Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.719
Peer-review started: May 18, 2021
First decision: July 8, 2021
Revised: July 20, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 27, 2022
Processing time: 338 Days and 20.4 Hours
The evaluation of periportal fibrosis (PPF) is essential for a prognostic assessment of patients with Schistosomiasis mansoni. The WHO Niamey Protocol defines patterns of fibrosis from abdominal ultrasonography, 1H-nuclear magnetic resonance (NMR)-based metabonomics has been employed to assess liver fibrosis in some diseases.
To build 1H-NMR-based metabonomics models (MM) to discriminate mild from significant periportal PPF and identify differences in the metabolite profiles.
A prospective cross-sectional study was performed on schistosomiasis patients at a University Hospital in Northeastern Brazil. We evaluated 41 serum samples from 10 patients with mild PPF (C Niamey pattern) and 31 patients with significant PPF (D/E/F Niamey patterns). MM were built using partial least squares-discriminant analysis (PLS-DA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA) formalisms.
PLS-DA and OPLS-DA resulted in discrimination between mild and significant PPF groups with R2 and Q2 values of 0.80 and 0.38 and 0.72 and 0.42 for each model, respectively. The OPLS-DA model presented accuracy, sensitivity, and specificity values of 92.7%, 90.3%, and 100% to discriminate significant PPF. The metabolites identified as responsible by discrimination were: N-acetylglucosamines, alanine, glycolaldehyde, carbohydrates, and valine.
MMs discriminated mild from significant PPF patterns in patients with Schistosomiasis mansoni through identification of differences in serum metabolites profiles.
Core Tip: In this study, we demonstrated a metabolic signatures and metabolic pathway disturbances that allowed to discriminate mild from significant periportal fibrosis in 41 patients with Schistosomiasis mansoni. Partial least squares-discriminant analysis (PLS-DA) and OPLS metabonomics models provided a clear separation between the groups. PLS-DA model presented accuracy, R2 and Q2 values equal to 0.85, 0.80 and 0.38, respectively, while OPLS model had R2 and Q2 values equal to 0.717 and 0.417, respectively. We also identified some metabolites responsible by discrimination which are associated with changes related to liver function and amino acids metabolism.
- Citation: Rodrigues ML, da Luz TPSR, Pereira CLD, Batista AD, Domingues ALC, Silva RO, Lopes EP. Assessment of periportal fibrosis in Schistosomiasis mansoni patients by proton nuclear magnetic resonance-based metabonomics models. World J Hepatol 2022; 14(4): 719-728
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/719.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.719
Schistosomiasis is a neglected disease that still occurs around the world and affects about 240 million people in 78 countries[1]. In Brazil, it is caused by Schistosoma mansoni. It has been considered an endemic disease in the state of Pernambuco, with cases reported in 102 of 185 cities[2,3].
Periportal fibrosis (PPF), known as Symmers’ fibrosis, is induced by helminth eggs deposition in the portal vein and its branches. This fibrosis can extend to the peripheral intrahepatic branches without promoting hepatocyte necrosis, making it one of the causes of non-cirrhotic portal hypertension[4].
Ultrasonography (US) scan is used for diagnosis and assessment of PPF by the Niamey-Belo Horizonte Protocol, the WHO Standard Protocol. This protocol classifies 6 PPF patterns from A (no-fibrosis) up F (very advanced fibrosis), plus mixed patterns, such as the D/C or E/C patterns[4-6].
Although the US exam enables PPF diagnosis and measurement, there are limitations for its use, including the inter-observer variation and the low sensitivity to the diagnosis of initial forms of the disease, especially if the examiner has no experience in applying the Niamey-Belo Horizonte protocol. Additionally, the device can be difficult to access in some poor regions. Due to these difficulties, alternative strategies are being studied. Some serum biomarkers, alone or in association (indexes), have been used for this purpose. Some authors reported an inversely proportional relationship between platelet count and PPF pattern, as well as a directly proportional relationship between liver enzymes serum levels and PPF patterns[5,6].
Metabonomics is an area of knowledge that uses multivariate statistical formalisms applied to spectra data of biofluids to obtain a multiparametric response to external stimuli, such as pathogens[7,8]. The biofluid, properly stored, can be analyzed at a center distant from the collection site. Batista et al[9] used 1H-nuclear magnetic resonance (NMR)-based metabonomics for liver fibrosis assessment in patients with chronic hepatitis C. The method proved to be useful in the diagnosis of significant and advanced fibrosis in these patients. Gardini et al[10] developed the profile of the serum metabolome of patients with hepatocellular carcinoma in early and advanced stages. They found that 1H-NMR metabolomics profiling could discriminate early from advanced hepatocellular carcinoma. The multivariate statistical formalisms most commonly used in metabonomics assays are: principal components analysis (PCA) for exploratory analyses, since it does not depend on class information and investigates if there are outlier samples; and partial least square-discriminant analyses (PLS-DA) or orthogonal PLS-DA (OPLS-DA). which use the class information to build metabonomics models (MM) that discriminate among samples from different groups[11]. In the present study, we aimed to build 1H-NMR-based MM to discriminate mild from significant PPF in patients with Schistosomiasis mansoni and identify differences in the profiles of the endogenous metabolites.
This is a phase II diagnostic validation test, a cross-sectional study performed with adult patients who were diagnosed with Schistosomiasis mansoni, aiming to assess PPF patterns by 1H-NMR-based metabonomics.
Patients aged 18 years or over diagnosed with Schistosomiasis mansoni were included from the Schistosomiasis Clinic of the Gastroenterology Service of the Hospital das Clínicas, Universidade Federal de Pernambuco (Recife, Pernambuco, Brazil), between March and December 2019. Schistosomiasis diagnosis was based on the clinical history of contact with water sources in endemic areas, report of previous treatment with praziquantel, and associated with finding of PPF by US scan. Exclusion clinical criteria were: presence of fatty liver disease, cirrhosis or hepatocellular carcinoma, portal vein thrombosis, HIV, hepatitis B or C virus coinfection, or history of drug-induced liver injury or alcohol abuse.
All patients were submitted to US scan after overnight fasting of about 8 h, by the same examiner. According to the Niamey-Belo Horizonte Protocol, PPF pattern was defined as follows: C (peripherical fibrosis), D (central fibrosis), E (advanced fibrosis) and F (very advanced fibrosis) patterns. Patients without or with a doubtful PPF (A and B pattern) were excluded of the study. All US exams were performed using a US Siemens Acuson S2000 instrument equipped with a 6C1 Ultrasound probe (Siemens Medical Solutions, Mountain View, CA, United States).
Blood samples were collected from a peripherical vein after US scan. Serum was obtained after centrifugation (3500 rpm) using a Centurion-Laborline equipment. Liver function tests, including alanine aminotransferase and aspartate aminotransferase, gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), lipid profile (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides), and glycemia, were carried out using Wiener Lab® (Wiener Lab Group, Santa Fé, Argentina) kits in a Wiener Lab® autoanalyzer (Wiener Lab Group, Santa Fé, Argentina). Part of the samples were stored at minus 40 °C until the NMR analysis.
To investigate the distribution of demographic and clinical or laboratory data between groups, univariate tests were performed using GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, United States) with unpaired Student's t-test, Mann-Whitney, and Fisher’s exact as appropriate. A P value < 0.05 was set as the level of statistical significance.
All 1H-NMR spectra were recorded using a VNMRSYS400 spectrometer operating at 400 MHz. After thawing, serum samples were prepared by mixing 400 μL of serum and 200 μL of D2O and placing in NMR tubes of 5 mm id. 1H-NMR spectra were performed using a sequence of radiofrequency pulses with presaturation of the water signal hyphenated to the Carr-Purcell-Meiboom-Gill pulse sequence, which was employed as a T2 filter. The following parameters were used: spectral window of 6.4 kHz, saturation delay of 2.0 s, acquisition time of 1.704 s, 90° RF pulse, temperature of 27 °C, 88 cycles, tau equal to 0.0004 s, bigtau equal to 0.07 s, and 128 scans. The line broadening used was 0.3 Hz. Baseline and phase distortions were corrected manually. The signal attributed to the methyl group of lactate (δ 1.33 ppm) was used as a chemical shift reference. Using MestreNova 9.0 software, the region between δ 4.004 and 0.772 ppm was binned into 808 bins (each 0.004 ppm-wide). The matrix was built with 41 rows (cases) and 809 variables (bins of 1H-NMR spectra plus class variable), and then was submitted to multivariate analysis. The models based on PCA, PLS-DA, and OPLS-DA were constructed using MetaboAnalyst online platform 4.0[12,13]. In the preprocessing step, each sample was normalized by sum (cumulative intensity of the spectrum). This was performed to compare the spectral data, avoiding problems with sample dilutions, for example[14]. In addition, data were pre-processed using autoscaling. The validation of the PLS-DA and OPLS-DA models was based on two methods: (1) the leave-one-out cross validation method (LOOCV), where the optimal number of latent variables for the PLS-DA model was determined, thus providing the basis for the computation of the predictive ability (Q2), determination coefficient (R2), and the classification accuracy of the model; and (2) the permutation test, which made 2000 permutations of the class label to verify the accuracy of metabonomics models. PLS-DA and OPLS-DA models provided a quantitative measure of the discriminating power of each spectral bin. Variable importance in the projection (VIP) score was used. VIP is a weighted sum of squares of the PLS loadings. These weights are based on the amount of explained variance of the dependent variable in each PLS dimension. A VIP score cut-off equal to 1 was used. Discriminatory signals were attributed to metabolites using Human Metabolome Database platform and also based on the literature[15-18]. Accuracy, sensitivity, and specificity values were obtained from a confusion matrix that was constructed considering classification of OPLS-DA model.
Forty-four patients were selected, but three were excluded because their samples proved to be outliers. Thus, 41 patients with PPF were included in the study: 10 patients with C, 12 patients with D, 17 patients with E, and 2 patients with F patterns, according to the Niamey-Belo Horizonte Protocol. These patients were divided into two group: mild PPF (C pattern) and significant PPF (D/E/F patterns)[19]. Table 1 shows clinical and demographic data of the patients.
| Characteristic | Total | Mild PPF (C pattern) | Significant PPF (D/E/F pattern) | P value |
| n | 41 | 10 | 31 | - |
| Age (yr) | 57 (18-80) | 48.1 (18-75) | 57.2 (25-80) | 0.0865a |
| Sex | 0.4820b | |||
| Male | 17 (41%) | 5 (40%) | 12 (39%) | |
| Female | 24 (59%) | 5 (50%) | 19 (61%) | |
| AST (U/L) | 29.0 ± 2.6 | 24.3 ± 1.9 | 30.0 ± 2.9 | 0.3328c |
| ALT (U/L) | 29.0 ± 2.2 | 26.2 ± 4.5 | 30.0 ± 3.2 | 0.4584c |
| ALP (U/L) | 262 ± 33 | 329 ± 113 | 238 ± 22 | 0.6379c |
| GGT (/LSN) | 62 ± 12 | 35 ± 16 | 71.2 ± 14.0 | 0.0013c |
| Platelets count (/mm3) | 131 ± 12 | 218 ± 15 | 102 ± 11 | 0.0001c |
| Total Cholesterol (mg/dL) | 169.0 ± 4.6 | 174.0 ± 7.2 | 167.8 ± 5.6 | 0.4626c |
| HDL (mg/dL) | 45.7 ± 2.0 | 49 ± 5.7 | 44.6 ± 2.0 | 0.4863c |
| LDL (mg/dL) | 105.0 ± 3.8 | 108 ± 4.7 | 104 ± 4.8 | 0.3760c |
| Glucose (mg/dL) | 93.6 ± 5.2 | 97 ± 14 | 92 ± 5.1 | 0.9451c |
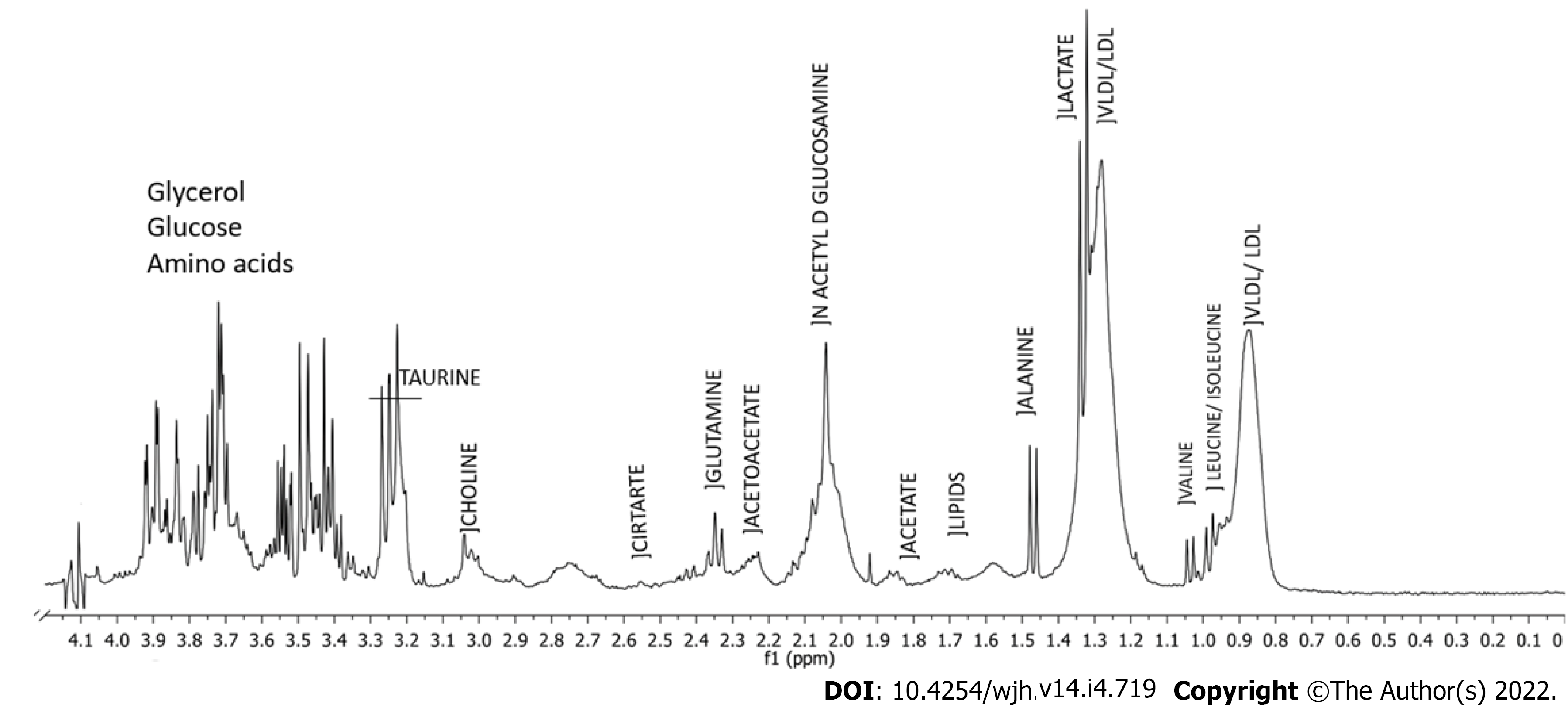
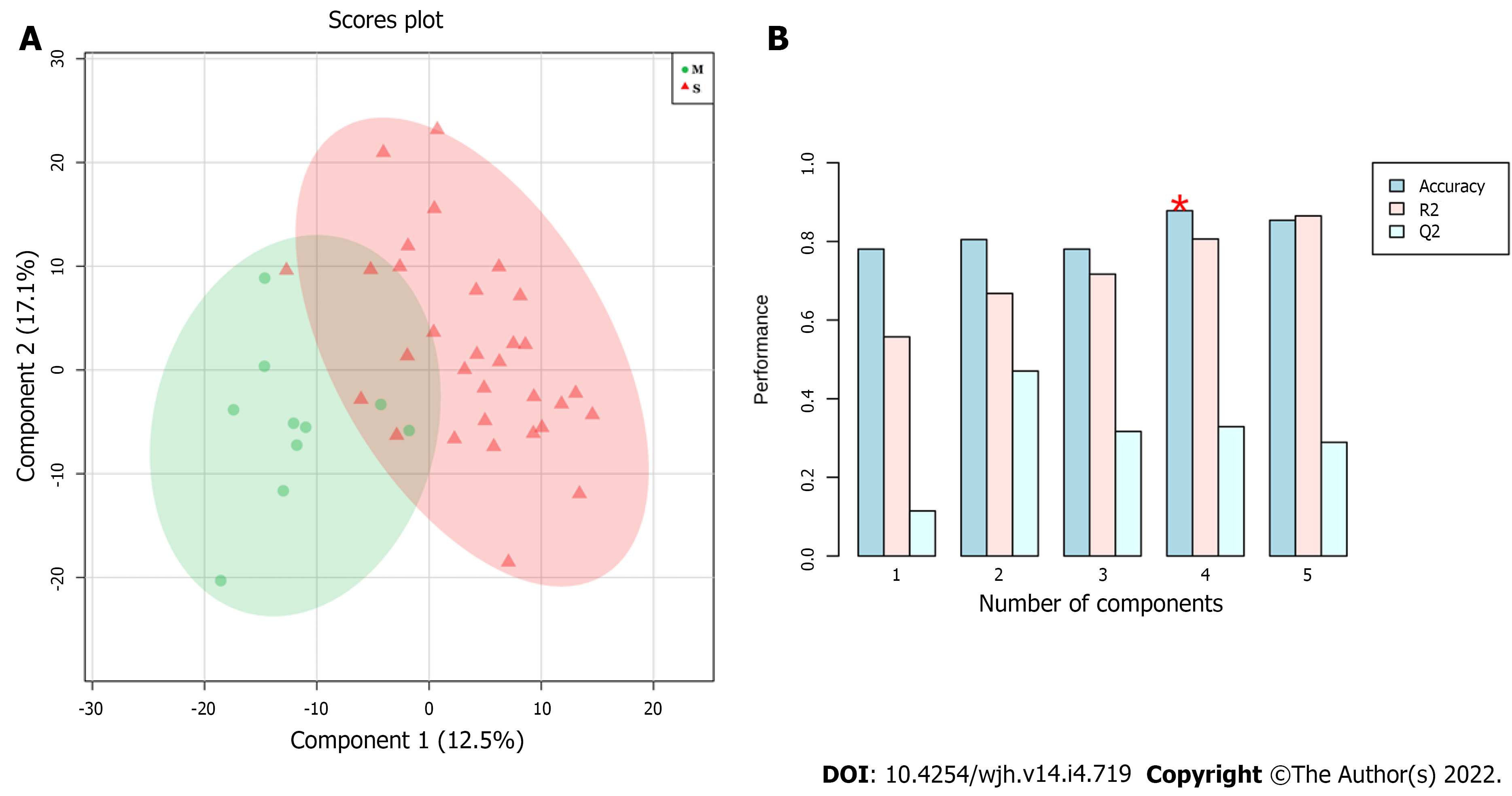
Figure 1 shows a typical 1H-NMR spectrum of serum obtained in the study with assigned peaks. Exploratory analyses by PCA failed to indicate separation between the groups (data not shown). Thus, MM were developed using supervised methods: PLS-DA and OPLS-DA formalisms. Figure 2 shows a score plot (A) and the performance of MM constructed using PLS-DA formalism (B). Regarding accuracy, the best performance was achieved when four latent variables were used, resulting in accuracy, R2 and Q2 values equal to 0.85, 0.80 and 0.38, respectively.
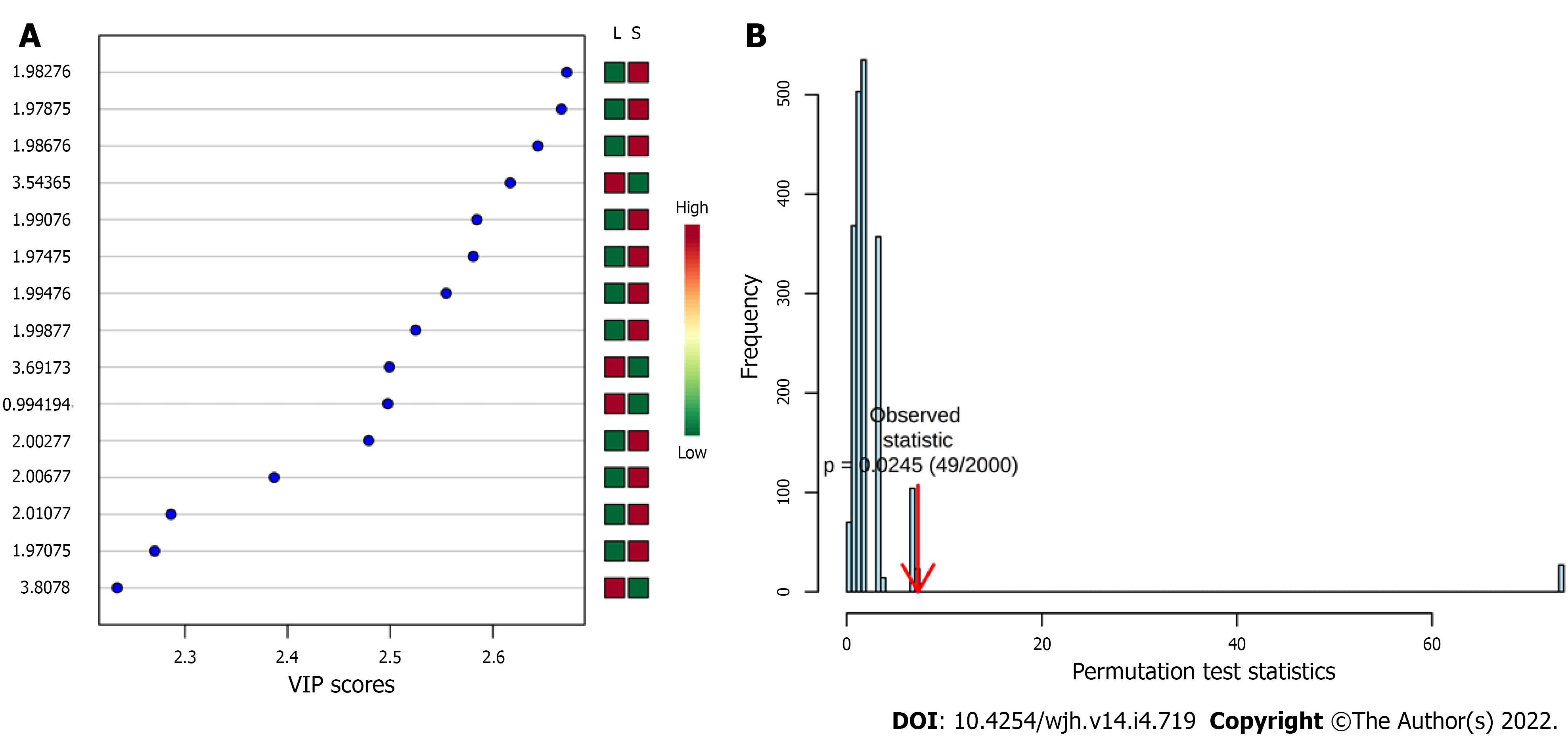
Figure 3 shows variables importance for projection (VIP) score plot (A) and the permutation test from PLS-DA model which presented P value equal to 0.0245 after 2000 classes permutations (B). The spectral region responsible for discrimination was between δ 1.975 and δ 2.011 ppm, which is attributed to the methyl group of N-acetylglucosamines. The serum level of N-acetylglucosamines is higher in the group with significant PPF (Significant PPF) than in the group with mild PPF (Mild PPF). In addition to this region, three more discriminatory bins can be observed: δ = 3.544, 3.692, and 3.808 ppm, which were assigned to carbohydrates. According to the VIP score plot, the serum level of these carbohydrates is higher in the Mild PPF group. The VIP score full table presents other discriminatory bins, such as δ 1.502 ppm (VIP score = 1.98) and δ 3.492 ppm (VIP score = 1.83), which were assigned to alanine and glycolaldehyde, both higher in the Significant PPF group.
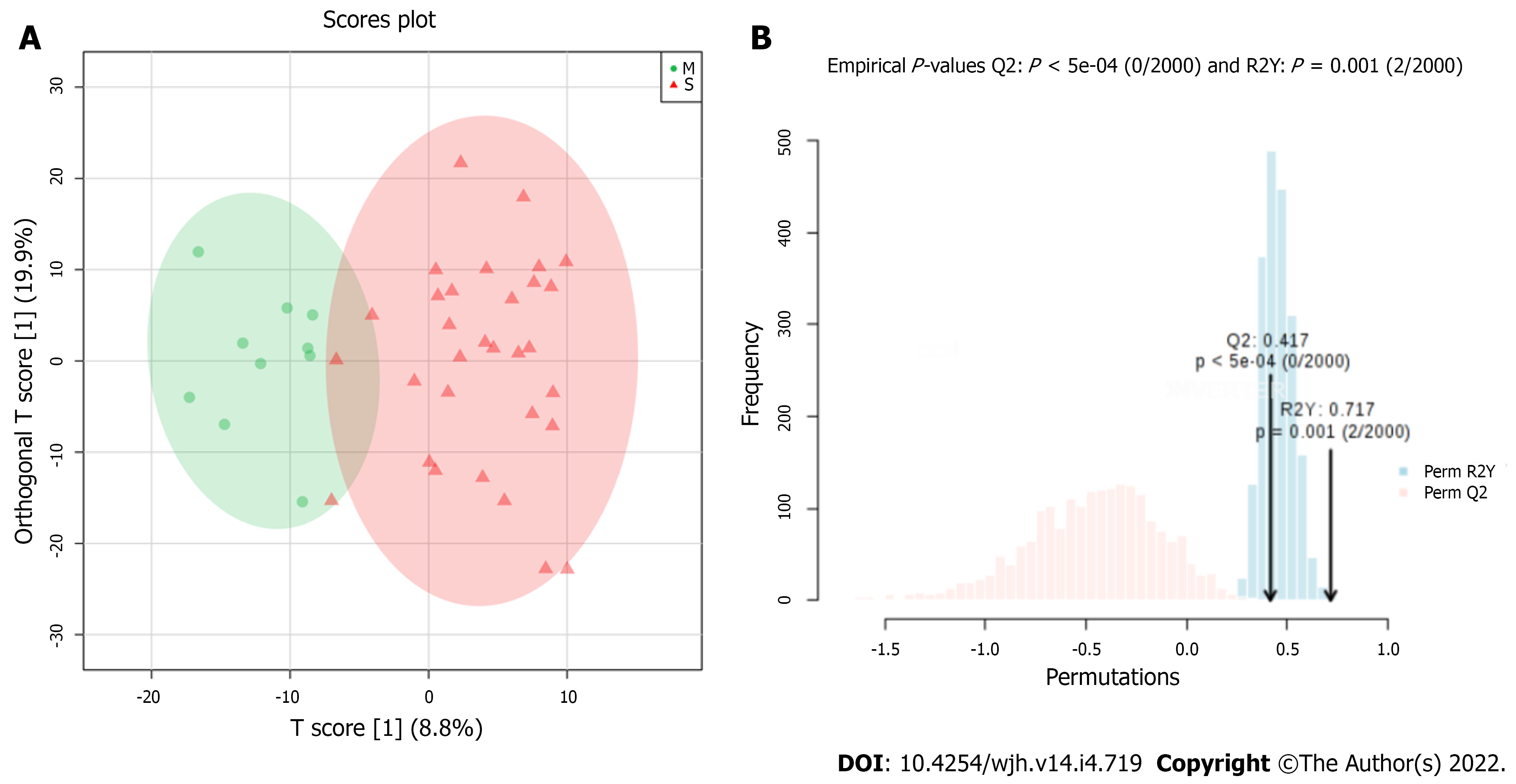
Figure 4 shows a score plot and the results of permutation test of metabonomics models using OPLS-DA formalism (B). The OPLS-DA MM presented R2Y and Q2 values equal to 0.717 and 0.417, respectively, with P values from permutation test less than 0.01. We identified four discriminatory bins, as follows: δ 1.030 ppm, assigned to valine; δ 1.046 ppm (not assigned); δ 1.446 ppm attributed to alanine; and δ 3.692 ppm assigned to carbohydrates. The serum levels observed for valine, carbohydrates, and the unidentified metabolite (signal at δ 1.046 ppm) were higher in the Mild PPF group, while the alanine serum level was higher in the Significant PPF group.
Table 2 presents a summary of metabolites that differentiated the Mild PPF samples from the Significant PPF samples, as well as the chemometric formalism employed, the chemical shift (δ) of each metabolite, and the group in which these metabolites had higher serum level.
| Metabolite | Chemical shift (δ/ppm) | Metabonomics formalism | Higher serum level |
| Valine | 1.030 | OPLS-DA | Mild PPF |
| No identified | 1.046 | OPLS-DA | Mild PPF |
| Alanine | 1.446 and 1.502 | PLS-DA and OPLS-DA | Significant PPF |
| N-acetylglucosamine | 1.975 up to 2.011 | PLS-DA | Significant PPF |
| Glycolaldehyde | 3.492 | PLS-DA | Significant PPF |
| Carbohydrates | 3.544; 3.692; and 3.808 | PLS-DA and OPLS-DA | Mild PPF |
Table 3 shows the confusion matrix obtained from the OPLS-DA MM. Accuracy, sensitivity, specificity, positive predictive and negative predictive values are equal to 92.7%, 90.3%, 100%, 100% and 76.9%, respectively.
| Classification from WHO Niamey Protocol | ||||
| Significant PPF | Mild PPF | P valuea | ||
| Metabonomics Model | Significant PPF | 28 | 0 | < 0.0001 |
| Mild PPF | 3 | 10 | ||
PPF is a mark of Schistosomiasis mansoni disease. Assessment of PPF intensity is crucial to determine disease morbidity and prognosis. In addition, significant PPF is associated with non-cirrhotic portal hypertension and its consequences. Generally, US scan and serum biomarkers have been used for PPF assessment in schistosomiasis patients[2,20]. Among serum biomarkers for PPF assessment, liver enzymes and platelet count alone or combined with ALP, as in the Coutinho-index, have been given importance in the literature[2,6,7]. In agreement with these authors, laboratory data found in this study indicate a higher serum level of GGT in particular, and lower platelet count in patients with significant PPF. Köpke-Aguiar et al[21] also reported higher serum levels of GGT in the more severe cases. Pereira et al[22] and Lambertucci[23] also reported lower platelets count, as well as increased spleen size in more severe Schistosomiasis mansoni disease.
Significant PPF in the schistosomiasis patients is induced by increase in number of eggs in the intrahepatic portal veins due repeated infections and by the host’s exacerbated immune response[24]. The presence of adult worms in mesenteric and portal vessels, as well as the presence of their eggs, promotes immunological stimulation and induces primary splenomegaly by reticuloendothelial system hyperplasia, which leads to pancytopenia by hypersplenism[19,22]. Splenomegaly also causes blood hyperflow in the splenic vein, which contributes to presinusoidal portal hypertension[25]. In addition, the changes in liver hemodynamics, triggered by portal hyperflow, could promote increase of GGT serum level, as observed in our study[26].
Usually, metabonomics studies begin with exploratory analyses by PCA. However, this initial analysis failed to discriminate between the groups. Therefore, discriminant analyses formalisms were employed, resulting in efficient separation between mild PPF and significant PPF groups. Serum levels of valine (δ 1.030 ppm) and alanine (δ 1.446 and 1.502 ppm) observed in the spectral data suggest that the changes in portal vein flow could trigger disorders of amino acids metabolism in hepatocytes, as reported by Li et al[27]. Changes in amino acid serum levels were important for discrimination in both the PLS-DA and OPLS-DA MM. These findings are in agreement with studies that correlate changes in serum levels of these amino acids in response to liver fibrogenesis caused by schistosomiasis in mice[15,28]. Balog et al[29] also reported an association between valine and alanine serum levels with the disease progression.
An increase of N-acetylglucosamines serum levels was observed in the significant PPF group, while the carbohydrate serum level was higher in the mild PPF group. Glucosamines are products of glucose metabolism, which are capable of suppressing the production of metalloproteinases. Therefore, the N-acetylglucosamines and glucose serum levels observed could be associated with liver damage, which requires glucose consumption and production of N-acetylglucosamines[30].
In clinical practice, the monitoring of patients with Schistosomiasis mansoni is done by US scan. Hence, it is necessary to transfer patients from rural zone to hospital unit or to bring the device to the field. In addition, an experienced examiner is necessary, since the US scan is operator-dependent.
The metabolic profile presented in this study can be strategic for monitoring the patients in endemic regions through blood samples collected and transported to a reference laboratory. 1H-NMR-based metabonomics produce a “metabolic fingerprint”, providing systemic metabolic information about patients. It can help to identify those with more severe forms of schistosomiasis. The main limitation of the study was the sample size and the disproportionate PPF pattern groups.
In the present study, we used 1H-NMR-based metabonomics from the serum of patients with Schistosomiasis mansoni to discriminate those with highest intensity of PPF. Moreover, the chemometric formalisms used enabled the identification of some metabolites associated with the discrimination, such as alanine, glycolaldehyde, and N-acetylglucosamines, which presented higher serum levels in the significant PPF group, while valine and carbohydrates presented lower serum levels in the most severe cases.
The 1H-NMR-based metabonomics models were able to discriminate mild from significant PPF patterns in patients with Schistosomiasis mansoni through identification of differences in serum metabolites profile. We intend to expand the study in the coming years in order to confirm the results and best understand the metabolic pathways associated to observed discrimination.
Classification of the pattern of periportal fibrosis (PPF) is essential in the prognostic evaluation of patients with Schistosomiasis mansoni.
There is a need for novel minimally invasive methods and new biomarkers for the diagnosis Schistosomiasis mansoni.
To develop metabolic models, based on 1H-nuclear magnetic resonance spectra, that allow the classification of the pattern of PPF and its associated metabolites in patients with Schistosomiasis mansoni.
Metabonomics models (MMs) were built to differentiate requirements with mild PPF and significant PPF. An analysis of the performance of MMs was performed for the prediction of PPF, using ultrasonography as a reference standard and the description of the main metabolites present in each PPF group and their relationship with serum markers.
The partial least squares-discriminant analysis (PLS-DA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA) formalisms discriminated spectral regions between the groups as follows: carbohydrates and valine, more concentrated in those of the group with mild FPP; N-Acetylglycosamines, Alanine, Glycolaldehyde more concentrated in the samples of the group with significant PPF. OPLS-DA showed accuracy, sensitivity, and specificity, were equal to 92.7%, 90.3%, and 100% for the diagnosis of significant PPF.
The constructed MMs were able to discriminate between mild and significant FPP in patients with schistosomiasis with good accuracy.
This technique will be able to detect even low-intensity infections, overcoming the limitations of current diagnostic techniques, with the use of a single serum sample. These models can be inserted in the propaedeutic arsenal in clinical practice for the measurement of PPF in remote areas.
The authors thank Analytical Central Laboratory, Universidade Federal de Pernambuco for the 1H-NMR spectra and to the Hospital das Clínicas, Universidade Federal de Pernambuco where patients were selected. They also thank Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University) by grammar review of English text.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mocan T, Romania S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Silva-Moraes V, Shollenberger LM, Siqueira LMV, Castro-Borges W, Harn DA, Grenfell RFQE, Rabello ALT, Coelho PMZ. Diagnosis of Schistosoma mansoni infections: what are the choices in Brazilian low-endemic areas? Mem Inst Oswaldo Cruz. 2019;114:e180478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Facchini LA, Nunes BP, Felisberto E, da Silva JAM, da Silva Junior JB, Tomasi E. Assessment of a Brazilian public policy intervention to address schistosomiasis in Pernambuco state: the SANAR program, 2011-2014. BMC Public Health. 2018;18:1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Graeff-Teixeira C, Favero V, Pascoal VF, de Souza RP, Rigo FV, Agnese LHD, Bezerra FSM, Coelho PMZ, Enk MJ, Favre TC, Katz N, Oliveira RR, Dos Reis MG, Pieri OS. Low specificity of point-of-care circulating cathodic antigen (POCCCA) diagnostic test in a non-endemic area for schistosomiasis mansoni in Brazil. Acta Trop. 2021;217:105863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Richter J, Domingues AL, Barata CH, Prata AR, Lambertucci JR. Report of the second satellite symposium on ultrasound in schistosomiasis. Mem Inst Oswaldo Cruz. 2001;96 Suppl:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Gunda DW, Mtui EF, Manyiri PM, Majinge DC, Kilonzo SB, Mazigo HD, Kidenya BR. Schistosoma mansoni-related periportal fibrosis; can we use APRI and PSDR levels in the real-time selection of patients for targeted endoscopy in a resource-limited setting? BMC Gastroenterol. 2021;21:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Hashim A, Berzigotti A. Noninvasive Assessment of Schistosoma-Related Periportal Fibrosis. J Ultrasound Med. 2021;40:2273-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008;455:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1368] [Cited by in RCA: 1385] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 8. | Garcia-Perez I, Posma JM, Serrano-Contreras JI, Boulangé CL, Chan Q, Frost G, Stamler J, Elliott P, Lindon JC, Holmes E, Nicholson JK. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat Protoc. 2020;15:2538-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Batista AD, Barros CJP, Costa TBBC, de Godoy MMG, Silva RD, Santos JC, de Melo Lira MM, Jucá NT, Lopes EPA, Silva RO. Proton nuclear magnetic resonance-based metabonomic models for non-invasive diagnosis of liver fibrosis in chronic hepatitis C: Optimizing the classification of intermediate fibrosis. World J Hepatol. 2018;10:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Casadei-Gardini A, Del Coco L, Marisi G, Conti F, Rovesti G, Ulivi P, Canale M, Frassineti GL, Foschi FG, Longo S, Fanizzi FP, Giudetti AM. 1H-NMR Based Serum Metabolomics Highlights Different Specific Biomarkers between Early and Advanced Hepatocellular Carcinoma Stages. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Worley B, Powers R. Multivariate Analysis in Metabolomics. Curr Metabolomics. 2013;1:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 12. | Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251-W257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2063] [Cited by in RCA: 2130] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 13. | Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2019;68:e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1496] [Article Influence: 299.2] [Reference Citation Analysis (0)] |
| 14. |
Loo RL, Lodge S, Kimhofer T, Bong SH, Begum S, Whiley L, et al Espectroscopia de RMN de diagnóstico quantitativo in vitro para medições de lipoproteínas e metabólitos em plasma e soro: recomendações para minimização de artefatos analíticos com referência especial a amostras COVID-19/SARS-CoV-2.
|
| 15. | Wang Y, Holmes E, Nicholson JK, Cloarec O, Chollet J, Tanner M, Singer BH, Utzinger J. Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc Natl Acad Sci U S A. 2004;101:12676-12681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Wishart DS. Computational strategies for metabolite identification in metabolomics. Bioanalysis. 2009;1:1579-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Li JV, Saric J, Wang Y, Keiser J, Utzinger J, Holmes E. Chemometric analysis of biofluids from mice experimentally infected with Schistosoma mansoni. Parasit Vectors. 2011;4:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Pontes TA, Barbosa AD, Silva RD, Melo-Junior MR, Silva RO. Osteopenia-osteoporosis discrimination in postmenopausal women by 1H NMR-based metabonomics. PLoS One. 2019;14:e0217348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Carvalho Santos J, Dória Batista A, Maria Mola Vasconcelos C, Souza Lemos R, Romão de Souza Junior V, Dessein A, Dessein H, Maria Lucena Montenegro S, Pessoa Almeida Lopes E, Lúcia Coutinho Domingues A. Liver ultrasound elastography for the evaluation of periportal fibrosis in schistosomiasis mansoni: A cross-sectional study. PLoS Negl Trop Dis. 2018;12:e0006868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1599] [Cited by in RCA: 1211] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 21. | Köpke-Aguiar LA, Martins JR, Passerotti CC, Toledo CF, Nader HB, Borges DR. Serum hyaluronic acid as a comprehensive marker to assess severity of liver disease in schistosomiasis. Acta Trop. 2002;84:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Pereira CLD, Santos JC, Arruda RM, Rodrigues ML, Siqueira ES, Lemos RS, Batista AD, Domingues ALC, Lopes EP. Evaluation of Schistosomiasis Mansoni Morbidity by Hepatic and Splenic Elastography. Ultrasound Med Biol. 2021;47:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Lambertucci JR. Revisiting the concept of hepatosplenic schistosomiasis and its challenges using traditional and new tools. Rev Soc Bras Med Trop. 2014;47:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Barbosa VS, E Guimarães RJ, Loyo RM, Barbosa CS. Modelling of the distribution of Biomphalaria glabrata and Biomphalaria straminea in the metropolitan region of Recife, Pernambuco, Brazil. Geospat Health. 2016;11:490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Coutinho A. Hemodynamic studies of portal hypertension in schistosomiasis. Am J Med. 1968;44:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Alves A, Fontes DA, De Melo VA, Machado MCC, Cruz JF, Santos EAS. Hipertensão portal esquistossomótica: Influência de fluxo sangüíneo portal nos níveis séricos das enzimas hepáticas. Arq Gastroenterol. 2003;40:203-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Li Y, Xie M, Men L, Du J. O-GlcNAcylation in immunity and inflammation: An intricate system (Review). Int J Mol Med. 2019;44:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Wu J, Xu W, Ming Z, Dong H, Tang H, Wang Y. Metabolic changes reveal the development of schistosomiasis in mice. PLoS Negl Trop Dis. 2010;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Balog CIA, Meissner A, Göraler S, Bladergroen MR, Vennervald BJ, Mayboroda OA, Deelder AM. Investigação metabonômica da infecção humana pelo Schistosoma mansoni. Mol Biosyst. 2011;7:1473-1480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Zhang B, Li MD, Yin R, Liu Y, Yang Y, Mitchell-Richards KA, Nam JH, Li R, Wang L, Iwakiri Y, Chung D, Robert ME, Ehrlich BE, Bennett AM, Yu J, Nathanson MH, Yang X. O-GlcNAc transferase suppresses necroptosis and liver fibrosis. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |