Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.754
Peer-review started: September 24, 2021
First decision: November 7, 2021
Revised: November 17, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: April 27, 2022
Processing time: 209 Days and 22.6 Hours
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent in people with diabetes with no available treatment.
To explore the effect of testosterone treatment on liver. Testosterone therapy improves insulin resistance and reduces total body fat, but its impact on the liver remains poorly studied.
This secondary analysis of a 40 wk, randomised, double-blinded, placebo-controlled trial of intramuscular testosterone undecanoate in men with type 2 diabetes and lowered serum testosterone concentrations evaluated the change in hepatic steatosis as measured by liver fat fraction on magnetic resonance imaging (MRI).
Of 88 patients enrolled in the index study, 39 had liver MRIs of whom 20 received testosterone therapy and 19 received placebo. All patients had > 5% hepatic steatosis at baseline and 38 of 39 patients met diagnostic criteria for NAFLD. Median liver fat at baseline was 15.0% (IQR 11.5%-21.1%) in the testosterone and 18.4% (15.0%-28.9%) in the placebo group. Median ALT was 34units/L (26-38) in the testosterone and 32units/L (25-52) in the placebo group. At week 40, patients receiving testosterone had a median reduction in absolute liver fat of 3.5% (IQR 2.9%-6.4%) compared with an increase of 1.2% in the placebo arm (between-group difference 4.7% P < 0.001). After controlling for baseline liver fat, testosterone therapy was associated with a relative reduction in liver fat of 38.3% (95% confidence interval 25.4%-49.0%, P < 0.001).
Testosterone therapy was associated with a reduction in hepatic steatosis in men with diabetes and low serum testosterone. Future randomised studies of testosterone therapy in men with NAFLD focusing on liver-related endpoints are therefore justified.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent disease with no current effective treatment. This study demonstrates a reduction in hepatic steatosis in men with type 2 diabetes and low testosterone who received testosterone therapy as part of a randomised controlled trial and provides justification for larger scale studies to assess the effects of testosterone therapy as a treatment for NAFLD.
- Citation: Apostolov R, Gianatti E, Wong D, Kutaiba N, Gow P, Grossmann M, Sinclair M. Testosterone therapy reduces hepatic steatosis in men with type 2 diabetes and low serum testosterone concentrations. World J Hepatol 2022; 14(4): 754-765
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/754.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.754
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis on imaging or histology in the absence of other secondary causes of hepatic steatosis[1]. It has an estimated overall global prevalence of 25%[2]. Patients with type 2 diabetes are at significantly higher risk of NAFLD due to a bidirectional pathophysiological link between the two disease entities[3,4]. A meta-analysis of 19 observational studies found that patients with NAFLD had a higher incidence of type 2 diabetes than those without, and that the incidence of type 2 diabetes further increased in patients with radiologically higher steatosis scores[5]. Furthermore, type 2 diabetes and features of the metabolic syndrome are known to be independent risk factors for liver fibrosis progression, cirrhosis, hepatocellular carcinoma and death in patients with NAFLD[6,7].
Low serum testosterone has been associated with an increased risk of NAFLD in men after adjustment for other metabolic risk factors[8-10]. A meta-analysis of 13721 men from cross-sectional, cohort and case-control studies reported that serum total testosterone (TT) concentrations were on average 2.8nmol/L lower in men with NAFLD than those without11].[ Subsequently, in a study of 159 men with NAFLD, lower testosterone concentrations were associated with a higher risk for the presence, and increasing severity, of non-alcoholic steatohepatitis, a recognised risk factor for liver disease progression in NAFLD[12].
Testosterone therapy has been shown to ameliorate hepatic steatosis and necroinflammation in animal models of male hypogonadism induced by castration [13,14] Only a small number of studies have examined the effect of exogenous testosterone in men with NAFLD and low testosterone concentrations, and these report mixed results. A placebo controlled study of obese men with severe obstructive sleep apnoea and testosterone concentrations that ranged from low normal to normal demonstrated that testosterone therapy reduced liver fat as measured by computed tomography[15]. A study of 21 men with low serum testosterone concentrations and NAFLD reported that treatment with a novel oral testosterone prodrug improved liver fat as measured by magnetic resonance imaging proton density fat fraction (MRI-PDFF) in 81% of patients[16]. Two small studies of testosterone therapy in men with type 2 diabetes and low testosterone concentrations and one in men with mobility limitation and low testosterone concentrations conversely showed no significant change in hepatic fat compared to placebo as measured by MRI[17-19].
The aim of this study was to evaluate the effect of testosterone therapy on liver fat fraction and to determine other factors associated with changes in liver fat in a population of men with low testosterone concentrations and type 2 diabetes.
The study design, eligibility and protocol of the trial is described in full in the original publication[20]. The trial was registered on the Australian and New Zealand Clinical Trials registry (trial number NCT00613782). This was a 40 wk, randomised, double-blind placebo-controlled trial conducted at single tertiary referral centre in Australia. Ethics approval for the study was granted by the Human Research Ethics Committee, Austin Health.
Eligible participants were men aged 35-70 years with a history of type 2 diabetes and a fasting, early morning TT concentration of ≤ 12.0 nmol/L (346 ng/dL), as measured by electrochemiluminescence immunoassay (ECLIA) and averaged across two readings.
Exclusion criteria for the trial were testosterone therapy within five years of randomisation, screening TT concentrations < 5.0 nmol/L (144 ng/dL), established pituitary or testicular disorder, luteinising hormone level > 1.5x upper limit of normal, prostate-specific antigen level > 4 µg/L, a history of urinary obstruction, prostate cancer or breast cancer, haematocrit > 0.50, uncontrolled hypertension (> 160/90 mmHg despite treatment), untreated obstructive sleep apnoea, estimated glomerular filtration rate < 30 mL/min, cardiac insufficiency, active malignancy, unstable psychiatric disease, weight > 135 kg, use of glucagon-like peptide-1 agonist therapy or very low-calorie diet, or an HbA1c level > 8.5% (69 mmol/mol).
Eligible participants were randomly assigned in a concealed 1:1 allocation to either testosterone or placebo therapy. Intramuscular testosterone undecanoate 1000 mg or a visually identical placebo was administered at 0, 6, 18 and 30 wk. Participants were followed up for a total of 40 wk.
In this post-hoc analysis we assessed as the primary outcome the change in liver fat fraction, as measured using MRI liver in-phase (IP) and opposed-phase (OP) T1 sequences and expressed as percentage fat. The primary outcome measure for the index study was the change across groups and time from baseline in the homeostasis model assessment index of insulin resistance using a computer-based calculation rather than the original linear equation.
Clinical and biochemical variables that were assessed in participants at baseline and 40 wk included body weight, body mass index (BMI), waist circumference, alanine aminotransferase (ALT), gamma-glutamyl transferase, alkaline phosphatase, bilirubin, albumin, international normalised ratio, TT, sex hormone-binding globulin, calculated free testosterone (cFT), fasting glucose, HbA1c and lipid profile. Free testosterone levels were calculated using Vermeulen’s formula, as previously described[21]. Additional measurements of total body mass, lean mass and fat mass were assessed by DXA scan (DXA Prodigy, Version 10.51; GE Lunar, Madison, WI) at 0 and 40 wk.
MRI scans of the abdomen were obtained at enrolment and after 40 wk of therapy using a 3 Tesla MRI scanner (Siemens, Erlangen, Germany). As part of the initial study, subcutaneous and visceral adipose tissue volume was calculated for each patient by analysis of five 10-mm slices around the L4 vertebral superior endplate using the SliceOmatic program software (version 4.2; Tomovision, Montreal, Canada) as previously described[20].
MRI images obtained before and after therapy were reanalysed for the present study by an expert liver radiologist who was blinded to treatment allocation. Liver fat fraction before and after therapy was calculated using conventional chemical shift imaging with IP and OP T1 sequences. An averaged signal intensity for IP and OP was obtained from three 5 to 10 cm2 regions within hepatic parenchyma of each scan. The fat fraction was calculated using the formula (IP - OP)/2 × IP. This technique has been previously validated to accurately estimate liver fat fraction to levels of up to 50%[22-27].
Continuous data are displayed as mean (standard deviation) for normally distributed data or median [interquartile range] for skewed data. Categorical data are presented as number (percentage). The Student t-test and Mann-Whitney test were used for normal and non-normal data, respectively. Exploratory data analysis included pairwise examination for correlation that may introduce multicollinearity into the final regression model. Linear regression modelling was used to identify variables associated with week 40 Liver fat proportion, which was the primary outcome variable, whilst controlling for baseline liver fat proportion (analysis of covariance). The outcome variable was natural log-transformed for these analyses as use of the untransformed values violated model assumptions. Univariate regression with all clinically relevant variables was performed and those with P values < 0.20 were selected for inclusion in the multivariable model. A manual elimination process was undertaken to arrive at the final model. The coefficients in the final model were back transformed to report relative change in liver fat proportion between groups (geometric mean). Standard regression diagnostics were performed to ensure non-violation of the underlying model assumptions. Analyses were performed in R v4.02[28].
A total of 39 men with type 2 diabetes and low serum testosterone were included in our analysis, of whom 20 received testosterone therapy and 19 received placebo. They represent a subset of 88 patients from the original trial who underwent MRI liver scanning both at the start and end of treatment. Remaining study participants did not undertake MRI scans either due to incompatible or unverifiable metal implants, inability to fit in the MRI machine or claustrophobia.
The baseline characteristics of our study participants are shown in Table 1 and are similar between the testosterone and placebo therapy group with the exception of lean mass which was lower in the testosterone group and HDL which was higher in the testosterone group compared to placebo. Compared to the participants in the index study who did not have MRI scans, our cohort had lower baseline BMI, visceral adiposity, waist circumference and higher cholesterol levels, but otherwise had comparable baseline characteristics as shown in Table 2. All patients had hereditary haemochromatosis and other causes of low testosterone excluded and no patients were taking medications were taking steatogenic medications during the study period. Five participants in the testosterone group and three in the placebo group were taking glucagon-like peptide-1 analogues or thiazolidinediones at baseline which continued throughout the study at stable doses. No other patients were taking medications known to directly influence liver fat (Supplementary Table 1). Median alcohol consumption was 3.5 (IQR 0-8.5) standard drinks per week in the testosterone group and 4 (IQR 0.5-7) standard drinks per week in the placebo group. One patient in the testosterone group had heavy alcohol consumption (defined as > 14 units per week), reporting 28 standard drinks per week during the study period. No patients in the placebo group had heavy alcohol consumption.
| Characteristic | Testosterone group (n = 20) | Placebo group (n = 19) | P value |
| Age (yr) | 62 (58-67) | 60.4 (6.5) | 0.5 |
| Duration of type 2 diabetes (yr) | 8 (6-14) | 7 (5-9) | 0.2 |
| Insulin therapy, n (%) | 3 (15) | 2 (10.5) | > 0.9 |
| Weight, kg | 92 (86-101) | 98 (92-105) | 0.15 |
| BMI, kg/m2 | 30.3 (27.3-31.9) | 32.0 (29.7-35.4) | 0.14 |
| Waist circumference, cm | 106 (102-116) | 112 (106-119) | 0.4 |
| Fat mass, g | 30788 (24875-38117) | 31758 (29332-35286) | 0.8 |
| Lean mass, g | 57090 (51658-62688) | 62140 (66226-65844) | 0.029 |
| ALT, IU/L | 34 (26-38) | 32 (25-52) | 0.8 |
| ALP, IU/L | 72 (60-82) | 58 (54-69) | 0.057 |
| GGT, IU/L | 30 (21-38) | 32 (25-45) | 0.5 |
| TT, nmol/L (ECLIA) | 9.8 (7.2-12.0) | 7.8 (6.0-10.8) | 0.2 |
| cFT, pmol/L (ECLIA) | 216 (151-272) | 176 (146-224) | 0.3 |
| SHBG, nmol/L | 28 (20-34) | 28 (23-32) | 0.8 |
| LH, IU/L | 4.5 (3.4-7.0) | 4.3 (3.4-6.0) | 0.7 |
| Fasting glucose, mmol/L | 8.9 (6.8-10.3) | 8.5 (7.7-9.8) | 0.6 |
| HbA1c, % | 6.6 (6.4-7.2) | 7.0 (6.7-7.3) | 0.071 |
| Cholesterol, mmol/L | 4.4 (4.0-5.0) | 4.6 (4.0-4.9) | > 0.9 |
| LDL, mmol/L | 2.4 (2.0-3.0) | 2.7 (2.0-3.0) | 0.7 |
| HDL, mmol/L | 1.2 (1.0-1.4) | 1.0 (0.7-1.2) | 0.03 |
| Characteristic | Non-MRI scan group (n = 49) | MRI scan group (n = 39) | P value |
| Age, yr | 62 (58-68) | 62 (58-67) | 0.6 |
| Duration of type 2 diabetes, yr | 9 (4-12) | 8 (5-11) | > 0.9 |
| Insulin therapy, n (%) | 13 (26.5) | 5 (12.8) | 0.19 |
| Weight, kg | 102 (89-111) | 95 (90-104) | 0.12 |
| BMI, kg/m2 | 33.4 (31.2-35.9) | 31.5 (28.2-35.3) | 0.012 |
| Waist circumference, cm | 116 (109-123) | 111 (104-118) | 0.024 |
| Fat mass, g | 32696 (29299-39109) | 31832 (26820-37809) | 0.2 |
| Lean mass, g | 62004 (58056-66170) | 60,049 (54278-64848) | 0.12 |
| ALT, IU/L | 34 (25-50) | 32 (25-42) | 0.7 |
| ALP, IU/L | 71 (56-82) | 66 (54-75) | 0.2 |
| GGT, IU/L | 30 (24-43) | 32 (24-43) | 0.8 |
| TT, nmol/L (ECLIA) | 8.5 (7.3-10.5) | 8.1 (6.7-11.4) | 0.8 |
| cFT, pmol/L (ECLIA) | 176 (151-230) | 194 (148-253) | 0.6 |
| SHBG, nmol/L | 28 (24-32) | 29 (22-34) | > 0.9 |
| LH, IU/L | 4.5 (3.5-6.2) | 4.5 (3.4-6.4) | 0.9 |
| Fasting glucose, mmol/L | 8.2 (7.0-10.6) | 8.6 (7.3-9.9) | > 0.9 |
| HbA1c, % | 7.1 (6.6-7.7) | 6.9 (6.5-7.3) | 0.4 |
| Cholesterol, mmol/L | 4.1 (3.5-4.7) | 4.6 (4.0-4.9) | 0.005 |
| LDL, mmol/L | 2.0 (1.6-2.4) | 2.7 (2.0-3.1) | < 0.001 |
| HDL, mmol/L | 1.0 (0.9-1.2) | 1.1 (0.9-1.2) | 0.5 |
| SAT, cm3 | 4095 (3526-5593) | 4661 (3137-5385) | 0.7 |
| VAT, cm3 | 4786 (3,642-5,617) | 3634 (2780-4823) | 0.044 |
All patients in the study had significant hepatic steatosis, defined as a liver fat fraction ≥ 5%. All patients but the one patient with heavy alcohol consumption had secondary causes of hepatic steatosis excluded and hence met diagnostic criteria for NAFLD as defined by the American Association for the Study of Liver Diseases[1]. Median liver fat fraction at baseline was 15.0% (IQR 11.5%-21.1%) in the testosterone group and 18.4% (IQR 15.0%-28.9%) in the placebo group (P = 0.14). The median values of all liver function tests were within normal limits for both groups at baseline (Table 1).
In the testosterone group, the median absolute reduction in liver fat fraction was 3.5% (IQR 2.9%-6.4%) and median relative reduction was 27.3% (IQR 18.0%-37.6%). Liver fat fraction increased in the placebo group, with a median absolute increase in liver fat fraction of 1.2% (IQR -2.6-3.0%) and median relative increase of 6.8% (IQR -7.3-15.3%). At week 40, the median liver fat fraction was 9.8% (IQR 8.6%-13.5%) in the testosterone group and 19.6% (IQR 17.8%-29.1%) in the placebo group.
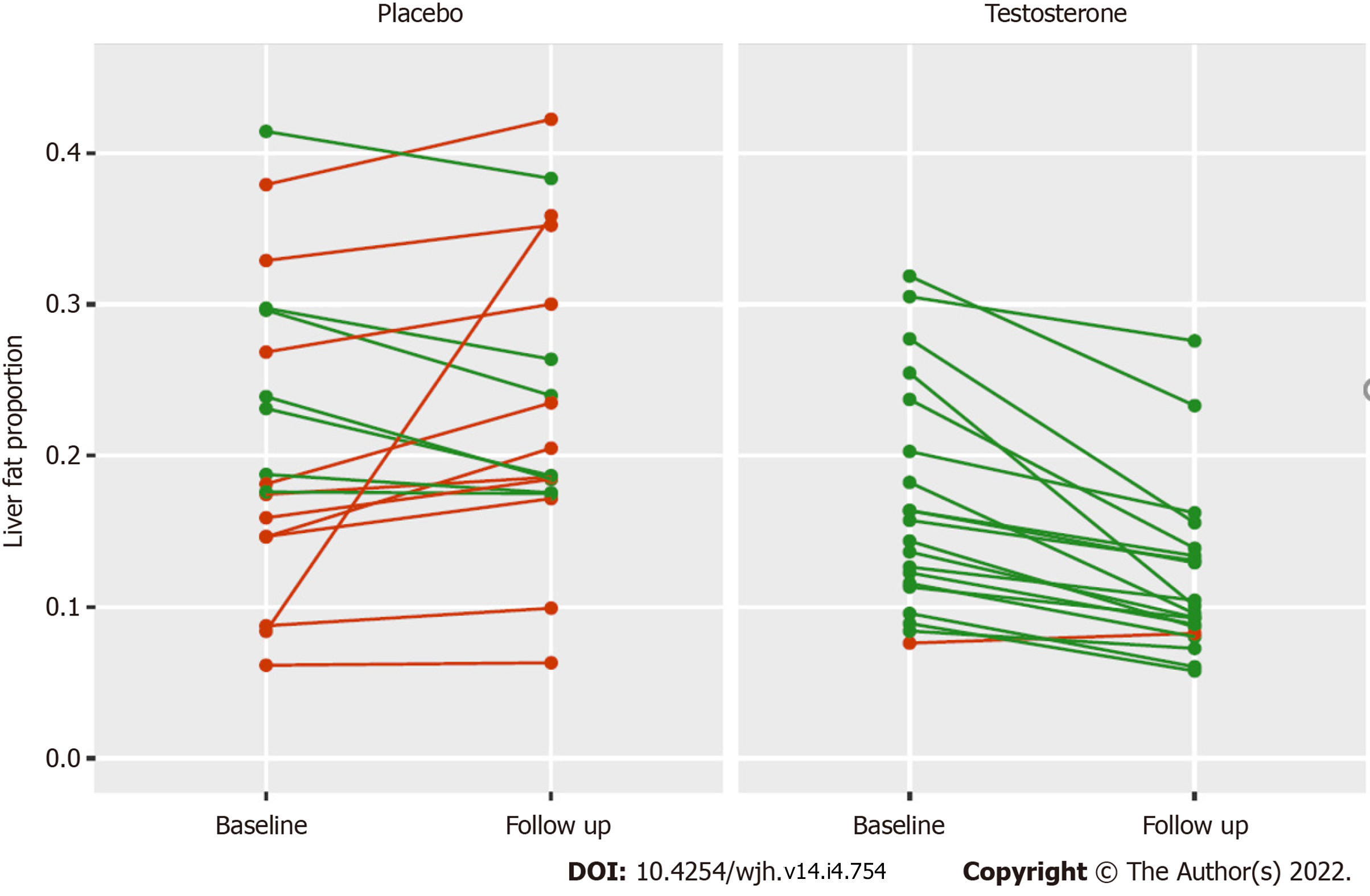
The change in absolute liver fat ranged from -15.3% to +3.6% in the testosterone group, with 18 of 20 individuals achieving a reduction in liver fat (Figure 1). The change in absolute liver fat ranged from -5.9% to +27.5% in the placebo group, with eight of 19 individuals achieving a reduction in liver fat (Figure 1). One patient from the placebo group was an outlier who had a 27.5% absolute increase in liver fat associated with a 32% increase in visceral adipose tissue (VAT) and a 7% increase in body weight. At week 40, there were no significant changes in liver function tests in either treatment group.
Univariate regression analysis found that testosterone therapy, BMI, baseline liver fat and week 40 TT and cFT concetrations were all significantly associated with changes in liver fat. Week 40 cFT and TT concentrations were, however, highly correlated ( hence a decision to use only cFT in the development of the multivariate model.
The multivariate model was significant (F(2,35)(54.13), R2 = 0.807, P < 0.001) and included treatment arm controlled for baseline liver fat proportion. In detail, those in the testosterone treatment group had a significantly lower follow-up liver fat proportion than those in the placebo group (P < 0.001). The Beta co-efficient of -0.48 (-0.67, -0.29) is on the log-transformed scale. Back-transformation of the co-efficient and associated 95% confidence interval returned values of -0.383 (-0.490, -0.254). Thus, in this model, testosterone therapy was associated with a 38.3% relative reduction in liver fat proportion (95% confidence interval 25.4% to 49.0% reduction) after controlling for baseline liver fat proportion compared to those receiving placebo. No other covariates reduced the unexplained residual variance in the model significantly to this estimate of between-group difference.
Baseline median TT concentrations as measured by ECLIA were 9.8 [7.2-12.00] nmol/L and 7.8 [5.9-10.8] nmol/L in the testosterone and placebo groups, respectively. After 40 wk of therapy, trough TT concentrations increased to 12.9 [11.9-15.0] nmol/L in the testosterone group and 8.8 [7.3-10.8] nmol/L in the placebo group (P < 0.001). Baseline median cFT concentrations were 216 [151-272] pmol/L and 176 [146-224] pmol/L in the testosterone and placebo groups respectively. After 40 wk of therapy cFT increased to 322 [231-392] pmol/L in the testosterone group and 193 [167-250] pmol/L in the placebo group (P < 0.001).
At 40 wk patients receiving testosterone therapy had no statistically significant changes in overall body weight, BMI, waist circumference, fasting glucose or HbA1c compared to placebo (Table 3).
| Testosterone group (n = 20) | Placebo group (n = 19) | P value | |
| Weight, kg | |||
| Baseline | 92 (86-101) | 98 (91-105) | 0.55 |
| 40 wk | 90 (86-100) | 96 (94-100) | |
| BMI, kg/m2 | |||
| Baseline | 30.3 (27.3-31.9) | 32.0 (29.7-35.4) | 0.64 |
| 40 wk | 29.3 (27.4-31.8) | 31.8 (30.0-34.4) | |
| Waist circumference, cm | |||
| Baseline | 106 (102-116) | 112 (106-118) | 0.15 |
| 40 wk | 108 (101-115) | 114 (110-118) | |
| Fasting glucose, mmol/L | |||
| Baseline | 8.9 (6.8-10.3) | 8.6 (7.7-9.8) | 0.93 |
| 40 wk | 8.3 (6.5-10.5) | 9.1 (7.7-9.9) | |
| HbA1c, % | |||
| Baseline | 6.6 (6.4-7.2) | 7.0 (6.8-7.4) | 0.95 |
| 40 wk | 7.0 (6.3-7.4) | 7.3 (7.0-7.7) | |
| Fat mass, g | |||
| Baseline | 30788 (24875-38117) | 31758 (29332-35286) | < 0.001a |
| 40 wk | 27272 (21948-34558) | 32089 (29869-34492) | |
| Lean mass, g | |||
| Baseline | 57090 (51658-62688) | 62140 (66226-65844) | < 0.001a |
| 40 wk | 58236 (55150-63077) | 61070 (57408-64833) | |
| SAT, cm3 | |||
| Baseline | 4445 (2928-5669) | 4302 (3828-5254) | 0.004a |
| 40 wk | 4140 (2769-5046) | 4330 (3741-5179) | |
| VAT, cm3 | |||
| Baseline | 3574 (2460-4711) | 3864 (3267-4703) | 0.63 |
| 40 wk | 3248 (2280-4634) | 3966 (3462-4699) | |
| ALT, U/L | |||
| Baseline | 34 (26-38) | 32 (25-52) | 0.27 |
| 40 wk | 32 (25-36) | 44 (29-61) | |
| GGT, U/L | |||
| Baseline | 30 (21-38) | 32 (25-45) | 0.28 |
| 40 wk | 28 (20-41) | 33 (28-56) | |
| ALP, U/L | |||
| Baseline | 72 (60-82) | 58 (54-69) | 0.04a |
| 40 wk | 70 (60-78) | 60 (58-72) | |
| Total cholesterol, mmol/L | |||
| Baseline | 4.4 (4.0-5.0) | 4.6 (4.0-4.9) | 0.008a |
| 40 wk | 3.7 (3.5-4.5) | 4.5 (4.1-5.1) | |
| LDL, mmol/L | |||
| Baseline | 2.4 (2.0-3.0) | 2.7 (2.0-3.0) | 0.21 |
| 40 wk | 2.1 (1.8-2.7) | 2.4 (1.9-3.2) | |
| HDL, mmol/L | |||
| Baseline | (1.01-1.40) | 1.02 (0.74-1.18) | 0.02a |
| 40 wk | 1.19 (0.93-1.36) | 0.98 (0.82-1.09) | |
| Triglycerides, mmol/L | |||
| Baseline | 1.6 (1.3-2.0) | 2.0 (1.4-2.4) | 0.25 |
| 40 wk | 1.4 (1.0-1.9) | 1.9 (1.6-3.0) |
At week 40, compared to placebo, patients receiving testosterone therapy had significantly increased lean mass (mean change 2111 g (1148-3073) relative to placebo, P < 0.001) and significantly decreased fat mass (mean change -2969 g (-3998 to -1941) relative to placebo, P < 0.001). All patients receiving testosterone therapy had reductions in fat mass and 19/20 patients receiving testosterone therapy had increases in lean mass (Supplementary Figure 1). Subcutaneous adipose tissue (SAT) was significantly decreased at week 40 in patients receiving testosterone therapy compared to placebo (mean change -359 cm3 (-570 to -147) relative to placebo, P = 0.002) but VAT was unchanged (Supplementary Figure 2).
Testosterone therapy was well-tolerated with rare serious adverse events that were not significantly different compared to placebo as outlined in Table 4 of the index study[20]. Patients receiving testosterone therapy had significant increases in haemoglobin and haematocrit at week 40 compared to placebo (P < 0.001 for both).
| Characteristic | n | Beta | 95%CI | P value |
| Testosterone | 381 | -0.66 | -1.0, -0.37 | < 0.001 |
| Age (yr) | 38 | -0.03 | -0.05, 0.00 | 0.075 |
| BMI (kg/m2) | 38 | 0.06 | 0.02, 0.10 | 0.003 |
| Baseline liver fat proportion | 38 | 4.6 | 3.3, 6.0 | < 0.001 |
| Baseline total testosterone (nmol/L) | 38 | -0.03 | -0.09, 0.03 | 0.3 |
| Baseline free testosterone (nmol/L) | 38 | 0.00 | 0.00, 0.00 | 0.5 |
| Baseline visceral fat (g) | 38 | 0.00 | 0.00, 0.00 | 0.4 |
| Follow up total testosterone (nmol/L) | 37 | -0.05 | -0.09, -0.02 | 0.006 |
| Follow up free testosterone (nmol/L) | 37 | 0.00 | 0.00, 0.00 | 0.005 |
| Follow up visceral fat (g) | 38 | 0.00 | 0.00, 0.00 | 0.2 |
In this study of men with type 2 diabetes and low testosterone concentrations, intramuscular testosterone undecanoate over 40 wk significantly reduced liver fat as measured by MRI in an adjusted model compared to placebo.
Our results are consistent with a recent study in which 32 men with low serum testosterone, of whom 8 (25%) had type 2 diabetes, were given a novel oral testosterone preparation LPCN 1144 for 16 wk. In this study, 21/32 patients met diagnostic criteria for NAFLD, and a mean relative reduction in liver fat of 33% as measured by MRI-PDFF was demonstrated in 17 of these 21 patients[16]. However, in contrast to our study, the LPCN 1144 study did not include a control group. Whilst three previous studies reported no change in liver fat in men with low testosterone concentrations after testosterone treatment[17-19], these all had some limitations including short follow-up time of six months, small sample size [17,18], and one study estimated liver fat changes by a less accurate method of measuring change in liver volume and relaxometry[19]. Additionally, patients in our study received intramuscular testosterone administered by study investigators, hence eliminating adherence issues whereas testosterone gel was used in two of the three studies, making adherence difficult to assess[17,19].
Secondary benefits of testosterone therapy in this study included increases in lean muscle mass and reduction in total fat mass, a result consistent with numerous previous testosterone trials across multiple population groups[29,30]. Interestingly, despite a reduction in subcutaneous adiposity, visceral adiposity was unchanged after testosterone therapy suggesting that testosterone may have a direct effect on hepatic steatosis. This is consistent with animal studies showing that the androgen receptor is expressed in the liver and testosterone has biological effects in the liver affecting lipid metabolism and glucose homeostasis[31]. Furthermore, animal models show that testosterone differentially regulates the expression of key targets of lipid and glucose metabolism in a tissue specific manner, with regional differences in action of testosterone on SAT compared to VAT[32]. Total cholesterol levels were also significantly lower in patients treated with testosterone therapy. NAFLD most frequently occurs in the context of the metabolic syndrome, with associated visceral adiposity, dyslipidaemia, and other cardiovascular risk factors[33]. The primary cause of death in such patients is cardiovascular disease, and thus the changes in body composition and lipid profile observed in our study may favourably change cardiovascular risk in patients with NAFLD, but this study was not powered or designed to assess this.
All patients in our study had significant steatosis, and 38/39 patients met diagnostic criteria for a diagnosis of NAFLD. The global prevalence of NAFLD in men with type 2 diabetes is 55%, markedly lower than our cohort’s prevalence[34]. This difference is unsurprising as our cohort consisted of older, obese men with a longstanding history of type 2 diabetes and low serum testosterone concentrations[8]. Given concurrent type 2 diabetes is the strongest risk factor for progression of NAFLD to significant fibrosis[35], demonstrating efficacy in this population represents an important finding with potentially important clinical implications.
Our study has several limitations. This study was a sub-analysis of a randomised controlled trial that was not designed to evaluate liver-related endpoints and not all index cases had liver MRI for fat assessment. However, those included in this study did not appear to significantly differ from those who were not, apart from our cohort having lower weight and waist circumference. Baseline and end of treatment median liver function tests were within the normal ranges for both treatment groups, suggesting that patients in our study did not have significant hepatic inflammation. Finally, fat fraction was assessed using conventional MRI-IP and OP T1 sequences rather than MRI-PDFF, which was not widely available at the time of the index study. Although changes in liver fat MRI-IP and OP T1 sequences do not have the same evidence for correlation with steatohepatitis improvement, it has been shown to accurately quantify liver fat up to 50%, a threshold which was not exceeded in our study[22-24].
Despite these limitations, we identified patients who met diagnostic criteria for NAFLD who had a significant reduction in hepatic steatosis with testosterone treatment while liver fat in most placebo patients increased. The change in hepatic steatosis was measured by MRI scan, which is increasingly recognised as a reasonable surrogate endpoint for histologic response[36]. The two cohorts were well matched with respect to medication use, in particular pertaining to diabetic agents that are known to impact hepatic steatosis and all 39 patients in our study maintained a stable diabetic regimen throughout the trial.
This study shows an association between testosterone treatment and a significant reduction in liver fat in men with type 2 diabetes and low testosterone concentrations. These data provide a strong rationale to perform large-scale randomised studies testing the effect of testosterone therapy in high-risk NAFLD patients with low serum testosterone, focusing on liver-specific endpoints including liver inflammation and fibrosis progression.
Testosterone levels are commonly low in men with type 2 diabetes and most men with type 2 diabetes have hepatic steatosis from non-alcoholic fatty liver disease (NAFLD). Animal models show that low testosterone states from castration results in hepatic steatosis and that testosterone replacement improves hepatic steatosis.
Hepatic steatosis occurs in NAFLD which currently has no readily available effective treatment. This study was conducted to provide rationale for future prospective studies of testosterone therapy for NAFLD.
To evaluate the effect of testosterone therapy on liver fat fraction as measured by magnetic resonance imaging (MRI) in a cohort of diabetic men with lowered testosterone levels. We further aimed to determine other factors associated with changes in liver fat in this population.
We performed a secondary analysis of a previous 40 wk, randomised, double-blinded, placebo-controlled trial of intramuscular testosterone undecanoate in men with type 2 diabetes and lowered serum testosterone levels. Liver fat as determined by MRI scan before and after therapy was analyzed in addition to blood tests and body composition scans.
Patients who received testosterone therapy had an absolute reduction of liver fat fraction by 3.5% and patients who received placebo had an absolute increase in liver fat fraction by 1.2%, with a between group difference of 4.7%, P < 0.001. After controlling for baseline liver fat, testosterone therapy was associated with a relative reduction in liver fat of 38.3% (P < 0.001).
Testosterone therapy was associated with a reduction in hepatic steatosis in a cohort of men with type 2 diabetes and lowered serum testosterone levels.
This study provides rationale for future prospective clinical trials of testosterone therapy for the treatment of NAFLD focusing on liver related endpoints.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen F, China; Ma JH, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4946] [Article Influence: 706.6] [Reference Citation Analysis (9)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 3. | Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 539] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019;1:312-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 5. | Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 314] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 6. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 7. | Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Kim S, Kwon H, Park JH, Cho B, Kim D, Oh SW, Lee CM, Choi HC. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 2012;12:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Yim JY, Kim J, Kim D, Ahmed A. Serum testosterone and non-alcoholic fatty liver disease in men and women in the US. Liver Int. 2018;38:2051-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Phan H, Richard A, Lazo M, Nelson WG, Denmeade SR, Groopman J, Kanarek N, Platz EA, Rohrmann S. The association of sex steroid hormone concentrations with non-alcoholic fatty liver disease and liver enzymes in US men. Liver Int. 2021;41:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Ann Hepatol. 2017;16:382-394. [PubMed] |
| 12. | Sarkar M, Yates K, Suzuki A, Lavine J, Gill R, Ziegler T, Terrault N, Dhindsa S. Low Testosterone Is Associated With Nonalcoholic Steatohepatitis (NASH) and Severity of NASH Fibrosis in Men With NAFLD. Clin Gastroenterol Hepatol. 2021;19:400-402.e2.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Nikolaenko L, Jia Y, Wang C, Diaz-Arjonilla M, Yee JK, French SW, Liu PY, Laurel S, Chong C, Lee K, Lue Y, Lee WN, Swerdloff RS. Testosterone replacement ameliorates nonalcoholic fatty liver disease in castrated male rats. Endocrinology. 2014;155:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Cai Z, Jiang X, Pan Y, Chen L, Zhang L, Zhu K, Cai Y, Ling Y, Chen F, Xu X, Chen M. Transcriptomic analysis of hepatic responses to testosterone deficiency in miniature pigs fed a high-cholesterol diet. BMC Genomics. 2015;16:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Albhaisi S, Kim K, Baker J, Chidambaram N, Patel MV, Charlton M, Sanyal AJ. LPCN 1144 Resolves NAFLD in Hypogonadal Males. Hepatol Commun. 2020;4:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Magnussen LV, Andersen PE, Diaz A, Ostojic J, Højlund K, Hougaard DM, Christensen AN, Nielsen TL, Andersen M. MR spectroscopy of hepatic fat and adiponectin and leptin levels during testosterone therapy in type 2 diabetes: a randomized, double-blinded, placebo-controlled trial. Eur J Endocrinol. 2017;177:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction After Testosterone Replacement in Men With Type 2 Diabetes. Diabetes Care. 2016;39:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 19. | Huang G, Bhasin S, Tang ER, Aakil A, Anderson SW, Jara H, Davda M, Travison TG, Basaria S. Effect of testosterone administration on liver fat in older men with mobility limitation: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37:2098-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience. Radiology. 2005;237:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 23. | Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:337-357, ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 24. | Koplay M, Sivri M, Erdogan H, Nayman A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:769-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 25. | Bahl M, Qayyum A, Westphalen AC, Noworolski SM, Chu PW, Ferrell L, Tien PC, Bass NM, Merriman RB. Liver steatosis: investigation of opposed-phase T1-weighted liver MR signal intensity loss and visceral fat measurement as biomarkers. Radiology. 2008;249:160-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Maruzzelli L, Parr AJ, Miraglia R, Tuzzolino F, Luca A. Quantification of hepatic steatosis: a comparison of computed tomography and magnetic resonance indices in candidates for living liver donation. Acad Radiol. 2014;21:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | Team RC. R v4.0.2: A Language and Environment for Statistical Computing. Vienna, Austria. [cited 20 July 2021]. Available from: https://www.r-project.org/. |
| 29. | Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, Grossmann M. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Grossmann M, Wierman ME, Angus P, Handelsman DJ. Reproductive Endocrinology of Nonalcoholic Fatty Liver Disease. Endocr Rev. 2019;40:417-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Kelly DM, Akhtar S, Sellers DJ, Muraleedharan V, Channer KS, Jones TH. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine. 2016;54:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 803] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 34. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1494] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 35. | Tada T, Toyoda H, Sone Y, Yasuda S, Miyake N, Kumada T, Tanaka J. Type 2 diabetes mellitus: A risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, Singh S, Fowler KJ, Sirlin CB, Loomba R. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2021;19:2274-2283.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |