Published online May 27, 2022. doi: 10.4254/wjh.v14.i5.984
Peer-review started: April 4, 2021
First decision: July 6, 2021
Revised: August 6, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: May 27, 2022
Processing time: 414 Days and 16.3 Hours
The utility of hepatitis C virus (HCV) organs has increased after the Food and Drug Administration approval of direct acting anti-viral (DAA) medications for the HCV treatment. The efficacy of DAA in treating HCV is nearly 100%.
To analyze the United Network for Organ Sharing (UNOS) database to compare the survival rates between the hepatitis C positive donors and negative recipients and hepatitis C negative donors and recipients.
We analyzed the adult patients in UNOS database who underwent deceased donor liver transplant from January 2014 to December 2017. The primary endpoint was to compare the survival rates among the four groups with different hepatitis C donor and recipient status: (Group 1) Both donor and recipient negative for HCV (Group 2) Negative donor and positive recipient for HCV (Group 3) Positive donor and negative recipient for HCV (Group 4) Both positive donor and recipient for HCV. SAS 9.4 software was used for the data analysis. Kaplan Meier log rank test was used to analyze the estimated survival rates among the four groups.
A total of 24512 patients were included: Group 1: 16436, Group 2: 6174, Group 3: 253 and Group 4: 1649. The 1-year (Group 1: 91.8%, Group 2: 92.12%, Group 3: 87%, Group 4: 92.8%), 2-year (Group 1: 88.4%, Group 2: 88.1%, Group 3: 84.3%, Group 4: 87.5%), 3-year (Group 1: 84.9%, Group 2: 84.3%, Group 3: 75.9%, Group 4: 83.2%) survival rates showed no statistical significance among the four groups. Kaplan Meier log rank test did not show any statistical significance difference in the estimated survival rates between Group 3 vs all the other groups.
The survival rates in hepatitis C positive donors and negative recipients are similar as compared to both hepatitis C negative donors and recipients. This could be due to the use of DAA therapy with cure rates of nearly 100%. This study supports the use of hepatitis C positive organs in the selected group of recipients with and without HCV infection. Further long-term studies are needed to further validate these findings.
Core Tip: Due to the limited availability of donor organs and high mortality rate on the transplant waiting list, newer strategies are needed. Use of direct acting anti-viral agents have led to high success rates for hepatitis C virus (HCV) treatment. Our study shows, the survival rates in hepatitis C positive donors and negative recipients are similar as compared to both hepatitis C negative donors and recipients. This study supports the use of hepatitis C positive organs in the selected group of recipients with and without HCV infection.
- Citation: Dhaliwal A, Dhindsa B, Ramai D, Sayles H, Chandan S, Rangray R. Impact of utilization of hepatitis C positive organs in liver transplant: Analysis of united network for organ sharing database. World J Hepatol 2022; 14(5): 984-991
- URL: https://www.wjgnet.com/1948-5182/full/v14/i5/984.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i5.984
In the United States, there has been increase in the number of cases in need for liver transplantations (LT) in the last decade while the availability of organs is unchanged[1]. The major contributing factor to this is limited availability of donor liver. Due to persistence of viable organ shortage in United States, it is of utmost importance that all transplantable organs are utilized to their maximum potential[2]. With the advent of direct acting anti-viral (DAA) therapy, the rate of cure of hepatitis C virus (HCV) has increased dramatically. This has been reflected by nonalcoholic fatty liver disease and alcoholic liver disease overtaking HCV as the leading cause for LT in the United States[1,2]. The rates of HCV-positive waitlisted patients and HCV-positive LT recipients have decreased by 8.2% and 7.6% respectively between 2006 and 2016[1].
Prior to approval of DAA therapy, recurrence of HCV after LT was the most common cause of graft failure and reduced recipient survival in those for were HCV positive as compared to HCV negative patients[3,4]. This recurrence of HCV greatly influenced the allocation of HCV positive donors leading to severe under-utilization of these organs, especially in HCV negative recipients[5-8]. Development of newer generation DAAs have resulted in interferon free regimens with high sustained virologic response (SVR) rates post LT[9-12].
These newer generation DAAs have high potency and low adverse event rates leading to increase in inclination for utilizing HCV positive donor organs, including those with high viral load. However, concerns exist regarding these medications as 5% of the patients have failure to achieve SVR after undergoing DAA therapy. This puts the patients at risk for developing chronic HCV or cholestatic hepatitis with increased possibility of need of re-transplantation.
The aim of this study is perform a comparative analysis on odds of survival between the HCV positive donors and negative recipients as compared to HCV negative donors and recipients, using United Network for Organ Sharing (UNOS) database.
We obtained data from the UNOS registry which contains data on all transplantations in the United States. Analysis was limited to records from April 1, 2014 or later, where both recipient and donor were at least 18 years or age, and HCV status was recorded for both recipient and donor. Some recipients appeared in the data set multiple times, but analysis for this study was limited to the first transplant recorded for each patient using a data element which recorded the number of previous transplants for each recipient. The remaining observations were classified into four groups based upon the HCV status of both donors and recipients: (Group 1) both donor and recipient negative, (Group 2) negative donor and positive recipient, (Group 3) positive donor and negative recipient, and (Group 4) both donor and recipient positive.
Descriptive statistics for each of the four groups, including demographic and clinical characteristics for both recipients and donors and causes of death were calculated using means and standard deviations for continuous measures and counts and percentages for categorical measures. The primary outcome was overall survival time with death indicated using the composite death indicator and censoring for those who did not die during the study period occurring at the date of last patient follow-up with the latest patient follow-up taking place on September 7, 2018. Note that all surviving patients were not censored at this date, this is just the latest date a patient was observed. Comparisons of overall survival between groups were made using log-rank tests and estimates of group survival at various time points following transplant. All analysis was completed using SAS v9.4 (The SAS Institute, Cary, NC).
We included a total of 24512 transplants in our analysis. Group 1 and 2 formed the majority of the transplants with 67.05% and 25.19% respectively. Group 3 consisted of 1.03% of transplants with 253 patients undergoing positive donor and negative recipient transplants while the remaining 6.73% were in Group 4. The percentages of males in the four groups were 62.87, 75.92, 66.80 and 76.53, respectively. The mean age (years) of recipients was comparable: Group 1 (54.66 ± 11.63), Group 2 (59.08 ± 6.82), Group 3 (56.13 ± 10.9) and Group 4 (58.92 ± 6.76). The mean age of donors was comparable as well: Group 1 (42.72 ± 15.60), Group 2 (42.96 ± 15.09), Group 3 (40.00 ± 12.41) and Group 4 (37.71 ± 11.82).
Cold ischemia times (hours) were similar in all the groups Group 1 (6.07 ± 2.23), Group 2 (6.20 ± 2.46), Group 3 (6.45 ± 2.03) and Group 4 (6.15 ± 2.51). The mean body mass index of the recipients were evenly matched as well: Group 1 (29.1 ± 6.12), Group 2 (28.67 ± 5.38), Group 3 (28.92 ± 6.08) and Group 4 (28.92 ± 6.08). The mean model for end-stage liver disease (MELD) scores were also similar in all groups: Group 1 (25.22 ± 10.86), Group 2 (19.41 ± 11.26), Group 3 (21.74 ± 8.39) and Group 4 (18.24 ± 7.95).
Anoxia was the most common cause of death (37.89%) followed by stroke (31.31%) and head trauma (28.4%). Majority of the transplants in all groups were whole LT: Group 1 (99.05%), Group 2 (99.14%), Group 3 (99.60%) and Group 4 (99.94%). These demographics can be seen in Tables 1 and 2. The most common primary diagnosis at listing was alcoholic cirrhosis/acute alcoholic hepatitis (26.26%) followed by HCV cirrhosis (20.92%), non-alcoholic steatohepatitis cirrhosis (15.36%) and hepatoma (12.28%). These can be seen in Table 3.
| Mean age-donor (yr) | Mean age-recipient (yr) | Males (%) | BMI-recipient | Cold ischemia time | MELD | |
| Group 1 | 42.72 ± 15.60 | 54.66 ± 11.63 | 62.87 | 29.1 ± 6.12 | 6.07 ± 2.23 | 25.22 ± 10.86 |
| Group 2 | 42.96 ± 15.09 | 59.08 ± 6.82 | 75.92 | 28.67 ± 5.38 | 6.20 ± 2.46 | 19.41 ± 11.26 |
| Group 3 | 40.00 ± 12.41 | 56.13 ± 10.9 | 66.8 | 28.92 ± 6.08 | 6.45 ± 2.03 | 21.74 ± 8.39 |
| Group 4 | 37.71 ± 11.82 | 58.92 ± 6.76 | 76.53 | 28.92 ± 6.08 | 6.15 ± 2.51 | 18.24 ± 7.95 |
| Donor cause of death | Type of liver procedure | |||||||
| Anoxia | Stroke | Head trauma | CNS tumor | Other | Whole | Partial | Split | |
| Group 1 | 5832 | 5360 | 4844 | 64 | 336 | 16280 | 5 | 151 |
| Group 2 | 2196 | 2007 | 1826 | 25 | 120 | 6121 | 2 | 51 |
| Group 3 | 182 | 33 | 33 | 0 | 5 | 252 | 0 | 1 |
| Group 4 | 1079 | 276 | 259 | 0 | 35 | 1648 | 1 | 0 |
| Primary diagnosis at listing | No. of patients | Percent |
| Alcoholic cirrhosis/acute alcoholic hepatitis | 6434 | 26.25 |
| Hepatitis C cirrhosis | 5128 | 20.92 |
| Non-alcoholic steatohepatitis cirrhosis | 3764 | 15.36 |
| Hepatoma | 3010 | 12.28 |
| Cryptogenic (idiopathic) cirrhosis | 1082 | 4.41 |
| Primary sclerosing cholangitis | 941 | 3.84 |
| Acute hepatic necrosis | 696 | 2.84 |
| Autoimmune cirrhosis | 650 | 2.65 |
| Primary biliary cirrhosis | 582 | 2.37 |
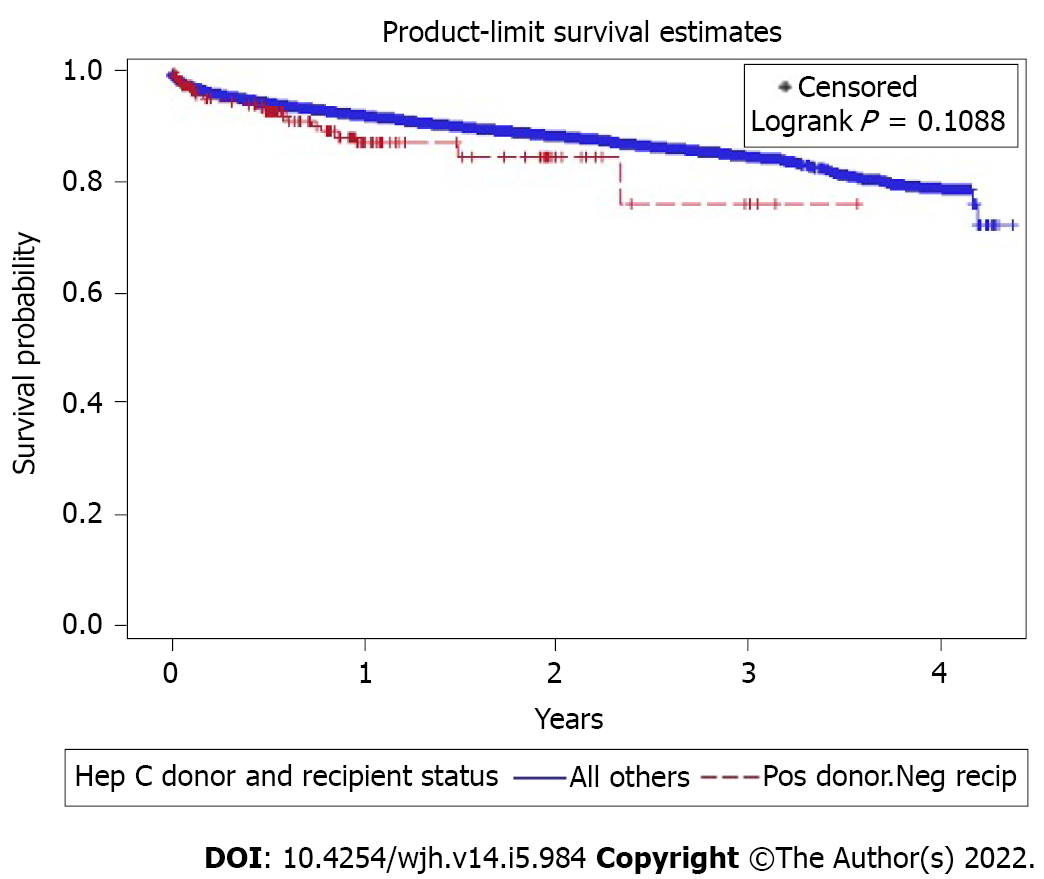
A log-rank test for survival differences between the four groups did not show any significance (P = 0.46). Observation of survival rates at 1-year (Group 1: 91.81%, Group 2: 92.13%, Group 3: 87.01%, Group 4: 92.89%), 2-year (Group 1: 88.4%, Group 2: 88.1%, Group 3: 84.3%, Group 4: 87.5%), 3-year (Group 1: 84.9%, Group 2: 84.3%, Group 3: 75.9%, Group 4: 83.2%) found that survival rates for Group 3 were lower at each point than the other three groups, which were all relatively close together. However, a second long-rank test comparing Group 3 vs all the other groups was also not significant (P = 0.11, Figure 1).
Despite the increasing number of LT over the years, the need for organ donors continues to outpace the availability of organs with estimated waitlist mortality of 20%[13].
In the United States, since 2000, the increased mortality related to the catastrophic opioid epidemic presented an opportunity to recruit more organ donors. However, the enthusiasm was curtailed by a relatively higher prevalence of HCV when compared to the general population[14]. Initially, the organs from HCV+ donors were primarily reserved for HCV+ recipients or those suffering from fulminant hepatic failure[15]. But there is still a reticence to use organs from HCV+ donors in HCV- recipients because of clinical and ethical considerations.
One of the biggest barriers to use HCV+ donor organs is the concern for increased risk of post-transplant HCV transmission. Another concern with the use of HCV+ donor liver grafts is because of the limited literature on post-transplantation outcomes. Lai et al[16] studying 99 recipients of HCV+ donor liver grafts demonstrated significantly higher unadjusted 1-year and 3-year rates of advanced fibrosis for recipients of HCV+ donor grafts (14% and 48%) vs HCV- donor grafts (7% and 33%, P = 0.01)[16]. Khapra et al[17] studying 29 recipients of HCV+ donor liver grafts showed significantly more fibrosis and a faster rate of progression compared with recipients of HCV- donor liver grafts[17]. Interestingly, when the analysis was stratified to mean donor age, both the studies showed poorer outcomes with older donors of age greater than 45 years and 50 years respectively[16,17]. But it must be noted that these studies were done in an era when antiviral therapy was initiated at later stages of fibrosis and there was low response to these regimens. At this time, more studies with longer follow up are needed to see if similar rates of fibrosis progression occur with early administration of antiviral therapy post-transplantation.
In terms of long-term graft and patient survival with HCV+ organs, the current literature is controversial depending on the type and success rate of HCV treatment regimens. With the advent of DAAs, recent studies from single-center experiences and large population-based databases such as UNOS and scientific registry of transplant recipients studying recipients with HCV+ and HCV- liver donors showed similar outcomes between the groups which are encouraging[18-20]. But it must be highlighted as there is no standardized protocol currently, these studies included recipients initiated on preemptive or prophylactic or both the treatment regimens.
With increasing wait-list mortality, higher mortality in patients with higher MELD (> 35 scores), and newer DAAs medications reaching clinical efficacy of nearly 96% it may be worth considering offering HCV+ donor organs to selected HCV- recipients where the risk-benefit outweighs the ethical considerations[21].
The data included in our study is from a large population-based study from an accepted UNOS database which includes a heterogeneous population from across the United States. Our study demonstrates that there is no statistically significant difference in the survival rates in HCV positive donors and negative recipients and HCV negative donors and recipients. In contrast to prior studies, which looked at the short-term outcomes such as survival rate at discharge, rejection rate prior to discharge, and 1-year mortality: Ours is the first study that shows that the survival rates were comparable in all group up to 3 years. Furthermore, the mean MELD score of the recipients in the group 3 was 21, suggesting that HCV+ donors can be used in recipients with high MELD score. Moreover, HCV positive organs were utilized in complicated cases with prolonged ischemia times and blood loss, showing tolerance to ischemia. This study supports the feasibility of expanding the general donor pool via the utilization of HCV positive organs for both HCV positive and negative recipients.
Due to the limitations of the data, we could not evaluate the reduction in time from listing to transplant, we can conclude that addition of HCV positive donor should decrease the organ shortage and reduce morbidity and mortality of all patients on the transplant list.
Although the outcomes of using hepatitis C positive donors are encouraging, there are some limitations of our study. The number of patients in group 3 were less, 253 out of total 24512 patient (1.03%). Even though we had estimates of survival rates up to 3 years (and longer for a small set of patients), long-term outcomes are still unknown. Additionally, it is still not clear whether preemptive or prophylactic HCV regimens are needed, and the length of HCV surveillance needed post-LT. Though the mean age of the donors were comparable in all groups, the medical co-morbidities of donors were unknown. In contrast to the study by Ballarin et al[22] this study was limited by the paucity of data on viral load and graft histology of both donors and recipients which may influence post LT outcomes. We also did not have information regarding the treatment status of HCV positive donors.
In summary, due to the mismatch in need and availability of donor organs and high mortality rate on the transplant waiting list, newer strategies are needed. DAA regimens have proved to be highly effective to treat HCV, even post-transplant and immunosuppressed patients. Effectiveness of these regimens can be exemplified by similar survival rates of HCV positive donor and negative recipients in comparison to HCV negative donors and recipients. Our study shows that HCV positive organs can be transplanted to recipients, irrespective of their HCV status. However, more studies are needed to confirm our findings.
Due to the mismatch in need and availability of donor organs and high mortality rate on the transplant waiting list, newer strategies are needed. In the era prior to direct acting anti-virals (DAAs), recurrence of hepatitis C virus (HCV) in post liver transplantations (LT) patients leading to decreased graft survival had greatly influenced the under-utilization of these organs, especially in HCV negative recipients. With the high sustained virologic response rate with DAAs both pre and post LT, this pool of organs can be utilized leading to more organ availability and decreased in mortality rate on transplant.
There is limited data evaluating outcomes of hepatitis C positive donor organs to HCV negative recipients in LT.
The aim of this study is perform a comparative analysis on odds of survival between the HCV positive donors and negative recipients as compared to HCV negative donors and recipients United Network for Organ Sharing (UNOS) database.
We included patients in UNOS database who underwent deceased donor LT over a period of three year. Data analysis was performed using SAS 9.4 software. Survival rates amongst groups were analyzed with help of Kaplan Meier log rank test.
Our study shows similar chance of survival of recipient, irrespective of HCV status of the donor.
Our study shows that HCV positive organs can be transplanted to recipients, irrespective of their HCV status due to the advent of DAA regimen.
More studies are needed to confirm findings of this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M, India; Maslennikov R, Russia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18 Suppl 1:172-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 2. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 3. | Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 829] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 4. | Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, Detre K, Hoofnagle J. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 428] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Gordon SC, Muir AJ, Lim JK, Pearlman B, Argo CK, Ramani A, Maliakkal B, Alam I, Stewart TG, Vainorius M, Peter J, Nelson DR, Fried MW, Reddy KR; HCV-TARGET study group. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol. 2015;62:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 7. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 8. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Scott J, Sinha R, Beumont-Mauviel M. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 9. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O'Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 633] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 10. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, Agarwal K, Angus P, Yoshida EM, Colombo M, Rizzetto M, Dvory-Sobol H, Denning J, Arterburn S, Pang PS, Brainard D, McHutchison JG, Dufour JF, Van Vlierberghe H, van Hoek B, Forns X; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 11. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 12. | Reau N, Kwo PY, Rhee S, Brown RS Jr, Agarwal K, Angus P, Gane E, Kao JH, Mantry PS, Mutimer D, Reddy KR, Tran TT, Hu YB, Gulati A, Krishnan P, Dumas EO, Porcalla A, Shulman NS, Liu W, Samanta S, Trinh R, Forns X. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology. 2018;68:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | NDOPaT. Network NDOPaT. 2018. [cited 25 March 2021]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. |
| 14. | Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 541] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 15. | Selzner N, Berenguer M. Should organs from hepatitis C-positive donors be used in hepatitis C-negative recipients for liver transplantation? Liver Transpl. 2018;24:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Lai JC, O'Leary JG, Trotter JF, Verna EC, Brown RS Jr, Stravitz RT, Duman JD, Forman LM, Terrault NA; Consortium to Study Health Outcomes in HCV Liver Transplant Recipients (CRUSH-C). Risk of advanced fibrosis with grafts from hepatitis C antibody-positive donors: a multicenter cohort study. Liver Transpl. 2012;18:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Khapra AP, Agarwal K, Fiel MI, Kontorinis N, Hossain S, Emre S, Schiano TD. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transpl. 2006;12:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Cholankeril G, Li AA, March KL, Yoo ER, Kim D, Snyder H, Gonzalez SA, Younossi ZM, Ahmed A. Improved Outcomes in HCV Patients Following Liver Transplantation During the Era of Direct-Acting Antiviral Agents. Clin Gastroenterol Hepatol. 2018;16:452-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, Pruett TL. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010;23:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Yoshida EM, Lilly LB, Marotta PJ, Mason AL, Bilodeau M, Vaillancourt M. Canadian national retrospective chart review comparing the long term effect of cyclosporine vs. tacrolimus on clinical outcomes in patients with post-liver transplantation hepatitis C virus infection. Ann Hepatol. 2013;12:282-293. [PubMed] |
| 21. | Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, Sulkowski MS, O'Leary JG, Koraishy F, Galati JS, Kuo AA, Vainorius M, Akushevich L, Nelson DR, Fried MW, Terrault N, Reddy KR. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090-1101. [RCA] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Ballarin R, Cucchetti A, Spaggiari M, Montalti R, Di Benedetto F, Nadalin S, Troisi RI, Valmasoni M, Longo C, De Ruvo N, Cautero N, Cillo U, Pinna AD, Burra P, Gerunda GE. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011;91:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |