Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.744
Peer-review started: May 10, 2021
First decision: July 6, 2021
Revised: July 14, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 27, 2022
Processing time: 347 Days and 5.7 Hours
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disease ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), through to advanced fibrosis and cirrhosis. Many patients with NAFLD remain undiagnosed and recognizing those at risk is very crucial. Although liver biopsy is the gold standard method for diagnosing and staging NAFLD, non-invasive imaging and lab modalities are also very promising in diagnosing these diseases.
To explore some of these non-invasive modalities in this context and assess how they hold up in terms of making a diagnosis while avoiding an invasive procedure like a liver biopsy.
This study was conducted on NAFLD/NASH patients (n = 73) who underwent Fibroscan examinations at Saint George Hospital University Medical Center over 17 mo in order to assess liver fibrosis. Obtained Fibroscan results were correlated to laboratory tests and calculated aspartate transaminase (AST)/alanine transaminase (ALT) ratio, AST platelet ratio index (APRI) score and Fibrosis-4 score.
A significant age difference was observed across fibrosis stages of investigated patients. The mean stiffness score was 9.48 ± 11.77 KPa. A significant negative correlation was observed between ALT, AST, Albumin, gamma-glutamyl transferase, cholesterol, LDL, HDL, triglycerides, and ALP when compared across fibrosis stages. On the other hand, a significant positive correlation was found between Bilirubin, PT INR, partial thromboplastin time, glucose, and Platelet count when compared across fibrosis stages, in addition to AST/ALT ratio, APRI, and Fib-4 scores.
This study showed that Ultrasound alone is not efficient in the assessment of advancement of liver disease. Furthermore, the high positive relation between AST/ALT ratio, APRI and Fib-4 scores with fibrosis stages in NAFLD patients suggests that they could be used clinically in combination with Fibroscan to predict significant fibrosis and cirrhosis and to avoid liver biopsy.
Core Tip: In this paper, we report on the correlation between aspartate transaminase/alanine transaminase ratio, aspartate transaminase platelet ratio index and Fib-4 scores with fibrosis stages in non-alcoholic fatty liver disease patients, suggesting that they could be used in combination with Fibroscan to predict significant fibrosis and cirrhosis. This is significant as this could be helpful in avoiding liver biopsy to assess liver fibrosis.
- Citation: Al Danaf L, Hussein Kamareddine M, Fayad E, Hussain A, Farhat S. Correlation between Fibroscan and laboratory tests in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis patients for assessing liver fibrosis. World J Hepatol 2022; 14(4): 744-753
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/744.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.744
The liver is the largest internal solid organ and gland in the human body, its major functions include bile production — consisting of bile salts, cholesterol, bilirubin, and electrolytes, and water, absorbing and metabolizing bilirubin, supporting blood clots, fat and carbohydrates metabolization, vitamin and mineral storage that helps metabolizing proteins for digestion, filtering the blood and removing compounds from the body, being involved in the immune activity and production of albumin. An organ as complex as the liver can experience a range of problems including fatty liver disease, hepatitis, cirrhosis, hepatocellular carcinoma and cholangiocarcinoma[1].
Milder form of liver disease is non-alcoholic fatty liver disease (NAFLD) where excess fat accumulates in the liver of non-alcoholic patients. A small group of NAFLD patients may develop non-alcoholic steatohepatitis (NASH) where fat accumulation is accompanied by hepatocellular inflammation and different degrees of scarring that may lead to severe liver scarring and cirrhosis, causing the liver to lose its proper function[2,3]. Since those with NASH-related cirrhosis generally have worse outcomes, it becomes imperative to identify patients with advanced fibrosis for screening for complications of cirrhosis and receive specific treatments aimed to reverse or prevent progression of fibrosis[4,5].
NAFLD is part of the metabolic syndrome characterized by insulin resistance (diabetes or pre-diabetes), BMI in the overweight or obese region, abnormal blood lipid levels, and hypertension. Common etiologies of chronic liver disease are alcoholic hepatitis, viral hepatitis alcohol abuse, hemochromatosis, and metabolic disorders that result in hepatocellular injury and consequently liver fibrosis, cirrhosis, and/or hepatocellular carcinoma. Accurate assessment of the disease severity is important treatment planning.
NAFLD patients may have right upper quadrant pain, fatigue, pruritis, and hepatomegaly, but they are often asymptomatic and stigmata of chronic liver disease are uncommon. Risk factors for NASH include age > 45 years, an aspartate transaminase (AST) level > alanine transaminase (ALT) level, insulin resistance, obesity, and portal hypertension[6].
Liver fibrosis is due to repetitive injury to the liver with the subsequent wound-healing[7]. Following hepatocyte damage (e.g., acute viral hepatitis), parenchymal cells regenerate and replace cells that have undergone apoptosis or necrosis. However, the process is accompanied by an inflammatory response and wound-healing process involving deposition of a limited amount of extracellular matrix (ECM) in the liver parenchyma. If the hepatocellular injury persists or continues, this process of liver regenerations is overwhelmed and fails, and the normal liver parenchyma is substituted with an abundant ECM rich in fibrillar collagen[8]. This ultimately leads to cirrhosis and its associated bad outcomes and high mortality rates. Progression to end-stage liver disease is variable, but typically slow, developing over 2-4 decades in those with chronic liver disease[9].
The gold standard for diagnosis of NAFLD involves a thorough clinical history with pathological correlations. This is then confirmed through the detection of steatosis on liver biopsy and the exclusion of all other causes, including alcohol consumption[10]. Liver biopsy is the most dependable and specific method of detecting and staging fibrosis, diagnosing the cause of fibrosis, and determining whether it had progressed to cirrhosis[11,12].
However, liver biopsy has many limitations including: high cost, sampling error as it only represents 1/50000 of the liver volume and therefore does not accurately reflect the entire liver’s architecture and fibrotic changes[13-16]. Consequently, biopsies from different areas depict varying stages of fibrosis and cirrhosis may be missed in up to 30% of patients, resulting in it not being an ideal prognostic indicator. Furthermore, variations of opinion between pathologists may lead to under staging of cirrhosis and this correlates with recounts of inter and intra-observer discrepancies of up to 20% in assessing fibrotic changes[17,18]. Given the prevalence of NAFLD and the invasive nature of a liver biopsy, it is not cost effective or practical to conduct this procedure on all patients at risk of NASH or fibrosis. Lastly, numerous risks and complications may occur with a 1% risk of significant complications post biopsy such as injury to adjacent organs, hemorrhage, bile leak and infection[13].
As a result of these limitations, the use of liver biopsies as a diagnostic tool has greatly reduced and led to the development of novel alternative noninvasive imaging modalities and laboratory tests for assessing liver fibrosis in NAFLD and NASH. These methods include AST/ALT ratio, AST platelet ratio index (APRI), Fibroscan, ultrasonography (US), and Fib-4 score which might be capable of overcoming the limitations of liver biopsy. They have been reported to be highly sensitive and specific in estimating liver fibrosis and predicting outcomes. In addition, they are liver specific, easy to perform, reliable, inexpensive, and are accurate tools for fibrosis staging and disease progression monitoring[16].
To assess the prevalence of fatty liver and detect moderate to severe fatty changes, non-invasive imaging modalities, such as US, are preferred[5,6]. It is recommended that this is the first-line imaging technique, as it is a reliable method for detection of moderate to severe steatosis in the liver. It is inexpensive, non-invasive, and readily available[16].
However, using ultrasound alone is an imperfect measure when staging fibrotic changes in the liver because of a lack of accuracy and reliability. Although ultrasound scoring systems have been proposed that assesses numerous factors to determine fibrosis stage such as evaluating liver edge bluntness, the size of the liver, the coarseness of the parenchyma and the nodularity of the liver surface, these findings are largely dependent on the equipment utilized[13,14]. Previously, clear correlations were not seen between the grayscale ultrasound findings and histological findings. However, recent developments in ultrasound technology have resulted in increased diagnostic accuracy when measuring hepatic fibrosis with the aforementioned ultrasound scoring system[15]. Nonetheless, ultrasound has various drawbacks as it still not able to distinguish NASH from simple steatosis or differentiate between steatosis and fibrosis and its accuracy when staging fibrosis is still questionable due to various influences[19,20].
Ultrasound is subject to intra-observer reproducibility and inter-observer variability that reduces the accuracy and reliability of pathological findings. Furthermore, factors such as patient body habitus may reduce its accuracy[16,17].
Using ultrasound-based techniques, Fibroscan was developed. It is one of the most extensively used noninvasive methods of assessing hepatic fibrosis[18]. It is simple, readily available, inexpensive, performed within a short procedure time (< 15 min), is able to provide accurate and immediate results, and can be performed at the bedside or in an outpatient clinic. This is through the usage of an ultrasound transducer probe, whereby an elastic shear wave is created through mild amplitude and low frequency vibrations that are transmitted through the hepatic tissue. Pulse-echo ultrasound is used to propagate the shear wave in order to measure the velocity (m/s) and provide an accurate liver stiffness measurement (LSM) within a specific volume of liver tissue The LSM is expressed in kilopascal (KPa) that correlate with fibrosis stage[19-21].
In several studies, the Fibroscan showed high sensitivity and specificity levels when predicting hepatic fibrosis and cirrhosis in patients with chronic liver disease[4]. With regards to cirrhosis, the specific and sensitivity of the Fibroscan approaches 90%, however when detecting liver fibrosis the sensitivity and specificity reduces to 70%-80%. Numerous variables can influence the Fibroscan as it utilizes ultrasound technology. For example, adipose tissue and the presence of fluid can alter the velocity of the shear wave. Furthermore, obesity, intercostal wall thickness, liver congestion, elevated portal vein pressure, operator inexperience, heart failure and ascites can all reduce the accuracy of the Fibroscan[14,18].
Liver function tests are used frequently in clinical settings to assist in diagnosing and monitoring hepatic pathologies and damage through measuring enzyme levels and protein in the blood. Although these investigations vary in range, normal liver function is also tested through its ability to produce protein and clear bilirubin. Other liver function tests measure the enzymes released by hepatic cells in response to damage due to hepatic pathologies or secondary processes. However, liver function tests may produce false positive and negative results and, therefore, do not always indicate disease.
Some common liver function tests include[22]: Alanine transaminase (ALT or SGPT), Aspartate transaminase (AST or SGOT), prothrombin index (PT), International Normalized ratio (INR), partial thromboplastin time (PTT), albumin, bilirubin, gamma-glutamyl transferase (GGT), platelet count, glucose, ALP, Triglycerides, LDL, HDL and Cholesterol are all relevant markers of fibrotic change[23].
Recently, as an alternative to liver biopsy, cost-effective, noninvasive and reliable laboratory assessments for monitoring chronic liver disease have been developed[24]. Although these tests cannot truly distinguish NASH vs simple steatosis, patients with significant fibrosis, will by definition also have NASH as simple steatosis is not associated with an acceleration in hepatic fibrosis. Research has emphasized these readily availabe markers in assessing those with more advanced fibrosis. Hence, they may help guide treatment decisions and prediction of cirrhosis complications. However, at this point, no available test or modality can completely take the place of a histological analysis[25]. These include: ALT: one of the first markers of assessing liver disease. Serum ALT has been shown to be highly sensitive and specific, and therefore valuable to measure[25].
AST/ALT ratio is another widely available test. In NAFLD, an AST/ALT ratio > 1 is usually associated with progressive liver fibrosis or cirrhosis[4].
Developed in 2003 by Wai et al[13], the APRI in 2003 and is calculated as such:

Multiple studies had shown that it is highly accurate in predicting advanced fibrosis in different forms of liver disease with a higher correlation coefficient than platelet count, or AST level alone[18]. In a meta-analysis including 40 studies, researchers showed that an APRI score > 1.0 had a 76% sensitivity and 72% specificity in predicting cirrhosis. Furthermore, the investigators concluded that APRI score > 0.7 had a 77% sensitivity and 72% specificity in predicting significant hepatic fibrosis[25].
The FIB-4 score is calculated as such:
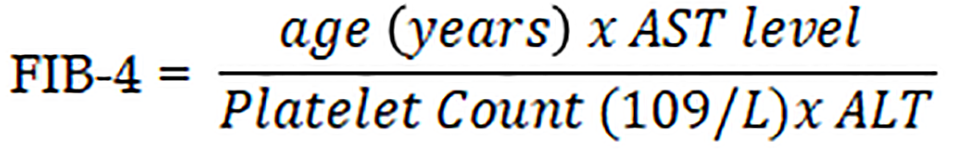
FIB-4 score less than 1.45 had a negative predictive value of 90% for advanced fibrosis. On the other hand, a FIB-4 score greater than 3.25 had a 97% specificity and 65% positive predictive value for advanced fibrosis[26].
This study was performed on 73 patients (45 males and 28 females) diagnosed with NAFLD/NASH based on Fibroscan examination and serum liver enzyme testing. Patients had undergone Fibroscan and Laboratory tests at Saint Georges Hospital University Medical Center, Ashrafieh, Beirut, Lebanon from 24 April 2018 to 6 September 2019. Patients with incomplete data or with ascites were excluded from analyses.
Fibroscans were performed on patients instructed to lay in the dorsal decubitus position along with the right arm put on the head. Estimations were made using a transducer probe starting in the right upper quadrant at the level of the right liver lobe. Up to 10 estimations were performed on every patient with outcomes expressed in KPa.
The following parameters were assessed for each patient: age, gender, total cholesterol, LDL, HDL, triglycerides, ALT, AST, albumin, bilirubin, GGT, PT, PTT, glucose, platelet counts. Relation between laboratory tests, age and gender and Fibroscan stages were analyzed.
APRI is calculated based on the following equation:
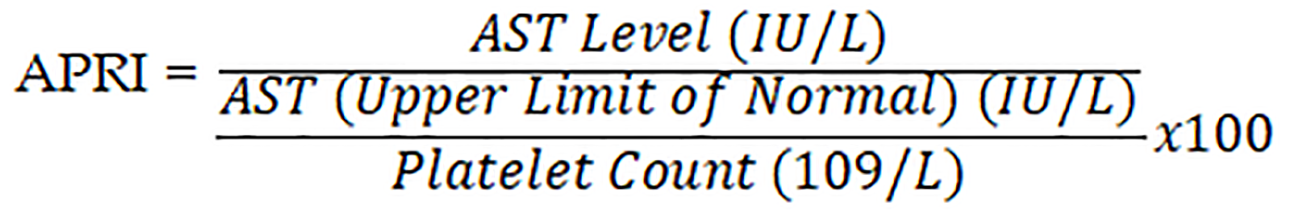
Fibrosis-4 is calculated based on the following equation:
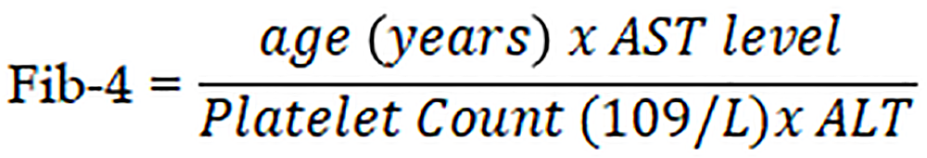
Where the laboratory reference normal range of serum ALT is 0-41 U/L. Normal upper serum ALT limits were defined as 40 U/L. The normal range of serum AST is 0-40 U/L, and the normal platelet count reference range is 150-450 × 103/mm3. AST/ALT ratio was also calculated. The results of APRI, Fib-4 and AST/ALT ratio were correlated to Fibroscan results in order to determine the ability of Liver Stiffness measurement to predict fibrosis in patients with chronic liver disease.
Statistical analysis was performed using Megastat12, an Excel add-on that enables advanced statistical analyses within an Excel workbook. Means and P value were calculated and significance between fibrosis stages, age, gender, Laboratory tests, and scores were assessed. The analyses of the patient characteristics were estimated using one-way ANOVA or chi-square test as appropriate. ANOVA test is a way to compare two means from two independent groups to find out if experiment results are significant (i.e., to reject the null hypothesis or accept the alternate hypothesis) by testing groups to see if there is a difference between them. However, a chi-squared test was used to determine whether a significant difference between expected and observed frequencies in one or more of the categories exists.
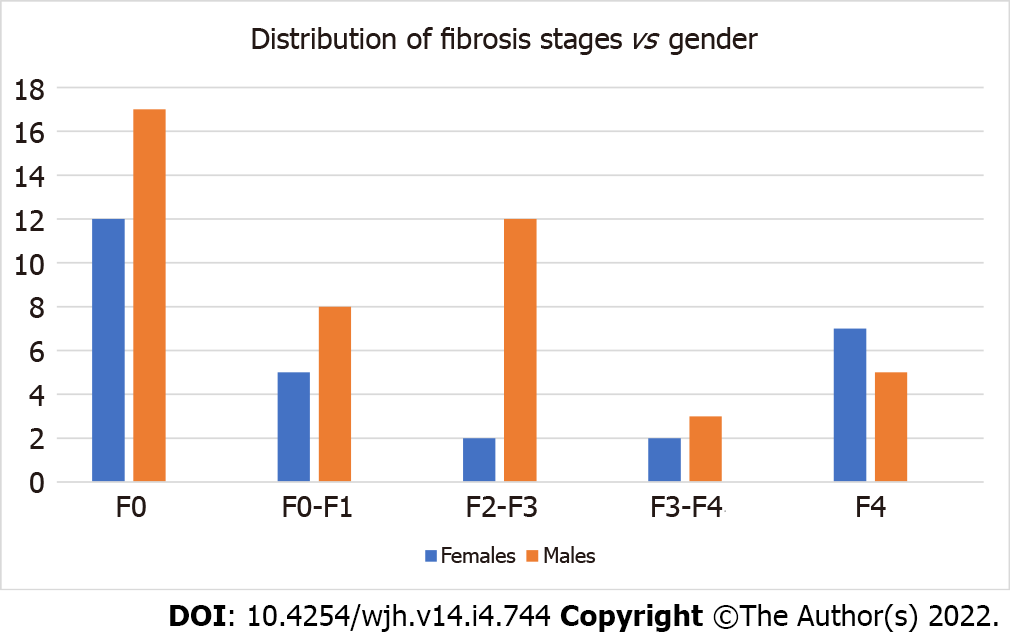
A total of 73 patients were identified, 45 males with mean age of 50.24 ± 15.71 and 28 females with mean age of 57.28 ± 15.07. The mean stiffness score was 9.48 ± 11.77 KPa, and the majority of patients did not exhibit fibrosis or advanced liver disease. According to the Metavir score, 29 patients were classified as F0 (Normal), 13 as F0-F1 (Normal-Mild Fibrosis stage), 14 as F2-F3 (Mild-Moderate Fibrosis stage), 5 as F3-F4 (Moderate-Severe Fibrosis stage) and 12 as F4 (Cirrhosis) (Table 1). Female patients (38% of all samples) exhibited higher stiffness scores than male patients (62% of all samples), but this difference was not significant 10.81 ± 15.42 vs 8.69 ± 9.02, respectively (P value = 0.23) (Figure 1).
| Stage of fibrosis | Number of patients (%) |
| F0 | 29 (40) |
| F0-F1 | 13 (18) |
| F2-F3 | 14 (19) |
| F3-F4 | 5 (7) |
| F4 | 12 (16) |
| Total | 73 (100) |
The total mean age for our sample was 52.5 years with a 15.5 standard deviation. A significant actual age difference exists across fibrosis stages (P value = 0.0036). Furthermore, the ages was classified into two groups (20-49 and > 50 years) in order to study the correlation between specific age groups and fibrosis stages. A significant difference exists across fibrosis stages and the two age groups with a P value = 0.0302. Table 2 summarizes the results for the relation between the two age groups and the fibrosis stages where patients above 50 years old are more prone to advanced stages of fibrosis.
| Patient’s age | Fibrosis stages | |||||
| F0 | F0-F1 | F2-F3 | F3-F4 | F4 | Total | |
| 20-49 | 17 | 7 | 7 | 0 | 2 | 33 |
| > 50 | 12 | 6 | 7 | 5 | 10 | 40 |
| Total | 29 | 13 | 14 | 5 | 12 | 73 |
Interestingly, a significant positive correlation was observed between Bilirubin, ALP, PT INR, PTT, Glucose, and Platelet count when compared to Fibrosis stages as statistically confirmed using Chi-squared Test (P value = 0.0001, 0.033, 0.0011, 0.0054, 0.0063, and 0.0001 respectively). However, a significant negative correlation was observed between ALT, AST, Albumin, GGT, Cholesterol, LDL, HDL and Triglycerides when compared to fibrosis stages as statistically confirmed using Chi-squared Test (P value = 0.71, 0.07, 0.44, 0.22, 0.22, 0.07, 0.68, and 0.57 respectively) although it has no clinical significance (Table 3).
| Characteristic | P value |
| Age | 0.0036 |
| Gender | 0.23 |
| ALT | 0.71 |
| AST | 0.07 |
| Albumin | 0.44 |
| Cholesterol | 0.22 |
| GGT | 0.22 |
| LDL | 0.07 |
| HDL | 0.68 |
| ALP | 0.033 |
| Triglycerides | 0.57 |
| Bilirubin | 0.0001 |
| Glucose | 0.0063 |
| Platelet count | 0.0001 |
| PT INR | 0.0011 |
| PTT | 0.0054 |
On the other hand, a positive correlation was also observed between the AST/ALT ratio, APRI, and Fib-4 scores when compared to Fibrosis stages (Table 4).
| < F2 (n = 42) | > F2 (n = 31) | P value | |
| AST/ALT ratio | 0.84 ± 0.47 | 1.31 ± 1.1 | 0.0029 |
| APRI score | 0.35 ± 0.26 | 1.08 ± 1.2 | 3.59 × 10-7 |
| Fib-4 score | 1.07 ± 0.73 | 3.41 ± 3.44 | 9.11 × 10-9 |
Data from patients at various stages of NAFLD were obtained in order to investigate if non-invasive biomarkers including AST/ALT ratio, APRI and Fib-4 scores can be used to assess liver fibrosis. The results confirmed that the fibrosis stages increased significantly with elevated AST/ALT ratio, Fib-4 and APRI scores.
Our data showed that based on Fibroscan exams, a high percentage of NAFLD patients had advanced stages of hepatic fibrosis. Furthermore, these findings were also supported by the strong parallel between the Fibroscan results and the FIB-4 scores, AST/ALT ratios, and APRI ratios.
A positive correlation between age and fibrosis stages was noted, while a negative correlation was observed between gender and fibrosis stages. Moreover, there was a negative correlation between both ALT and AST levels and fibrosis stages, which means that it is not effective to rely only on ALT and AST levels when clinically diagnosing patients with NAFLD. Studies conducted on the general population have demonstrated that ALT levels increase with age[27,28]. However, our results did not display a relationship between ALT levels and age. Moreover, studies have shown that a significant amount of NAFLD patients had normal or near-normal liver enzyme levels[29-31].
All patients in this cohort underwent baseline abdominal US that demonstrated steatosis; however as discussed earlier, US alone is not an ideal assessment tool of liver disease advancement. This further encourages the use of readily available biomarkers and Fibroscan plus abdominal US in the assessment of NAFLD instead of liver biopsy. Fibroscan is a helpful instrument that was recently developed to assess transient liver elasticity and expresses liver stiffness in KPa. Several studies have shown a direct relationship between liver stiffness on Fibroscan and fibrosis staging with liver biopsy. In a study conducted by Sandrin et al[32], the median hepatic elasticity was 4.2 KPa for F0 fibrosis score, 4.5-6.25 KPa for F1 fibrosis score, 5.5-7.8 KPa for F2 fibrosis score, 8.0-13.7 KPa for F3 fibrosis score, and 21–34 KPa for a F4 fibrosis score.
This study’s main finding is that AST/ALT ratio, APRI and Fib-4 score have high positive relation with advanced fibrosis in patients with NAFLD. This suggests that they could be clinically used to avoid liver biopsy. A large numbers of patients with NAFLD are being referred for evaluation, and these non-invasive tests could help reduce the quantity of liver biopsies performed. This would benefit patients in the way of cost staving as well as by directing liver biopsies to patients more likely to be exhibiting advanced disease. Fallatah et al[23] showed results that are similar to this study where a strong positive correlation was observed between AST/ALT ratio, APRI and Fib-4 scores and fibrosis scores[26].
Liver enzyme levels in NAFLD patients fluctuate. When they are elevated, the increase is often mild and usually restricted to one or both of AST and ALT. Nevertheless, it is important to emphasize that although elevated ALT is generally associated with histological NASH/NAFLD, a large number of NASH/NAFLD patients had normal or near-normal liver enzyme levels. Therefore, ALT level alone cannot be used to rule out significant liver disease in patients suspected of having NASH/NAFLD.
This study also showed that platelet count, Bilirubin, PT INR, PTT, and Glucose levels are significantly related to fibrosis stages and can be used as independent predictors for fibrosis.
The obvious major advantage of using these simple scoring systems is that the labs they are derived from are readily available. Clearly, AST/ALT ratio is the simplest to calculate. Fib-4 and APRI scores require more complex calculation, but the relevant details can easily be entered onto many of the medical calculator smartphone applications with instantaneous results. Therefore, introduction of the use of these tests into daily practice should be relatively simple and will not result in extra costs.
The limitations of the study were that it took place in a tertiary hospital where there may be selection bias, a higher number of patients will be required for future studies, and no correlation with liver biopsy, the gold standard of NAFLD diagnosis, was possible at that time.
Based on this study, I would recommend the use of Fibroscan in combination with Laboratory tests and non-invasive Laboratory biomarkers including AST/ALT ratio, APRI and Fib-4 scores for assessing liver fibrosis in patients with early fibrosis stages, for their great advantages and cost-effective benefits, and limiting the use of liver biopsy for patients with advanced fibrosis stages.
In conclusion, this study showed that Ultrasound alone is not efficient in assessing the advancement of liver disease. Furthermore, the high positive correlation of AST/ALT ratio, APRI and Fib-4 scores from one side when compared to fibrosis stages in patients with NAFLD suggests that they could be clinically used in combination with Fibroscan to predict significant fibrosis and cirrhosis and to avoid liver biopsy. This benefits to patients with cost savings and less invasive procedures.
As a future perspective, the use of these simple scoring systems that are derived from readily available clinical and laboratory tests, using a pre-designed Excel sheet, can give an instant result, therefore, introducing these tests into daily practice should be rather simple and will not result in extra costs[32].
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disease ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), through to advanced fibrosis and cirrhosis. Many patients with NAFLD remain undiagnosed and recognizing those at risk is very crucial. Although liver biopsy is the gold standard method for diagnosing and staging NAFLD, non-invasive imaging and lab modalities are also very promising in diagnosing these diseases.
The main motivation for this research was to objectively assess existing non-invasive modalities alone or in combination and determine whether they could accurately help in diagnosing and staging liver disease, foregoing the need for invasive diagnostics such as liver biopsy.
The objective of this research was to combine clinical, lab, and imaging data and assess their ability to accurately diagnose and stage NAFLD without invasive diagnostics such as liver biopsy.
This study was conducted on NAFLD/NASH patients (n = 73) who underwent Fibroscan examinations at Saint George Hospital University Medical Center over 17 mo in order to assess liver fibrosis. Obtained Fibroscan results were correlated to laboratory tests and calculated aspartate transaminase (AST)/alanine transaminase (ALT) ratio, AST platelet ratio index (APRI) score and Fibrosis-4 score.
A significant age difference was observed across fibrosis stages of investigated patients. The mean stiffness score was 9.48 ± 11.77 KPa. A significant positive correlation was found between Bilirubin, PT INR, partial thromboplastin time, glucose, and platelet count when compared across fibrosis stages, in addition to AST/ALT ratio, APRI, and Fib-4 scores.
We conclude that ultrasound alone is not efficient in the assessment of the advancement of liver disease. Furthermore, the high positive relation between AST/ALT ratio, APRI and Fib-4 scores with fibrosis stages in NAFLD patients suggests that they could be used clinically in combination with Fibroscan to predict significant fibrosis and cirrhosis and to avoid liver biopsy.
More research and data is required to make better recommendations. As more and more fields of clinical medicine forego invasive diagnostics in favor of their non-invasive counterparts, the data for such a shift in the diagnosis and staging of NAFLD is encouraging.
We would like to express our special appreciation and thanks to Malaeb B, MD, Khoury S, MD, and Al Faraj A, PhD who offered a great help and support. Special thanks to Ms. Othman H who helped in statistical analysis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tolunay HE, Turkey S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Newman T. What does the liver do?. Medical News Today. 2 March 2018. Available from: https://www.medicalnewstoday.com/articles/305075.php Cited 12 January 2021. |
| 2. | Micelle Biopharma. (2018). Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. [cited 12 January 2021]. In: Micelle biopharma [Internet]. Available from: https://micellebiopharma.com/non-alcoholic-fatty-liver-disease-and-non-alcoholic-steatohepatitis-sc410/. |
| 3. | Alkhouri N, Kay MH. Non Alcoholic Fatty Liver Disease (NAFLD). [cited 12 January 2021]. In: American College of Gasteroenterology [Internet]. Available from: https://gi.org/topics/fatty-liver-disease-nafld/. |
| 4. | Kang JS, Lee MH. Noninvasive Diagnostic and Prognostic Assessment Tools for Liver Fibrosis and Cirrhosis in Patients with Chronic Liver Disease. In: Tsoulfas G. Liver Cirrhosis. Georgios Tsoulfas, IntechOpen, 2017. |
| 5. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 681] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 6. | Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (3)] |
| 8. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4116] [Article Influence: 205.8] [Reference Citation Analysis (3)] |
| 9. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1375] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 10. | Hannah WN Jr, Harrison SA. Nonalcoholic fatty liver disease and elastography: Incremental advances but work still to be done. Hepatology. 2016;63:1762-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 11. | Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Afzal S, Masroor I, Beg M. Evaluation of Chronic Liver Disease: Does Ultrasound Scoring Criteria Help? Int J Chronic Dis. 2013;2013:326231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3242] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 14. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 15. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 16. | Westin J, Lagging LM, Wejstål R, Norkrans G, Dhillon AP. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver. 1999;19:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Fallatah HI. Noninvasive Biomarkers of Liver Fibrosis: An Overview. Adv Hepatol. 2014;2014:357287. |
| 18. | Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? J Hepatol. 1997;27:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD, Chen CH, Huang WS, Changchien CS. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol. 2003;38:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Nishiura T, Watanabe H, Ito M, Matsuoka Y, Yano K, Daikoku M, Yatsuhashi H, Dohmen K, Ishibashi H. Ultrasound evaluation of the fibrosis stage in chronic liver disease by the simultaneous use of low and high frequency probes. Br J Radiol. 2005;78:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1447] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 22. | Pimpalwar Y, Rao A. Liver fibrosis assessment: a correlation of fibro scan values with gray scale assessment of portal vein. Int J Res Med Sci. 2018;6:317-320. |
| 23. | Fallatah HI, Akbar HO, Fallatah AM. Fibroscan Compared to FIB-4, APRI, and AST/ALT Ratio for Assessment of Liver Fibrosis in Saudi Patients With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2016;16:e38346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N Y). 2012;8:605-607. [PubMed] |
| 25. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 26. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 27. | Davis CP. Liver Blood Tests (Normal, Low, and High Ranges & Results). [cited 12 January 2021]. In: MedicineNet [Internet]. Available from: https://www.medicinenet.com/Liver_blood_tests/article.htm#what_are_the_basic_functions_of_the_liver. |
| 28. | Chen ZW, Chen LY, Dai HL, Chen JH, Fang LZ. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. J Zhejiang Univ Sci B. 2008;9:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2292] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 30. | Pradat P, Alberti A, Poynard T, Esteban JI, Weiland O, Marcellin P, Badalamenti S, Trépo C. Predictive value of ALT levels for histologic findings in chronic hepatitis C: a European collaborative study. Hepatology. 2002;36:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 791] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 32. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3555] [Article Influence: 187.1] [Reference Citation Analysis (0)] |