Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.696
Peer-review started: June 21, 2021
First decision: September 2, 2021
Revised: September 14, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: April 27, 2022
Processing time: 304 Days and 14 Hours
Schistosomiasis mansoni is a neglected disease and key public health problem, mainly due to its high prevalence, the scarcity of public policies, and the severity of some clinical forms. Periportal fibrosis (PPF) is the commonest complication of chronic schistosomiasis mansoni and its diagnosis requires different techniques. Even though wedge biopsy of the liver is considered the gold standard, it is not justified in non-surgical patients, and percutaneous liver biopsy may be informative but does not have sufficient sensitivity. Noninvasive PPF tests mostly include biological (serum biomarkers or combined scores) or physical assessments (imaging assessment of fibrosis pattern or tissue stiffness). Moreover, imaging techniques, such as ultrasound, computed tomography, magnetic resonance imaging, and elastography are applied not only to support the diagnosis of schistosomiasis, but also to assess and detect signs of portal hypertension and organ damage due to chronic schistosomiasis. A combination between a comprehensive history and physical examination with biomarkers for liver fibrosis and imaging methods seems to offer the best approach for evaluating these patients. In addition, understanding their strengths and limitations will allow a more accurate interpretation in the clinical context and can lead to greater accuracy in estimating the degree of fibrosis in patients with Schistosomiasis mansoni (S. mansoni) infection. This review will discuss the different noninvasive methods that are currently available for the evaluation of PPF in S. mansoni infection, and their application, advantages, and limitations in clinical practice.
Core Tip: Schistosomiasis mansoni is a neglected and key public health problem and periportal fibrosis (PPF) is its commonest complication. Noninvasive PPF tests mostly include biological or physical assessments. Imaging techniques have been currently applied to assess and detect liver damage due to chronic schistosomiasis. A combination between these biomarkers, a comprehensive history and physical examination, laboratory tests, and imaging methods seems to offer the best approach for evaluating these patients. We herein discuss the different noninvasive methods that are currently available for evaluating PPF in Schistosomiasis mansoni infection, and their application, advantages, and limitations in clinical practice.
- Citation: Santos JC, Pereira CLD, Domingues ALC, Lopes EP. Noninvasive diagnosis of periportal fibrosis in schistosomiasis mansoni: A comprehensive review. World J Hepatol 2022; 14(4): 696-707
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/696.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.696
Schistosomiasis mansoni is a neglected parasitic disease of chronic evolution and a key public health problem, mainly due to its high prevalence, the scarcity of public policies, and the severity of some clinical forms. As it is endemic in over 78 resource-constrained countries, schistosomiasis is considered one of the indicators of poverty[1,2].
The World Health Organization (WHO) estimates that at least 241 million people required preventive treatment[3]. In Brazil, the economic burden of schistosomiasis mansoni is high and results in loss of productivity, and it is a big challenge to public health[4]. Approximately 200 thousand deaths are attributed to this disease annually and they occur in up to 29% of those who present bleeding varices even with full hospital care. Community-based studies suggest that periportal fibrosis (PPF), which is the term more frequently used after the advent of ultrasonography in schistosomiasis, is the commonest complication of Schistosomiasis mansoni (S. mansoni) chronic infection[5].
Additionally, Symmers fibrosis or “pipestem fibrosis” is the advanced PPF form that occurs in patients with hepatosplenic schistosomiasis (HSS) and it is defined as a moderate to advanced PPF, with or without hepatomegaly, but always with splenomegaly, on ultrasound (US). In endemic areas, it is possible to find patients with advanced PPF (Symmers fibrosis) without splenomegaly, also known as the hepatic form of the disease. On the other hand, a few individuals with splenomegaly without PPF can also be found, which is due to other causes of splenomegaly, and if there were no US, these patients would be misdiagnosed, based on the clinical examination, as HSS.
Hence, Symmers fibrosis is the most prominent feature of liver pathology in schistosomiasis mansoni and it is represented by a process of portal fibrosis that extends from the smallest to the largest portal spaces[6]. Granulomas around the parasite eggs can be seen in abundance in the portal spaces, at the beginning of the disease, which disappear when it becomes chronic and the fibrosis remains. There are also obstructive vascular lesions secondary to granulomas, thrombosis, phleboscleorosis, and fibrous intimal thickening. The major liver pathology of egg granulomas results from physical obstruction and tissue compression, while splenomegaly results from both chronic passive congestion and reactive hyperplasia of the reticuloendothelial system due to immune dysregulation[7]. The main clinical manifestation of HSS is portal hypertension (non-cirrhotic) and porto-systemic collateral circulation, notably esophageal varices[8,9].
The hepatic parenchyma maintains its usual acinar structure, and this is reflected in patients exhibiting a normal hepatocyte function despite the signs of portal hypertension, without stigmata of chronic liver disease, unlike cirrhosis. However, in some cases, compensated HSS may turn into decompensated HSS with the presence of stigmatas, ascites, muscular loss, and hepatic failure[8]. These cases of progression from schistosomiasis to liver cirrhosis, with the capacity of the synthesis of hepatocytes being impaired, are sometimes observed in clinical practice in patients who have recurrent episodes of digestive bleeding and repeated necrosis of hepatocytes.
In these decompensated cases, it should also be remembered that there is the possibility of co-infection of schistosomiasis with hepatitis B, C, and E viruses, with the non-alcoholic steatohepatitis or chronic alcohol abuse[10-12].
Schistosomiasis mansoni is commonly diagnosed due to detecting parasite eggs in stool using the Kato-Katz technique. Immunological methods are favored for monitoring communities in areas with low infection rates, and for patients with mild and chronic infections where parasitological tests are negative. Polymerase chain reaction-based diagnostic techniques are more sensitive, but expensive. The Point-of-care Circulating Cathodic Antigen test method appeared to be a promising feature in the diagnosis, but many false positive cases were reported in low endemic areas[2,3,13].
Recently, studies in low prevalence areas for schistosomiasis in Brazil have shown that the primary diagnostic approach performed by the Schistosomiasis Control Country Program may underestimate the real prevalence of this infection. In addition, there have been reports of a high proportion of infected individuals in the urban area, the presence of infected snails, a random distribution of vectors, and the absence of an association between classical risk factors and the human infection[14]. In low endemic countries, the determination of infected patients should combine different methods and algorithms for accurately estimating the prevalence and the indicators that could be used in the control of schistosomiasis[2].
Moreover, diagnosing PPF requires different techniques. Wedge liver biopsy is considered the gold standard, but it is not justified in non-surgical patients. Alternatively, percutaneous liver biopsy may be informative but does not have sufficient sensitivity[15,16]. Imaging techniques like US, computed tomography (CT), magnetic resonance imaging (MRI), and elastography, on the other hand, are used not only to support the diagnosis of schistosomiasis but also to assess and detect signs of portal hypertension and organ damage due to chronic schistosomiasis disease[17-20]. A combination between a comprehensive history and physical examination with biomarkers for liver fibrosis, and imaging methods seems to offer the best approach for evaluating these patients[7,17].
In this review, we will discuss the different noninvasive methods that are currently available for evaluating PPF in chronic schistosomiasis mansoni, and their application, advantages, and limitations in clinical practice.
Noninvasive markers of liver fibrosis can be divided into two groups: Serum biomarkers and imaging techniques. Table 1 summarizes the main features of these methods. Besides the clear advantage of being noninvasive, some of them can offer a more objective interpretation of numerical test results and may overcome the intra- and inter-observer variability of US techniques.
| Feature | Liver biopsy | Serum markers | Imaging techniques | |||
| Wedged | Percutaneous | US | pSWE | TE | ||
| Invasiveness | High | High | Minimal | None | None | None |
| Post-procedural risk | Possible | Possible | Minimal | None | None | None |
| Accuracy for PPF prediction | High | Low | Medium to high | High | Good | Good |
| Sensitivity | High | Low | Medium | High | Medium | Medium |
| Interpretation | Subjective | Subjective | Objective | Subjective | Objective | Objective |
| Observer variability | High | High | Low | High | Not yet evaluated | Not yet evaluated |
| Costs | High | Medium | Depends | Medium | Medium | Medium |
| Limitations by anthropometric features | High | High | None | Medium | Medium | Medium |
| Suitability for monitoring PPF | Low | Low | High | High | High | High |
Serological biomarkers of liver fibrosis are frequently classified as direct or indirect biomarkers. Whereas direct biomarkers reflect extracellular matrix (ECM) turnover and the changes in the fibrogenic cell type, indirect biomarkers mostly estimate the degree of fibrosis[21].
In advanced stages of fibrosis, the liver contains approximately six times more ECM components than normal, including collagens (I, III, and IV), fibronectin, undulin, elastin, laminin, hyaluronic acid (HA), and proteoglycans[22]. Therefore, qualitative and quantitative ECM changes in liver fibrosis can be measured in the blood or urine using these serological biomarkers[23].
Direct and indirect markers may be used alone or in combination to produce composite scores, which can be relatively simple or can be based on complicated formulas[21]. These markers should be reliable and sensitive so as to indicate advanced lesions and also have prognostic value for identifying early disease[17]. Table 2 summarizes the major advantages and limitations of these markers.
| PFF markers | Advantages | Limitations |
| Direct markers | ||
| PICP | Elevated levels in patients not treated yet with praziquantel and related to the stage of fibrosis and necroinflammation | Not reliable for establishing fibrosis grade |
| P3NP | Use for complicated patients who developed hypertension and with more severe liver diseases | Low sensitivity in mild cases |
| Serum type VI collagen | Correlated with liver fibrosis, splenomegaly, portal vein dilatation and the presence of portosystemic collaterals | Low sensitivity |
| Hyaluronic acid | Marker for the initial phase of liver fibrosis and it is able to assess the severity of liver disease | High levels in different etiologies of liver disease, barely accessible |
| Indirect markers | ||
| APRI | Low cost, good sensitivity, high diagnostic accuracy for cirrhosis | Interference of hepatic comorbidities |
| Blood platelet count | Low cost and sensitive marker. It is a marker of portal hypertension and inversely correlated with advanced PPF and the diameter of the spleen | Interference of coagulopathies, some drugs and other live disorders |
| GGT | Low cost. Correlated with more advanced PPF, faster fibrosis progression rate and indicates intrahepatic alterations | Interference of hepatobiliary alterations |
| Coutinho Index | Simplicity of calculation and low cost | Requires more tests for use in mild and moderate fibrosis |
Direct markers are usually fragments of the components of the ECM of the liver produced by hepatic stellate cells during the ECM remodeling process and they usually reflect its deposition or removal[24]. However, they do not indicate the extent of ECM protein distribution[21] and since they are not liver specific, they have a tendency to be more elevated when associated with high inflammatory activity and tend not to be detected in the presence of minimal inflammation[24].
These biomarkers are classified into three groups: Those that measure matrix deposition [procollagen I carboxy terminal peptide, procollagen III amino terminal peptide, tissue inhibitors of metalloproteinase (MMP), transforming growth factor beta and tenascin], those that reflect matrix removal or degradation (procollagen IV C peptide, procollagen IV N peptide, collagen IV, MMP, undulin, urinary desmosine, and hydroxylysylpyridinoline), and those that cannot clearly determine the relationship to the matrix deposition or removal (HA, chitinase-3-like protein 1, and laminin)[23].
Even though direct markers are less useful in clinical practice due to their high cost and difficult procedures, their potential role in the diagnosis and assessment of PPF in schistosomiasis warrants further investigation. The most promising and studied serum markers for the evaluation of PPF are HA, collagen type III, chitinase-3-like protein 1, and laminin[17,23,25].
Since the latest studies referring to these markers have been previously described in complete review articles[17,23,24], the present paper will not be well on them but rather will concentrate on the most recent markers.
Indirect markers include molecules synthesized, regulated, excreted by the liver or released into the blood in response to liver inflammation, or impairment of the liver function[24].
These markers include common clinical biochemistry tests, such as enzymes, proteins, platelets, and coagulation factors, which do not necessarily reflect ECM turnover or fibrogenic cell changes[24]. Moreover, most indirect biomarkers of fibrosis are integrated with one or more biomarker panels that predict fibrosis.
The most prevalent markers to assess PPF are the platelet count, aspartate aminotransferase to platelet ratio index (APRI)[26,27], Fibrosis-4 (FIB-4)[4], gamma glutamyl transferase (GGT)[17,28], and the Coutinho Index[29,30]. Table 3 summarizes the performance of these markers that has been reported in the literature.
| Marker | Parameters | Performance in S. mansoni infected patients | |||||
| Severe PPF | Mild/significant PPF | ||||||
| Cut-off | Sn (%) | Sp (%) | Cut-off | Sn (%) | Sp (%) | ||
| Platelet count[17,26] | Platelet count/mm3 | 141000[17] | 78.5 | 60 | 171000[17], 108500[26] | 80, 91 | 91.7, 85 |
| APRI[17,26] | (AST/ULN)/platelet count | 1.066 | 58.5 | 71.1 | 0.349[17], 0.440[26] | 90, 96 | 83.3, 85 |
| GGT[17] | GGT/ULN | > 1.55 | 60.0 | 75.6 | > 0.84 | 74.6 | 83.3 |
| Coutinho index[29,30] | (ALP/ULN)/platelet count | ≥ 0.330[29], ≥ 0.316[30] | 98, 67.4 | 94.7, 68.3 | ≥ 0.300, ≥ 0.228 | 70.8, 68.6 | 89.5, 46.3 |
In addition, Nascimento et al[4] (2018) noted a correlation tendency between the scores for APRI and FIB-4 (Spearman r = 0.87, P < 0.0001), transient elastography (TE) and FIB-4 (r = 0.70, P = 0.01), and TE and GGT (r = 0.68, P < 0.01) in a small sample of 17 patients with schistosomiasis. Therefore, further studies with a larger sample are required to confirm these results and to determine if this correlation can be used clinically.
The Coutinho index is the alkaline phosphatase (ALP) to platelet ratio {[ALP/upper limit of normality (ULN)]/platelet count (106/L) × 100}. This index was developed to identify patients with more advanced fibrosis (Niamey’s US classification D, E, and F patterns) in endemic areas, without the need for US. These patients, once recruited, were evaluated and, if necessary, sent to a referral hospital. Barreto et al[29] initially studied 120 patients at the referral hospital in Pernambuco, Brazil. After analyzing different enzymes that change during the course of this disease, they developed this index. Subsequently, in another study, this index was validated in an endemic area of the same state, with 378 patients who took a parasitological test that was positive for S. mansoni[30].
Moreover, Gunda et al[31] have also indicated a positive correlation between the presence of esophageal varices and higher APRI levels (> 1.5 m/s, P < 0.001) with a higher sensitivity (82.5%) and specificity (80.1%) in discriminating varices among patients with PPF due to schistosomiasis mansoni. The most common diagnostic approaches to stratify patients based on the risk of variceal bleeding include the use of US and platelet count[32].
In this context, Agha et al[33] in a case-control study in Saudi Arabia, and Xu et al[34] in a cohort in China, investigated, independently, the platelet count/spleen diameter ratio and reported high specificity (83% to 92%) and sensitivity (85% to 100%) for the presence of esophageal varices in HSS patients.
Although the results of some of these studies are promising with regard to evaluating PPF with these indices, more studies with a larger number of patients will be necessary in order to demonstrate the validity of applying these indices in clinical practice.
The early detection of serious complications is vital in the management of chronic schistosomiasis. Thus, making use of diagnostic imaging modalities, such as US, CT, and MRI, is very important as each of them has a vital role in diagnosing and assessing the severity of target organ involvement[35,36].
Since US is a convenient and reliable method, it is routinely used in the diagnosis and evaluation of patients with schistosomiasis and it has become the most well-established tool for evaluating PPF. It can be applied to demonstrate classical features of schistosomal hepatic damage and to grade schistosomiasis disease patterns and status, based on criteria published by the WHO[37].
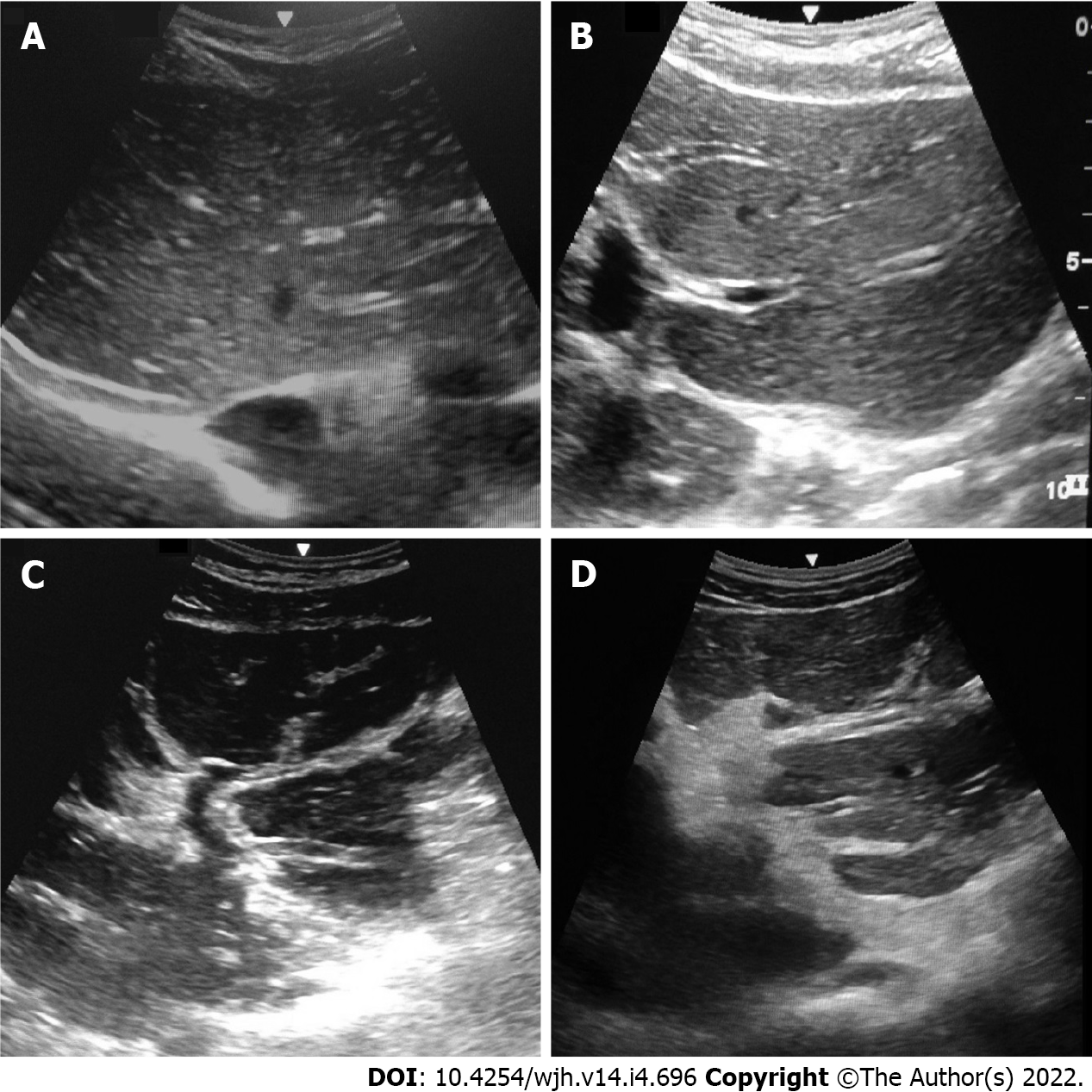
US is a simple, inexpensive, and safe tool and the technique is more sensitive with respect to diagnosing advanced PPF (Symmers fibrosis) than its milder stages. Since the advent of the WHO protocol[37] and later the Niamey-Belo-Horizonte Protocol (2001), US has become the gold standard in the diagnosis of PPF and in the classification of its intensity, which is mainly based on the use of pictorial image patterns (Table 4 and Figure 1). Other previously used protocols, such as Cairo and Managil, were abandoned due to flaws in classifying mild forms of the disease[38,39].
| IP | Description |
| A | Normal |
| B | Diffuse echogenic foci (“starry sky”), minimal wall thickening of portal and segmental branches |
| C | Ring echoes around vessels in cross-section; pipe-stems parallel with portal vessels |
| D | Echogenic ruff around portal bifurcation and main stem; main portal vessel wall thickening |
| E | Hyper-echogenic patches expanding into parenchyma |
| F | Echogenic bands and streaks extending from main portal vein and its bifurcatin to liver surface; may retract the surface of the organ |
| X | Cirrhosis |
| Y | Fatty liver |
| Z | Other hepato-biliary diseases |
Santos et al[14] demonstrated moderate to substantial reproducibility in the PPF classification according to the WHO protocol, mainly using image patterns. In field studies, using these patterns is reported to be simpler and more reproducible than applying other measurements, such as the thickness of the wall of the portal branch and the diameter of the portal, which are also recommended in the WHO protocol[40,41].
Furthermore, the imaging patterns B, C, and D can also be found in other diseases, such as congenital liver fibrosis, viral and autoimmune hepatitis, primary sclerosing cholangitis, and liver cirrhosis. Consequently, a more rigorous analysis by the observer is required to find other signs that will lead to a differential diagnosis of schistosomiasis liver fibrosis[42].
More advanced portable US equipment, like color Doppler US, can characterize portal vein perfusion to predict disease prognosis and with this, decisions can be made on the best treatment options for portal hypertension complications, such as portal vein thrombosis[18].
In our opinion, as US is an operator-dependent technique, the previous experience of the ultrasonographist is very important in evaluation of the image patterns and therefore, to obtain more consistent results. That is the reason why it is unsuitable for the PPF evaluation by US scan to be performed by non-physicians in rural areas, even if they are well trained. Well-trained physicians who know about other diseases that may affect the liver would apply the Niamey protocol in the evaluation of PPF in patients with S mansoni. However, in endemic areas, this protocol is not widely applied by every physician that usually performs US, but only by the few who are trained and interested in research projects.
CT and MRI can also be applied to diagnose PPF. The main features of CT in the fibrotic liver with HSS are round, low-density periportal zones enhanced after contrast administration, and linear bands in longitudinal sections of portal veins[43]. On the other hand, MRI is described as a more sensitive imaging technique than US in the diagnosis of Symmers fibrosis, because it can differentiate fibrosis from fatty liver disease and inflammatory infiltrate.
However, these techniques are not routinely used for schistosomiasis diagnosis in resource-poor settings, due to the use of contrast and the associated risks and the costs[44-46]. In addition, some places do not have an adequate structure for installing these sophisticated devices.
Nevertheless, Voieta et al[47] aimed to classify schistosomal PPF intensity and observed a moderate agreement between imaging techniques (US and MRI) (κ = 0.41). However, it was only after re-grouping the grades (absent and slight vs moderate and intense) that it was observed that the concordance was substantial (κ = 0.63). On the other hand, the agreement between US and MRI and histology was poor.
Moreover, Silva et al[15] described a poor correlation between US and MRI in PPF assessment using WHO patterns (κ = 0.14), and even after grouping image patterns such as “A-D”, “Dc-E” and “Ec-F”, the agreement between US and MRI remained weak (κ = 0.39). The authors recommended that new patterns should be constructed to better reflect MRI findings. Accordingly, Scortegagna et al[48] reported that MRI presented a good reproducibility in the evaluation of PPF in later stages of schistosomiasis, with a global interobserver agreement of 70%. However, the correlation between MRI and US was poor, showing only a 30% agreement.
Recently, elastography-based imaging techniques, such as TE and point shear wave elastography (pSWE) using acoustic radiation force imaging (ARFI), have received substantial attention with respect to the non-invasive assessment of the mechanical properties of tissue. These techniques have emerged as complementary to US images in the study of liver fibrosis in many hepatic diseases[49,50] including schistosomiasis mansoni[19,20,51-54].
These techniques take advantage of changes in the elasticity of soft tissue according to the involvement of certain organs, including the liver and spleen, as this yields qualitative and quantitative information that could be used for diagnostic and prognostic purposes[55].
Recent studies have suggested an association between the degree of liver fibrosis in chronic hepatitis C virus (HCV) infection, reflected by the shear wave speed in the liver, and the severity of liver disease (advanced, compensated, decompensated, or hepatocellular carcinoma). Additionally, in 358 patients with schistosomiais mansoni, Carvalho Santos et al[19] evaluated the PPF by hepatic pSWE (ARFI), thus revealing that this technique could be useful in the diagnosis of advanced forms of schistosomiasis mansoni. The ARFI was able to accurately differentiate mild PPF from significant PPF. Table 5 summarizes the performance reported in the literature of noninvasive PPF imaging techniques in S. mansoni infected patients.
| Marker | Parameters | Performance in schistosoma infected patients | ||
| Severe PPF | Mild/significant PPF | |||
| Cut-off | Cut-off | |||
| HS | HSS | HIS | ||
| US[37] | Image interpretation (Niamey sonographic protocol) | D | E/F | - |
| TE[20,51,53,57] | Wave propagation speed (kPa) | - | 9.6 kPa[57], 8.9 kPa[51], 9.7 kPa[20], 9.5 kPa[53] | - |
| Pswe[19,51,42] | Wave propagation speed (m/s; kPa) | 1.33 m/s[51] | 1.39 m/s[19], 1.53 m/s[52] | 1.11 m/s[19], 1.29 m/s[52] |
| 2D-SWE[54] | Wave propagation speed (m/s; kPa) | - | 14.9 kPa[54] | - |
Similarly, an association has also been reported between splenic elastography and signs of portal hypertension, including the presence of esophageal varices, in some chronic liver diseases[56]. However, these are still initial studies in assessing schistosomiasis portal hypertension[52].
In fact, recent studies have shown that the measurement of spleen stiffness by TE and pSWE correlates with that of liver stiffness and of portal hypertension in patients with HSS[20,52]. Accordingly, spleen stiffness may also be a predictor of variceal bleeding in HSS and may be further investigated for predicting HSS complications[57].
Furthermore, it is relevant to highlight that, in schistosomiasis, massive splenomegaly occurs due to the hyperplasia of the reticuloendothelial system. Hence, there is an increase in blood flow in the splenic vein and also obstruction for the venous flow by intra-hepatic granulomas resulting in portal hypertension[9,58]. The pathophysiology of portal hypertension in (non-cirrhotic) schistosomiasis should contribute to more changes in splenic parenchyma stiffness compared to cirrhosis, since the spleen undergoes passive congestion (without hyperplasia). As a result, splenic elastography should be more accurate than liver elastography for evaluating disease morbidity and portal hypertension signs in patients with schistosomiasis mansoni[59,60]. Table 6 summarizes the advantages and limitations of noninvasive PPF techniques that are frequently used in clinical practice with regard to S. mansoni infected patients.
| Techniques | Advantages | Limitations |
| Blood Platelet Count | Low cost, routine laboratory test, easy access | Difficult to diagnose patients with initial PPF |
| APRI | Low cost, based on routine laboratory tests, easy access | More frequently used to diagnose patients with portal hyperpertension and esophageal varices, less sensitive for PPF |
| Coutinho index | Low cost, based on routine laboratory tests (alkaline phosphatase and platelet count), easy access, lets advanced PPF be identified | These tests need to be validated in other centers |
| Ultrasound | Low cost, safe and based on the Niamey-WHO protocol | Operator dependent |
| MRI/CT | MRI is more sensitive than ultrasound at diagnosing PPF | Expensive, use of radiation, not available in endemic areas, no relation with the Niamey-WHO protocol |
| Liver elastography | Good accuracy, distinguishes mild from significant PPF | Expensive, not available in endemic areas |
| Spleen elastography | Related to portal hypertension | Expensive, not available in endemic areas, needs further studies |
| Wedge liver biopsy | Gold standard used to diagnose Symmers fibrosis | Only for surgical patients |
| Percutaneous liver biopsy | Can be used in differential diagnosis between schistosomiasis and other liver diseases | Insufficiently sensitive and so may fail to diagnose PPF |
Despite their increased use in clinical practice, Non-invasive tests were not designed to follow longitudinal changes in fibrosis or disease activity nor to reflect the dynamic process of fibrogenesis and differentiate between adjacent disease stages regardless of their increased application in clinical practice. Comprehending their strengths and limitations will result in a more accurate interpretation in the clinical context[61].
Therefore, liver fibrosis biological markers allow an objective interpretation of results and significantly reduce the risk of bias due to variability that occurs in liver biopsy, in addition to which these markers have the advantage of not being invasive. Associating the various non-invasive methods can lead to greater accuracy in estimating the degree of fibrosis in patients with schistosomiasis mansoni disease[4].
Crossan et al[62] in a cost-effectiveness study of non-invasive tests for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease in the United Kingdom, reported that high-quality studies with a low risk of bias are required to allow sufficient validation of specific cut-offs to stage fibrosis in different disease etiologies.
Moreover, Crossan et al[62] suggested that, for HCV and hepatitis B virus (HBeAg-negative) infected patients, treating all patients without prior diagnostic testing and regardless of fibrosis level was the most cost-effective option. Nevertheless, the findings from these models may not be transferable to a resource setting where funds are limited and the ability to treat all patients is not a realistic option[63].
In rural areas with few resources, it becomes very difficult to implement imaging methods, such as elastography, for example, or even for there to be a trained physician available to perform US and define the fibrosis pattern using the Niamey-Belo Horizonte protocol. Certainly, the use of serum markers, especially the platelet count and the Coutinho index, should be the most cost-effective, straightforward, and objective noninvasive way to identify and select patients who may have the most advanced forms of PPF.
By undertaking this literature review, we have observed that noninvasive PPF tests mostly include biological (serum biomarkers, indices, or combined algorithms) or physical assessments (imaging assessment of the fibrosis pattern or tissue stiffness). Even though currently available approaches have shown some advantages with respect to overcoming the limitations set out in the previous section, the reason for requesting a test is what will determine the best one for each case.
To date, the platelet count and the Coutinho index appear to be the most applicable noninvasive tests for evaluating PPF in endemic zones where schistosomiasis mansoni occurs. These tests are simple and inexpensive and thus are used to select the cases that will need more accurate evaluation (US assessment of the fibrosis pattern or tissue stiffness). However, they are not always available in endemic areas. Therefore, in such cases, hepatosplenic patients should be referred, if necessary, to hospitals where more specialized imaging tests, such as MRI and CT, can be conducted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen HQ, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Karunamoorthi K, Almalki MJ, Ghailan KY. Schistosomiasis: A Neglected Tropical Disease of Poverty: A Call for Intersectoral Mitigation Strategies for Better Health. J Heal Res Rev. 2018;5:1-12. [DOI] [Full Text] |
| 2. | Silva-Moraes V, Shollenberger LM, Siqueira LMV, Castro-Borges W, Harn DA, Grenfell RFQE, Rabello ALT, Coelho PMZ. Diagnosis of Schistosoma mansoni infections: what are the choices in Brazilian low-endemic areas? Mem Inst Oswaldo Cruz. 2019;114:e180478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. PCT Databank Schistosomiasis; 2021. [cited 1 March 2021]. Available from: https://www.who.int/teams/control-of-neglected-tropical-diseases/preventive-chemotherapy/pct-databank/schistosomiasis. |
| 4. | Nascimento MAJ, Palomares Filho G, Andrade ML, Reis AA VO, Baiao KMR, do Nascimento TVSB, Pacheco MS. Correlation Among Three Non-Invasive Methods (Apri, Fib-4 and Transient Elastography) To Evaluate Liver Function and Stiffness in Patients With Viral Hepatitis C or Schistosomiasis Mansoni. J Trop Pathol. 2018;47:100-110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Gunda DW, Kilonzo SB, Manyiri PM, Peck RN, Mazigo HD. Morbidity and Mortality Due to Schistosoma mansoni Related Periportal Fibrosis: Could Early Diagnosis of Varices Improve the Outcome Following Available Treatment Modalities in Sub Saharan Africa? Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Andrade ZA. Schistosomal hepatopathy. Mem Inst Oswaldo Cruz. 2004;99:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Lambertucci JR. Revisiting the concept of hepatosplenic schistosomiasis and its challenges using traditional and new tools. Rev Soc Bras Med Trop. 2014;47:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Andrade ZA. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009;31:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Coutinho A. Hemodynamic studies of portal hypertension in schistosomiasis. Am J Med. 1968;44:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Van-Lume DS, Albuquerque Mde F, Souza AI, Domingues AL, Lopes EP, Morais CN, Montenegro SM. Association between Schistosomiasis mansoni and hepatitis C: systematic review. Rev Saude Publica. 2013;47:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Passos-Castilho AM, de Sena A, Domingues AL, Lopes-Neto EP, Medeiros TB, Granato CF, Ferraz ML. Hepatitis E virus seroprevalence among schistosomiasis patients in Northeastern Brazil. Braz J Infect Dis. 2016;20:262-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Gasim GI, Bella A, Adam I. Schistosomiasis, hepatitis B and hepatitis C co-infection. Virol J. 2015;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 13. | Graeff-Teixeira C, Favero V, Pascoal VF, de Souza RP, Rigo FV, Agnese LHD, Bezerra FSM, Coelho PMZ, Enk MJ, Favre TC, Katz N, Oliveira RR, dos Reis MG, Pieri OS. Low specificity of point-of-care circulating cathodic antigen (POC-CCA) diagnostic test in a non-endemic area for schistosomiasis mansoni in Brazil. Acta Trop. 2021;217:105863. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Santos IGA, Bezerra LP, Cirilo TM, Silva LO, Machado JPV, Lima PD, Bispo MRS, Gomes SDC, Silva GILD, Alencar VJB, Damasceno IA, Carvalho MMV, Gomes DS, Ramos RES, Santos Júnior EG, Alves LC, Brayner FA. New epidemiological profile of schistosomiasis from an area of low prevalence in Brazil. Rev Soc Bras Med Trop. 2020;53:e20200335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Silva LC, Andrade LM, Queiroz LC, Voieta I, Azeredo LM, Antunes CM, Lambertucci JR. Schistosoma mansoni: magnetic resonance analysis of liver fibrosis according to WHO patterns for ultrasound assessment of schistosomiasis-related morbidity. Mem Inst Oswaldo Cruz. 2010;105:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 17. | Domingues AL, Medeiros TB, Lopes EP. Ultrasound versus biological markers in the evaluation of periportal fibrosis in human Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2011;106:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Olveda DU, Olveda RM, Lam AK, Chau TN, Li Y, Gisparil AD 2nd, Ross AG. Utility of Diagnostic Imaging in the Diagnosis and Management of Schistosomiasis. Clin Microbiol. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Carvalho Santos J, Dória Batista A, Maria Mola Vasconcelos C, Souza Lemos R, Romão de Souza Junior V, Dessein A, Dessein H, Maria Lucena Montenegro S, Pessoa Almeida Lopes E, Lúcia Coutinho Domingues A. Liver ultrasound elastography for the evaluation of periportal fibrosis in schistosomiasis mansoni: A cross-sectional study. PLoS Negl Trop Dis. 2018;12:e0006868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Veiga ZST, Villela-Nogueira CA, Fernandes FF, Cavalcanti MG, Figueiredo FA, Pereira JL, Pereira GH, Moraes Coelho HS, Peralta JM, Marques CE, Perez RM, Fogaça HS. Transient elastography evaluation of hepatic and spleen stiffness in patients with hepatosplenic schistosomiasis. Eur J Gastroenterol Hepatol. 2017;29:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Nielsen MJ, Leeming DJ, Karsdal MA, Krag A. Biomarkers of extracellular matrix remodeling in liver disease. In: Patel VB, Preedy VR. Biomarkers in disease: methods, discoveries and applications. Dordrecht: Springer, 2017: 221-246. |
| 22. | Olveda DU, Ross AGP. Chronic schistosomiasis. In: Jamieson BGM. Schistosoma: biology, pathology, and control. 8th ed. Boca Raton: CRC press, 2016: 360-378. |
| 23. | Olveda DU, Olveda RM, McManus DP, Cai P, Chau TN, Lam AK, Li Y, Harn DA, Vinluan ML, Ross AG. The chronic enteropathogenic disease schistosomiasis. Int J Infect Dis. 2014;28:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Silva CC, Domingues AL, Lopes EP, Morais CN, Santos RB, Luna CF, Nader HB, Martins JR. Schistosomiasis mansoni: ultrasound-evaluated hepatic fibrosis and serum concentrations of hyaluronic acid. Ann Trop Med Parasitol. 2011;105:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Lambertucci JR, Silva LC, Antunes CM. Aspartate aminotransferase to platelet ratio index and blood platelet count are good markers for fibrosis evaluation in schistosomiasis mansoni. Rev Soc Bras Med Trop. 2007;40:599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Medeiros TB, Domingues AL, Luna CF, Lopes EP. Correlation between platelet count and both liver fibrosis and spleen diameter in patients with schistosomiasis mansoni. Arq Gastroenterol. 2014;51:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Leite LA, Pimenta Filho AA, Martins da Fonseca CS, Santana dos Santos B, Ferreira Rde C, Montenegro SM, Lopes EP, Domingues AL, Owen JS, Lima VL. Hemostatic dysfunction is increased in patients with hepatosplenic schistosomiasis mansoni and advanced periportal fibrosis. PLoS Negl Trop Dis. 2013;7:e2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Barreto AV, Alecrim VM, Medeiros TB, Domingues AL, Lopes EP, Martins JR, Nader HB, Diniz GT, Montenegro SM, Morais CN. New index for the diagnosis of liver fibrosis in Schistosomiasis mansoni. Arq Gastroenterol. 2017;54:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Barreto AVMS, Domingues ALC, Diniz GTN, Cavalcanti AMS, Lopes EP, Montenegro SML, Morais CNL. The Coutinho index as a simple tool for screening patients with advanced forms of Schistosomiasis mansoni: a validation study. Trans R Soc Trop Med Hyg. 2022;116:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Gunda DW, Mtui EF, Manyiri PM, Majinge DC, Kilonzo SB, Mazigo HD, Kidenya BR. Schistosoma mansoni-related periportal fibrosis; can we use APRI and PSDR levels in the real-time selection of patients for targeted endoscopy in a resource-limited setting? BMC Gastroenterol. 2021;21:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Tamarozzi F, Fittipaldo VA, Orth HM, Richter J, Buonfrate D, Riccardi N, Gobbi FG. Diagnosis and clinical management of hepatosplenic schistosomiasis: A scoping review of the literature. PLoS Negl Trop Dis. 2021;15:e0009191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Agha A, Abdulhadi MM, Marenco S, Bella A, Alsaudi D, El-Haddad A, Inferrera S, Savarino V, Giannini EG. Use of the platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices in patients with schistosomiasis. Saudi J Gastroenterol. 2011;17:307-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Xu XD, Xu CF, Dai JJ, Qian JQ, Pin X. Ratio of platelet count/spleen diameter predicted the presence of esophageal varices in patients with schistosomiasis liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Sah VK, Wang L, Min X, Rizal R, Feng Z, Ke Z, Deng M, Li L, Li H. Human schistosomiasis: a diagnostic imaging focused review of a neglected disease. Radiol Infect Dis. 2015;2:150-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Weifeng G, Joseph WM, Zhisheng D, Yumin Z, Wei H. Advances in diagnosis of schistosomiasis. Microbiol Curr Res. 2018;2:3-8. [DOI] [Full Text] |
| 37. | World Health Organization. Ultrasound in schistosomiasis: a practical guide to the standard use of ultrasonography for assessment of schistosomiasis-related morbidity. In: Richter J, Hatz C, Campagne G, Bergquist NR, Jenkins JM, editors. Second international workshop; 1996 Oct 22-26; Niamey, NE. Geneva: World Health Organization, 2000: 1-49. |
| 38. | Friis H, Ndhlovu P, Kaondera K, Franke D, Vennervald BJ, Christensen NO, Doehring E. Ultrasonographic assessment of Schistosoma mansoni and S haematobium morbidity in Zimbabwean schoolchildren. Am J Trop Med Hyg. 1996;55:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Nooman ZM, Hassan AH, Mishrirky AM, Ragheb M, Abu-Saif AN, Abaza SM, Serwah AA, Kamal M, Fouad M. The use and limitations of ultrasonography in the diagnosis of liver morbidity attributable to Schistosoma mansoni infection in community-based surveys. Mem Inst Oswaldo Cruz. 1995;90:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | King CH, Magak P, Salam EA, Ouma JH, Kariuki HC, Blanton RE; World Health Organization. Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Int Health. 2003;8:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 41. | Berhe N, Geitung JT, Medhin G, Gundersen SG. Large scale evaluation of WHO's ultrasonographic staging system of schistosomal periportal fibrosis in Ethiopia. Trop Med Int Health. 2006;11:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Pinto-Silva RA, Queiroz LC, Azeredo LM, Silva LC, Lambertucci JR. Ultrasound in schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 2010;105:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Fataar S, Bassiony H, Satyanath S, Rudwan MA, Khaffaji S, el Magdy W, Al-Ansari AG, Hanna R. CT of hepatic schistosomiasis mansoni. AJR Am J Roentgenol. 1985;145:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Bezerra AS, D'Ippolito G, Caldana RP, Cecin AO, Ahmed M, Szejnfeld J. Chronic hepatosplenic schistosomiasis mansoni: magnetic resonance imaging and magnetic resonance angiography findings. Acta Radiol. 2007;48:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Manzella A, Ohtomo K, Monzawa S, Lim JH. Schistosomiasis of the liver. Abdom Imaging. 2008;33:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 46. | Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the Diagnosis of Human Schistosomiasis. Clin Microbiol Rev. 2015;28:939-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 47. | Voieta I, de Queiroz LC, Andrade LM, Silva LC, Fontes VF, Barbosa A Jr, Resende V, Petroianu A, Andrade Z, Antunes CM, Lambertucci JR. Imaging techniques and histology in the evaluation of liver fibrosis in hepatosplenic schistosomiasis mansoni in Brazil: a comparative study. Mem Inst Oswaldo Cruz. 2010;105:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Scortegagna E, Leão ARDS, Santos JEM, Sales DM, Shigueoka DC, De Aguiar LAK, Brant PE, Neto RC, Borges DR, D’Ippolito G. Agreement between magnetic resonance imaging and ultrasonography in the classification of schistosomal periportal fibrosis, according to Niamey’s criteria. Radiol Bras. 2007;40:303-308. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich CF; EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 50. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 580] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 51. | Lima LMSTB, Lacet CMC, Parise ER. Evaluation of hepatic fibrosis by elastography in patients with schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 2020;114:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Pereira CLD, Santos JC, Arruda RM, Rodrigues ML, Siqueira ES, Lemos RS, Batista AD, Domingues ALC, Lopes EP. Evaluation of Schistosomiasis Mansoni Morbidity by Hepatic and Splenic Elastography. Ultrasound Med Biol. 2021;47:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Sinkala E, Vinikoor M, Miyanda Siyunda A, Zyambo K, Besa E, Nsokolo B, Wandeler G, Foster GR, Kelly P. Hepatosplenic schistosomiasis in Zambian adults is characterized by increased liver stiffness: A nested case-control study. Heliyon. 2020;6:e04534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Veiga ZST, Perazzo H, Fernandes FF, Pereira GH, Cavalcanti MG, Peralta JM, Perez RM, Villela-Nogueira CA. 2-D Shear Wave Elastography for the Evaluation of Liver Fibrosis in Hepatosplenic Schistosomiasis: Reliability of a Single Measurement and Inter-Hepatic Lobe Variability. Am J Trop Med Hyg. 2020;104:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1090] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 56. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 57. | Silva CF, Nardelli MJ, Barbosa FA, Galizzi HO, Cal TCMF, Ferrari TCA, Faria LC, Couto CA. Liver stiffness is able to differentiate hepatosplenic Schistosomiasis mansoni from liver cirrhosis and spleen stiffness may be a predictor of variceal bleeding in hepatosplenic schistosomiasis. Trans R Soc Trop Med Hyg. 2022;116:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 708] [Article Influence: 101.1] [Reference Citation Analysis (2)] |
| 59. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Gibiino G, Garcovich M, Ainora ME, Zocco MA. Spleen ultrasound elastography: state of the art and future directions - a systematic review. Eur Rev Med Pharmacol Sci. 2019;23:4368-4381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 62. | Crossan C, Tsochatzis EA, Longworth L, Gurusamy K, Davidson B, Rodríguez-Perálvarez M, Mantzoukis K, O'Brien J, Thalassinos E, Papastergiou V, Burroughs A. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19:1-409, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Crossan C, Tsochatzis EA, Longworth L, Gurusamy K, Papastergiou V, Thalassinos E, Mantzoukis K, Rodriguez-Peralvarez M, O'Brien J, Noel-Storr A, Papatheodoridis GV, Davidson B, Burroughs AK. Cost-effectiveness of noninvasive liver fibrosis tests for treatment decisions in patients with chronic hepatitis B in the UK: systematic review and economic evaluation. J Viral Hepat. 2016;23:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |