Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.827
Peer-review started: July 5, 2021
First decision: November 11, 2021
Revised: November 22, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 27, 2022
Processing time: 291 Days and 0.2 Hours
Natriuretic peptides are involved in the cascade of pathophysiological events occurring in liver cirrhosis, counterbalancing vasoconstriction and anti-natriuretic factors. The effects of natriuretic peptides as treatment of cirrhotic ascites have been investigated only in small studies, and definitive results are lacking.
To examine the effects and safety of natriuretic peptides in cirrhosis patients with ascites.
We searched MEDLINE, Web of Science, Scopus, Cochrane Library and Embase for all available studies applying intravenous administration of any natriuretic peptide to patients suffering from cirrhotic ascites. Inclusion was not limited by treatment duration or dose, or by follow-up duration. Both randomised controlled trials and non-randomised studies were eligible for inclusion. The primary outcome was change in renal sodium excretion. Secondary outcomes included safety measures and changes in renal water excretion, plasma aldosterone concentration, and plasma renin activity.
Twenty-two studies were included. Atrial natriuretic peptide (ANP) was the only intensively studied treatment. Sodium excretion increased in response to continuous ANP infusion and was more pronounced when infusion rates of > 30 ng/kg/min were administered compared with ≤ 30 ng/kg/min (P < 0.01). Moreover, natriuresis was significantly higher in study subgroups with mild/moderate ascites compared with moderate/severe and refractory ascites (P < 0.01). ANP infusions increased renal water excretion, although without reaching a statistically significant dose-response gradient. Plasma aldosterone concentration and plasma renin activity were significantly lower at baseline in study subgroups achieving a negative sodium balance in response to an ANP administration compared with treatment non-responders (P < 0.01). Blood pressure decreases occurred less frequently when ANP doses ≤ 30 ng/kg/min were applied. The quality of evidence for a natriuretic response to ANP was low, mainly due to small sample sizes and considerable between-study heterogeneity. Data were sparse for the other natriuretic peptides; B-type natriuretic peptide and urodilatin.
Intravenous ANP infusions increase sodium excretion in patients with cirrhotic ascites. Continuous infusion rates > 30 ng/kg/min are the most effective. However, safety increases with infusion rates ≤ 30 ng/kg/min.
Core Tip: Pharmacotherapies for cirrhotic ascites have remained largely unchanged for the past four decades. 5%-10% of cirrhosis patients with ascites become refractory to available treatments, and the majority of these patients require frequent large-volume paracenteses. This justifies a continued search for new and improved treatments for ascites. This is the first systematic review and meta-analysis to investigate the effects and safety of natriuretic peptides as treatment of cirrhotic ascites. We demonstrate a significant natriuretic effect of intravenously administered atrial natriuretic peptide and summarise the safety findings.
- Citation: Gantzel RH, Kjær MB, Jepsen P, Aagaard NK, Watson H, Gluud LL, Grønbæk H. Effects and safety of natriuretic peptides as treatment of cirrhotic ascites: A systematic review and meta-analysis. World J Hepatol 2022; 14(4): 827-845
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/827.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.827
The cirrhosis-derived health-care burden is growing globally[1]. Patients suffering from liver cirrhosis may develop portal hypertension with substantial neuro-hormonal impacts. Portal hypertension directly induces vasodilation of the splanchnic vascular system with blood pooling in the splanchnic bed. This reduces the central blood volume, leading to arterial hypotension. When these pathophy-siological regulatory events become manifest, vasoconstrictor and anti-natriuretic factors are activated, ultimately resulting in retention of sodium and water[2,3]. Ascites formation is the most common manifestation of decompensation due to cirrhosis with portal hypertension. After the appearance of ascites, patients experience a poor prognosis, frequent hospital contacts, and an impaired quality of life[3,4]. In general, dietary sodium restriction, anti-aldosterone agents, and loop-diuretics are effective treatments to manage ascites[4,5]. However, 5%-10% progress to refractory ascites[6]. Invasive treatments, such as liver transplantation, transjugular intrahepatic portosystemic shunt, or implantation of an automated low-fluid ascites pump (Alfapump®), are restricted to a minor proportion of patients with refractory ascites, while the majority require frequent hospitalizations for large-volume paracentesis[3,4]. New pharmacotherapies to counteract the mechanisms involved in ascites development, as well as to treat established ascites and reduce the need for paracenteses in refractory ascites, are highly warranted.
Atrial natriuretic peptide (ANP) is the most extensively studied member of the natriuretic peptide family, which also comprises B-type natriuretic peptide (BNP) and two active forms of C-type natriuretic peptide (CNP), as well as the renal paracrine peptide urodilatin[7,8]. Urodilatin is identical to ANP except for an N-terminal extension of 4-amino acids, which makes it less vulnerable to degradation by the renal neutral endopeptidase[8,9]. Therefore, the renal effects of urodilatin are stronger than those of ANP[10].
The majority of patients with cirrhotic ascites have marked activation of vasoconstrictor systems[11-14]. Moreover, plasma concentrations of ANP and BNP are elevated in patients with cirrhosis and ascites[15,16], consistent with a physiological attempt to counterbalance vasoconstriction and anti-natriuretic factors. However, in advanced cirrhosis, endogenous natriuretic peptides cannot override the pathophysiological events leading to ascites formation[11].
Earlier studies, that have investigated natriuretic peptides as treatment of cirrhotic ascites, have been too small to provide a definitive result. Thus, a review of the literature is justified. This systematic review and meta-analysis evaluates the benefits and harms of intravenous administration of the natriuretic peptides, ANP, BNP, or urodilatin in patients with liver cirrhosis and ascites. We aim to perform a meta-analysis of available data, especially regarding the effects on natriuresis, to determine the therapeutic potential of natriuretic peptides in cirrhotic ascites. Moreover, we intend to identify patient subpopulations for which treatment with natriuretic peptides may be particularly beneficial.
This systematic review was performed in accordance with the PRISMA guidelines and the Cochrane Handbook[17,18]. The preregistered review protocol (CRD42020195619) is available in PROSPERO.
We included trials evaluating mono-therapy of ANP, BNP, urodilatin, or any synthetic equivalent of these in patients with cirrhosis and ascites. Randomised controlled trials (RCTs) and non-randomised studies, e.g., trials investigating the pharmacodynamics of natriuretic peptides in cirrhosis patients with ascites and dose-finding trials, were considered for inclusion.
Trials were included regardless of dose and duration of the intervention or follow-up. Treatment administration had to be intravenous. As sodium retention is a main characteristic of decompensated cirrhosis, and existing pharmacological treatments aim to increase renal sodium excretion, the primary outcome in this systematic review was the maximum difference in natriuresis from baseline. To further evaluate renal and systemic treatment effects, secondary outcomes comprised the maximum difference in diuresis, plasma osmolality, urine osmolality, plasma creatinine concentration, plasma aldosterone concentration, and plasma renin activity from baseline. Change in bodyweight and waist circumference were included as objective surrogate measures of ascites burden. Non-serious and serious adverse events (AEs) were assessed focusing on the expected risk of blood pressure decreases and hypotension.
Trials were eligible for inclusion regardless of publication status, publication year, or language. The databases MEDLINE, Web of Science, Scopus, Cochrane Library and Embase were searched for eligible publications on the 13th of August 2020 (Supplementary material, page 1). References were managed in the reference sorting tool Covidence® (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia).
Study screening and data extraction to a spreadsheet with predefined variables were performed independently by two reviewers (RHG and MBK). Discrepancies were resolved through discussion before analyses. To characterise study populations, we collected background details including age, gender, cirrhosis aetiology, ascites severity, dietary sodium restriction, and diuretic withdrawal prior to treatment. We extracted data concerning the intervention including the name of the experimental intervention (generic and international non-proprietary name), comparator intervention, duration and dose of treatment, and whether a bolus or continuous infusion was applied. Finally, we collected information on study design, size of the trial, and follow-up duration.
Outcomes measured at baseline, during the intervention and at the end of follow-up were extracted from the text, tables or figures using a measuring tool from Adobe Acrobat 2017 (Adobe Inc., California, United States). The baseline outcome value was selected at the time point closest to the intervention exposure. For data concerning primary and secondary outcomes, the peak response or concentration measured during the intervention was used for trials administering a continuous intervention, while the peak response or concentration measured after the intervention was used for bolus infusion trials. Analyses were made using means and standard deviations (assumptions regarding units and unit conversions are described in Supplementary material, page 2).
Risk of bias assessments were performed at study level using the Cochrane risk of bias tool version 2 (RoB2)[19] for RCTs. An overall risk of bias judgment; low, moderate, or high was independently conducted by RHG and MBK. Non-randomised studies were assessed using the Newcastle-Ottawa-Scale (NOS)[20] (Supplementary material, pages 2 and 3) with a score from 0 (lowest) to 9 (highest). The score was transformed to generate a RoB2-comparable entity; NOS-scores 8-9 were classified as “low”, 5-7 as “moderate”, and ≤ 4 as “high”. The Grading of Recommendations Assessment and Evaluation was used to judge the quality of evidence for the study questions[21].
The effects of natriuretic peptides were summarised as overall peak mean and compared with overall baseline mean. This was performed for all trials and for study subgroups stratified by type of natriuretic peptide and continuous vs bolus infusions. When non-randomised studies outnumbered RCTs, baseline sodium excretion and peak sodium excretion were meta-analysed separately. We were unable to gather data allowing meta-analyses of safety data. Safety outcomes were therefore reviewed without statistical analyses.
The statistical software Stata, version 16.1, (StataCorp Lp, TX, United States) was used for data analyses. We applied a random-effects model with a DerSimonian-Laird tau2 estimator for all meta-analyses. I2 was used as a consistency measure. In situations with high levels of heterogeneity, meta-regressions were performed to elucidate study moderators. Moreover, the reasons for heterogeneity were addressed through subgroup analyses and leave-one-out analyses.
Small study effects were assessed using the Egger regression-based test. Funnel plots were included for graphical illustration of publication bias, while Trim-and-fill analysis were performed to estimate and account for this.
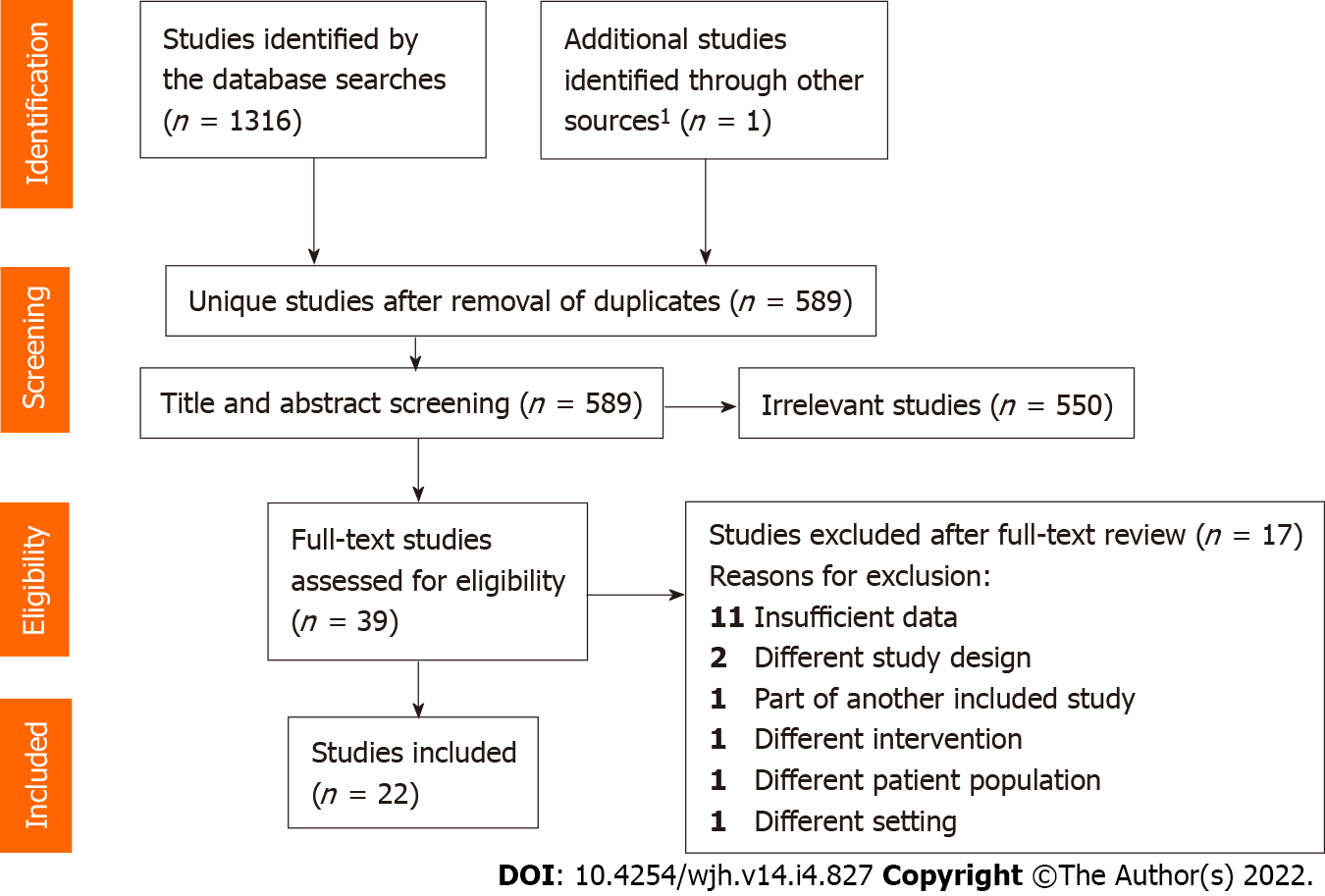
The literature search identified a total of 589 unique references. Ultimately, 22 publications describing 22 studies were included for data extraction (Figure 1)[17] covering 34 subgroups eligible for review and analyses (Table 1). ANP was studied in 19 studies (215 patients)[11-15,22-35], BNP in one study (seven patients)[16], and urodilatin in two studies (21 patients)[36,37].
| Ref. | Country | Study type | Patient n | Drug | Comparator | Adm | Dose (ng/kg/min or ng/kg) | Duration (min) | Post-infusion follow-up (min) | Age (mean) | Male (%) | Ascites severity: Mild/moderate/ severe/refractory | S- or P-sodium (mmol/L) mean ± SE | Quality assessments (risk of bias) | |
| NOS | RoB2 | ||||||||||||||
| Abraham et al[31], 1995 | United States | Non-random | 6 | ANP | None | Cont | 150 | 180 | 180 | 44.5 | 67 | 6/0/0/0 | NR | Low | - |
| 6 | ANP | None | Cont | 150 | 180 | 180 | 49.5 | 100 | 1/3/2/0 | ||||||
| Ando[32], 1991 | Japan | Non-random | 10 | ANP | None | Bolus | 1000 | NA | 90 | 57.1 | 80 | 0/5/5/0 | 138.3 ± 0.7 | Low | - |
| 6 | ANP | None | Bolus | 1000 | NA | 90 | 58.7 | 67 | 0/3/3/0 | 134.2 ± 0.2 | |||||
| Badalamenti et al[33], 1992 | Italy | Non-random | 9 | ANP | None | Bolus | 1000 | NA | 90 | 57.8 | 78 | 0/5/4/0 | 134.7 ± 1.6 | Low | - |
| 4 | ANP | None | Cont | 20 | 60 | 0 | 63.3 | 75 | 0/3/1/0 | Moderate | |||||
| Carstens et al[36], 1998 | Denmark | RCT | 15 | Urodilatin | Placebo | Cont | 20 | 60 | 60 | 50.6 | 60 | 15/0/0/0 | 137.0 ± 0.5 | - | Low |
| Carstens et al[37], 2007 | Denmark | RCT | 6 | Urodilatin | Placebo | Cont | 20 | 90 | 90 | 54.0 | 67 | 0/0/0/6 | 133.3 ± 2.0 | - | Low |
| Ferrier et al[14], 1989 | Switzerland | Non-random | 7 | ANP | None | Cont | 36 | 60 | 60 | 54.3 | 57 | 0/7/0/0 | NR | Moderate | - |
| Fried et al[34], 1990 | United States/Switzerland | RCT | 11 | ANP | Placebo | Cont | 15 | 120 | 90 | NR | 82 | 11/0/0/0 | NR | - | Moderate |
| RCT | 8 | ANP | Placebo | Cont | 30 | 120 | 90 | NR | 88 | 8/0/0/0 | |||||
| RCT | 9 | ANP | Placebo | Cont | 60 | 120 | 90 | NR | 89 | 9/0/0/0 | |||||
| Gerbes et al[35], 1988 | Germany | Non-random | 4 | ANP | None | Cont | 50 | 30 | 90 | NR | NR | NR | NR | Moderate | - |
| Ginès et al[22], 1992 | Spain | Non-random | 5 | ANP | None | Cont | 50 | 60 | 0 | 57.0 | 60 | 0/5/0/0 | NR | Moderate | - |
| 11 | ANP | None | Cont | 50 | 60 | 0 | 73 | 0/11/0/0 | |||||||
| Heim et al[23], 1990 | Germany | Non-random | 8 | ANP | None | Bolus | 500 | NA | 60 | 54.5 | 75 | 0/8/0/0 | NR | Low | - |
| Jespersen et al[12], 1995 | Denmark | Non-random | 9 | ANP | None | Bolus | 2000 | NA | 120 | 49.0 | 89 | 0/7/2/0 | NR | Low | - |
| Laffi et al[15], 1989 | Italy | Non-random | 8 | ANP | None | Bolus | 1000 | NA | 900 | 56.4 | 75 | 0/0/0/8 | 131.8 ± 2.6 | Low | - |
| Laffi et al[24], 1989 | Italy | Non-random | 5 | ANP | None | Cont | 100 | 45 | 75 | 58.0 | 60 | 0/5/0/0 | NR | Low | - |
| 4 | ANP | None | Cont | 100 | 45 | 75 | 56.4 | 25 | 0/4/0/0 | ||||||
| 6 | ANP | None | Cont | 100 | 45 | 75 | 54.0 | 83 | 0/6/0/0 | ||||||
| La Villa et al[16], 1995 | Italy | Non-random | 7 | BNP | None | Cont | 13.861 | 60 | 60 | 56.0 | 57 | 0/7/0/0 | 135.0 ± 1.0 | Low | - |
| Legault et al[25], 1993 | Canada | Non-random | 5 | ANP | None | Cont | 15 | 120 | 120 | NR | 80 | 5/0/0/0 | 139.0 ± 2.0 | Low | - |
| 7 | ANP | None | Cont | 15 | 120 | 120 | NR | 71 | 7/0/0/0 | 133.0 ± 1.0 | |||||
| Miyase et al[26], 1990 | Japan | Non-random | 6 | ANP | None | Bolus | 500 | NA | 90 | 58.0 | NR | 6/0/0/0 | NR | Low | - |
| Morali et al[13], 1991 | Canada | Non-random | 5 | ANP | None | Cont | 15 | 120 | 60 | 54.0 | 80 | 5/0/0/0 | 133.0 ± 1.0 | Low | - |
| 12 | ANP | None | Cont | 15 | 120 | 60 | 53.0 | 67 | 0/0/0/12 | 138.0 ± 1.0 | |||||
| Morali et al[11], 1992 | Canada | Non-random | 6 | ANP | None | Cont | 15 | 120 | 0 | 56.8 | 100 | 0/0/0/6 | NR | Low | - |
| 4 | ANP | None | Cont | 15 | 120 | 0 | 64.3 | 100 | 0/0/0/4 | ||||||
| Salerno et al[27], 1988 | Italy | Non-random | 7 | ANP | Placebo | Bolus | 1000 | NA | 60 | 56.6 | 100 | 0/2/5/0 | NR | Low | - |
| Tobe et al[28], 1993 | Canada | Non-random | 8 | ANP | None | Cont | 15 | 120 | 0 | 52.4 | 100 | 8/0/0/0 | NR | Low | - |
| Tobe et al[29], 1993 | Canada | Non-random | 6 | ANP | None | Cont | 15 | 120 | 0 | 58.0 | 67 | 0/0/0/6 | NR | Low | - |
| Wong et al[30], 1993 | Canada | RCT | 3 | ANP | Albumin | Cont | 15 | 120 | 60 | 58.0 | 67 | 3/0/0/0 | 135.0 ± 5.0 | - | Low |
| 10 | ANP | Albumin | Cont | 15 | 120 | 60 | 54.0 | 70 | 0/0/0/10 | 134.0 ± 1.0 | |||||
The 22 included studies were conducted in eight countries and published between 1988 and 2007. Eighteen studies were non-randomised without a comparator, while four were cross-over RCTs using either placebo (3) or albumin (1) as a comparator. As the majority of included trials were non-randomised studies, we were unable to perform paired comparisons to compute effect sizes in the subsequent data analysis, except for urodilatin which was assessed in two RCTs. Fifteen studies investigated a continuous drug infusion, six studies applied a bolus injection, and one study assessed both a continuous infusion and a bolus injection (Table 1).
We classified ascites as mild, moderate, severe, or refractory. In a few situations, necessary assumptions were made regarding treatment dose and ascites severity to permit comparability (Table 1 and Supplementary material, pages 3 and 4).
Two hundred and forty-three patients with cirrhosis and ascites were treated with intravenous natriuretic peptide (Table 1). The overall ratio of male and female participants was 3:1. Mean age ranged between 44.5 and 64.3 years (median 56.2 years). Ascites severity was reported in 21 of the included studies, covering 239 participants. Eighty-four (35%) had mild ascites, 81 (34%) had moderate ascites, 22 (9%) had severe/tense ascites, and 52 (22%) had refractory ascites. Only one study did not withdraw loop-diuretics and anti-aldosterone agents prior to the intervention[23]. The time from discontinuation of diuretics until intervention in the remaining 21 studies ranged between one day and 14 d (median 7 d). Finally, a sodium restrictive diet was applied before the intervention in 18 studies ranging from 15 to 150 mmol/d (median 20 mmol/d). The duration of continuous infusions ranged from 30 to 180 min (median 120 min) with post-infusion follow-up period ranging from 0 to 180 min (median 60 min). Post-bolus follow-up ranged from 60 to 900 min (median 90 min). For studies administering a continuous infusion, the time point for peak natriuresis in general coincided with the treatment duration. For studies using a bolus injection, a peak response after 30 min was most commonly observed.
We found a substantial inter-study variation in definitions of responder and non-responder subgroups. As the majority of included trials introduced dietary sodium restriction prior to intervention, we redefined treatment responsiveness as the achievement of a negative sodium balance during or after the intervention.
One study investigated the natriuretic effect of continuous BNP infusion. No significant change in natriuresis was observed, nor any effect on diuresis and blood pressure[16]. Therefore, due to the lack of effect on natriuresis, BNP did not receive further attention in our analyses.
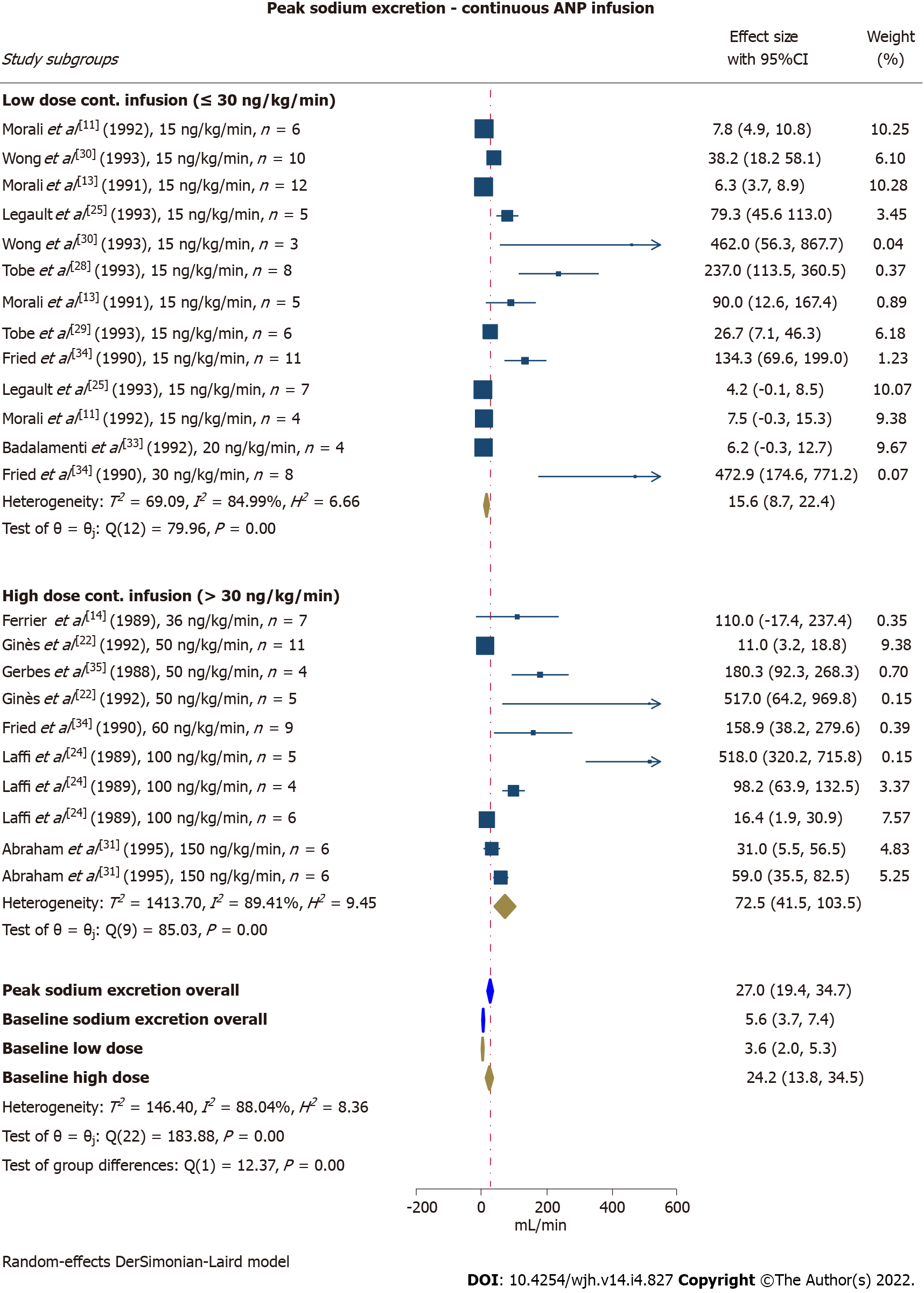
Continuous infusion of ANP increased sodium excretion from 5.6 μmol/min [95% confidence interval (CI): 3.7-7.4] pre-intervention (baseline) to 27.0 μmol/min (95%CI: 41.5-103.5) at peak response. Interestingly, the peak response was significantly higher in study subgroups receiving an infusion rate of > 30 ng/kg/min compared with ≤ 30 ng/kg/min (P < 0.01) (Figure 2). The between-study heterogeneity was considerable (peak I2= 88%, baseline I2= 89%). Leave-one-out analyses did not recognise a single study responsible for the large I2. To search for interactions to explain the heterogeneity we performed subgroup analyses exploring the effects of infusion duration, ascites severity, and dietary sodium restriction. No statistically significant differences were observed between subgroups exposed to short-term or long-term infusions (threshold at 60 min) (P = 0.07), nor between subgroups with a marked or moderate restriction of dietary sodium intake (threshold at 20 mmol/d) (P = 0.11). The baseline and peak sodium excretion were higher in subgroups with mild/moderate ascites compared with moderate/severe and refractory ascites (P < 0.01). Meta-regression found that the effect size was moderated by treatment dose and ascites severity, although adjusting for these parameters had only limited influence on the overall I2. Effect size was not affected by quality assessment score, mean age, dietary sodium intake, and treatment duration. Study subgroups exposed to a bolus ANP injection were heterogeneous (peak I2= 94%, baseline I2= 93%) affecting the interpretability of the results. However, overall sodium excretion increased marginally (Supplementary Figure 1). Leave-one-out analyses did not identify any single study responsible for the heterogeneity. As only seven studies (covering eight study subgroups) applied a bolus injection, subgroup analyses and meta-regression were not justified. However, we performed a sensitivity analysis excluding the single study that did not withdraw diuretics prior to ANP injection. This manoeuvre slightly lowered the baseline and peak natriuresis.
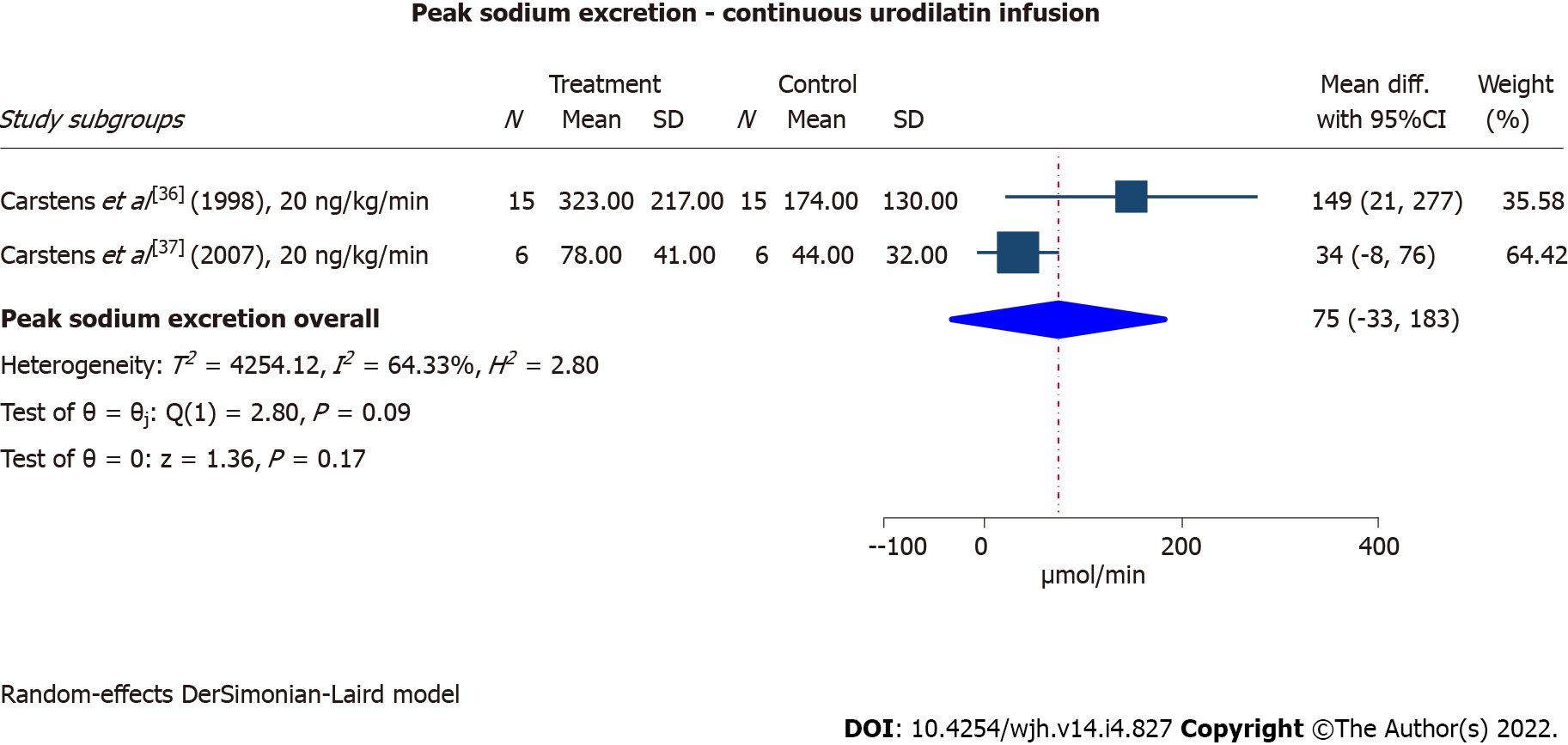
Two cross-over RCTs investigated the natriuretic effect of urodilatin in cirrhosis patients with ascites[36,37], originating from the same study site. The patients in the earliest and larger trial had mild/moderate ascites and received 60 min infusion with 20 ng/kg/min, while the patients in the latest trial had refractory ascites and received 90 min of infusion with 20 ng/kg/min. Both studies reported a significant increase in sodium excretion as a response to the treatment compared with placebo, although the response was most pronounced in the larger trial investigating cirrhosis patients with mild/moderate ascites (Figure 3). The results suffered from substantial heterogeneity (I2= 64%).
A summary of the findings with quality of evidence are listed in Table 2. For natriuresis, the primary outcome of this systematic review, the quality of evidence was assessed as low for ANP and very low for urodilatin. These conclusions were primarily based on few RCTs being available, resulting in the dominance of non-randomised studies contributing to considerable heterogeneity, inconsistency, and imprecision due to small sample sizes.
| Outcomes | Anticipated absolute effects (95%CI) | No of participants (study subgroups) | Publication bias | Quality of evidence (GRADE) | Comments | |||
| Baseline | Peak | Egger test | Trim-and-fill1 | |||||
| Baseline | Peak | |||||||
| Atrial natriuretic peptide | ||||||||
| Natriuresis (µmol/min) | Low2,3 | |||||||
| Continuous infusion | 5.6 (3.7-7.4) | 27.0 (19.4-34.7) | 152 (23) | P < 0.0001 | 4.2 (2.1-6.1) | 22.9 (14.3-31.5) | ||
| Low dose (≤ 30 ng/kg/min) | 3.6 (2.0-5.3) | 15.6 (8.7-22.4) | 89 (13) | |||||
| High dose (> 30 ng/kg/min) | 24.2 (13.8-34.5) | 72.5 (41.5-103.5) | 63 (10) | |||||
| Bolus injection | 10.3 (4.3-16.2) | 18.4 (8.8-28.0) | 63 (8) | P < 0.0001 | 8.0 (1.2-14.8) | 5.1 (-6.9-17.1) | ||
| Diuresis (mL/min) | Very low2,4 | |||||||
| Continuous infusion | 1.3 (1.0-1.6) | 1.8 (1.2-2.3) | 62 (8) | P < 0.0001 | 1.0 (0.7-1.3) | 1.8 (1.3-2.4) | ||
| Low dose (≤ 30 ng/kg/min) | 1.0 (0.7-1.3) | 15.6 (8.7-22.4) | 89 (13) | |||||
| High dose (> 30 ng/kg/min) | 1.8 (1.2-2.4) | 2.8 (1.8-3.8) | 63 (10) | |||||
| Bolus injection | 1.1 (0.8-1.5) | 2.3 (1.3-3.2) | 63 (8) | P < 0.001 | 0.7 (0.2-1.1) | 2.0 (1.1-2.9) | ||
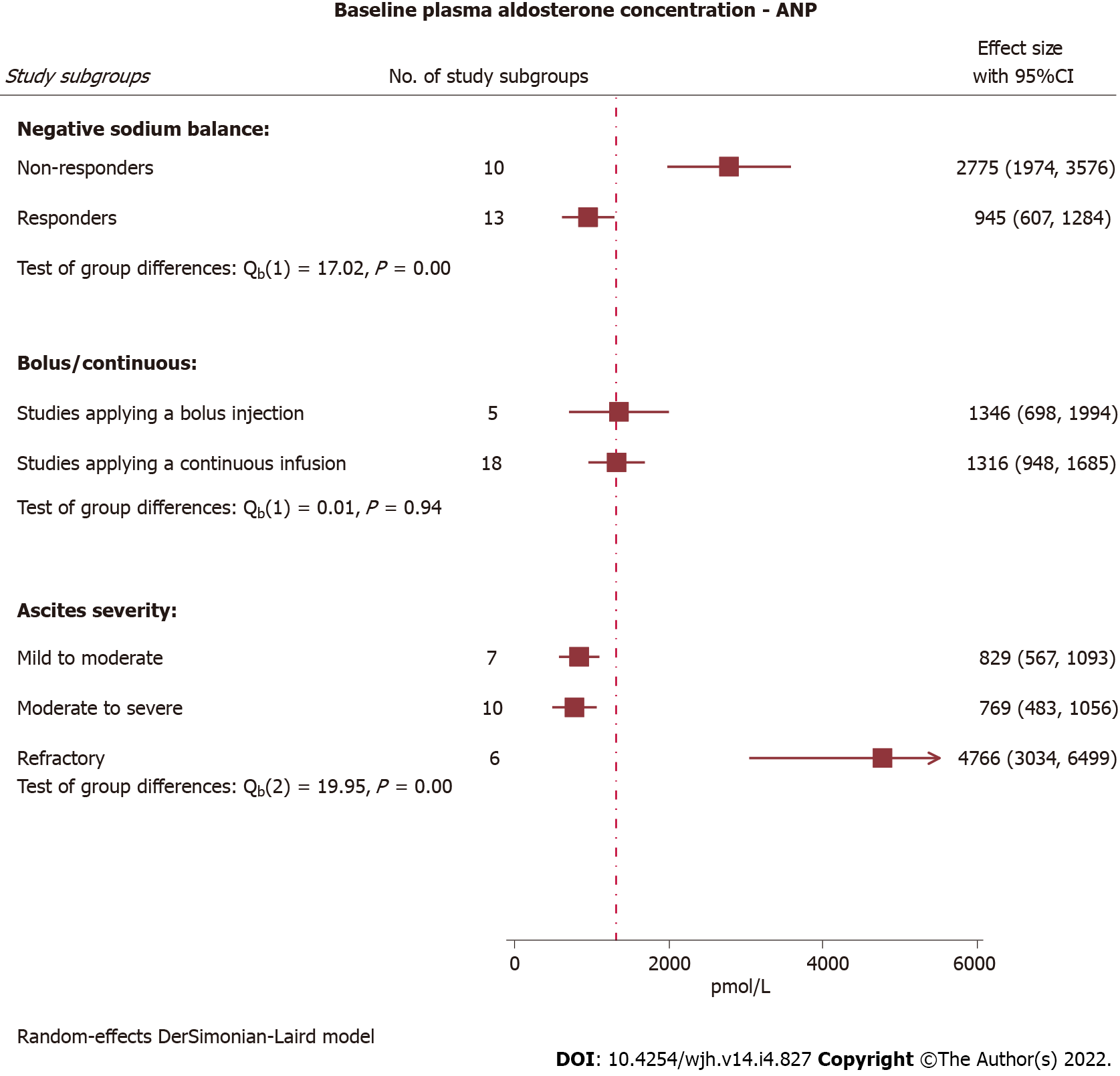
| P-Aldosterone (pmol/L) | Very low2,4 | |||||||
| Overall | 1182 (879-1484) | 739 (553-925) | 148 (21) | P < 0.0001 | 1110 (802-1418) | 497 (306-689) | ||
| Continuous infusion | ||||||||
| Low dose (≤ 30 ng/kg/min) | 1895 (929-2861) | 1110 (670-1551) | 56 (8) | |||||
| High dose (> 30 ng/kg/min) | 753 (491-1015) | 607 (412-801) | 50 (8) | |||||
| Bolus injection | 1095 (449-1741) | 435 (58-812) | 42 (5) | |||||
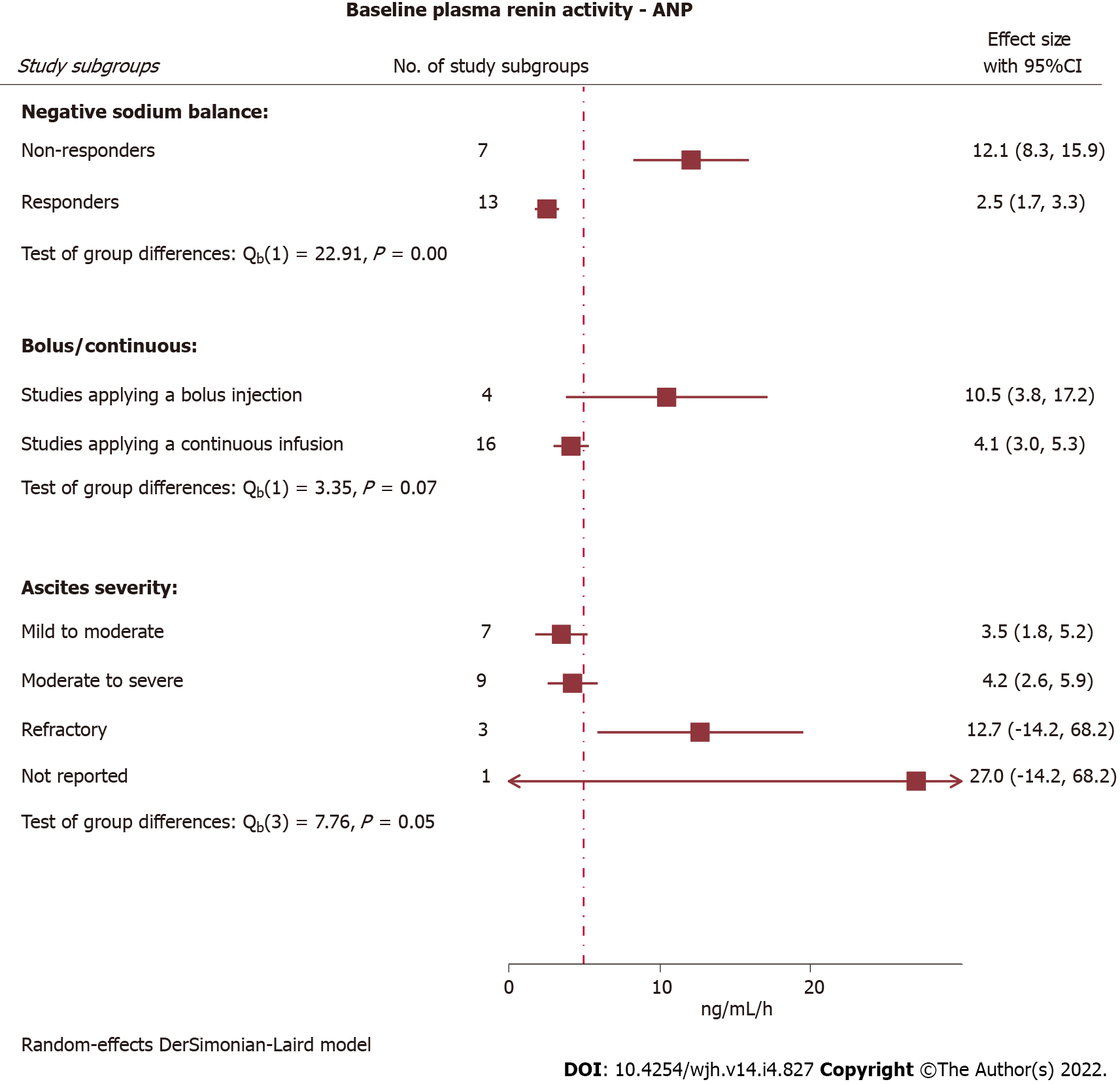
| P-Renin activity (ng/mL/h) | Very low2,4 | |||||||
| Overall | 5.0 (3.7-6.2) | 5.6 (4.2-6.9) | 137 (20) | P < 0.0001 | 4.4 (3.2-5.6) | 3.2 (1.8-4.6) | ||
| Continuous infusion | ||||||||
| Low dose (≤ 30 ng/kg/min) | 5.5 (2.8-8.2) | 4.0 (2.2-5.8) | 50 (7) | |||||
| High dose (> 30 ng/kg/min) | 2.6 (1.8-3.4) | 6.5 (3.7-9.2) | 54 (9) | |||||
| Bolus injection | 10.5 (3.8-17.2) | 11.0 (2.9-19.1) | 33 (4) | |||||
| Bodyweight | NA | NA | NA | NA | NA | NA | NA | Data too sparse for synthesis and quality assessment |
| Waist circumference | NA | NA | NA | NA | NA | NA | NA | No available data |
| P-Osmolality | NA | NA | NA | NA | NA | NA | NA | Data too sparse for synthesis and quality assessment |
| U-Osmolality | NA | NA | NA | NA | NA | NA | NA | No available data |
| P-Creatinine | NA | NA | NA | NA | NA | NA | NA | Data too sparse for synthesis and quality assessment |
| AEs | Risk of any AEs: 20 AEs/70 participants = 29% | 70 (10) | NA | NA | NA | NA5 | 20 AEs were reported in two studies covering 6 study subgroups. 3 studies (4 subgroups) observed no AEs | |
| BP reduction | Subgroups reporting BP drops | NA | NA | NA | NA6 | Details outlined in Supplementary Table 2 | ||
| Continuous infusion | 53% | 125 (19) | ||||||
| Bolus injection | 100% | 63 (8) | ||||||
| B-type natriuretic peptide | ||||||||
| NA | NA7 | Data too sparse for synthesis and quality assessment | ||||||
| Urodilatin8 | ||||||||
| Natriuresis (µmol/min) | Mean difference (Urodilatin vs Placebo): 75 (-33-183) | 21 (2) | NA | Mean difference; NA | Very low2,4 | |||
| Diuresis (mL/min) | Mean difference (Urodilatin vs Placebo): 2.5 (-0.9-5.8) | 21 (2) | NA | Mean difference; NA | Very low2,4 | |||
| AEs | Risk of any AEs: 6 AEs/21 participants = 29% | 21 (2) | NA | NA | NA5 | 6 AEs were reported in the two studies | ||
| BP reduction | No BP drops observed for subgroups | 21 (2) | NA | NA | NA6 | Details outlined in Supplementary Table 2 | ||
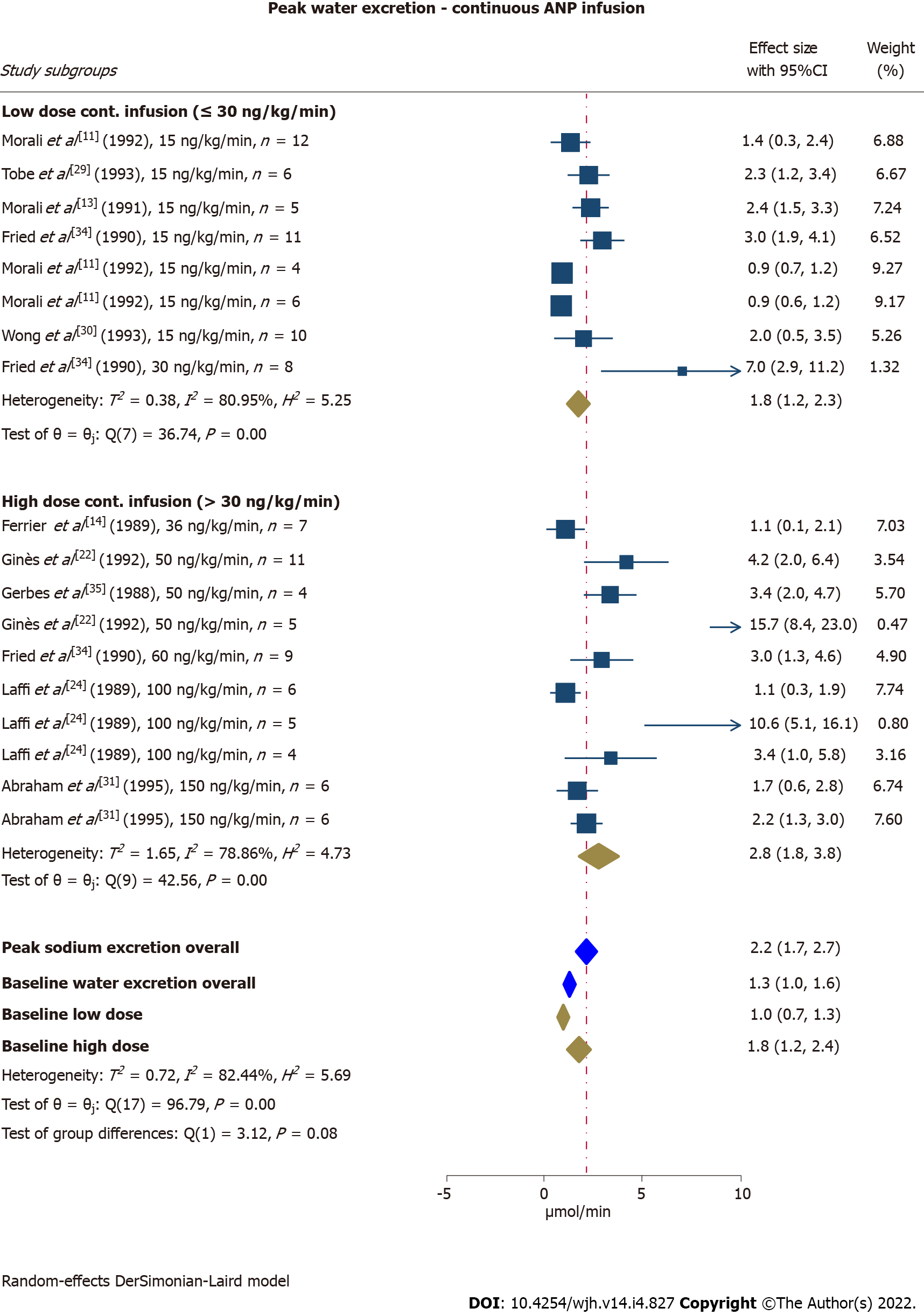
Water excretion increased from baseline 1.3 mL/min (95%CI: 1.0-1.6) to 2.2 mL/min (95%CI: 1.7-2.7) in study subgroups receiving a continuous ANP infusion. The peak response tended to be higher in study subgroups receiving an infusion rate of > 30 ng/kg/min compared with ≤ 30 ng/kg/min (P = 0.08) (Figure 4). In our subgroup analysis, we found higher baseline and peak water excretion in study subgroups with non-refractory ascites compared with refractory ascites (P < 0.01). Dose, ascites severity, and quality assessment score explained between-study heterogeneity to some extent and especially in the low-dose group. When we adjusted for these factors overall heterogeneity remained substantial (I2= 77%), but was reduced to moderate in the low-dose group (I2= 54%). We were unable to identify parameters explaining heterogeneity in the high-dose group. Effect size was unaffected by mean age and treatment duration. Leave-one-out analyses also failed to identify any single study to explain the heterogeneity for this outcome.
Study subgroups exposed to bolus ANP injections had a tendency to increase diuresis with an estimated effect size similar to continuous infusion (Supplementary Figure 2). However, there was also considerable heterogeneity in this analysis (peak I2= 94%, baseline I2= 90%).
Urodilatin significantly increased urine production in the first and larger RCT[36] compared with placebo. However, in the most recent RCT with patients suffering from refractory ascites, water excretion was largely unaltered by urodilatin compared with placebo[37] (Supplementary Figure 3).
Data were sparse on bodyweight and non-existent on waist circumference, thus these outcomes did not receive further evaluation. Two studies measured urine osmolality[14,24]. Laffi et al[24] reported a reduction in urine osmolality at the end of ANP-infusion and at the end of the recovery period compared with baseline for a subgroup of four participants. An increase was observed for five participants in another subgroup, while urine osmolality was unaffected in a third subgroup of six participants. Ferrier et al[14] reported no significant change in urine osmolality; however, a tendency towards a reduction was observed in the recovery period compared with baseline. None of the included studies published data on changes in plasma osmolality. Plasma creatinine was unaffected in the seven subjects investigated by Ferrier et al[14].
Repeated measurements of plasma aldosterone concentration were performed in 13 studies covering 21 study subgroups exposed to intravenous ANP, while another two studies covering three study subgroups restricted aldosterone concentration measurements to baseline. Plasma renin activity was measured before and after initiation of the intervention with ANP in 20 study subgroups originating from 12 studies. Two additional studies with three study subgroups measured plasma renin activity exclusively at baseline. Hence, for evaluation of ANP effects on these two parameters we defined peak response as the value that deviated the most from baseline.
We obtained an impression of decreased aldosterone concentration when exposed to ANP, which was similar for low- and high-dose continuous infusions as well as bolus injections (Supplemen
Only the largest study with urodilatin reported characteristics on plasma renin activity and aldosterone concentration. Plasma renin activity during the intervention decreased insignificantly from baseline levels, while plasma aldosterone concentration significantly decreased during the intervention compared with baseline.
Specific AEs (Supplementary Table 1) were reported in five of the included trials[16,24,34,36,37]. Three studies stated that AEs were absent[12,25,26], while no information on AEs was provided by 14 studies[11,13-15,22,23,27-33,35]. The most frequently reported AEs were facial flushing and hypotensive episodes. The latter were reported on an individual level in studies without any effect on blood pressure at study subgroup level.
In general, the included studies presented thorough illustrations or descriptions of the blood pressure course before, during and after the intervention, with only three studies[28-30] not mentioning this variable. Since the studies were of small size, we considered both statistical significance and trends as occurrence of blood pressure reductions. Blood pressure was unaffected in the three trials investigating urodilatin and BNP[16,36,37] (Supplementary Table 2).
Eighteen study subgroups exposed to ANP, including all eight subgroups that received a bolus injection, showed reductions in either mean arterial blood pressure or mean systolic blood pressure during the intervention while the blood pressure remained stable in nine study subgroups. Change in blood pressure was unreported in four study subgroups. Full recovery in the post-infusion follow-up period was reported in six study subgroups, all exposed to a bolus infusion. Partial recovery occurred in five study subgroups. No recovery was observed in one study subgroup subsequent to a continuous infusion. The remaining six study subgroups lacked follow-up data (Supplementary Table 2). Regarding the effect on blood pressure, our results clearly indicated continuous infusions of ≤ 30 ng/kg/min as well-tolerated, while higher doses almost guaranteed blood pressure reductions with a reduced likelihood of recovery in the follow-up period (Supplementary Table 2).
For all outcomes, Egger tests indicated statistically significant publication bias (Table 2). For peak natriuresis induced by ANP, funnel plots showed large asymmetry (Supplementary Figure 6). This may be explained by only small sized studies being available. Nevertheless, for continuous effect parameters such as sodium and water excretion, large standard errors for small effects are unlikely. However, to overcome this disadvantage of funnel plots, we included trim-and-fill analyses to adjust for publication bias and the adjusted effect values are presented in Table 2. Baseline and peak values of the effect parameters decreased equally leaving our meta-findings unchanged.
We present here the first systematic review and meta-analysis of the benefits and harms of natriuretic peptides in patients with cirrhosis and ascites. In an attempt to investigate alternative treatments for cirrhotic ascites, this systematic review demonstrated a clinically relevant natriuretic effect in addition to diuretic and hormonal effects of natriuretic peptides. However, the levels of evidence are judged as low or very low, mainly due to considerable between-study heterogeneity, inconsistency, and imprecision due to small sample sizes. The most robust data exists for natriuresis induced by intravenous ANP administration, and especially when given by continuous infusion. Moreover, high-dose continuous infusions induce stronger sodium excretion than low-dose infusions, and subgroups with mild/moderate ascites have the most pronounced responses. Based on results from one single study, BNP is probably ineffective in this setting, although the patients included in the trial had marked sodium retention and activation of vasoconstrictor systems, and thus may have limited response to any natriuretic therapy[16]. Results from two studies investigating continuous urodilatin infusion suggest a clinically relevant natriuretic effect.
Since the effects of ANP were discovered in the early 1980’s[38], investigations have been conducted to determine the therapeutic potential of natriuretic peptides in a broad range of diseases[39,40]. Through binding to specific natriuretic peptide receptors, ANP increases renal sodium and water excretion, increases glomerular filtration rate, inhibits aldosterone secretion, suppresses the release of arginine vasopressin, and reduces vascular resistance by smooth muscle relaxation[41-45]. These effects on fluid, electrolyte, and neuro-hormonal homeostasis reduce vasoconstriction and the intravascular volume, which may be beneficial for patients with fluid-accumulating disorders, including liver cirrhosis which is frequently complicated by ascites and peripheral oedema. Mild and moderate degrees of ascites are effectively treated with salt restriction and diuretics, but when ascites becomes refractory even to high doses of diuretics, treatment options are restricted to invasive procedures. Advances in diagnostic and therapeutic measures for refractory ascites remain unmet needs. Although a range of drugs, intended to alter essential events in the pathophysiological cascade, have undergone clinical investigations to relieve refractory ascites, none are currently approved.
Fyhrquist et al[40] were the first to report on the natriuretic and diuretic effects induced by intravenous ANP in refractory cirrhotic ascites and in the following decades further investigations with natriuretic peptides administered to cirrhotic patients were conducted. However, the trials sought primarily to elucidate the pharmacodynamics of natriuretic peptides in patients with cirrhosis and ascites, and some used ANP responsiveness to select eligible patients for other experiments e.g., combined treatment of ANP and norepinephrine to stabilize blood pressure[33], compare ANP responsiveness with responsiveness to head-out water immersion[25], compare ANP responsiveness with sympathetic nerve activity[13], test the inhibitory potential of Angiotensin-II on ANP responsiveness[28], and compare ANP responsiveness before and after establishment of a peritoneovenous shunt[29]. Thus, the general limitations of the trials are the small number of participants, different study designs, short-term interventions, varying treatment doses, and differences in ascites severity of participants. These limitations manifest as considerable heterogeneity in the current meta-analysis and reduce the level of evidence obtained for all outcomes. Eight studies investigating a bolus ANP injection were also included in this work. Although natriuresis may be induced with this setup, due to a short half-life bolus injection as therapy has limited clinical potential. As continuous intravenous infusions are more effective and applicable, this may be the preferred method of administration for future trials.
We included the outcomes bodyweight and waist circumference in our protocol since they may function as surrogate markers of ascites burden. Unfortunately, only a few trials weighed their participants and none measured girth; thus, data on these parameters are too sparse for analysis. A maximum ascites reabsorption capacity of less than one litre per day[46] probably explains why these parameters were neglected for the majority of studies investigating short-term infusions of natriuretic peptides. However, due to the absence of these data the clinical relevance of natriuresis is still to be demonstrated. Serum sodium and potassium concentrations were not included as outcomes in the present study, although these electrolytes may be affected by natriuretic peptides. Baseline sodium concentration was measured in 12 study subgroups (Table 1). In only one study a follow-up measurement after ANP infusion, which was unchanged from baseline, was reported. The baseline serum sodium concentration generally reflects the disease severity, with the lowest concentrations in study subgroups characterised by refractory ascites, and thus, may be a potential predictor of ANP responsiveness. Exclusively at baseline, serum potassium concentration was measured in six study subgroups, which showed similar results. A RCT investigating longer-term urodilatin infusion is currently recruiting patients, and includes bodyweight, waist circumference, plasma sodium and plasma potassium as outcomes[47].
A notable finding of this meta-analysis is the observation that non-responders unable to achieve a negative sodium balance when treated with a natriuretic peptide were characterised by high baseline plasma aldosterone concentrations and renin activity. Plasma levels of these parameters indicate the severity of renin-angiotensin-aldosterone (RAAS)-activation, which directly parallels the degree of cirrhosis decompensation with portal hypertension. Therefore, we hypothesise that a threshold exists where RAAS-activation is too severe for ANP to counteract. Future trials may benefit from inclusion of these hormones to validate their ability to identify treatment responders and non-responders, and further investigate if a threshold predicting responsiveness can be estimated.
AEs and especially effects on blood pressure were assessed in this systematic review. The results indicated the incidence of any AE in every fourth patient receiving treatment with either ANP or urodilatin, and the AEs described were predominantly subjective measures, which may easily be explained by the well-known physiological effects of natriuretic peptides. Blood pressure is sensitive to intravenous administration of natriuretic peptides, which is well-documented in other diseases, e.g., decompensated heart failure[48]. Our results clearly indicate that bolus ANP injections guarantee reductions in blood pressure, although with a high chance of rapid recovery. For continuous ANP infusions, doses ≤ 30 ng/kg/min are less likely to affect blood pressure compared with higher doses. Future trials are needed before a definite conclusion on the tolerability of natriuretic peptides in cirrhotic patients with ascites can be made.
The present study is limited to the inclusion of cirrhosis patients with ascites treated with either ANP, BNP, or urodilatin. Thus, we have not included results from studies investigating natriuretic peptide effects in cirrhosis patients without ascites. Furthermore, we have excluded studies focusing on interventions intended for blockade of natriuretic peptide degradation. One trial has tested the effects of sinorphan, an enkephalinase inhibitor, in patients with cirrhosis and ascites. Enkephalinase is a neutral membrane endopeptidase with a broad presence in plasma and tissues including the kidneys[49]. By blocking this enzyme, plasma levels of ANP and the second messenger cyclic guanosine mono-phosphate increase. Sixteen cirrhosis patients with persistent ascites received treatment with sinorphan in a double-blind, placebo-controlled, cross-over trial. Plasma concentrations of ANP increased significantly after drug administration and sodium excretion doubled (mean increase 17.2 μmol/min) in the 2-h post-administration period[49]. Interestingly, mean arterial blood pressure was unaffected. Therefore, blockage of ANP degradation may be of relevance for future trials, potentially with limited effects on blood pressure.
Another limitation of the present study is the age of the included studies. All but one of the studies were performed and published more than 20 years ago. The distribution of cirrhosis aetiologies has changed during the past two decades, particularly due to an increased frequency of cirrhosis due to metabolic disease. However, we focus here on cirrhosis decompensation with ascites, which occurs late in the disease course, allowing translation of the results to the present.
Ultimately, this systematic review and meta-analysis provides new perspectives into future medico-pharmaceutical research in patients with advanced cirrhosis and elucidates the course for future research in the field. A clear natriuretic and diuretic effect of intravenous ANP is evident from our results, and we suggest that future trials would benefit from a RCT design with a comparator group, longer-term infusion, and clinical follow-up. The preferred dose has to be determined on the basis of larger natriuretic and diuretic effects with doses > 30 ng/kg/min, but better tolerability of doses ≤ 30 ng/kg/min. Although the natriuretic and diuretic effects are modest in patients with severe or refractory ascites, new treatments are most needed for this indication. This argues for a particular focus on these patients in future trials. To increase the treatment duration and maintain increased natriuresis, alternative methods of administration must be considered e.g., continuous subcutaneous infusion. Finally, clinicians may find a prolongation of the interval between paracentesis as a sufficiently meaningful treatment effect and not necessarily a reversion from refractory ascites to treatment responsive ascites.
Ascites formation is the most frequent event of cirrhosis decompensation with no approved non-invasive treatment for patients with refractory ascites. However, natriuretic peptides may induce beneficial effects in patients with cirrhosis and ascites, by counterbalancing vasoconstriction and anti-natriuretic factors.
Only small studies with a broad pallet of designs, have investigated the renal and systemic effects and safety of natriuretic peptides in patients with cirrhosis and ascites. We were motivated to compile results regarding natriuretic peptides as treatment for ascites to advance pharmaceutical research concerning this disease.
We aimed to systematically review the effects and safety of natriuretic peptides in patients with cirrhosis and ascites. We collected results regarding changes in renal sodium and water excretion, plasma aldosterone concentration, and plasma renin activity, and to perform meta-analyses providing overall estimates of the effects of natriuretic peptide infusions. Safety assessments, in particular blood pressure decreases, were included and descriptively summarised.
We adhered to the PRISMA guidelines and the Cochrane Handbook. A review protocol was preregistered at PROSPERO. The databases MEDLINE, Web of Science, Embase, Scopus, and Cochrane Library were searched and references reporting the renal and systemic effects of mono-therapy with atrial natriuretic peptide (ANP), B-type natriuretic peptide, urodilatin, or any synthetic equivalent in patients with cirrhosis and ascites were considered for inclusion. Treatment administration had to be intravenous, but trials were included regardless of dose, follow-up duration, and whether a continuous infusion or bolus injection was administered. The study screening and data extraction were performed independently by two reviewers. A random-effects model was applied for the meta-analysis. Reasons for heterogeneity were explored through subgroup and leave-one-out analyses.
Twenty-two studies were included and short-term ANP infusion was the only intensively studied treatment. Renal sodium and water excretion increased in response to continuous ANP infusion and was most pronounced when infusion rates > 30 ng/kg/min were applied. Furthermore, sodium excretion was higher in study subgroups with mild/moderate ascites compared with refractory ascites. The baseline plasma aldosterone concentration and renin activity were significantly lower in subgroups achieving a negative sodium balance compared with treatment non-responders, and may be relevant response predictors to include in future trials. Blood pressure decreases occurred less frequently with doses ≤ 30 ng/kg/min.
Intravenous infusions of natriuretic peptides induce meaningful sodium and water excretion in patients with cirrhotic ascites. Continuous ANP infusions > 30 ng/kg/min induce the most pronounced renal effects, but with a higher risk of blood pressure decreases than doses ≤ 30 ng/kg/min.
Future larger-scale clinical trials are justified to determine the therapeutic potential of natriuretic peptides in patients with cirrhotic ascites.
The authors thank librarian Karen Sigaard at AU Library for professional assistance in generating the search string and for performing the literature search.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HC, Taiwan S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2286] [Article Influence: 381.0] [Reference Citation Analysis (0)] |
| 2. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 3. | Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1812] [Article Influence: 258.9] [Reference Citation Analysis (2)] |
| 5. | Pérez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, Rivera F, Rodés J. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84:961-968. [PubMed] |
| 6. | Bernardi M, Laffi G, Salvagnini M, Azzena G, Bonato S, Marra F, Trevisani F, Gasbarrini G, Naccarato R, Gentilini P. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1688] [Cited by in RCA: 1639] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 8. | Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of "urodilatin", a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 174] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Abassi ZA, Golomb E, Agbaria R, Roller PP, Tate J, Keiser HR. Hydrolysis of iodine labelled urodilatin and ANP by recombinant neutral endopeptidase EC. 3.4.24.11. Br J Pharmacol. 1994;113:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hildebrandt DA, Mizelle HL, Brands MW, Hall JE. Comparison of renal actions of urodilatin and atrial natriuretic peptide. Am J Physiol. 1992;262:R395-R399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Morali GA, Tobe SW, Skorecki KL, Blendis LM. Refractory ascites: modulation of atrial natriuretic factor unresponsiveness by mannitol. Hepatology. 1992;16:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Jespersen B, Eiskjaer H, Jensen JD, Mogensen CE, Sørensen SS, Pedersen EB. Effects of high dose atrial natriuretic peptide on renal haemodynamics, sodium handling and hormones in cirrhotic patients with and without ascites. Scand J Clin Lab Invest. 1995;55:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Morali GA, Floras JS, Legault L, Tobe S, Skorecki KL, Blendis LM. Muscle sympathetic nerve activity and renal responsiveness to atrial natriuretic factor during the development of hepatic ascites. Am J Med. 1991;91:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Ferrier C, Beretta-Piccoli C, Weidmann P, Gnädinger MP, Shaw S, Suchecka-Rachon K, Saxenhofer H. Hypotension and renal impairment during infusion of atrial natriuretic factor in liver cirrhosis with ascites. Am J Nephrol. 1989;9:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Laffi G, Marra F, Pinzani M, Meacci E, Tosti-Guerra C, De Feo ML, Gentilini P. Effects of repeated atrial natriuretic peptide bolus injections in cirrhotic patients with refractory ascites. Liver. 1989;9:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | La Villa G, Riccardi D, Lazzeri C, Casini Raggi V, Dello Sbarba A, Tosti Guerra C, Fronzaroli C, Foschi M, Laffi G, Gentilini P. Blunted natriuretic response to low-dose brain natriuretic peptide infusion in nonazotemic cirrhotic patients with ascites and avid sodium retention. Hepatology. 1995;22:1745-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47118] [Article Influence: 2944.9] [Reference Citation Analysis (0)] |
| 18. | Higgens JPT, Thomas J, Chandler J, Cumpston M, Li T, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). [cited 20 June 2021]. Available from: https://training.cochrane.org/handbook. |
| 19. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15138] [Article Influence: 2523.0] [Reference Citation Analysis (0)] |
| 20. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 20 June 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 21. | Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4813] [Cited by in RCA: 7088] [Article Influence: 472.5] [Reference Citation Analysis (0)] |
| 22. | Ginès P, Titó L, Arroyo V, Llach J, Salmerón JM, Ginès A, Jiménez W, Badalamenti S, Rivera F, Rodés J. Renal insensitivity to atrial natriuretic peptide in patients with cirrhosis and ascites. Effect of increasing systemic arterial pressure. Gastroenterology. 1992;102:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Heim JM, Gottmann K, Weil J, Schiffl H, Lauster F, Loeschke K, Gerzer R. Effects of a small bolus dose of ANF in healthy volunteers and in patients with volume retaining disorders. Klin Wochenschr. 1990;68:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Laffi G, Pinzani M, Meacci E, La Villa G, Renzi D, Baldi E, Cominelli F, Marra F, Gentilini P. Renal hemodynamic and natriuretic effects of human atrial natriuretic factor infusion in cirrhosis with ascites. Gastroenterology. 1989;96:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Legault L, Warner LC, Leung WM, Logan AG, Blendis LM, Skorecki KL. Assessment of atrial natriuretic peptide resistance in cirrhosis with head-out water immersion and atrial natriuretic peptide infusion. Can J Physiol Pharmacol. 1993;71:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Miyase S, Fujiyama S, Chikazawa H, Sato T. Atrial natriuretic peptide in liver cirrhosis with mild ascites. Gastroenterol Jpn. 1990;25:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Salerno F, Badalamenti S, Incerti P, Capozza L, Mainardi L. Renal response to atrial natriuretic peptide in patients with advanced liver cirrhosis. Hepatology. 1988;8:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Tobe SW, Blendis LM, Morali GA, Warner LC, Logan AG, Skorecki KL. Angiotensin II modulates atrial natriuretic factor-induced natriuresis in cirrhosis with ascites. Am J Kidney Dis. 1993;21:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Tobe SW, Morali GA, Greig PD, Logan A, Blendis LM. Peritoneovenous shunting restores atrial natriuretic factor responsiveness in refractory hepatic ascites. Gastroenterology. 1993;105:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Wong F, Tobe S, Legault L, Logan AG, Skorecki K, Blendis LM. Refractory ascites in cirrhosis: roles of volume expansion and plasma atrial natriuretic factor level elevation. Hepatology. 1993;18:519-528. [PubMed] |
| 31. | Abraham WT, Lauwaars ME, Kim JK, Peña RL, Schrier RW. Reversal of atrial natriuretic peptide resistance by increasing distal tubular sodium delivery in patients with decompensated cirrhosis. Hepatology. 1995;22:737-743. [PubMed] |
| 32. | Ando M. [Renal tubular function in cirrhotic patients with ascites: special reference to lithium clearance following the human atrial natriuretic peptide administration]. Nihon Jinzo Gakkai Shi. 1991;33:791-801. [PubMed] |
| 33. | Badalamenti S, Borroni G, Lorenzano E, Incerti P, Salerno F. Renal effects in cirrhotic patients with avid sodium retention of atrial natriuretic factor injection during norepinephrine infusion. Hepatology. 1992;15:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Fried T, Aronoff GR, Benabe JE, Brunner HR, DiBona GF, Fleischhauer T, Lam M, Lawton WJ, Luft FC, Martinez-Maldonado M. Renal and hemodynamic effects of atrial natriuretic peptide in patients with cirrhosis. Am J Med Sci. 1990;299:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Gerbes AL, Arendt R, Xie Y, Knorr HJ, Riedel A, Paumgartner G. [Pathophysiologic and clinical relevance of atrial natriuretic factor in patients with cirrhosis of the liver]. Z Kardiol. 1988;77 Suppl 2:99-103. [PubMed] |
| 36. | Carstens J, Greisen J, Jensen KT, Vilstrup H, Pedersen EB. Renal effects of a urodilatin infusion in patients with liver cirrhosis, with and without ascites. J Am Soc Nephrol. 1998;9:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Carstens J, Grønbaek H, Larsen HK, Pedersen EB, Vilstrup H. Effects of urodilatin on natriuresis in cirrhosis patients with sodium retention. BMC Gastroenterol. 2007;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2358] [Cited by in RCA: 2212] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 39. | Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;341-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 40. | Fyhrquist F, Tötterman KJ, Tikkanen I. Infusion of atrial natriuretic peptide in liver cirrhosis with ascites. Lancet. 1985;2:1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Weidmann P, Hasler L, Gnädinger MP, Lang RE, Uehlinger DE, Shaw S, Rascher W, Reubi FC. Blood levels and renal effects of atrial natriuretic peptide in normal man. J Clin Invest. 1986;77:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 232] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Chartier L, Schiffrin E, Thibault G, Garcia R. Atrial natriuretic factor inhibits the stimulation of aldosterone secretion by angiotensin II, ACTH and potassium in vitro and angiotensin II-induced steroidogenesis in vivo. Endocrinology. 1984;115:2026-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Dillingham MA, Anderson RJ. Inhibition of vasopressin action by atrial natriuretic factor. Science. 1986;231:1572-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Currie MG, Geller DM, Cole BR, Boylan JG, YuSheng W, Holmberg SW, Needleman P. Bioactive cardiac substances: potent vasorelaxant activity in mammalian atria. Science. 1983;221:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 561] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Needleman P, Greenwald JE. Atriopeptin: a cardiac hormone intimately involved in fluid, electrolyte, and blood-pressure homeostasis. N Engl J Med. 1986;314:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 335] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Shear L, Ching S, Gabuzda GJ. Compartmentalization of ascites and edema in patients with hepatic cirrhosis. N Engl J Med. 1970;282:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 101] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Gantzel RH, Meyer M, Mazgareanu S, Aagaard NK, Jepsen P, Holzmeister J, Watson H, Grønbæk H. Ularitide as treatment of refractory ascites in cirrhosis- a study protocol for a randomised trial. Dan Med J. 2021;68. [PubMed] |
| 48. | Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, Kobalava Z, Nitsche K, Forssmann WG, Lüss H, Meyer M. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006;27:2823-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Dussaule JC, Grangé JD, Wolf JP, Lecomte JM, Gros C, Schwartz JC, Bodin F, Ardaillou R. Effect of sinorphan, an enkephalinase inhibitor, on plasma atrial natriuretic factor and sodium urinary excretion in cirrhotic patients with ascites. J Clin Endocrinol Metab. 1991;72:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |