Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.778
Peer-review started: September 30, 2021
First decision: December 4, 2021
Revised: December 30, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 27, 2022
Processing time: 204 Days and 5 Hours
As survival has been prolonged owing to surgical and medical improvements, liver failure has become a prognostic determinant in patients with congestive heart diseases. Congestive hepatopathy, an abnormal state of the liver as a result of congestion, insidiously proceed toward end-stage liver disease without effective biomarkers evaluating pathological progression. Regular measurements of shear wave elastography cannot qualify liver fibrosis, which is a prognosticator in any type of chronic liver disease, in cases of congestion because congestion makes the liver stiff without fibrosis. We hypothesized that the effects of congestion and fibrosis on liver stiffness can be dissociated by inducing architectural deformation of the liver to expose structural rigidity.
To establish a strategy measuring liver stiffness as a reflection of architectural rigidity under congestion.
Two-dimensional shear wave elastography (2dSWE) was measured in the supine (Sp) and left decubitus (Ld) positions in 298 consecutive cases as they were subjected to an ultrasound study for various liver diseases. Regions of interest were placed at twelve sites, and the median and robust coefficient of variation were calculated. Numerical data were compared using the Mann-Whitney U or Kruskal-Wallis test followed by Dunn's post-hoc multiple comparisons. The inferior vena cava (IVC) diameters at different body positions were compared using the Wilcoxon matched pairs signed rank test. The number of cases with cardiothoracic ratios greater than or not greater than 50% was compared using Fisher’s exact test. A correlation of 2dSWE between different body positions was evaluated by calculating Spearman correlation coefficients.
The IVC diameter was significantly reduced in Ld in subjects with higher 2dSWE values in Ld (LdSWE) than in Sp (SpSWE) (P = 0.007, (average ± SD) 13.9 ± 3.6 vs 13.1 ± 3.4 mm) but not in those with lower LdSWE values (P = 0.32, 13.3 ± 3.5 vs 13.0 ± 3.5 mm). In 81 subjects, SpSWE was increased or decreased in Ld beyond the magnitude of robust coefficient of variation, which suggests that body postural changes induced an alteration of liver stiffness significantly larger than the technical dispersion. Among these subjects, all 37 with normal SpSWE had a higher LdSWE than SpSWE (Normal-to-Hard, SpSWE - LdSWE (∆2dSWE): (minimum-maximum) -0.74 - -0.08 m/sec), whereas in 44 residual subjects with abnormal SpSWE, LdSWE was higher in 27 subjects (Hard-to-Hard, -0.74 - -0.05 m/sec) and lower in 17 subjects (Hard-to-Soft, 0.04 - 0.52 m/sec) than SpSWE. SpSWE was significantly correlated with ∆2dSWE only in Hard-to-Soft (P < 0.0001). ∆2dSWE was larger in each lobe than in the entire liver. When Hard-to-Hard and Hard-to-Soft values were examined for each lobe, fibrosis-4 or platelet counts were significantly higher or lower only for Hard-to-Soft vs Normal-to-Hard cases.
Gravity alters the hepatic architecture during body postural changes, causing outflow blockage in hepatic veins. A rigid liver is resistant to structural deformation. Stiff-liver softening in the Ld position suggests a fibrous liver.
Core Tip: Medical progress ironically makes the liver a prognostic determinant in patients with congenital heart diseases because there are no effective biomarkers to evaluate pathological progression in congestive hepatopathy. A canonical liver stiffness measurement cannot screen for fibrous liver under congestion because congestion itself makes the liver stiff without fibrosis. Here, we report a simple strategy of liver stiffness measurement to identify clues to liver fibrosis even under congestion. The basic data presented in this report provide insights not only for the clinical application of liver stiffness in patients with congestive heart diseases but also for the physiological components and mechanisms underlying liver stiffness.
- Citation: Suda T, Sugimoto A, Kanefuji T, Abe A, Yokoo T, Hoshi T, Abe S, Morita S, Yagi K, Takahashi M, Terai S. Gravity assistance enables liver stiffness measurements to detect liver fibrosis under congestive circumstances. World J Hepatol 2022; 14(4): 778-790
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/778.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.778
The survival of children and adolescents undergoing the Fontan procedure continues to improve as various modifications of this operation have been applied since 1968[1-3]. In conjunction with technological advancements in the pathophysiological evaluation of the liver, the frequency of encountering the spectrum of liver disease is increasing in patients with heart diseases. The frequency of nonalcoholic cirrhosis is reported to be greater than 4% among hospital admissions of patients with a single functional ventricle, whereas it is approximately 0.3% of hospitalizations for patients without congenital heart diseases[4]. The pathophysiology is termed congestive hepatopathy, which is not restricted to the postoperative condition of the Fontan procedure but arises from chronically elevated hepatic venous pressures secondary to biventricular or isolated right-sided heart failure. Low cardiac output itself may also accelerate fibrosis pathways by reducing circulating blood flow to the liver. To determine a specific patient’s prognosis, screening and management strategies (including candidacy for isolated heart or combined heart-liver transplantation), the detection of fibrous progression in the liver is critical. Unfortunately, there is a growing awareness that fibrosis biomarkers, such as serum tests, fibrosis calculators, and liver stiffness, are not reliable in congestive hepatopathy[5-7]. Even liver biopsy is unlikely to stage fibrosis and predict clinical outcomes accurately because the heterogeneity of fiber deposition is quite large in congestive hepatopathy[5].
Liver stiffness is a useful surrogate marker in viral hepatitis and alcoholic and nonalcoholic fatty liver diseases to assess the degree of fibrous accumulation in the liver[8-11], which is a good prognostic indicator irrespective of the etiologies for chronic liver diseases. Because liver stiffness is directly measured in the liver as a physical property, this value is fundamentally spared from systemic disparity. Based on its noninvasive nature, the value can be repeatedly measured from various sites, especially in shear wave elastography using acoustic radiation force impulse technology or in magnetic resonance elastography. On the other hand, the clinical feasibility may be limited in magnetic resonance imaging, as many patients with congestive hepatopathy have non-magnetic resonance compatible cardiac devices. Furthermore, congestion itself increases liver stiffness and causes overestimation of the amount of fibrosis, as was reported in transient elastography[12].
This study aims to establish a strategy that enables the evaluation of fibrous accumulation in the liver with respect to architectural rigidity under congestive circumstances by measuring shear wave elastography. After assessing the impacts of interstitial tissue pressure on shear wave elastography, the effects of body postural changes on the diameter of the inferior vena cava (IVC) and liver stiffness were evaluated. Based on the different reactions of shear wave elastography upon changing body positions, the patients were hypothetically divided into three groups: normal liver, congestive liver, and congestive liver with fiber accumulation. The Fibrosis-4 Index (FIB4) and its constituents were compared among groups to endorse the significance of hypothetical classification. The possibility of dissociating fibrosis from underlying congestion using a gravity aid to induce architectural deformity of the liver is discussed.
Two-dimensional shear wave elastography (2dSWE) was measured in both the supine and left decubitus positions in 298 consecutive patients, who were subjected to 2dSWE measurements for the evaluation of various diseases, including nonalcoholic fatty liver disease (NAFLD). The patients’ characteristics are summarized in Table 1. All studies were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008. Routine blood biochemistry was measured in the clinical laboratories of our hospital, where quality control of each test was regularly performed every day. NAFLD was diagnosed based on the criteria proposed by the Asia-Pacific Working Party on NAFLD[13]. Fatty liver was diagnosed by abdominal US as defined by an increased echogenicity of the liver along with the presence of any two of the following three findings: liver-kidney contrast, vascular blurring, and deep attenuation of echo-beam[14].
| Background | |||
| Sex (F:M) | 142:156 | ||
| Age | 62.31 | years old | 49.6-71.42 |
| BMI | 22.91 | kg/m2 | 20.8-25.52 |
| Liver diseases | |||
| Alcoholic liver disease | 28 | ||
| HBV | 38 | ||
| HCV | 40 | ||
| Nonalcoholic fatty liver diseases | 56 | ||
| Hepatocellular carcinoma | 12 | ||
| Other chronic liver dysfunction | 69 | ||
| Miscellaneous | 55 | Total | 298 |
| Shear wave elastography | |||
| 2dSWE (supine) | 1.52 (6.93)1 | m/sec (kPa) | 1.43-1.672 |
| (6.13-8.37) | |||
| 2dSWE (left decubitus) | 1.57 (7.39)1 | m/sec (kPa) | 1.46-1.742 |
| (6.39-9.08) | |||
| Wilcoxon matched-pairs signed rank test, P < 0.0001 | |||
| %CVRsup | 9.73 | % | 5.74 |
| %CVRsup ≤ Δ2dSWE% | 81 (27.2%) | ||
To clarify the relationship between liver stiffness and interstitial tissue pressure, virtual touch quantification of point shear wave elastography was measured before and after cardiac surgery in a different cohort consisting of 41 cases (19 males and 22 females, 5.5 (1.7-61.0) years old (median (interquartile range))) with disorders, including 10 valvular and 31 congenital heart diseases. No patients were treated or followed for chronic liver diseases. HBsAg negativity, anti-HCV antibody negativity, and no alcohol abuse were confirmed. Physical properties with respect to cardiac function were evaluated using ultrasound, chest X-ray, and cardiac catheterization. The data are shown in supplementary digital content Figure 1 and referenced in the discussion section.
The review boards of the Uonuma Institute of Community Medicine and Niigata University Medical and Dental Hospital approved the study measuring liver stiffness in our main cohort consisting of 298 cases with various diseases in two body positions and another cohort of 41 patients undergoing cardiac surgery. These studies did not require informed consent because they were retrospective studies using only medical records or noninvasive imaging examinations.
Shear wave elastography (SWE) evoked by acoustic radiation force impulse was measured as point shear wave elastography using an ACUSON S2000 ultrasound system (Siemens Healthcare, Eriangen, Germany) or as 2dSWE using an Aplio 500 (Canon Medical System Corporation, Ohtawara, Japan). SWE was measured thrice in each segment (posterior, anterior, medial, and lateral) with a transient breath hold at a neutral cycle after one-night of fasting followed by a 30 min or longer rest while the patient was in the supine position. A region of interest (ROI) was set between 1 and 5 cm beneath the liver capsule. In the case of 2dSWE measurements, the size of the ROI was approximately 30 mm × 30 mm square, and 3 measurements were achieved in each ROI by placing an acquisition circle 2 mm in diameter after confirming the proper propagation of shear waves in a “wavefront” style display. When 2dSWE was measured at two body positions, the measurements were performed again in the liver at 12 sites in the left decubitus position. SWE was measured in the cohort consisting of 298 or 41 cases by 7 ultrasonographers or 2 medical doctors, respectively, who had conducted SWE measurements every day for more than 2 years or ultrasonography of the abdomen for more than 2 decades and SWE measurements for more than 3 years.
To define the cutoff value of 2dSWE suggesting the least fiber accumulation in the liver, 2dSWE was measured in 480 voluntary annual medical checkup visitors who had been diagnosed with NAFLD one year prior. Because median 2dSWE values in the 480 visitors fit well on a Gaussian distribution represented by an average of 1.324 m/sec (5.26 kPa) with a standard deviation of 0.0847 m/sec (0.022 kPa, r2 = 0.98), a cutoff value to distinguish the liver with fiber accumulation was statistically defined and reported as the average plus standard deviation of 1.41 m/sec (5.96 kPa) [15].
A robust counterpart to the standard deviation was calculated as follows. First, the median absolute deviation was calculated as the median of the difference in the absolute values between each SWE and the median of 12 measurements; thereafter, a constant factor of 1.4826 was multiplied. Finally, the robust coefficient of variation (CVR) was calculated by dividing the robust standard deviation by the median and expressed as a percentage. The inter- or intraobserver variation was not evaluated. Numerical data from independent cases were compared using the Mann-Whitney U or Kruskal-Wallis test followed by Dunn's post-hoc multiple comparisons between two groups or among three groups, respectively. IVC diameters at different body positions in each case were compared using the Wilcoxon matched pairs signed rank test. A correlation of 2dSWE between different body positions was evaluated by calculating Spearman correlation coefficients. The number of cases with cardiothoracic ratios greater than or not greater than 50% was compared using Fisher’s exact test. The statistical methods of this study were reviewed by Professor Kohei Akazawa from the Department of Medical Informatics, Niigata University Medical and Dental Hospital. All statistical analyses were conducted with GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, USA), and two-sided P values less than 0.05 were considered statistically significant.
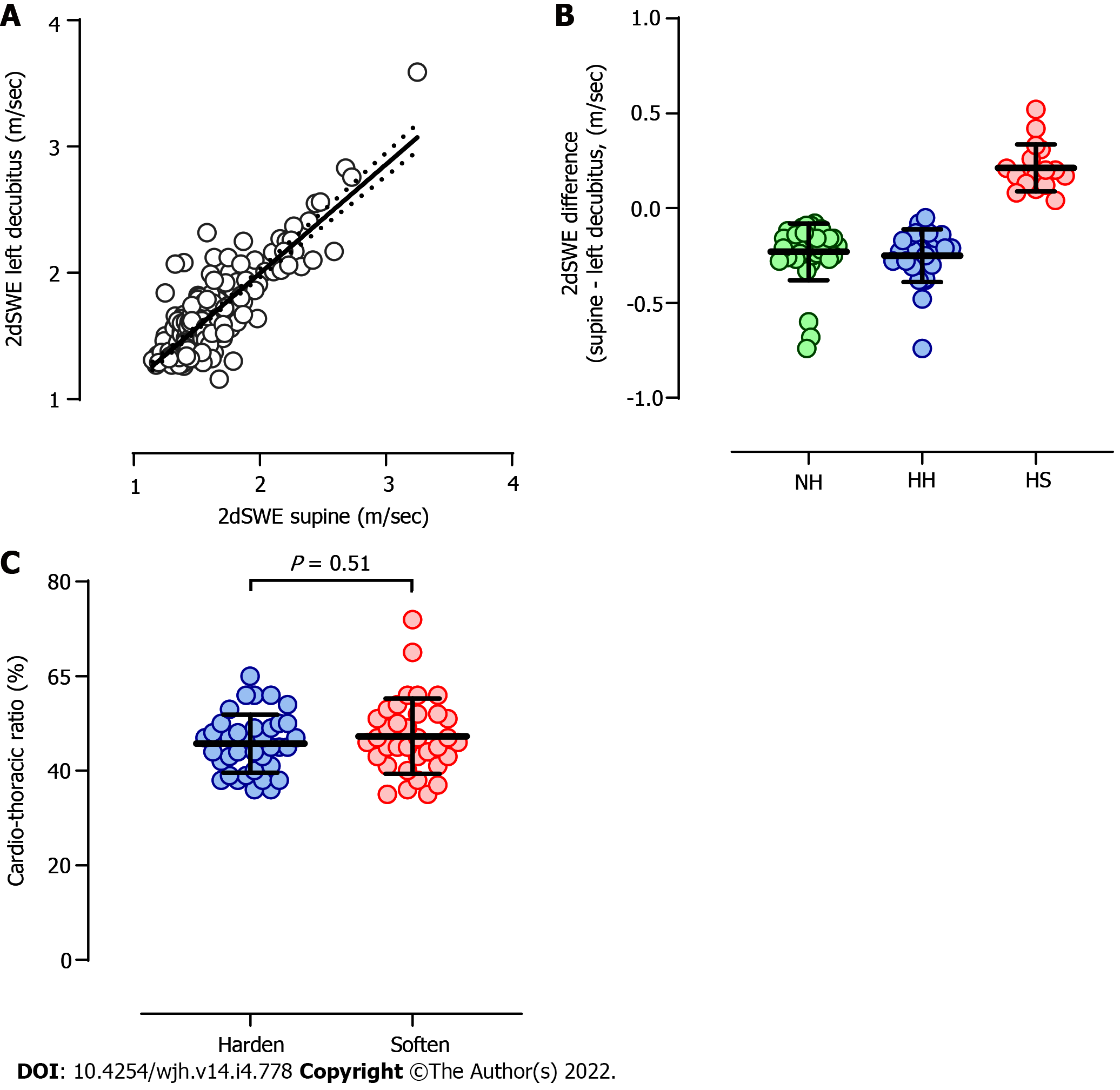
When 2dSWE was measured for both supine (SpSWE) and left decubitus (LdSWE) positions, the values revealed a significant positive correlation, as shown in Figure 1A (P < 0.0001, r = 0.68). Because 12 values of 2dSWE in each liver were dispersed on a case-by-case basis, it is reasonable to assume that 2dSWE is substantially affected by changing body positions only when the difference between SpSWE and LdSWE (∆2dSWE; SpSWE - LdSWE) is greater than the dispersion of SpSWE, which is a robust coefficient of variation (CVR). Among 298 cases, LdSWE increased or decreased from SpSWE over the magnitude of CVR in 81 cases (27.2%). These 81 cases can be classified into four groups based on SpSWE normality and positive/negative ∆2dSWE values. For 37 cases in which SpSWE was lower than the upper normal limit of 1.41 m/sec (5.96 kPa, see Methods), ∆2dSWE was negative in all the cases (Normal-to-Hard: NH), as shown in Figure 1B. On the other hand, in 44 cases with stiff livers in the supine position, ∆2dSWE was negative (Hard-to-Hard: HH) or positive (Hard-to-Soft: HS) in 27 and 17 cases, respectively. The 2dSWE values in each group at different body positions are summarized in Table 2.
| Shear wave elastography | |||
| Group (n) | Supine | ||
| Median | Inter quartile range | ||
| Normal-to-Hard (37) | 1.35 (5.47) | m/sec (kPa) | 1.30 (5.07)-1.38 (5.71) |
| Hard-to-Hard (27) | 1.56 (7.30) | m/sec (kPa) | 1.47 (6.48)-1.64 (8.07) |
| Hard-to-Soft (17) | 1.62 (7.87) | m/sec (kPa) | 1.57 (7.39)-1.84 (10.16) |
| Left decubitus | |||
| Median | Inter quartile range | ||
| Normal-to-Hard (37) | 1.53 (7.02) | m/sec (kPa) | 1.46 (6.39)-1.63 (7.97) |
| Hard-to-Hard (27) | 1.79 (9.61) | m/sec (kPa) | 1.68 (8.47)-1.99 (11.88) |
| Hard-to-Soft (17) | 1.52 (6.93) | m/sec (kPa) | 1.33 (5.31)-1.64 (8.07) |
To assess the possibility that ∆2dSWE is determined by cardiac function, the cardiothorax ratio was compared between cases with negative and positive ∆2dSWE. As shown in Figure 1C, the cardiothorax ratio was not significantly different between the two groups (P = 0.51). The number of cases showing a cardiothoracic ratio larger than 50% was 11 out of 35 ∆2dSWE-positive cases and 6 out of 37 ∆2dSWE-negative cases and was not significantly different between the two groups (P = 0.17).
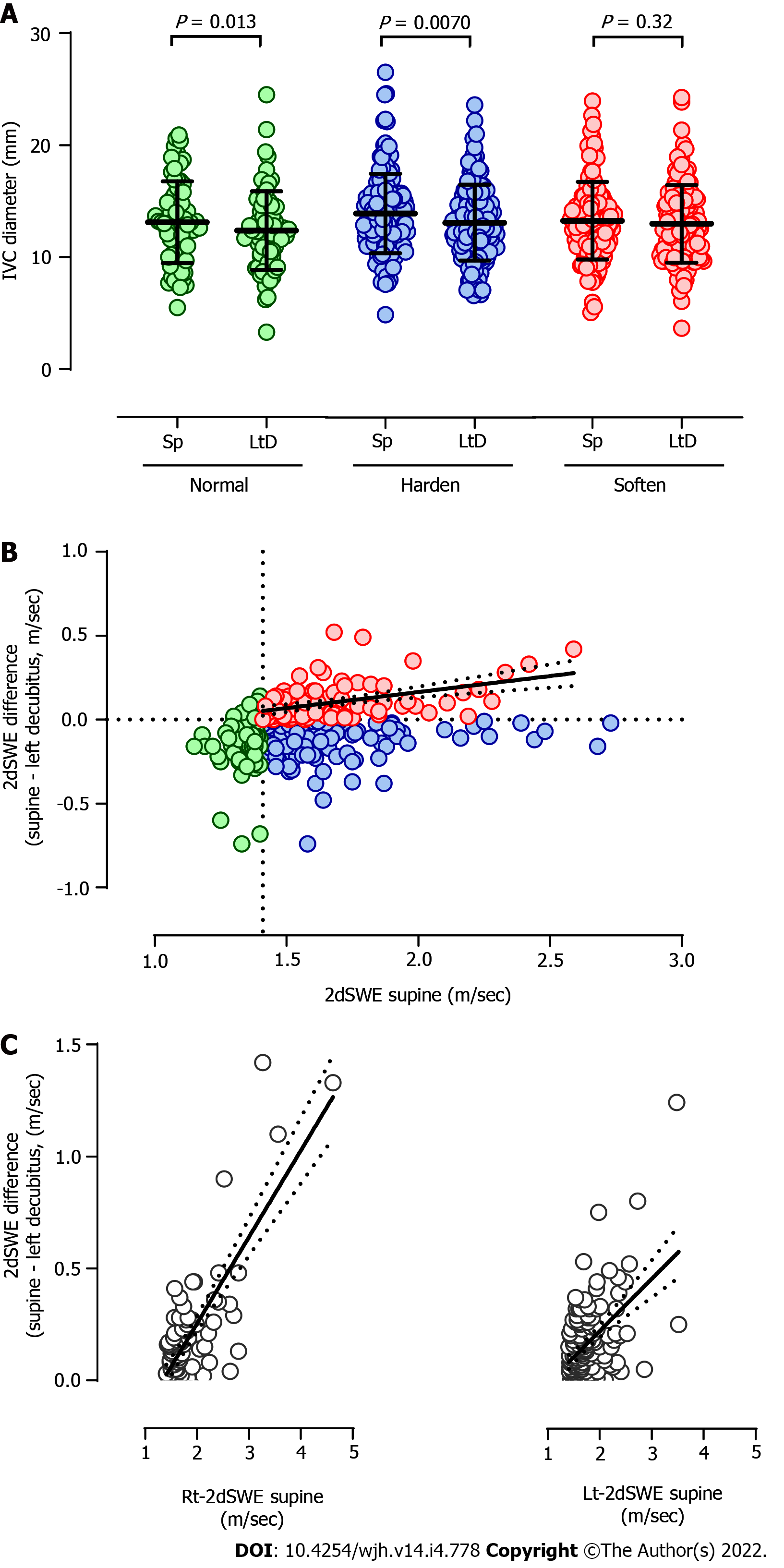
Next, the effects of body position on IVC diameter were evaluated irrespective of whether the ∆2dSWE scale was beyond or within the CVR. In the results, the diameter of the IVC in the left decubitus position was significantly reduced compared with that in the supine position in the cases showing normal liver stiffness in the supine position, as shown in the left panel of Figure 2A (P = 0.013). Consistently, the IVC diameter was also shortened in the cases with a stiff liver in the supine position that hardened further in the left decubitus position (Figure 2A middle panel, P = 0.0070). On the other hand, the IVC diameters in the supine and left decubitus positions were not significantly different in the cases with a stiff liver in the supine position that softened in the left decubitus position (Figure 2A right panel, P = 0.32).
To understand the implications of the pressure connection between the liver and IVC, the correlation between SpSWE and ∆2dSWE was evaluated. As shown in Figure 2B, a significant correlation was not observed in the cases showing normal liver stiffness in the supine position (P = 0.56) or the cases with a stiff liver in the supine position that hardened farther in the left decubitus position (P = 0.88). In contrast, SpSWE and ∆2dSWE revealed a significant positive correlation in the cases with a stiff liver in the supine position that softened in the left decubitus position (P < 0.0001, r = 0.38), suggesting a direct connection between the IVC pressure and the interstitial pressure of the liver. When the same relation was separately evaluated in the right or left lobe, as shown in Figure 2C, the correlation was clearly tighter in the right lobe (P < 0.0001, r = 0.48) than in the left lobe (P < 0.0001, r = 0.31).
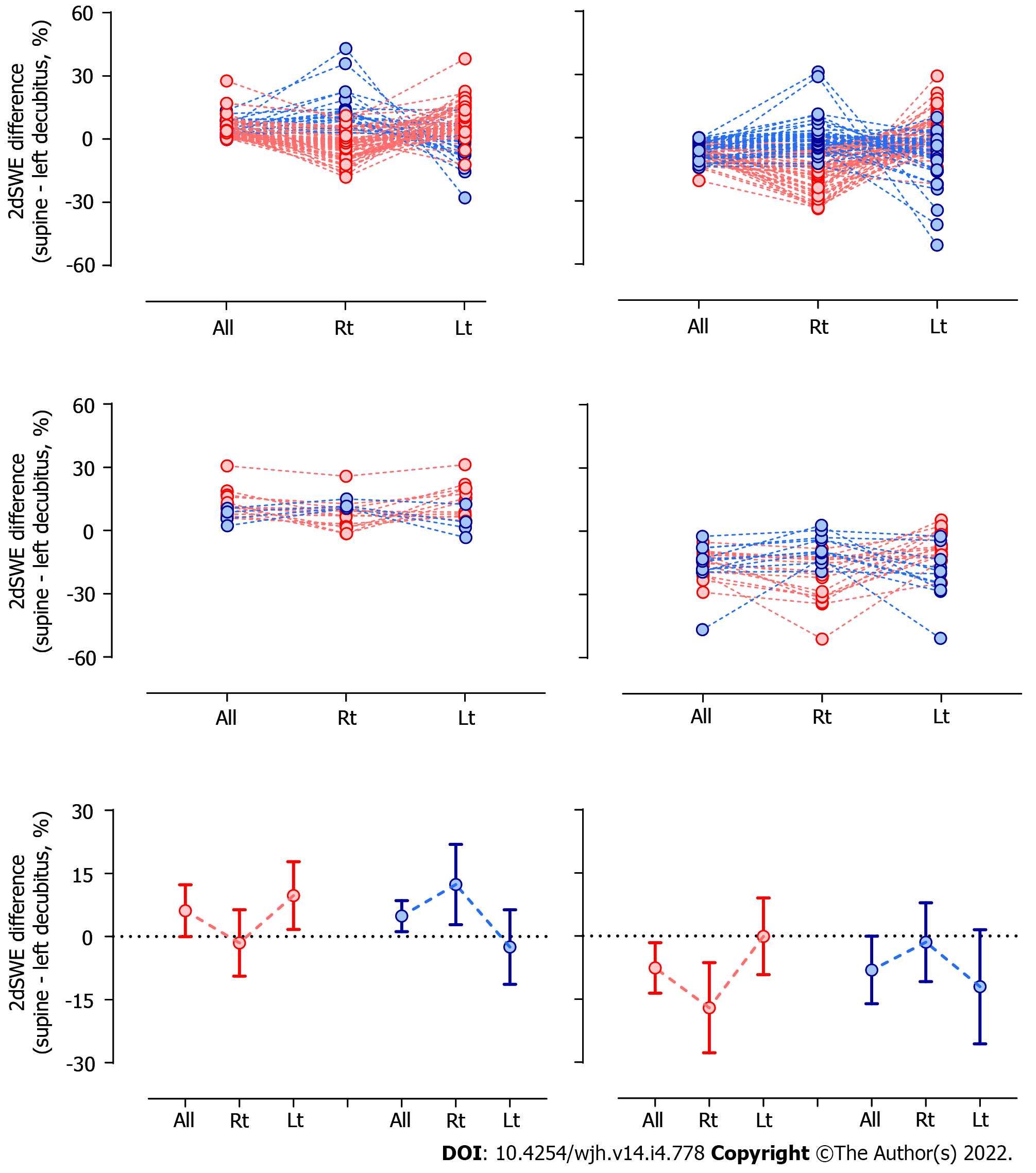
The paradoxical increment/shrinkage of LdSWE/IVC in the left decubitus position indicates that pressure thresholds exist between the hepatic veins and IVC, where outflow blocks would be built under architectural deformation of the liver during postural changes. Given that postural changes may not evenly impact the liver architecture, ∆2dSWE was separately evaluated in the right and left lobes. As shown in Figure 3, larger differences in ∆2dSWE were noted between the right and left lobes in cases with positive or negative ∆2dSWE values in the entire liver. When ∆2dSWE is positive or negative in the entire liver, ∆2dSWE in a single lobe is reciprocally negative or positive, respectively, suggesting that the impact of postural change on liver architecture would be detected much more easily in a single lobe than in the entire liver.
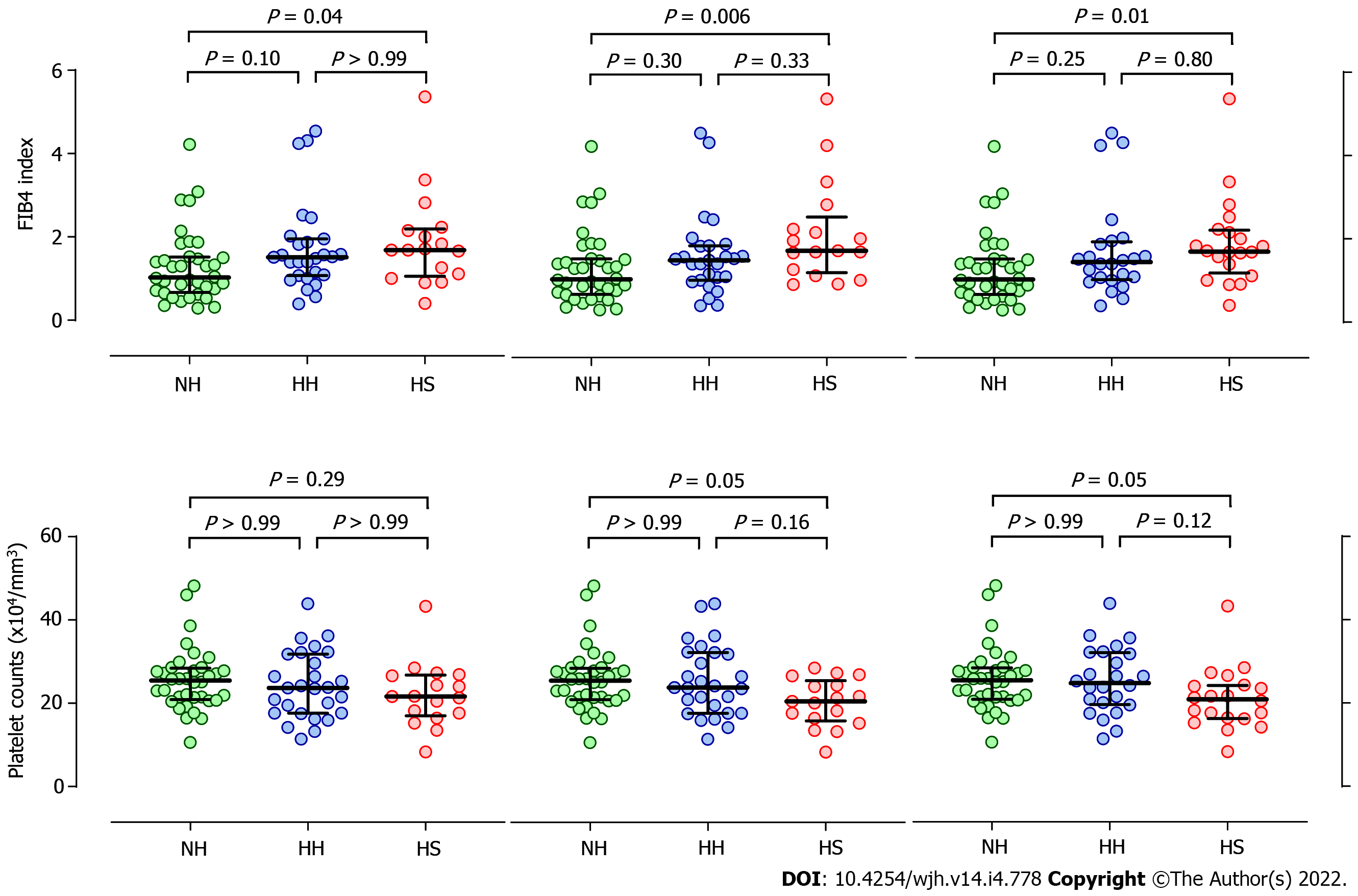
To infer the relationship between pathological differences of the liver and ∆2dSWE, FIB4 and its constituents, platelet count, age, and alanine aminotransferase, were compared among Normal-to-Hard, Hard-to-Hard, and Hard-to-Soft cases. As shown in Figure 4, FIB4 and platelet counts revealed significantly higher and lower values, respectively, in Hard-to-Soft than in Normal-to-Hard cases, especially when a Hard-to-Soft texture was not judged in the entire liver but in a single lobe on the right or left (judged in the entire liver, right lobe, left lobe; (FIB4) P = 0.04, P = 0.006, P = 0.01; (platelet counts) P = 0.29, P = 0.05, P = 0.05, respectively). In terms of age and alanine aminotransferase, no significant differences were noted between Hard-to-Soft and Normal-to-Hard cases even when Hard-to-Soft values were determined in each lobe. No significant differences were noted between the Normal-to-Hard and Hard-to-Hard groups in terms of FIB4, platelet counts, age, or alanine aminotransferase levels.
It has been reported that the IVC diameter and area decrease significantly from the right lateral to the supine position and further to the left lateral position in a healthy population[16]. The height of the IVC relative to the right ventricle, compression of the IVC between the liver and spine, different levels of venous return and/or splanchnic blood pooling are thought to cause postural differences in IVC size[16,17]. Consistently, the IVC diameter was significantly reduced in cases with normal liver stiffness when the body positions were changed from supine to left decubitus in our cohort. Liver stiffness is clearly correlated with IVC pressure/diameter in the supine position, as shown in Supplementary Figure 1A and B. Thus, if the pressure is equilibrated between the IVC and hepatic veins during body position changes, liver stiffness should be reduced in the left decubitus position. However, our study clearly revealed that IVC diameter and liver stiffness exhibited paradoxical changes. The liver hardened, whereas the IVC diameter was reduced. These findings suggest that a pressure threshold exists between the IVC and hepatic veins in the left decubitus position in livers with normal stiffness. Given that intra-abdominal organs relocate along with postural change[18], it is reasonable to assume that the hepatic veins are vented and twisted against the IVC in the left decubitus position, establishing an outflow block. Furthermore, it is anticipated that a rigid liver is less deformed after a body position change. A minimal outflow block keeps the efflux from the liver to the IVC and obviates the shrinkage of the IVC. Therefore, we hypothesized that a stiff liver in the supine position would soften in the left decubitus position if substantial fiber accumulation was present. Otherwise, the liver will further harden (Supplementary Figure 2).
Because IVC pressure strikingly affects liver stiffness[12], as shown in Supplementary Figure 1A and B, the correlation of liver stiffness before and after changing of the IVC pressure strongly indicates a direct connection between the IVC and hepatic veins (Supplementary Figure 1C). Along with the body position changes from the supine to left decubitus position, a significant correlation between SpSWE and ∆2dSWE was only observed in cases with a liver that softened in the left decubitus position. These results strongly support the notion that pressure thresholds generally exist between the IVC and hepatic veins in the left decubitus position, but fewer pressure differences are noted between the IVC and hepatic veins in cases with a stiff liver that softens in the left decubitus position. Furthermore, the correlation coefficients were substantially different between the lobes. In addition, ∆2dSWE revealed large differences between the right and left lobes. These values are reciprocally negative and positive, suggesting that poor venous drainage in the left decubitus position heterogeneously occurs in the liver and is compensated through the area where gravity generates less impact. It is well known that if the flow volume is reduced from the portal vein, the arterial flow instantly compensates, and vice versa[19]. In a similar way, if venous drainage is hindered in a certain area, congestion is avoided by opening latent vascular connections toward the outside of the burden area, as noted in the case of Budd-Chiari syndrome[20].
The different anatomical connections between the IVC and hepatic veins are one reason for the uneven impacts of gravity on the lobes among cases[21]. Given that liver stiffness is measured in two different body positions, it is assumed that a separate evaluation of each lobe should have a higher probability of detecting the different architectural rigidities. In fact, higher probabilities were calculated when the groups for the comparison of FIB4 were assessed in each lobe. One limitation of our study is the relatively smaller number of cases and selection bias. The limited number of enrollments may have caused inadequate assessment of the biological variability. In particular, the efficacy as a prognostic indicator of liver stiffness measurements in supine and left decubitus postures has to be validated in a cohort of congestive heart diseases to guide decisions with respect to the burden of liver diseases. Although the significance of our hypothesis was supported by FIB4 and platelet counts of surrogates for liver fibrosis, there is no standardized indicator for liver fibrosis in congestive hepatopathy referred to in a validation study. A longitudinal observation would be necessary. Furthermore, the gravitational effects on the liver architecture were proposed but not visualized or quantified in this study. To obtain direct evidence, SWE should be measured at two body positions coupled with a quantitative evaluation of structural deformation of the liver.
In this report, a strategy was proposed for measuring shear wave elastography that enables evaluation of architectural deformity under congestive circumstances. With the help of gravity, the impacts on architectural rigidity and interstitial tissue pressure are dissociated when measuring liver stiffness. The basic data presented in this report provide insights not only for the clinical application of liver stiffness in patients with congestive heart diseases but also for the physiological components and mechanisms defining liver stiffness.
Congestive hepatopathy, an abnormal state of the liver as a result of congestion, has become a prognostic determinant by insidiously proceeding toward end-stage liver disease without effective biomarkers in patients with congestive heart diseases as survival has been prolonged owing to surgical and medical improvements. Although liver stiffness is generally a useful surrogate marker for liver fibrosis, which is a universal prognosticator in any type of chronic liver disease, regular measurements of shear wave elastography cannot qualify liver fibrosis in cases of congestion because congestion makes the liver stiff without fibrosis. A noninvasive biomarker is demanded for the managements of patients with congestive heart diseases.
When it is difficult to clearly visualize some area of the liver in ultrasound study, we ask patients to change body postures from supine to such as left decubitus position. At that time, we realized that shear wave elastography values substantially changed in some case. We hypothesized that the effects of congestion and fibrosis on liver stiffness may be dissociated by measuring shear wave elastography in different body positions.
To establish a strategy that enables the evaluation of fibrous accumulation in the liver with respect to architectural rigidity under congestive circumstances by measuring shear wave elastography.
Two-dimensional shear wave elastography was measured in the supine and left decubitus positions in 298 consecutive cases as they were subjected to an ultrasound study for various liver diseases. To clarify the relationship between liver stiffness and interstitial tissue pressure, virtual touch quantification of point shear wave elastography was measured before and after cardiac surgery in a different cohort consisting of 41 cases. Regions of interest were placed at twelve sites, and the median and robust coefficient of variation were calculated. The liver stiffness values and clinicopathological data such as cardiothoracic ratio and the Fibrosis-4 Index were statistically analyzed.
The inferior vena cava diameter was significantly reduced in left decubitus (Ld) position in subjects with higher 2-dimensional shear wave elastography (2dSWE) value in Ld (LdSWE) than the 2dSWE value (SpSWE) in supine (Sp) (P = 0.007) but not in those with lower LdSWE values (P = 0.32). Among 81 patients, in whom SpSWE was increased or decreased in Ld beyond the magnitude of robust coefficient of variation, all 37 with normal SpSWE had a higher LdSWE than SpSWE (Normal-to-Hard), whereas in 44 residual subjects with abnormal SpSWE, LdSWE was higher in 27 subjects (Hard-to-Hard) and lower in 17 subjects (Hard-to-Soft) than SpSWE. SpSWE was significantly correlated with the difference between 2dSWE values in Sp and Ld (∆2dSWE) only in Hard-to-Soft (P < 0.0001). ∆2dSWE was larger in each lobe than in the entire liver. When Hard-to-Hard and Hard-to-Soft values were examined for each lobe, fibrosis-4 or platelet counts were significantly higher or lower only for Hard-to-Soft vs Normal-to-Hard cases.
With the help of gravity during body postural changes, the impacts on architectural rigidity and interstitial tissue pressure are dissociated when measuring liver stiffness. Because a rigid liver is resistant to structural deformation, stiff-liver softening in left decubitus position suggests fiber accumulation of the liver. In this report, a simple strategy of liver stiffness measurement is proposed to identify clues to liver fibrosis even under congestive circumstances.
Because there is no standardized indicator for liver fibrosis in congestive hepatopathy, a longitudinal observation would be only the way to validate the efficacy of liver stiffness measurements in supine and left decubitus postures as a decision guidance strategy with respect to the burden of liver diseases in a cohort of congestive heart diseases. Furthermore, synergistic studies that measure shear wave elastography and quantify structural deformation of the liver in different body positions will help understand the physiological components and mechanisms defining liver stiffen.
The authors are grateful to Imai R, Miyashita H, Yanagi M, Maruyama N, Kobayashi Y, Kawakami M and Miyashita H for the performance of 2dSWE measurements, especially in laborious manner involving two body positions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 022956; The Japan Society of Hepatology, No. 000295; Japan Gastroenterological Endoscopy Society, No. 41330637; The Japan Society of Portal Hypertension, No. 527-982-2252; Liver Cancer Study Group of Japan, No. 11311814; The Japanese Society of Internal Medicine, No. 1991; Japanese Caner Association, No. 032155; and American Society of Gene and Cell Therapy, No. 12450.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cebula M, Poland; Liaqat M, Pakistan; Manesis EK, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2003] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 2. | Downing TE, Allen KY, Glatz AC, Rogers LS, Ravishankar C, Rychik J, Faerber JA, Fuller S, Montenegro LM, Steven JM, Spray TL, Nicolson SC, Gaynor JW, Goldberg DJ. Long-term survival after the Fontan operation: Twenty years of experience at a single center. J Thorac Cardiovasc Surg. 2017;154:243-253.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, Cetta F. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66:1700-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 4. | Krieger EV, Moko LE, Wu F, Landzberg MJ, Valente AM, Assenza GE, Ukomadu C, Opotowsky AR. Single ventricle anatomy is associated with increased frequency of nonalcoholic cirrhosis. Int J Cardiol. 2013;167:1918-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Louie CY, Pham MX, Daugherty TJ, Kambham N, Higgins JP. The liver in heart failure: a biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod Pathol. 2015;28:932-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Ford RM, Book W, Spivey JR. Liver disease related to the heart. Transplant Rev (Orlando). 2015;29:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Bradley E, Hendrickson B, Daniels C. Fontan Liver Disease: Review of an Emerging Epidemic and Management Options. Curr Treat Options Cardiovasc Med. 2015;17:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 9. | Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 927] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 10. | Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Osaki A, Kubota T, Suda T, Igarashi M, Nagasaki K, Tsuchiya A, Yano M, Tamura Y, Takamura M, Kawai H, Yamagiwa S, Kikuchi T, Nomoto M, Aoyagi Y. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol. 2010;16:2918-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK, Mueller S. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia-as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Suda T, Kanefuji T, Abe A, Nagayama I, Hoshi T, Morita S, Yagi K, Hatakeyama S, Hayatsu M, Hasegawa N, Terai S. A cut-off value of shear wave speed to distinguish nonalcoholic steatohepatitis candidates. Medicine (Baltimore). 2019;98:e13958. [PubMed] [DOI] [Full Text] |
| 16. | Nakao S, Come PC, McKay RG, Ransil BJ. Effects of positional changes on inferior vena caval size and dynamics and correlations with right-sided cardiac pressure. Am J Cardiol. 1987;59:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Mookadam F, Warsame TA, Yang HS, Emani UR, Appleton CP, Raslan SF. Effect of positional changes on inferior vena cava size. Eur J Echocardiogr. 2011;12:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Deukmedjian AR, Le TV, Dakwar E, Martinez CR, Uribe JS. Movement of abdominal structures on magnetic resonance imaging during positioning changes related to lateral lumbar spine surgery: a morphometric study: Clinical article. J Neurosurg Spine. 2012;16:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Yamashita H, Hachisuka Y, Kotegawa H, Fukuhara T, Kobayashi N. Effects of posture change on the hemodynamics of the liver. Hepatogastroenterology. 2004;51:1797-1800. [PubMed] |
| 20. | Tang W, Zhang XM, Yang L, Mitchell DG, Zeng NL, Zhai ZH. Hepatic caudate vein in Budd-Chiari syndrome: depiction by using magnetic resonance imaging. Eur J Radiol. 2011;77:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Joshi SD, Joshi SS, Siddiqui AU. Anatomy of retrohepatic segment of inferior vena cava and termination of hepatic veins. Indian J Gastroenterol. 2009;28:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |