki M, Pavlaki KI, Drakos E, Kehagias E, Tzardi M, Papadaki HA. Aggressive recurrence of Non-Hodgkin's Lymphoma after successful clearance of hepatitis C virus with direct acting antivirals. Ann Hepatol. 2021;21:100141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 163. | Kim M, Lee YK, Park B, Oh DJ, Choi HG. Hepatitis virus B and C infections are associated with an increased risk of non-Hodgkin lymphoma: A nested case-control study using a national sample cohort. J Med Virol. 2020;92:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 164. | Alkrekshi A, Kassem A, Park C, Tse W. Risk of Non-Hodgkin's Lymphoma in HCV Patients in the United States Between 2013 and 2020: A Population-Based Study. Clin Lymphoma Myeloma Leuk. 2021;21:e832-e838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 165. | Tsai Y-F, Liu Y-C, Yang C-I, Chuang T-M, Ke Y-L, Yeh T-J, et al. Poor Prognosis of Diffuse Large B-Cell Lymphoma with Hepatitis C Infection. Journal of Personalized Medicine. 2021;11: 1-13. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 166. | Sagnelli E, Sagnelli C, Russo A, Pisaturo M, Camaioni C, Astorri R, Coppola N. Impact of DAA-Based Regimens on HCV-Related Extra-Hepatic Damage: A Narrative Review. Adv Exp Med Biol. 2021;1323:115-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 167. | Defrancesco I, Zerbi C, Rattotti S, Merli M, Bruno R, Paulli M, Arcaini L. HCV infection and non-Hodgkin lymphomas: an evolving story. Clin Exp Med. 2020;20:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 168. | Mazzaro C, Maso LD, Visentini M, Ermacora A, Andreone P, Gattei V, et al. Hepatitis C virus‐associated indolent B‐cell lymphomas: A review on the role of the new direct antiviral agents therapy. Hematological Oncology. 2021;39:439-447. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 169. | Zhang M, Gao F, Peng L, Shen L, Zhao P, Ni B, et al. Distinct clinical features and prognostic factors of hepatitis C virus-associated non-Hodgkin’s lymphoma: a systematic review and meta-analysis. Cancer Cell International. 2021;21:1-15. [DOI] [Full Text] |
| 170. | Zaimi Y, Ayari M, Cherifi W, Ksontini FL, Ayadi S, Mabrouk EBH, et al. Primary hepatic large B-cell lymphoma following direct-acting antiviral treatment for hepatitis C. BMJ Open Gastroenterology Hepatology. 2021;8:1-4. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 171. | Amodio P, Salari L, Montagnese S, Schiff S, Neri D, Bianco T, et al. Hepatitis C virus infection and health-related quality of life. World J Gastroenterol. 2012;18:2295-2299. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 172. | Tagliapietra M, Monaco S. Neuroimaging Findings in Chronic Hepatitis C Virus Infection: Correlation with Neurocognitive and Neuropsychiatric Manifestations. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 173. | Faccioli J, Nardelli S, Gioia S, Riggio O, Ridola L. Neurological and psychiatric effects of hepatitis C virus infection. World J Gastroenterol. 2021;27:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (3)] |
| 174. | Abrantes J, Torres DS, Brandão-Mello CE. Patients with Hepatitis C Infection and Normal Liver Function: A Neuropsychological and Neurophysiological Assessment of Cognitive Functions. Int J Hepatol. 2021;2021:8823676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 175. | Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 176. | Moretti R, Gazzin S, Crocè LS, Baso B, Masutti F, Bedogni G, Tiribelli C. Rapid identification system of frontal dysfunction in subclinical hepatic encephalopathy. Ann Hepatol. 2016;15:559-567. [PubMed] [DOI] [Full Text] |
| 177. | Salama II, Sami SM, Abdellatif GA, Mohsen A, Rasmy H, Kamel SA, et al. Plasma microRNAs biomarkers in mild cognitive impairment among patients with type 2 diabetes mellitus. PLoS One. 2020;15:e0236453. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 178. | Abrantes J, Torres DS, de Mello CE. Patients with hepatitis C infection and normal liver function: an evaluation of cognitive function. Postgrad Med J. 2013;89:433-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 179. | Adinolfi LE, Nevola R, Lus G, Restivo L, Guerrera B, Romano C, Zampino R, Rinaldi L, Sellitto A, Giordano M, Marrone A. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21:2269-2280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 180. | Monaco S, Ferrari S, Gajofatto A, Zanusso G, Mariotto S. HCV-related nervous system disorders. Clin Dev Immunol. 2012;2012:236148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 181. | Huckans M, Fuller BE, Olavarria H, Sasaki AW, Chang M, Flora KD, et al. Multi-analyte profile analysis of plasma immune proteins: altered expression of peripheral immune factors is associated with neuropsychiatric symptom severity in adults with and without chronic hepatitis C virus infection. Brain Behav. 2014;4:123-142. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 182. | Yeoh SW, Holmes ACN, Saling MM, Everall IP, Nicoll AJ. Depression, fatigue and neurocognitive deficits in chronic hepatitis C. Hepatol Int. 2018;12:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 183. | Weissenborn K, Ennen JC, Bokemeyer M, Ahl B, Wurster U, Tillmann H, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55:1624-1630. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 184. | Boscarino JA, Lu M, Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, Schmidt MA, Vijayadeva V, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: the Chronic Hepatitis Cohort Study (CHeCS). Hepatology. 2015;61:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 185. | Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. 2013;54:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 186. | Zacks S, Beavers K, Theodore D, Dougherty K, Batey B, Shumaker J, et al. Social stigmatization and hepatitis C virus infection. J Clin Gastroenterol. 2006;40:220-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 187. | Golden J, O'Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 188. | Machado MO, Oriolo G, Bortolato B, Köhler CA, Maes M, Solmi M, Grande I, Martín-Santos R, Vieta E, Carvalho AF. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J Affect Disord. 2017;209:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 189. | Bell AM, Wagner JL, Barber KE, Stover KR. Elbasvir/grazoprevir:a review of the latest agent in the fight against hepatitis C. Int J Hepatol. 2016;2016:3852126. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 190. | Bertino G, Ardiri A, Proiti M, Rigano G, Frazzetto E, Demma S, Ruggeri MI, Scuderi L, Malaguarnera G, Bertino N, Rapisarda V, Di Carlo I, Toro A, Salomone F, Malaguarnera M, Bertino E. Chronic hepatitis C: This and the new era of treatment. World J Hepatol. 2016;8:92-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 191. | Greig SL. Sofosbuvir/Velpatasvir: A Review in Chronic Hepatitis C. Drugs. 2016;76:1567-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 192. | Younossi ZM, Stepanova M, Feld J, Zeuzem S, Sulkowski M, Foster GR, Mangia A, Charlton M, O'Leary JG, Curry MP, Nader F, Henry L, Hunt S. Sofosbuvir and Velpatasvir Combination Improves Patient-reported Outcomes for Patients With HCV Infection, Without or With Compensated or Decompensated Cirrhosis. Clin Gastroenterol Hepatol. 2017;15:421-430.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 193. | Nardelli S, Riggio O, Rosati D, Gioia S, Farcomeni A, Ridola L. Hepatitis C virus eradication with directly acting antivirals improves health-related quality of life and psychological symptoms. World J Gastroenterol. 2019;25:6928-6938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 194. | Younossi ZM, Stepanova M, Feld J, Zeuzem S, Jacobson I, Agarwal K, Hezode C, Nader F, Henry L, Hunt S. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: Results from ASTRAL-1 placebo-controlled trial. J Hepatol. 2016;65:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 195. | Kleefeld F, Heller S, Ingiliz P, Jessen H, Petersen A, Kopp U, Kraft A, Hahn K. Interferon-free therapy in hepatitis C virus (HCV) monoinfected and HCV/HIV coinfected patients: effect on cognitive function, fatigue, and mental health. J Neurovirol. 2018;24:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 196. | Younossi ZM, Stepanova M, Henry L, Nader F, Hunt S. An In-Depth Analysis of Patient-Reported Outcomes in Patients With Chronic Hepatitis C Treated With Different Anti-Viral Regimens. Am J Gastroenterol. 2016;111:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 197. | Ridola L, Riggio O, Gioia S, Faccioli J, Nardelli S. Clinical management of type C hepatic encephalopathy. United European Gastroenterol J. 2020;8:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |