Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.812
Peer-review started: June 9, 2021
First decision: July 6, 2021
Revised: July 18, 2021
Accepted: March 7, 2022
Article in press: March 7, 2022
Published online: April 27, 2022
Processing time: 316 Days and 15.8 Hours
Von-Willebrand factor (vWF) disposes certain prognostic value in patients with liver cirrhosis, but its relation to other prognostic indicators has not been fully investigated.
To analyze the relation between vWF and other prognostic indicators in cirrhotic patients and to evaluate its prognostic value for mortality.
This analytic prospective study was carried out in a tertiary center and initially enrolled 71 patients with liver cirrhosis and portal hypertension. It analyzed the relation between vWF and the stage of the disease and several inflammatory and prognostic indicators. The prospective analysis, performed on a sample of 63 patients, evaluated the association between the selected variables [vWF, Model for End-stage Liver Disease (MELD) score, C-reactive protein (CRP), ferritin, vitamin D, activated partial thromboplastin time, thrombin time, D-dimer concentration] and the survival time as well as their predictive value in terms of 3-mo, 6-mo and 1-year mortality.
vWF was significantly higher in patients with higher Child-Turcotte-Pugh class (P = 0.0045), MELD group (P = 0.0057), ferritin group (P = 0.0278), and D-dimer concentration (P = 0.0232). vWF significantly correlated with D-dimer concentration, ferritin, CRP, International Normalized Ratio, and MELD, Child-Turcotte-Pugh, Sequential Organ Failure Assessment, and CLIF-consortium organ failure (CLIF-C OF) scores. vWF, MELD score, and CRP were significantly associated with death and were significant predictors of 3-mo, 6-mo, and 1-year mortality. Each vWF unit significantly increased the probability for 3-mo mortality by 1.005 times (P = 0.008), for 6-mo mortality by 1.006 times (P = 0.005), and for 1-year mortality by 1.007 times (P = 0.002). There was no significant difference between the diagnostic performance of vWF and MELD score and also between vWF and CRP regarding the 3-mo, 6-mo, and 1-year mortality.
In patients with liver cirrhosis, vWF is significantly related to other prognostic indicators and is a significant predictor of 3-mo, 6-mo, and 1-year mortality similar to MELD score and CRP.
Core Tip: The prognostic value of von-Willebrand factor (vWF) in cirrhotic patients has been previously evaluated, but its relation to other inflammatory and prognostic indicators has not been fully investigated. The study confirmed that vWF was significantly associated with the stage of liver disease, D-dimer concentration, ferritin, and survival and that vWF was a significant predictor of 3-mo, 6-mo, and 1-year mortality similar to Model for End-stage Liver Disease score and C-reactive protein. These data reflect the important prognostic role of the complex and dynamic interaction between endothelial dysfunction, systemic inflammation, and cirrhosis-related coagulopathy in cirrhotic patients.
- Citation: Curakova Ristovska E, Genadieva-Dimitrova M. Prognostic value of von-Willebrand factor in patients with liver cirrhosis and its relation to other prognostic indicators. World J Hepatol 2022; 14(4): 812-826
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/812.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.812
The high mortality rate in cirrhotic patients and the shortage of organs worldwide are constantly raising the issue of mortality prediction in patients with end-stage liver disease in terms of differentiating the most critically ill patients with the highest short-term mortality[1]. This leads to continuous and intense research towards defining new biological variables that possess certain prognostic potential in cirrhotic patients. Chronic liver disease is inevitably related to portal hypertension (PH), an entity that closely accompanies and often defines the natural course of the disease. Its prognostic significance derives from the fact that PH is closely related to several severe, life-threatening complications that are associated with high morbidity and mortality[2]. PH is diagnosed and quantified with the hepatic venous pressure gradient, but since it is an invasive, expensive, and not widely available procedure, there is a need for alternative relevant and noninvasive indicators of PH.
Recent research emphasizes the significant contributing role of endothelial dysfunction (ED) in the pathogenesis and progression of PH and its relation to poor prognosis in cirrhotic patients[3]. Intrahepatic ED is considered a major determinant of the increased hepatic vascular tone of the cirrhotic liver, and systemic ED due to endotoxemia is the cause of increased nitric oxide production, the major determinant of the hyperdynamic circulation [3]. Considering its important contributing role in the pathogenesis of PH, von-Willebrand factor (vWF) has recently gained some attention as a prognostic indicator in cirrhotic patients. The increased vWF production due to ED favors hypercoagulable state, formation of platelet-induced thromboses in the hepatic microcirculation, and gradual thrombotic vascular obliteration[4,5]. It is considered that the increased vWF concentration and the cirrhosis-related procoagulant imbalance are the two crucial predisposing events responsible for the progressive vascular occlusion of the portal circulation[4,5]. Also, ED is the major cause of many complex hemostatic abnormalities that occur in cirrhotic patients. The imbalance in the secretion of pro-coagulant, anticoagulant, fibrinolytic, and antifibrinolytic substances due to ED in different clinical settings may have a different hemostatic phenotype. On the other hand, short-term prognosis in cirrhotic patients largely depends on the accompanying liver-related events that temporarily worsen the liver function[6]. Recent data also emphasize the important contributing role of systemic inflammation (SI) in the pathogenesis of the majority of the acute events in cirrhotic patients. It has been established that SI is common and almost a persistent state, especially in advanced liver disease, that it has a crucial role in the course of the disease, and that SI is related to adverse outcomes in cirrhotic patients[6-8].
It seems that ED, SI, and liver-related coagulopathy have an important role in the natural course of chronic liver disease[7,9-11]. The involvement of vWF as an indicator of ED plays a substantial role in the progression of PH, which explains the significant and relevant prognostic potential of vWF in cirrhotic patients. Still, the relation between vWF and other inflammatory and prognostic indicators has not been completely investigated. The aim of the study was to evaluate the relation between vWF and liver cirrhosis, its relation to other relevant prognostic indicators in cirrhotic patients, and the prognostic value of vWF in terms of 3-mo, 6-mo, and, 1-year mortality.
This analytic monocentric prospective study initially enrolled 71 patients with liver cirrhosis and PH. Data regarding demographic and clinical characteristics of patients (age, gender, etiology, disease duration, data regarding previous complications, related diagnostic/therapeutic interventions) were collected, and a number of imaging and laboratory investigations were performed in order to determine the stage of the disease, to register the present complications of PH, and to assess the mortality risk. Besides the basic biochemical and hemostatic analyses, the concentration of vWF was also measured. Afterward, by using the American Society of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) criteria, the presence of systemic inflammatory response syndrome (SIRS) was determined, and the Child-Turcotte-Pugh (CTP) score, Model for End-stage Liver Disease (MELD) score, Sequential Organ Failure Assessment (SOFA) score, CLIF-consortium organ failure (CLIF-C OF) scores, and Simplified Acute Physiology Score II (SAPS II) were calculated. After enrollment, patients were prospectively monitored for 1 year, and every 3 mo a regular control examination was performed. During every regular visit clinical examination (physical and abdomen examination), vital parameters measurement (blood pressure, heart rate, respiratory rate, blood oxygenation, body temperature), abdominal ultrasound examination with color Doppler of the portal vein, complete blood count, and biochemical analysis of blood sample and urine sediment was performed. In case of some clinical deterioration during the follow-up, an additional investigation was performed (patients were provided with phone communication with the study doctor), after which the patients went back to the regular study protocol. During the follow-up period, 8 patients dropped out (occurrence of some of the exclusion criteria, transplanted or noncompliant patients). Within the prospective analysis performed in 63 patients, the predictive value of vWF and several parameters of interest [MELD score, C-reactive protein (CRP), ferritin, vitamin D, activated partial thromboplastin time (aPTT), thrombin time (TT), D-dimer concentration] were analyzed in terms of 3-mo, 6-mo, and 1-year mortality. All patients signed an informed consent form for participation in the study. The research and the study protocol were in line with the ethical principles of the Helsinki declaration.
The study enrolled patients with clinically evident liver cirrhosis and portal hypertension with no significant preexisting comorbidities (systemic, infective, cardiovascular, metabolic, or neoplastic disease) and without active alcohol consumption, previous thrombotic event, blood transfusion, or interferon, antiplatelet, or anticoagulant therapy. Some patients were enrolled after hospitalization at the University Clinic for Gastroenterohepatology in Skopje, and some were enrolled during the outpatient follow-up.
At enrollment and during every regular visit, a complete blood count and biochemical blood analysis [glucose, blood urea nitrogen, creatinine, bilirubin, protein profile (albumin, globulin), sodium, potassium, calcium, iron, total iron-binding capacity, lipid profile (cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides), CRP, ferritin, vitamin D, urinary sediment, alpha fetoprotein], hemostasis [prothrombin time (PT), International Normalized Ratio (INR), aPTT, TT], D-dimer concentration, urinary sediment, and gas analyses from capillary blood sample were performed. The vWF concentration was measured in platelet-rich plasma aliquoted after centrifugation of sodium citrate blood sample. The analysis was performed by using the immunoturbidimetric method (vWF Ag Test Kit, Siemens, Munich, Germany) on an automatic coagulometer (BCS XP System-Siemens Healthiness Global device). The normal range of vWF was 50%-150%. According to the obtained score values, patients were classified in three CTP classes (Class A, B and C), in three MELD groups [group 1 (MELD ≤ 9), group 2 (MELD 10-19), and group 3 (MELD ≥ 20)], in three serum ferritin (SF) groups [group 1 (SF < 200 ng/mL), group 2 (SF 200-400 ng/mL), and group 3 (SF > 400 ng/mL)], in two D-dimer groups (below/above 500 µg/mL), and in two vitamin D groups (below/above 20 ng/mL).
The statistical analysis was performed by using the SPSS software package, version 22.0 for Windows (IBM Corp., Armonk, NY, United States). Descriptive statistics were provided as mean ± standard deviation, median, and interquartile range (IQR). Mann Whitney U test and Kruskal-Wallis H test were used to test the significance of the difference between the numeric variables without normal distribution. Correlation between vWF and other variables was analyzed by Spearman’s correlation. Univariate Cox proportional model was used in order to evaluate the association between the selected variables and the survival time, and the univariate logistic regression analysis was used to determine the significant predictors of mortality. The diagnostic performance of the significant mortality predictors was assessed by the receiver operating characteristic (ROC) analysis and the area under the curve (AUC) values of two independent ROC curves that were compared using the Z test. P values < 0.05 were considered statistically significant.
The mean age in the group was 58.8 ± 10.7 years [95% confidence interval (CI): 54.4-59.1], and there was a significant male predominance [56 (78.87%) men and 15 (21.13%) women; (gender ratio 3.73:1)]. Regarding etiology, alcoholic liver disease was the most prevalent entity (36 patients, 50%). According to the CTP classification, most patients were in class C (28, 39.40%), 25 patients (35.20%) in class B, and 18 patients (25.30%) in class А [CTP score 8.9 ± 2.9 (5-15); IQR = 9 (6-11)]. MELD score was 19.7 ± 9.9 (6-59); IQR = 18 (11-25). The CRP concentration was 21.1 ± 27.5 mg/L and SIRS was registered in 43 (60.60%) patients. The ferritin concentration was 290.45 ± 354.33 ng/mL [SF < 200 ng/mL in 39 (62.9%) patients, SF 200-400 ng/mL in 5 (8.1%), and SF > 400 ng/mL in 18 (29.0%) patients]. The vitamin D concentration was 17.65 ± 13.31 ng/mL, and the prevalence of vitamin D deficiency was 48.9% (Table 1).
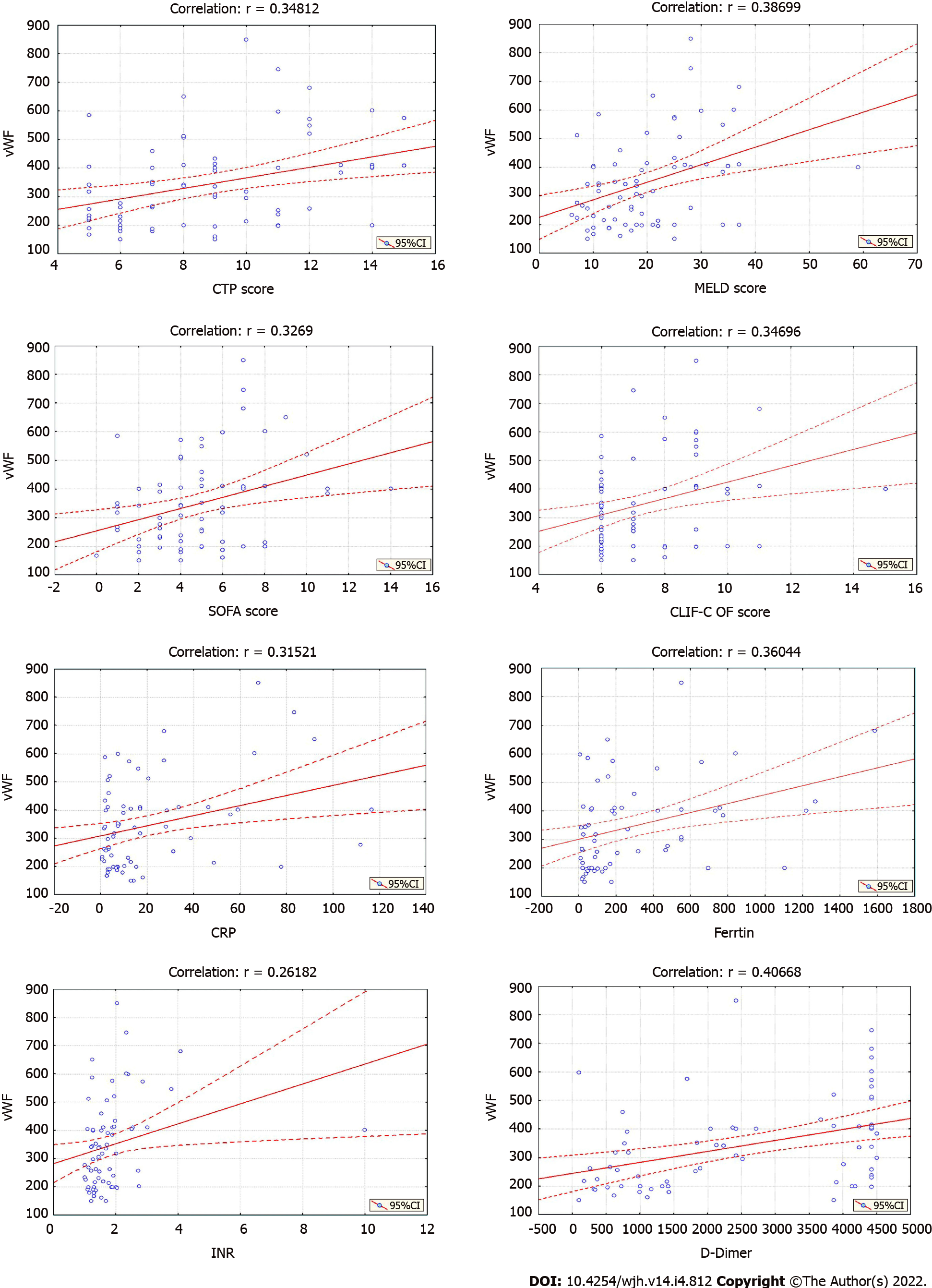
The mean vWF concentration in the group was 346.18 ± 155.97% (150-850), IQR = 318.40% (214.00-410.10) (Table 1). The analysis confirmed significantly higher vWF values in higher CTP class [Kruskal-Wallis H test: Chi-square (2) = 10.8177; P = 0.0045], MELD group [Kruskal-Wallis H test: Chi-square (2) = 10.3357; P = 0.0057], and SF group [Kruskal-Wallis H test: Chi-square (2) = 7.1653; P = 0.0278] and in patients with elevated (> 500 µg/mL) D-dimer concentration (Mann-Whitney U test: Z = 2.6407; P = 0.0083) (Table 2). The analysis did not show a significant difference between the vWF values regarding vitamin D deficiency (Mann-Whitney U test: Z = -1.6916; P = 0.0907) and platelet count [(above/below 100×109/L), (Mann-Whitney U test: Z = -0.02898; P = 0.9769)], (Table 2). The correlation analysis confirmed a strong significant positive linear correlation only between vWF and D-dimer concentration (r = 0.407) and a moderate significant positive linear correlation between vWF and CTP score, MELD score, SOFA score, CLIF-C OF score, CRP, ferritin, and INR (r = 0.348, 0.387, 0.327, 0.328, 0.315, 0.360 and 0.262, respectively) (Figure 1). The correlation between vWF and the other selected parameters (SAPS II score, vitamin D, Alveolar-Arterial Oxygen Gradient, hemoglobin, platelet count, WBC, PT, aPTT, TT) was weak or not significant (Figure 2).
| Parameter | n | mean ± SD | Min | Max | Percentiles | ||
| 25th | 50th | 75th | |||||
| CTP score | 71 | 8.94 ± 2.95 | 5.00 | 15.00 | 6.00 | 9.00 | 11.00 |
| MELD score | 71 | 19.72 ± 9.86 | 6.00 | 59.00 | 11.00 | 18.00 | 25.00 |
| vWF (%) | 71 | 346.18 ± 155.97 | 150 | 850 | 214.00 | 318.40 | 410.10 |
| CRP (mg/L) | 71 | 21.15 ± 27.50 | 0.60 | 116.50 | 3.30 | 9.70 | 27.40 |
| Ferritin (ng/mL) | 62 | 290.45 ± 354.33 | 7.20 | 1586.10 | 47.50 | 149.40 | 464.30 |
| Vitamin D (ng/mL) | 46 | 17.65 ± 13.31 | 3.00 | 62.24 | 9.12 | 11.89 | 24.82 |
| D-dimer (µg/mL) | 69 | 2558.4 ± 1645.1 | 99.00 | 4500.00 | 969.90 | 2420.70 | 4427.00 |
| PT (s) | 71 | 20.50 ± 14.71 | 11.60 | 133.20 | 14.70 | 17.57 | 21.26 |
| aPTT (s) | 70 | 43.99 ± 14.32 | 23.56 | 120.00 | 35.53 | 41.94 | 48.07 |
| TT (s) | 70 | 24.10 ± 7.08 | 16.00 | 59.00 | 19.22 | 22.94 | 22.93 |
| PLT (109/L) | 71 | 105.51 ± 60.52 | 18.00 | 311.00 | 62.00 | 91.00 | 127.00 |
| WBC (109/L) | 71 | 6.62 ± 3.43 | 1.34 | 23.20 | 4.70 | 6.20 | 7.50 |
| Bilirubin (µg/mL) | 71 | 84.73 ± 119.64 | 8.00 | 611.00 | 25.30 | 39.30 | 83.00 |
| Albumin (µg/mL) | 71 | 29.68 ± 7.88 | 12.00 | 46.00 | 24.00 | 29.00 | 35.00 |
| Sodium (µg/mL) | 71 | 135.65 ± 4.77 | 117.00 | 141.00 | 134.00 | 137.00 | 138.00 |
| Creatinine (µg/mL) | 71 | 106.02 ± 96.15 | 41.00 | 530.00 | 61.30 | 72.00 | 105.40 |
| Parameter | vWF | P value | ||||||
| n | mean ± SD | Min | Max | Percentiles | ||||
| 25th | 50th | 75th | ||||||
| CTP score | ||||||||
| Class A | 18 | 258.0 ± 104.2 | 150 | 586 | 190.0 | 228.0 | 276.0 | Kruskal-Wallis H test: Chi-square (2) = 10.8177; P = 0.0045a |
| Class B | 25 | 336.5 ± 122.5 | 150 | 650 | 262.0 | 341.0 | 400.0 | |
| Class C | 28 | 411.5 ± 182.2 | 198 | 850 | 246.0 | 402.8 | 560.0 | |
| Class А/B = Mann-Whitney U test: Z = -2.191; P = 0.028a | ||||||||
| Class А/C = Mann-Whitney U test: Z = -3.028; P = 0.002a | ||||||||
| Class B/C = Mann-Whitney U test: Z = -1.639; P = 0.101 | ||||||||
| MELD score | ||||||||
| Group 1 | 9 | 271.5 ± 106.3 | 150 | 513 | 225.0 | 257.0 | 276.0 | Kruskal-Wallis H test: Chi-square (2) = 10.3357; P = 0.0057a |
| Group 2 | 33 | 301.0 ± 115.9 | 161 | 650 | 200.0 | 296.0 | 350.0 | |
| Group 3 | 29 | 420.8 ± 179.7 | 150 | 850 | 258.0 | 409.0 | 548.0 | |
| Group 1/2 = Mann-Whitney U test: Z = -0.690; P = 0.507 | ||||||||
| Group 1/3 = Mann-Whitney U test: Z = -0.031; P = 0.029a | ||||||||
| Group 2/3 = Mann-Whitney U test: Z = -2.942; P = 0.003a | ||||||||
| SIRS score | ||||||||
| SIRS (+) | 43 | 339.2 ± 142.0 | 150 | 680 | 200.0 | 336.5 | 409.0 | Mann-Whitney U test: Z = -0.3529; P = 0.7241 |
| SIRS (-) | 28 | 356.9 ± 177.5 | 150 | 850 | 216.0 | 309.2 | 410.8 | |
| Ferritin (ng/mL) | ||||||||
| < 200 | 39 | 310.0 ± 140.7 | 150.0 | 650.0 | 198.0 | 262.0 | 405.0 | Kruskal-Wallis H test: Chi-square (2) = 7.1653; P = 0.0278a |
| 200-400 | 5 | 343.3 ± 91.2 | 253.0 | 458.9 | 258.0 | 336.5 | 410.1 | |
| > 400 | 18 | 423.9 ± 171.0 | 199.7 | 850.0 | 300.0 | 400.5 | 548.0 | |
| < 200/200-400 = Mann-Whitney U test: Z = -0.292; P = 0.311 | ||||||||
| < 200/> 400 = Mann-Whitney U test: Z = -2.584; P = 0.010a | ||||||||
| 200-400/> 400 = Mann-Whitney U test: Z = -0.820; P = 0.446 | ||||||||
| Vitamin D (ng/mL) | ||||||||
| ≤ 20 | 22 | 279.8 ± 108.9 | 150.0 | 513.0 | 199.7 | 241.0 | 344.0 | Mann-Whitney U test: Z = -1.6916; P = 0.0907 |
| > 20 | 23 | 372.0 ± 179.8 | 161.0 | 850.0 | 214.0 | 385.0 | 458.9 | |
| PLT (109/ L) | ||||||||
| ≤ 100 | 40 | 350.8 ± 171.4 | 161 | 850 | 207.2 | 312.7 | 412.1 | Mann-Whitney U test: Z = -0.02898; P = 0.9769 |
| > 100 | 31 | 340.2 ± 136.0 | 150 | 598 | 239.0 | 337.0 | 405.0 | |
| D-dimer (µg/mL) | ||||||||
| ≤ 500 | 6 | 205.2 ± 38.5 | 150 | 262 | 187.2 | 203.5 | 225.0 | Mann-Whitney U test: Z = 2.6407; P = 0.0083a |
| > 500 | 63 | 355.9 ± 156.2 | 150 | 850 | 216.0 | 337.0 | 411.5 | |
The Cox proportional model and the univariate logistic regression analysis showed that vWF, MELD score, and CRP were significantly associated with the event (death) and significant predictors of mortality in all three follow-up periods. The Cox proportional model showed that vWF, MELD score, CRP, and aPTT were significantly associated with 3-mo survival; that vWF, MELD score, CRP, and vitamin D were significantly associated with 6-mo survival; and that vWF, MELD score, CRP, vitamin D, ferritin, and aPTT were significantly associated with 1-year survival (Table 3). The univariate logistic regression analysis showed that vWF, MELD score, CRP, and D-dimer concentration were significant predictors of 3-mo mortality; that vWF, MELD score, CRP, and vitamin D were significant predictors of 6-mo mortality, and that vWF, MELD score, CRP, and ferritin were significant predictors of 1-year mortality (Table 3).
| Parameter | Univariate Cox proportional model | Univariate logistic regression analysis | ||||||||||
| 3-mo | 6-mo | 1-yr | 3-mo | 6-mo | 1-yr | |||||||
| Sig. | Exp (B) | Sig. | Exp (B) | Sig. | Exp (B) | Sig. | Exp (B) | Sig. | Exp (B) | Sig. | Exp (B) | |
| vWF | 0.004a | 1.004 | 0.005a | 1.006 | 0.000a | 1.004 | 0.008a | 1.005 | 0.005a | 1.006 | 0.002a | 1.007 |
| MELD | 0.000a | 1.144 | 0.000a | 1.157 | 0.000a | 1.116 | 0.000a | 1.191 | 0.000a | 1.157 | 0.000a | 1.176 |
| CRP | 0.000a | 1.029 | 0.001a | 1,044 | 0.000a | 1.025 | 0.001a | 1.044 | 0.001a | 1.044 | 0.002a | 1.046 |
| Vitamin D | 0.077 | 0.923 | 0.013a | 0.877 | 0.040a | 0.939 | 0.096 | 0.918 | 0.013a | 0.877 | 0.061 | 0.931 |
| Ferritin | 0.119 | 1.001 | 0.333 | 1.001 | 0.016a | 1.001 | 0.104 | 1.001 | 0.333 | 1.001 | 0.015a | 1.003 |
| aPTT | 0.000a | 1.05 | 0.068 | 1.052 | 0.000a | 1.051 | 0.059 | 1.049 | 0.068 | 1.052 | 0.067 | 1.055 |
| TT | 0.258 | 1.034 | 0.588 | 1.02 | 0.426 | 1.021 | 0.292 | 1.041 | 0.588 | 1.02 | 0.529 | 1.023 |
| D-dimer | 0.061 | 1 | 0.014a | 1 | 0.059 | 1 | 0.003a | 1.001 | 0.014 | 1 | 0.008 | 1 |
| Dependent variable-survival in days; significant for a | Dependent variable-mortality no/yes; significant for a | |||||||||||
Regarding the association between vWF and survival in cirrhotic patients, the analysis showed that vWF was significantly associated with survival in all three follow-up periods and that each vWF unit significantly increased the daily association with death by 0.4% regarding 3-mo [Exp(B) hazard ratio (HR) = 1.004], by 0.6% regarding 6-mo [Exp(B) (HR) = 1.006], and by 0.4% [Exp(B) (HR) = 1.004] regarding 1-year survival. Regarding mortality, the analysis confirmed that each vWF unit significantly increased the probability for 3-mo mortality by 1.005 (P = 0.008) times, for 6-mo mortality by 1.006 (p = 0.005) times, and for 1-year mortality by 1.007 (P = 0.002) times (Table 3).
Regarding the association between CRP and survival in cirrhotic patients, the analysis showed that CRP was significantly associated with survival in all three follow-up periods and that each CRP unit significantly increased the daily association with the event (death) by 2.9% [Exp(B) (HR) = 1.029] regarding the 3-mo, by 4.4% [Exp(B) (HR) = 1.044] regarding the 6-mo, and by 2.5% [Exp(B) (HR) = 1.025] regarding the 1-year survival. More importantly, we confirmed that CRP was a significant predictor of mortality in patients with liver cirrhosis and that each CRP unit significantly increased the probability for 3-mo mortality by 1.044 (P = 0.001) times, for 6-mo mortality by 1.044 (P = 0.001) times, and for 1-year mortality by 1.046 (P = 0.002) times (Table 3).
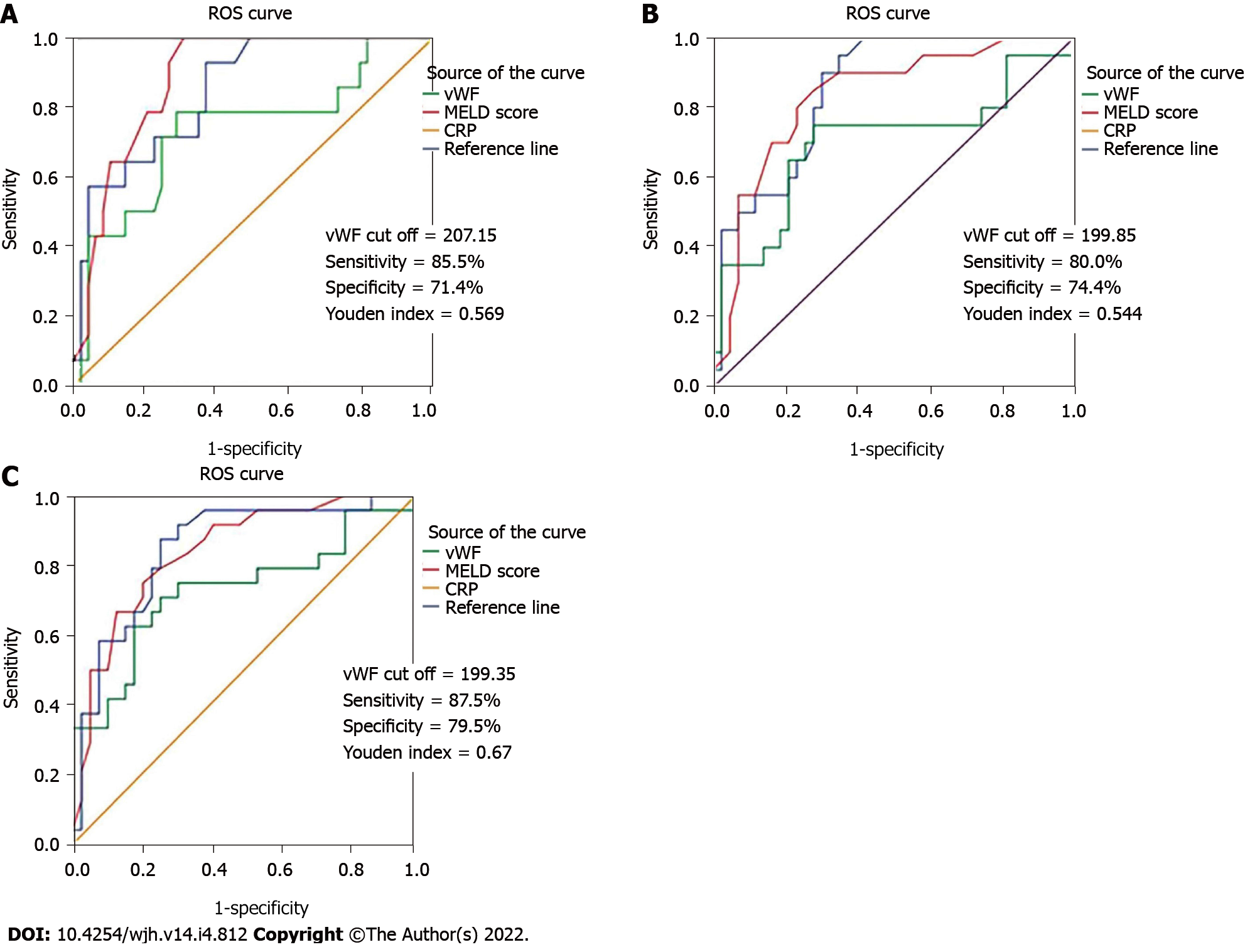
The ROC analysis did not confirm a statistically significant difference between the AUC values of the ROC curves for vWF and MELD score (Z = 1.459; P = 0.1444) and between the AUC values for vWF and CRP (Z = 1.063; P = 0.2876) regarding the 3-mo mortality [vWF-AUC = 0.734 (95%CI: 0.571-0.897), P = 0.008; MELD score-AUC = 0.884 (95%CI: 0.803-0.966), P = 0.000 and CRP-AUC = 0.848 (95%CI: 0.744-0.953), P = 0.000], between the AUC values for vWF and MELD score (Z = 1.385;P = 0.1662) and between the AUC values for vWF and CRP (Z = 1.601; P = 0.547) regarding the 6-mo mortality [vWF-AUC = 0.700 (95%CI: 0.544-0.856), P = 0.011; MELD score- AUC = 0.833 (95%CI: 0.725-0.940), P = 0.000 and CRP-AUC = 0.851 (95%CI: 0.758-0.943), P = 0.000], and between the AUC values for vWF and MELD score (Z = 1.276; P = 0.20192) and between the AUC values for vWF and CRP (Z = 1.366; P = 0.1718) regarding the 1-year mortality [vWF – AUC = 0.729 (95%CI: 0.590-0.868), P = 0.002; MELD score – AUC = 0.841 (95%CI: 0.742-0.941), P = 0.000 and CRP- AUC = 0.848 (95%CI: 0.747-0.948), P = 0.000] (Figure 3). According to the ROC curve, we received vWF cut off = 207.15; sensitivity = 85.5%; specificity = 71.4%, Youden index = 0.569 for 3-mo mortality, vWF cut off = 199.85; sensitivity = 80.0%; specificity = 74.4%, Youden index = 0.544 for 6-mo mortality, and vWF cut off = 199.35; sensitivity = 87.5%; specificity = 79.5%; Youden index = 0.67 for 1-year mortality.
Our study confirmed a significant relation between vWF and liver cirrhosis, CRP, ferritin, and D-dimer concentration. The study also confirmed that in patients with liver cirrhosis vWF, MELD score and CRP were significantly related to death and significant predictors of 3-mo, 6-mo, and 1-year mortality. Regarding mortality, our study did not confirm a significant difference between the diagnostic performance of vWF and MELD score and between the diagnostic performance of vWF and CRP.
Regarding its pronounced ability for short-term outcomes prediction, MELD score is the most widely accepted and currently the most useful indicator of liver function. Its wide scale provides high precision and good discriminating ability in assessing the death risk in cirrhotic patients[12]. However, the variability due to different laboratory methodologies[13], the low ability for prediction of post-transplant outcomes[14], the lower discriminating power of the lower MELD values[15], and its poor usefulness in compensated disease and in acute decompensation are the most pointed limitations of MELD score. Considering these facts many studies investigated the predictive value of other potential prognostic indicators in cirrhotic patients and compared it to the prognostic value of MELD score.
Most studies that evaluated the predictive value of vWF in cirrhotic patients confirmed significantly higher vWF values in patients with an advanced stage of disease[5,16-18] and in uncensored patients[18,19] and also a significant predictive value of vWF for mortality[18]. We also confirmed a significantly higher vWF level in patients with higher CTP class and in higher MELD group. Still, considering the fact that vWF does not always adequately correlate with the indicators of liver dysfunction, it seems that higher vWF concentration in advanced disease is probably more directly related to the degree of PH than with the level of liver dysfunction. One of the most important findings of our research was that along with MELD score and CRP, vWF was significantly associated with death and that vWF was a significant predictor of mortality in all follow-up periods.
Since MELD score is currently the most reliable short-term mortality predictor in cirrhotic patients, we compared the diagnostic efficacy of vWF to the diagnostic efficacy of MELD score. The ROC analysis in the study did not confirm a significant difference between the diagnostic performances of the two parameters, suggesting that the predictive value of vWF for mortality is similar to the predictive value of MELD score. Most studies in the literature that compared the diagnostic performance of the two parameters for mortality came across similar results[16,18]. The study of Kalambokis et al[17] demonstrated that the predictive performance of vWF for new-onset ascites and for variceal bleeding was stronger than that of MELD score, suggesting that in terms of liver disease complications, the procoagulant state could be a stronger determining factor than the severity of the liver disease. Previous research investigating the prognostic role of vWF defined cut-off values with the best sensitivity and specificity discriminating patients with significantly different prognoses[16-18]. Ferlitsch et al[18] defined a vWF cut-off value of 315% that stratifies patients with completely different survival. La Mura et al[16] defined vWF value of 216 U/dL, differencing two groups of patients with significantly different probability of survival without the occurrence of clinical events related to death and transplantation. Kalambokis et al[17] defined a vWF cut-off value of 392%, indicating significantly higher 3-year mortality in patients with liver cirrhosis. According to the ROC curves, we also defined cut-off values for mortality (207.15% for 3-mo, 199.85% for 6-mo, and 199.35% for 1-year mortality) that did not differ much between each other.
Regarding the complex pathogenesis of PH and its influence on liver disease progression, we tried to make a deeper insight into the role of ED and SI, into their mutual interaction, and also into their interaction with the numerous and complex hemostatic abnormalities within coagulopathy associated with chronic liver disease. In this context, we analyzed the relation between vWF and some biological variables that reflect SI or that are considered to have some prognostic potential in patients with liver cirrhosis. Our study showed that vWF was not only associated with CTP and MELD score, but it was also significantly associated with some other variable and prognostic indicators in these patients.
Since our analysis confirmed a strong significant correlation only between vWF and D-dimer concentration, we wanted to analyze this relation more profoundly. D-dimer concentration is a specific indicator of fibrin turnover and the most widely used indicator of active coagulation and fibrinolysis. Hyperfibrinolysis is present in approximately one-third of cirrhotic patients[20], and in some of them, low-grade disseminated intravascular coagulation has also been registered[21]. It has been established that the abnormalities in the fibrinolytic system were more pronounced in patients with advanced, decompensated cirrhosis[20,22]. Still, the main dilemma regarding hyperfibrinolysis in these patients is whether it occurs mainly as a primary phenomenon or is induced secondarily as a response to activated coagulation, most commonly within disseminated intravascular coagulation. Previously reported data related to the prognostic relevance of D-dimer levels in cirrhotic patients have confirmed a significant association between elevated D-dimer concentration and liver dysfunction[22]. Although some authors suggest that the intense ascites reabsorption stimulates hyperfibrinolysis in patients with advanced disease[23,24] still, endotoxemia is probably the key factor that induces hyperfibrinolysis through endothelial activation and release of fibrinolytic substances[25]. It seems that the crucial role of ED in these developments may explain the relationship between elevated vWF and D-dimer concentration. In addition to the strong correlation, we also registered significantly higher vWF values in patients with elevated (> 500 µg/mL) D-dimer levels (355.9 ± 156.2 vs 205.2 ± 38.5, P = 0.0232). More importantly, our study also confirmed that elevated D-dimer levels were a significant predictor of 3-mo mortality (P = 0.003). Some previous studies have proven that in patients with liver cirrhosis elevated D-dimer levels were related to poor outcomes and high short-term mortality[26,27]. Still, as far as we are aware, elevated D-dimer levels have not been specifically related to 3-mo mortality previously. These findings confirm the important role of ED underlying the hemostatic abnormalities as well as the relation between ED, procoagulant tendency, and short-term mortality in cirrhotic patients.
Taking into account the important prognostic role of SI, especially in advanced disease, we analyzed the SIRS occurrence, its relation to CRP as SIRS indicator, and its relation to vWF as an indicator of ED. Considering the fact that ED and SI coexist and support each other, we assumed that SIRS would be accompanied by higher vWF values. On the contrary, the analysis did not confirm a significant difference between vWF values in patients with and without SIRS (P = 0.7241). The positive linear correlation between CRP and vWF (r = 0.315) and the absent relation between vWF and SIRS mainly indicates that the applied ACCP / SCCM criteria for SIRS do not reflect the presence of SI adequately. Some previous studies have shown that ACCP/SCCM criteria are generally not suitable for use in cirrhotic patients[28,29], which has raised interest in CRP as an indicator of SIRS and also as a prognostic indicator in cirrhotic patients. According to some findings, elevated CRP in cirrhotic patients is not only a reliable indicator of active bacterial infection[30], but it may also reflect persistent low-grade SI even outside the context of active infection[6]. Moreover, one of the most significant limitations of MELD score is that the formula does not include a variable that reflects inflammation, such as leukocyte count or CRP, suggesting that MELD score does not take into account the presence of SI, a condition that from a prognostic point of view has great importance in cirrhotic patients[31]. Regarding the predictive value of CRP for mortality, our study confirmed that along with vWF and MELD score, CRP has been significantly associated with death and that CRP has been a significant mortality predictor in all three follow-up periods, which was the most important finding regarding this issue. The ROC analysis comparing the corresponding AUC values for mortality did not show a significant difference between the diagnostic efficacy of vWF and CRP, indicating that in cirrhotic patients vWF and CRP were a significant mortality predictor with a similar predictive value, which, according to our knowledge on this topic, has not been reported previously.
Elevated SF is registered in about 30% of patients with advanced liver disease, and it is mainly due to the release of ferritin from the damaged hepatocytes[32,33]. Previous research has shown a significant association between SF and almost all known predictors of poor outcome in decompensated patients (MELD score, CTP score, leukocyte count, sodium level, ACLF stages, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome)[34], but as far as we are aware, the relation between vWF and SF in cirrhotic patients has not been previously evaluated. Except for the significant positive correlation between vWF and ferritin (r = 0.360, P = 0.04), we also confirmed significantly higher vWF levels in patients with higher SF concentration (P = 0.0278). Despite the well-known relation between SF and liver cirrhosis, several studies have also confirmed the significant prognostic value of SF for mortality[32,33]. Walker et al[33] confirmed that SF was higher than 400 µg/L in all uncensored cirrhotic patients and that SF above 500 µg/L was an accurate predictor of 6-mo and 1-year mortality. The exact pathophysiological mechanism that explains this relation is not completely understood. It is presumed that an increased hepatic iron concentration promotes additional oxidative hepatocellular injury and also stellate cell activation, which can explain the ferritin involvement in the progression of liver disease and the relation between SF and mortality in cirrhotic patients[32]. Regarding mortality prediction, our study confirmed that in patients with liver cirrhosis SF was significantly associated with 1-year survival (P = 0.016) and that SF was a significant predictor of 1-year mortality (P = 0.015). Unlike some studies[32], our research did not confirm an association between SF and 3-mo and 6-mo mortality and a significant predictive value for short-term mortality in cirrhotic patients.
It is known that chronic liver disease is related to high prevalence of vitamin D deficiency that according to some data might reach up to 90%[35]. It is also considered that vitamin D deficiency additionally worsens liver dysfunction; it is related to decompensation and has a negative impact on the prognosis and survival especially in advanced liver disease[36,37]. Our study confirmed that vitamin D concentration was significantly associated with 6-mo and 1-year survival and that vitamin D was a significant predictor of 6-mo mortality (P = 0.013). However, according to some data, the cut-off value that defines vitamin D deficiency (20 ng/mL) does not appear to be a significant risk factor in cirrhotic patients[36]. In this context, one study showed that mortality was significantly affected only when a vitamin D cut-off value of 6 ng/mL was applied[36]. Despite the well-established predictive value of vitamin D in cirrhotic patients, our study did not confirm a relationship between vWF and vitamin D deficiency. We did not show a significant correlation between vWF and vitamin D (r = 0.064) or significantly higher vWF values in patients with vitamin D deficiency (P = 0.0907). The negative prognostic influence of vWF in cirrhotic patients is mainly due to its prothrombotic potential, which is a factor for progression of PH. On the other hand, the prognostic potential of vitamin D is mostly due to its effect on the immune system. Hence, it seems that in this case, these two parameters are involved differently in the pathogenesis of liver disease progression, which may explain the absence of a direct association between them.
In patients with liver cirrhosis, vWF is elevated and significantly related to the stage of the disease and other prognostic and inflammatory indicators. vWF is significantly associated with death and is a significant predictor of 3-mo, 6-mo, and 1-year mortality similar to MELD score and CRP. The significant prognostic value of CRP in cirrhotic patients confirms the important prognostic role of SI in these patients and highlights the importance of recognizing the condition for more accurate mortality prediction. Although generally reflecting an increased prothrombotic state, hyperfibrinolysis and elevated D-dimer levels in these patients should be analyzed in relation to clinical presentation, stage of disease, and other hemostatic parameters. The significant interaction between the variables analyzed in the study has reflected the complex and dynamic interaction between ED, SI, and cirrhosis-related coagulopathy that occurs in patients with liver cirrhosis.
Endothelial dysfunction (ED) and systemic inflammation (SI) play an important role in the pathogenesis of portal hypertension (PH). Von-Willebrand factor (vWF) is an indicator of ED that favors a prothrombotic state, and hence it is directly involved in the progression of PH. Although previous research confirmed its prognostic value in cirrhotic patients, its relation to other prognostic indicators has not been properly evaluated. By analyzing the relation between vWF and other biological variables with certain prognostic potential, our research provides an insight into the complex relation between ED, SI, and liver-disease related coagulopathy in cirrhotic patients.
Although Model for End-stage Liver Disease (MELD) score is the most widely used prognostic score in cirrhotic patients, it does not take into account the presence of circulatory dysfunction or SI and it does not assess the coagulopathy properly. This raises the need for further research towards identifying new biological variables with certain prognostic potential in cirrhotic patients and evaluating their prognostic value for mortality. This could lead toward defining new prognostic scores or improve the predictive value of those currently in use. Recent researchers have suggested that some biological variables such as vWF, C-reactive protein (CRP), ferritin, and vitamin D possess certain prognostic potential in cirrhotic patients, but this area has not been widely investigated.
We tried to analyze the relation between vWF and liver cirrhosis and the relation between vWF and several inflammatory indicators and other variables that have certain prognostic potential in cirrhotic patients. We also tried to evaluate the prognostic value of vWF and several parameters in terms of 3-mo, 6-mo, and 1-year mortality.
We conducted an analytic prospective study that enrolled 71 patients with liver cirrhosis and portal hypertension. At enrollment, we performed detailed examinations (abdominal ultrasound, complete blood count, biochemical blood analysis, basic hemostasis, D-dimer, vWF concentration) in order to assess the stage of the liver disease after which we followed the patients for 1 year. We analyzed the relation between vWF and chronic liver disease and between vWF and several prognostic and inflammatory indicators. We prospectively evaluated the prognostic value of vWF and several other variables (MELD score, CRP, ferritin, vitamin D, activated partial thromboplastin time, thrombin time, D-dimer concentration) in terms of 3-mo, 6-mo, and 1-year mortality, and we compared the diagnostic efficacy of vWF for morality to other significant mortality predictors.
Our study confirmed a significant relation between vWF and the stage of liver disease, CRP, ferritin, and D-dimer concentration. The study also confirmed that in patients with liver cirrhosis vWF, MELD score, and CRP were significantly related to 3-mo, 6-mo, and 1-year survival and significant predictors of 3-mo, 6-mo, and 1-year mortality. Our study did not confirm a significant difference between the diagnostic performance for mortality of vWF and MELD score and between the diagnostic performance of vWF and CRP.
In patients with liver cirrhosis, vWF is a significant and relevant mortality predictor similar to MELD score and CRP, which highlights the important role of the ED in the pathogenesis of PH. Elevated CRP is a significant mortality predictor in patients with liver cirrhosis, which emphasizes the importance of recognizing the presence of SI for accurate mortality prediction. The relation between vWF and D-dimer concentration, ferritin, and CRP reflects the complex and dynamic interaction between ED, SI, and cirrhosis-related coagulopathy that occurs in patients with liver cirrhosis.
Future research should be focused on identifying specific clinical settings in which vWF would have more accurate prognostic value.
The authors are very grateful to Jane Misevski, MD for critical revision of the manuscript for important intellectual content, to Violeta Neceva, MD, PhD from the Hemostasis Laboratory of Institute of Transfusional Medicine for the technical assistance, to Vesna Velic-Stefanovska MD, PhD for the assistance with the statistical analysis, and to Lence Danevska for proofreading the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; European Society of Gastrointestinal Endoscopy; European Society for Clinical Nutrition and Metabolism; and World Gastroenterology Organization.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: North Macedonia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de la Pinta C, Spain S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wu RR
| 1. | Di Martino V, Weil D, Cervoni JP, Thevenot T. New prognostic markers in liver cirrhosis. World J Hepatol. 2015;7:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 337] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Wannhoff A, Müller OJ, Friedrich K, Rupp C, Klöters-Plachky P, Leopold Y, Brune M, Senner M, Weiss KH, Stremmel W, Schemmer P, Katus HA, Gotthardt DN. Effects of increased von Willebrand factor levels on primary hemostasis in thrombocytopenic patients with liver cirrhosis. PLoS One. 2014;9:e112583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol. 2015;7:1974-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 8. | Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Curakova Ristovska E. Endothelial dysfunction and systemic inflammation in the pathogenesis and progression of portal hypertension. In: Qi X. Portal hypertension-Recent advances. London: IntechOpen; 2021. [DOI] [Full Text] |
| 10. | Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol. 2015;7:443-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Li CJ, Yang ZH, Lu FG, Shi XL, Liu DL. Clinical significance of fibrotic, haemostatic and endotoxic changes in patients with liver cirrhosis. Acta Gastroenterol Belg. 2018;81:404-409. [PubMed] |
| 12. | Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, Burroughs AK. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Trotter JF, Brimhall B, Arjal R, Phillips C. Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl. 2004;10:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Yoo HY, Thuluvath PJ. Short-term postliver transplant survival after the introduction of MELD scores for organ allocation in the United States. Liver Int. 2005;25:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Huo TI, Lin HC, Wu JC, Lee FY, Hou MC, Lee PC, Chang FY, Lee SD. Different model for end-stage liver disease score block distributions may have a variable ability for outcome prediction. Transplantation. 2005;80:1414-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | La Mura V, Reverter JC, Flores-Arroyo A, Raffa S, Reverter E, Seijo S, Abraldes JG, Bosch J, García-Pagán JC. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut. 2011;60:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Kalambokis GN, Oikonomou A, Christou L, Kolaitis NI, Tsianos EV, Christodoulou D, Baltayiannis G. von Willebrand factor and procoagulant imbalance predict outcome in patients with cirrhosis and thrombocytopenia. J Hepatol. 2016;65:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, Payer BA, Trauner M, Peck-Radosavljevic M, Ferlitsch A. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology. 2012;56:1439-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Curakova Ristovska E, Genadieva-Dimitrova M, Caloska-Ivanova V, Misevski J. Von-Willebrand factor as a predictor of three-month mortality in patients with liver cirrhosis compared to MELD score. Acta Gastroenterol Belg. 2019;82:487-493. [PubMed] |
| 20. | Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Violi F, Ferro D, Basili S, Saliola M, Quintarelli C, Alessandri C, Cordova C. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Cong YL, Wei YX, Zhang LW, Yin ZJ, Bai J. [The relationship between hemostatic changes in liver cirrhosis patients with different degrees of liver lesions in reference to Child-Pugh scores]. Zhonghua Gan Zang Bing Za Zhi. 2005;13:31-34. [PubMed] |
| 23. | Saray A, Mesihovic R, Gornjakovic S, Vanis N, Mehmedovic A, Nahodovic K, Glavas S, Papovic V. Association between high D-dimer plasma levels and ascites in patients with liver cirrhosis. Med Arch. 2012;66:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Agarwal S, Joyner KA Jr, Swaim MW. Ascites fluid as a possible origin for hyperfibrinolysis in advanced liver disease. Am J Gastroenterol. 2000;95:3218-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Hanss M, Collen D. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by cultured human endothelial cells: modulation by thrombin, endotoxin, and histamine. J Lab Clin Med. 1987;109:97-104. [PubMed] |
| 26. | Primignani M, Dell'Era A, Bucciarelli P, Bottasso B, Bajetta MT, de Franchis R, Cattaneo M. High-D-dimer plasma levels predict poor outcome in esophageal variceal bleeding. Dig Liver Dis. 2008;40:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Li Y, Qi X, Li H, Dai J, Deng H, Li J, Peng Y, Liu X, Sun X, Guo X. D-dimer level for predicting the in-hospital mortality in liver cirrhosis: A retrospective study. Exp Ther Med. 2017;13:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Lan P, Wang SJ, Shi QC, Fu Y, Xu QY, Chen T, Yu YX, Pan KH, Lin L, Zhou JC, Yu YS. Comparison of the predictive value of scoring systems on the prognosis of cirrhotic patients with suspected infection. Medicine (Baltimore). 2018;97:e11421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Curakova Ristovska E, Genadieva-Dimitrova M, Caloska-Ivanova V, NikolovskaE, Joksimovic N, Todorovska B, Milichevik I, Isahi U. The SIRS score relevance for assessment of systemic inflammation compared to C-reactive protein in patients with liver cirrhosis. Mac Med Preview. 2019;73:24-30. |
| 30. | Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599-607. [PubMed] |
| 31. | Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, Vanlemmens C, Thevenot T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015;21:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, Rastogi A, Bihari C, Vashisht C, Sarin SK. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol. 2014;61:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Walker NM, Stuart KA, Ryan RJ, Desai S, Saab S, Nicol JA, Fletcher LM, Crawford DH. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010;51:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Kowdley KV, Brandhagen DJ, Gish RG, Bass NM, Weinstein J, Schilsky ML, Fontana RJ, McCashland T, Cotler SJ, Bacon BR, Keeffe EB, Gordon F, Polissar N; National Hemochromatosis Transplant Registry. Survival after liver transplantation in patients with hepatic iron overload: the national hemochromatosis transplant registry. Gastroenterology. 2005;129:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 36. | Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, Obermayer-Pietsch B, Stauber RE. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |