Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.623
Peer-review started: October 19, 2021
First decision: December 3, 2021
Revised: December 19, 2022
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 27, 2022
Processing time: 156 Days and 13.6 Hours
Fibroblast growth factor 19 (FGF-19) is one of the founding members of the endocrine FGF subfamily. Recently, it has been the subject of much interest owing to its role in various physiological processes affecting glucose and lipid metabolism and the regulation of bile acid secretion as well as cell proliferation, differentiation, and motility. Additionally, FGF-19 secretion in an autocrine style has reportedly contributed to cancer progression in various types of malignancies including hepatocellular carcinoma (HCC).
To estimate the serum FGF-19 concentrations in HCC cases and assess its diagnostic performance for the detection of HCC.
We recruited 90 adult participants and divided them into three equal groups: Healthy controls, cirrhosis patients, and HCC patients. Serum FGF-19 concentrations were measured using the Human FGF-19 ELISA kit.
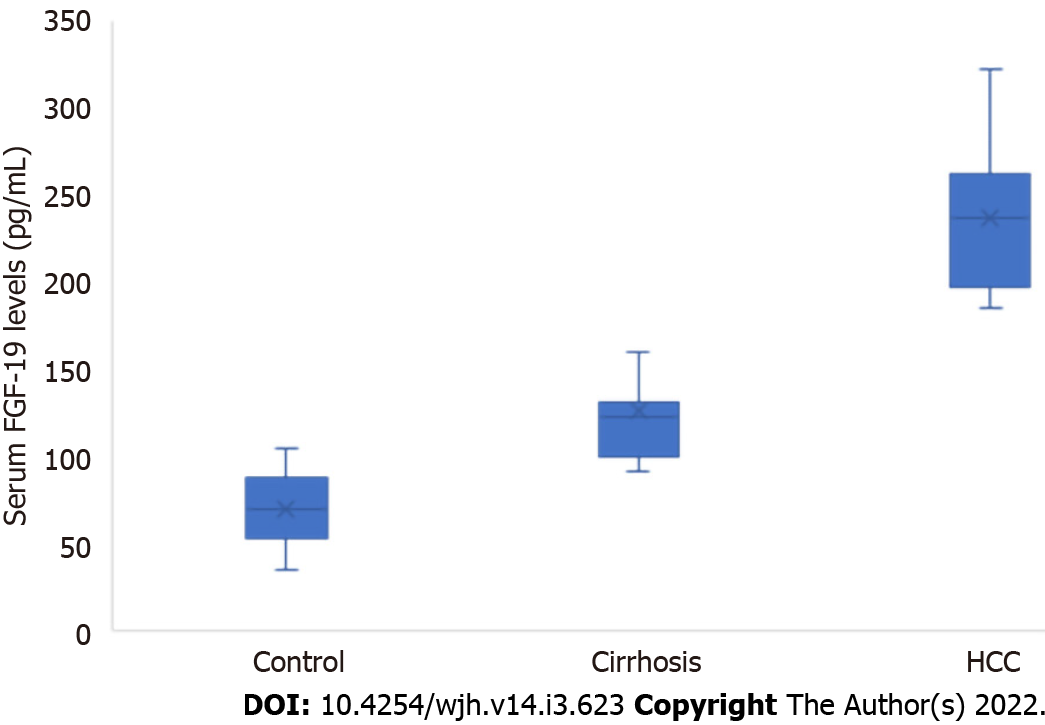
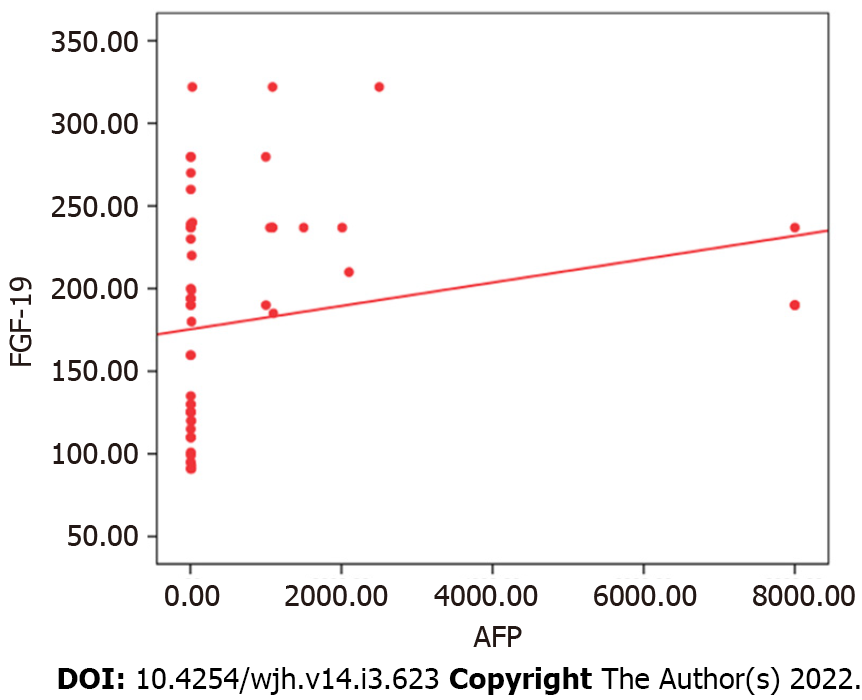
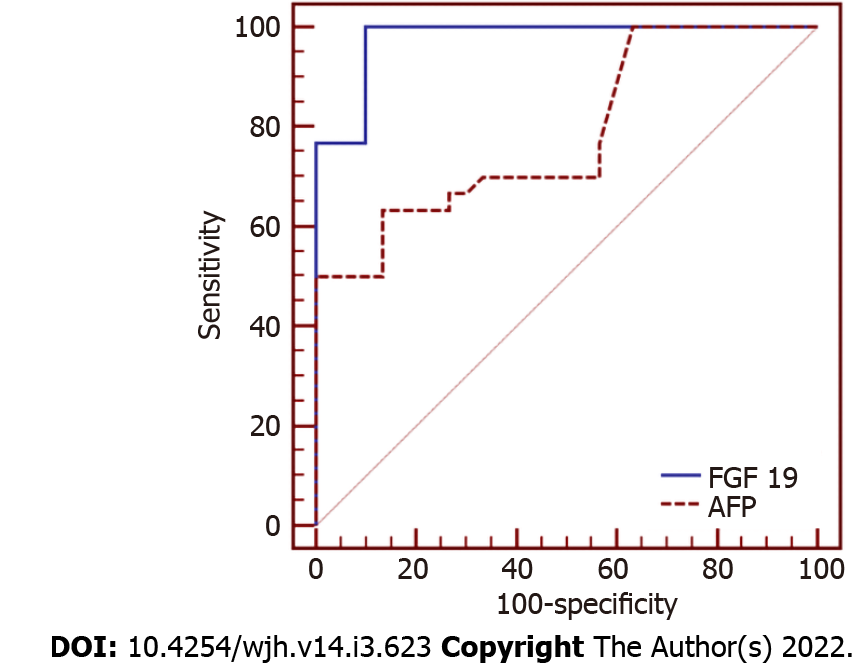
We detected a high statistically significant difference in serum FGF-19 levels among the three groups. The highest level was observed in the HCC group, followed by the cirrhosis and control groups (236.44 ± 40.94 vs 125.63 ± 31.54 vs 69.60 ± 20.90 pg/mL, respectively, P ≤ 0.001). FGF-19 was positively correlated with alpha fetoprotein (AFP; r = 0.383, P = 0.003) and international normalised ratio (r = 0.357, P = 0.005), while it was negatively correlated with albumin (r = -0.500, P ≤ 0.001). For the detection of HCC, receiver operating characteristic curve analysis showed that the best cut-off point of AFP was > 8.2 ng/mL with an area under the curve (AUC) of 0.78, sensitivity of 63.33%, specificity of 83.33%, positive predictive value (PPV) of 79.2%, negative predictive value (NPV) of 69.4%, and total accuracy of 78%. However, FGF-19 at a cut-off point > 180 pg/mL had an AUC of 0.98, sensitivity of 100%, specificity of 90.0%, PPV of 90.0%, NPV of 100%, and total accuracy of 98%.
FGF-19 represents a possible novel non-invasive marker for HCC. It may improve the prognosis of HCC patients due to its utility in several aspects of HCC detection and management.
Core Tip: We recruited 90 adult participants and divided them into three equal groups: Healthy controls, cirrhosis patients, and hepatocellular carcinoma (HCC) patients. We detected a high statistically significant difference in fibroblast growth factor 19 (FGF-19) levels among the three groups, with the highest level occurring in the HCC group, followed by the cirrhosis and control groups (236.44 ± 40.94 vs 125.63 ± 31.54 vs 69.60 ± 20.90 pg/mL, respectively, P ≤ 0.001). For the detection of HCC, receiver operating characteristic curve analysis showed that FGF-19 demonstrated a better diagnostic performance than alpha fetoprotein (area under the curve = 0.98 vs 0.78). Consequently, we can conclude that FGF-19 represents a possible novel non-invasive marker for HCC.
- Citation: Mohamed GA, Nashaat EH, Fawzy HM, ElGhandour AM. Assessment of fibroblast growth factor 19 as a non-invasive serum marker for hepatocellular carcinoma. World J Hepatol 2022; 14(3): 623-633
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/623.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.623
Fibroblast growth factor 19 (FGF-19) is one of the founding members of the endocrine FGF subfamily[1]. Recently, it has been the subject of much interest owing to its role in various physiological processes affecting glucose and lipid metabolism and bile acid secretion as well as cell proliferation, differentiation, and motility[2-4]. Additionally, FGF-19 secretion in an autocrine style has reportedly contributed to cancer progression in various types of malignancies including hepatocellular carcinoma (HCC)[5-9].
FGF-19 has a restricted pattern of expression. It is mostly expressed in the terminal ileum in response to the bile-acid-stimulated intestinal Farnesoid X receptor (FXR)[10], and then, through the portal circulation to the liver, it attaches to its receptor, fibroblast growth factor receptor 4 (FGFR4), and a co-factor known as β-klotho. This action initiates the transcription of various genes that negatively regulate bile acid synthesis through the downregulation of CYP7A1[11].
Although FGF-19 is formed principally in the ileum and FGF-19 expression is almost absent in the human liver under normal conditions, current studies propose that FGF-19 may be autocrined by human hepatocytes under cholestatic conditions, peritumoral tissue cirrhosis, and HCC. The secretion of FGF-19 in these conditions demonstrates the protective negative feedback of FGF-19 in order to guard hepatocytes from the cytotoxicity of bile acids[12-14] and the promotion of the development and progression of HCC by bile acids through mTOR dependent mechanisms[15]. This beneficial effect of the FGF-19 pathway has also been proposed in other studies in FXR-/-knock out mice that developed hepatic malignancies, which were inhibited by the expression of an FXR transgene in the intestine[16]. This effect indicates the protective aspect of Fgf15 (the mouse homolog of human FGF-19) in relation to hepatic malignancies. Additionally, Fgf15/FGF19 mediated hepatic regeneration in mice in other studies[17,18].
However, the higher expression of FGF-19 in HCC patients has been found to promote tumour cell survival and has antiapoptotic impacts that are applied through the FGFR4-glycogen synthase kinase (GSK)3β-Nrf2 signalling pathway[19]. Moreover, Kang et al[20] showed that a distinctive molecular subtype of FGF-19 is correlated with a poor prognosis in HCC patients. In addition, Cui et al[21] and Zhao et al[22] reported that Fgf15 and FGF-19, respectively, promoted the progression of HCC by stimulating epithelial-mesenchymal transition and Wnt/β-catenin cascade, which is linked to tumour aggression and mortality. Furthermore, previous data has pointed to FGF-19 as a promoter of liver stem cells in HCC patients, as noted in the robust association between FGF-19 and EpCAM, which is a moderator of cell adhesion and signalling and a special biomarker for liver cancer stem cells[23,24]. Additionally, confirmation of the role of FGF-19 signalling in HCC progression arises from the tumour-preventing effect of the selective FGFR4 inhibitor BLU9931 in a mouse HCC model with implanted FGF-19-producing, FGFR4-expressing hepatic cells[25]. These results suggest that FGF-19 may be implicated in tumour development in HCC cases.
Since FGF-19 is a serum protein secreted by HCC cells in an autocrine loop style, and systemic concentrations of FGF-19 have been found to reflect its portal concentrations[14,26], we aimed to estimate the serum FGF-19 concentrations in HCC cases and assess the diagnostic performance of FGF-19 for the detection of HCC.
This observational study was conducted at Ain Shams University Hospitals in Cairo, Egypt from March 2021 to September 2021. This study was performed in accordance with the ethics principles of the Declaration of Helsinki and was authorised by the ethics board of the Faculty of Medicine, Ain Shams University (No. FMASU MS 66/2021). Written informed approval was obtained from all the participants before they were enrolled in the study.
We consecutively recruited 90 adult participants and divided them into three equal groups: Healthy controls, cirrhosis patients, and HCC patients. Patients with any malignant disease other than HCC were excluded. None of the HCC cases had either neoadjuvant chemotherapy or radiotherapy.
Cirrhosis was diagnosed according to laboratory parameters, clinical manifestations, and/or histological criteria[27]. HCC was identified through contrast-enhanced imaging studies and/or histological criteria as per the practice guidelines[28].
The serum FGF-19 concentrations were measured using the Human FGF-19 ELISA kit (SunRed Biological Technology Co. Ltd., Shanghai, China, Catalogue # 201-12-2199) with a sensitivity of 2.032 pg/mL, assay range of 2.5-700 pg/mL, intra-assay coefficient of variability (CV) < 10%, and inter-assay CV < 12%.
Data were analysed using the Statistical Package for Social Science (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). The qualitative variables are shown as numbers and percentages, while the quantitative variables are shown as the mean, standard deviation, or median and interquartile range, as appropriate. The differences among the groups were calculated using the Chi-square test, Fisher exact test, independent t-test, one-way ANOVA test, or Kruskal-Wallis test, as appropriate. A receiver operating characteristic (ROC) curve analysis was applied to assess the diagnostic performance of FGF-19 and alpha fetoprotein (AFP) for HCC detection. A P value of less than 0.05 was considered statistically significant.
This study included 90 participants divided into control, cirrhosis, and HCC groups. The HCC group was comprised of 19 males (63.3%) and 11 females (36.7%), with a mean age of 57.37 years. In the cirrhotic group, there were 20 males (66.7%) and 10 (33.3%) females, with a mean age of 53.57 years. The control group included 18 males (60%) and 12 females (40%), with a mean age of 51.07 years (Table 1). According to the Child-Pugh class, 14 of the HCC cases (46.7%) belonged to Class C, while 18 (60%) of the cirrhotic cases belonged to Class A (P = 0.002, Table 1). There were statistically significant differences among the three groups concerning AFP, haemoglobin, platelets, alanine aminotransferase, aspartate aminotransferase (AST), albumin, international normalised ratio (INR), fasting blood glucose, and bilirubin (Table 1).
| Control (n = 30) | Cirrhosis (n = 30) | HCC (n = 30) | P value | Post-hoc analysis | ||
| Age (yr) | 51.07 ± 12.38 | 53.57 ± 10.48 | 57.37 ± 10.25 | 0.091 | ||
| Sex | Female | 12 (40%) | 10 (33.3%) | 11 (36.7%) | 0.866 | |
| Male | 18 (60%) | 20 (66.7%) | 19 (63.3%) | |||
| Aetiology of hepatic disease | HCV (n = 18, 60%) | HCV (n = 25, 83.33%) | 0.691 | |||
| HBV (n = 7, 23.3%) | HBV (n = 3, 10%) | |||||
| Others (n = 5, 16.6%) | Others (n = 2, 6.66%) | |||||
| Child-Pugh Class | Class A | 18 (60%) | 5 (16.7%) | 0.002 | ||
| Class B | 6 (20%) | 11 (36.7%) | ||||
| Class C | 6 (20%) | 14 (46.7%) | ||||
| Fibroblast growth factor 19 (pg/mL) | 69.60 ± 20.90 | 125.63 ± 31.54 | 236.44 ± 40.94 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 ≤ 0.001 | ||||||
| Alpha fetoprotein (ng/mL) | 3.35 (2.5 – 4.5) | 6.4 (4 – 6.9) | 513.5 (5.6 – 1500) | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 ≤ 0.001 | ||||||
| Haemoglobin (g/dL) | 13.16 ± 1.24 | 10.68 ± 1.11 | 10.49 ± 1.59 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 0.588 | ||||||
| White blood cells (109/L) | 7.09 ± 2.01 | 6.37 ± 2.27 | 5.86 ± 2.43 | 0.109 | ||
| Platelets (109/L) | 288.10 ± 92.79 | 144.17 ± 48.27 | 136.13 ± 43.78 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 0.636 | ||||||
| Alanine aminotransferase (U/L) | 20.67 ± 7.02 | 65.47 ± 33.00 | 52.97 ± 23.25 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 0.044 | ||||||
| Aspartate aminotransferase (U/L) | 23.23 ± 12.69 | 49.87 ± 24.78 | 45.93 ± 20.02 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 0.444 | ||||||
| Creatinine (mg/dL) | 0.90 ± 0.22 | 0.99 ± 0.36 | 1.11 ± 0.51 | 0.112 | ||
| Urea (mg/dL) | 21.70 ± 7.37 | 30.10 ± 18.82 | 32.97 ± 25.17 | 0.057 | ||
| Albumin (g/dL) | 3.96 ± 0.34 | 3.33 ± 0.53 | 2.65 ± 0.43 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 ≤ 0.001 | ||||||
| INR | 1.09 ± 0.11 | 1.54 ± 0.24 | 1.85 ± 0.36 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 ≤ 0.001 | ||||||
| Bilirubin (mg/dL) | 0.75 ± 0.26 | 1.80 ± 0.74 | 1.97 ± 0.42 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 0.211 | ||||||
| Fasting blood glucose (µmoI/L) | 5.19 ± 0.19 | 4.46 ± 0.28 | 4.46 ± 0.28 | ≤ 0.001 | P1 ≤ 0.001 | |
| P2 ≤ 0.001 | ||||||
| P3 = 1.000 | ||||||
We detected a high statistically significant difference in the FGF-19 levels of the three groups. The highest level occurred in the HCC group, followed by the cirrhosis and control groups (236.44 ± 40.94 vs 125.63 ± 31.54 vs 69.60 ± 20.90 pg/mL, respectively, P ≤ 0.001; Table 1, Figure 1). There were seven HCC patients with negative AFP; however, they had elevated FGF-19 levels (> 180 pg/mL). Serum FGF-19 levels were not significantly different according to the Child–Pugh class in the cirrhosis and HCC groups (Table 2).
| Child-Pugh Class | Cirrhosis (n = 30) | HCC (n = 30) | |
| Fibroblast growth factor 19 (pg/mL) | Class A | 129.311 (± 38.01) | 223.320 (± 37.39) |
| Class B | 123.383 (± 21.51) | 230.209 (± 30.96) | |
| Class C | 116.833 (± 15.69) | 246.029 (± 48.71) | |
| P value | 0.7046 | 0.479 |
The tumour characteristics of the HCC cases are shown in Table 3. Serum FGF-19 levels were higher in relation to the size of the tumour, the presence of portal vein thrombosis, jaundice, lower limb oedema, and weight loss; however, these differences did not reach statistical significance (Table 4). FGF-19 was positively correlated with AFP (r = 0.383, P = 0.003) and INR (r = 0.357, P = 0.005), while it was negatively correlated with albumin (r = -0.500, P ≤ 0.001; Table 5, Figure 2).
| HCC (n = 30) | ||
| Size | < 2 cm | 3 (10%) |
| 2-3 cm | 17 (56.7%) | |
| > 5 cm | 10 (33.3%) | |
| Number of tumour foci | Single | 10 (33.3%) |
| 2-3 | 9 (30%) | |
| Multiple | 11 (36.7%) | |
| Portal vein thrombosis | No | 21 (70%) |
| Yes | 9 (30%) | |
| Metastasis | No | 27 |
| Yes | 3 | |
| FGF-19 pg/mL (mean ± SD) | P value | ||
| Size | < 2 cm | 219.9 ± 51.79 | 0.254 |
| 2-3 cm | 229.2 ± 36.06 | ||
| > 5 cm | 253.72 ± 44.39 | ||
| Number | Single | 234.17 ± 36.38 | 0.885 |
| 2 - 3 | 242.28 ± 45.69 | ||
| Multiple | 233.74 ± 44.22 | ||
| Portal vein thrombosis | No | 230.55 ± 39.13 | 0.235 |
| Yes | 250.2 ± 44.08 | ||
| Right upper quadrant pain | No | 237.171 ± 41.026 | 0.885 |
| Yes | 234.744 ± 43.163 | ||
| Weight loss | No | 229.132 ± 34.285 | 0.106 |
| Yes | 256.550 ± 52.793 | ||
| Pruritus | No | 239.518 ± 39.170 | 0.505 |
| Yes | 227.988 ± 47.214 | ||
| Jaundice | No | 226.182 ± 29.468 | 0.118 |
| Yes | 249.86 ± 50.48 | ||
| Fever | No | 237.668 ± 40.531 | 0.834 |
| Yes | 234.33 ± 43.54 | ||
| Oedema | No | 228.945 ± 37.054 | 0.16 |
| Yes | 251.44 ± 46.12 | ||
| AFP | FGF-19 | |||
| r | P value | r | P value | |
| AFP | – | – | 0.383 | 0.003 |
| FGF-19 | 0.383 | 0.003 | – | – |
| Age | 0.062 | 0.640 | 0.125 | 0.343 |
| Haemoglobin | -0.196 | 0.133 | -0.060 | 0.651 |
| White blood cells | -0.064 | 0.627 | -0.144 | 0.272 |
| Platelets | 0.018 | 0.893 | -0.151 | 0.248 |
| Alanine aminotransferase | 0.036 | 0.786 | -0.151 | 0.249 |
| Aspartate aminotransferase | 0.040 | 0.764 | -0.024 | 0.855 |
| Creatinine | -0.164 | 0.211 | 0.093 | 0.480 |
| Urea | -0.022 | 0.867 | 0.012 | 0.929 |
| Albumin | -0.213 | 0.102 | -0.500 | 0.000 |
| INR | -0.001 | 0.993 | 0.357 | 0.005 |
| Bilirubin | -0.093 | 0.479 | 0.008 | 0.952 |
| Fasting blood glucose | -0.135 | 0.477 | 0.056 | 0.767 |
For the detection of HCC, the ROC curve analysis showed that the best cut-off point of AFP was > 8.2 ng/mL with an area under the curve (AUC) of 0.78, sensitivity of 63.33%, specificity of 83.33%, positive predictive value (PPV) of 79.2%, negative predictive value (NPV) of 69.4%, and total accuracy of 78%. However, FGF-19 at a cut-off point > 180 pg/mL had an AUC of 0.98, sensitivity of 100%, specificity of 90.0%, PPV of 90.0%, NPV of 100%, and total accuracy of 98% (Table 6, Figure 3).
| Cut-off point | AUC | Sensitivity | Specificity | PPV | NPV | |
| FGF-19 | > 180 pg/mL | 0.98 | 100% | 90% | 90% | 100% |
| AFP | > 8.2 ng/mL | 0.78 | 63.33% | 83.33% | 79.2% | 69.4% |
HCC is the third highest cause of tumour death globally, with a 5-year survival rate of approximately 20% despite the developments in imaging technologies and therapeutic methodologies[29]. Unfortunately, the majority of HCC patients are diagnosed at an advanced stage of disease; therefore, early recognition of the disease is crucial to improving the prognosis and overall survival of patients[24].
Tumour markers have commonly been utilised for numerous objectives, such as diagnosis, follow-up care after treatment, optimisation of therapeutic effectiveness, and prediction of prognosis. Earlier studies have identified various serum markers for HCC which can be applied as diagnostic and prognostic markers for HCC. Although the assessment of these biomarkers is not essential for establishing a conclusive diagnosis of HCC as per the guidelines, these biomarkers play a key role in HCC diagnosis and monitoring[28,30,31]. However, it has been found that AFP, which is the most studied marker, may remain in the normal range not only in the early stages, but also in the advanced stages of HCC[32]. Moreover, an increase of AFP is occasionally detected in cirrhotic patients. Considering these two facts, alternative serum markers with high levels of sensitivity and specificity are needed.
It has previously been reported that FGF-19 may be associated with the pathogenesis and clinical characteristics of HCC[12,24]. Thus, we aimed to investigate the diagnostic utility of FGF-19 in HCC cases. We observed significantly higher serum FGF-19 levels in the HCC group compared to the control and cirrhosis groups. Serum FGF-19 levels were also higher in relation to the size of the tumour and presence of portal vein thrombosis; however, these differences did not reach statistical significance owing to the small sample size.
In accordance with our results, Maeda et al[12] detected higher serum levels of FGF-19 in their HCC group (214.5 pg/mL) compared to the cirrhosis group (100.1 pg/mL, P < 0.001) and the control group (78.8 pg/mL, P = 0.002). However, no statistically significant difference was detected between the cirrhotic cases and controls in their study.
Similar to the current results, Li et al[24] detected significantly higher serum FGF-19 levels in the HCC group compared to the control group (145.57 ± 118.72 vs 90.18 ± 13.88 pg/mL, P = 0.044). They also reported that FGF-19 levels were significantly raised in the HCC tissues (57.80 ± 4.39 pg/10 mg total protein) in comparison to both healthy control tissues (33.29 ± 1.53 pg/10 mg total protein, P < 0.001) and paired peritumoral tissues (46.33 ± 2.53 pg/10 mg total protein, P = 0.032). Additionally, FGF-19 mRNA expression was significantly raised in the HCC tissues in comparison to paired peritumoral tissues (3.30 ± 1.82 vs 2.25 ± 0.82, respectively, P = 0.025). Moreover, FGF-19 expression increased significantly with a strong positive correlation (r = 0.968) consistent with the histological severity of hepatic disease, showing a trend in samples with steatosis (224.13 ± 115.68, P = 0.087), steatohepatitis (413.99 ± 159.55, P = 0.002), cirrhosis (613.35 ± 157.29, P < 0.001), and HCC (2507.28 ± 831.10, P = 0.001) in comparison to the paired peritumoral tissues (142.96 ± 41.32).
Our results are also consistent with those of Sun et al[33], who detected higher FGF-19 levels in the HCC and diabetes-HCC groups than in the control and diabetes groups (220.5, 185.1, 115.8, and 70.4 pg/mL, respectively, P < 0.001). All these results indicate that FGF-19 may have a role in the pathogenesis of HCC.
In line with the results of the current research, Sun et al[33] detected a positive association between FGF-19 and AFP in HCC patients (P < 0.05). However, Maeda et al[12] found no significant association between serum FGF-19 concentrations and AFP. Moreover, in partial agreement with the present study, Wunsch et al[34] observed that serum and hepatic concentrations of FGF-19 were associated with the severity of hepatic disease, as measured by laboratory parameters including albumin (r = -0.408, P = 0.007), haemoglobin (r = -0.394, P = 0.01), AST (r = 0.328, P = 0.03), and total bilirubin (r = 0.577, P < 0.001).
For HCC detection, in the study by Maeda et al[12], the ROC curve analysis determined a cut-off point of FGF-19 of 200 pg/mL, which had an AUC of 0.795, sensitivity of 53.2%, specificity of 95.1%, PPV of 95.9%, and NPV of 48.7%. This result was comparable to those of AFP (AUC = 0.827). However, in the current study, FGF-19 had a better diagnostic performance at a cut-off > 180 pg/mL with an AUC of 0.98, sensitivity of 100%, specificity of 90%, PPV of 90%, and NPV of 100%.
The current study was limited by a small sample size and a high ratio of patients with advanced HCC. Further studies are needed to investigate the clinical applications of the current results. FGF-19 could serve as a predictor of prognosis and a marker for follow-up after HCC treatment. Additionally, the FGF-19 pathway has received increased interest as a possible therapeutic target in chronic liver diseases[5,35-37]. In fact, anti-FGF-19 antibody therapy has been described as inhibiting HCC evolution in FGF-19 transgenic mice[38].
FGF-19 could be a possible novel non-invasive marker for HCC. It may improve the prognosis of HCC patients due to its utility in several aspects of HCC detection and management.
Fibroblast growth factor 19 (FGF-19) is one of the founding members of the endocrine FGF subfamily. Recently, it has been the subject of much interest owing to its role in various physiological processes affecting glucose and lipid metabolism and bile acid secretion as well as cell proliferation, differentiation, and motility. Additionally, FGF-19 secretion in an autocrine style has reportedly contributed to cancer progression in various types of malignancies including hepatocellular carcinoma (HCC).
Tumour markers for HCC with a high sensitivity and specificity are necessary.
We aimed to estimate the serum FGF-19 concentrations in HCC cases and assess the diagnostic performance of FGF-19 for the detection of HCC.
We recruited 90 adult participants and divided them into three equal groups: Healthy controls, cirrhosis patients, and HCC patients. Serum FGF-19 concentrations were measured using the Human FGF-19 ELISA kit.
We detected a high statistically significant difference in the FGF-19 levels between the three groups, with the highest level occurring in the HCC group, followed by the cirrhosis and control groups (236.44 ± 40.94 vs 125.63 ± 31.54 vs 69.60 ± 20.90 pg/mL, respectively, P ≤ 0.001). For the detection of HCC, ROC curve analysis showed that FGF-19 produced a better diagnostic performance than alpha fetoprotein with an AUC of 0.98 vs 0.78.
FGF-19 may be a possible novel non-invasive marker for HCC.
FGF-19 could serve as a predictor of prognosis and a marker for follow-up after HCC treatment. Furthermore, the FGF-19 pathway may be a therapeutic target for the management of HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kanno H, Tong GD S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Rysz J, Gluba-Brzózka A, Mikhailidis DP, Banach M. Fibroblast growth factor 19-targeted therapies for the treatment of metabolic disease. Expert Opin Investig Drugs. 2015;24:603-610. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Zhang J, Li H, Bai N, Xu Y, Song Q, Zhang L, Wu G, Chen S, Hou X, Wang C, Wei L, Xu A, Fang Q, Jia W. Decrease of FGF19 contributes to the increase of fasting glucose in human in an insulin-independent manner. J Endocrinol Invest. 2019;42:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Babaknejad N, Nayeri H, Hemmati R, Bahrami S, Esmaillzadeh A. An Overview of FGF19 and FGF21: The Therapeutic Role in the Treatment of the Metabolic Disorders and Obesity. Horm Metab Res. 2018;50:441-452. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, Valentí V, Salvador J, Giralt M, Villarroya F, Frühbeck G. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017;36:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Repana D, Ross P. Targeting FGF19/FGFR4 Pathway: A Novel Therapeutic Strategy for Hepatocellular Carcinoma. Diseases. 2015;3:294-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Shimokawa T, Furukawa Y, Sakai M, Li M, Miwa N, Lin YM, Nakamura Y. Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res. 2003;63:6116-6120. [PubMed] |
| 7. | Zaharieva BM, Simon R, Diener PA, Ackermann D, Maurer R, Alund G, Knönagel H, Rist M, Wilber K, Hering F, Schönenberger A, Flury R, Jäger P, Fehr JL, Mihatsch MJ, Gasser T, Sauter G, Toncheva DI. High-throughput tissue microarray analysis of 11q13 gene amplification (CCND1, FGF3, FGF4, EMS1) in urinary bladder cancer. J Pathol. 2003;201:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Gowardhan B, Douglas DA, Mathers ME, McKie AB, McCracken SR, Robson CN, Leung HY. Evaluation of the fibroblast growth factor system as a potential target for therapy in human prostate cancer. Br J Cancer. 2005;92:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Ruohola JK, Viitanen TP, Valve EM, Seppänen JA, Loponen NT, Keskitalo JJ, Lakkakorpi PT, Härkönen PL. Enhanced invasion and tumor growth of fibroblast growth factor 8b-overexpressing MCF-7 human breast cancer cells. Cancer Res. 2001;61:4229-4237. [PubMed] |
| 10. | Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, Williamson C, Walters JR. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol. 2013;304:G940-G948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Maeda T, Kanzaki H, Chiba T, Ao J, Kanayama K, Maruta S, Kusakabe Y, Saito T, Kobayashi K, Kiyono S, Nakamura M, Ogasawara S, Suzuki E, Ooka Y, Nakamoto S, Nakagawa R, Muroyama R, Kanda T, Maruyama H, Kato N. Serum fibroblast growth factor 19 serves as a potential novel biomarker for hepatocellular carcinoma. BMC Cancer. 2019;19:1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Lin ZZ, Hsu C, Jeng YM, Hu FC, Pan HW, Wu YM, Hsu HC, Hu MC, Cheng AL. Klotho-beta and fibroblast growth factor 19 expression correlates with early recurrence of resectable hepatocellular carcinoma. Liver Int. 2019;39:1682-1691. [PubMed] |
| 14. | Johansson H, Mörk LM, Li M, Sandblom AL, Björkhem I, Höijer J, Ericzon BG, Jorns C, Gilg S, Sparrelid E, Isaksson B, Nowak G, Ellis E. Circulating Fibroblast Growth Factor 19 in Portal and Systemic Blood. J Clin Exp Hepatol. 2018;8:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Gao L, Lv G, Li R, Liu WT, Zong C, Ye F, Li XY, Yang X, Jiang JH, Hou XJ, Jing YY, Han ZP, Wei LX. Glycochenodeoxycholate promotes hepatocellular carcinoma invasion and migration by AMPK/mTOR dependent autophagy activation. Cancer Lett. 2019;454:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Degirolamo C, Modica S, Vacca M, Di Tullio G, Morgano A, D'Orazio A, Kannisto K, Parini P, Moschetta A. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology. 2015;61:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Kong B, Huang J, Zhu Y, Li G, Williams J, Shen S, Aleksunes LM, Richardson JR, Apte U, Rudnick DA, Guo GL. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G893-G902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Uriarte I, Fernandez-Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S, Vespasiani-Gentilucci U, Morini S, Vicente E, Concepcion AR, Medina JF, Marin JJ, Berasain C, Prieto J, Avila MA. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut. 2013;62:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Teng Y, Zhao H, Gao L, Zhang W, Shull AY, Shay C. FGF19 Protects Hepatocellular Carcinoma Cells against Endoplasmic Reticulum Stress via Activation of FGFR4-GSK3β-Nrf2 Signaling. Cancer Res. 2017;77:6215-6225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Kang HJ, Haq F, Sung CO, Choi J, Hong SM, Eo SH, Jeong HJ, Shin J, Shim JH, Lee HC, An J, Kim MJ, Kim KP, Ahn SM, Yu E. Characterization of Hepatocellular Carcinoma Patients with FGF19 Amplification Assessed by Fluorescence in situ Hybridization: A Large Cohort Study. Liver Cancer. 2019;8:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Cui G, Martin RC, Jin H, Liu X, Pandit H, Zhao H, Cai L, Zhang P, Li W, Li Y. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J Exp Clin Cancer Res. 2018;37:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Zhao H, Lv F, Liang G, Huang X, Wu G, Zhang W, Yu L, Shi L, Teng Y. FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3β/β- catenin signaling cascade via FGFR4 activation. Oncotarget. 2016;7:13575-13586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64:935-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Li Y, Zhang W, Doughtie A, Cui G, Li X, Pandit H, Yang Y, Li S, Martin R. Up-regulation of fibroblast growth factor 19 and its receptor associates with progression from fatty liver to hepatocellular carcinoma. Oncotarget. 2016;7:52329-52339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N, Shutes A, Winter C, Lengauer C, Kohl NE, Guzi T. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov. 2015;5:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 26. | Koelfat KV, Bloemen JG, Jansen PL, Dejong CH, Schaap FG, Olde Damink SW. The portal-drained viscera release fibroblast growth factor 19 in humans. Physiol Rep. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762. [PubMed] |
| 28. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6050] [Article Influence: 864.3] [Reference Citation Analysis (3)] |
| 29. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 30. | Aboelfotoh AO, Foda EM, Elghandour AM, Teama NM, Abouzein RA, Mohamed GA. Talin-1; other than a potential marker for hepatocellular carcinoma diagnosis. Arab J Gastroenterol. 2020;21:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3023] [Article Influence: 431.9] [Reference Citation Analysis (3)] |
| 32. | Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Sun Y, Zhu M, Zhao H, Ni X, Chang R, Su J, Huang H, Cui S, Wang X, Yuan J, OuYang R, Zhang R, Chen W, Gu Y, Sun Y. Serum Fibroblast Growth Factor 19 and Total Bile Acid Concentrations Are Potential Biomarkers of Hepatocellular Carcinoma in Patients with Type 2 Diabetes Mellitus. Biomed Res Int. 2020;2020:1751989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Wunsch E, Milkiewicz M, Wasik U, Trottier J, Kempińska-Podhorodecka A, Elias E, Barbier O, Milkiewicz P. Expression of hepatic Fibroblast Growth Factor 19 is enhanced in Primary Biliary Cirrhosis and correlates with severity of the disease. Sci Rep. 2015;5:13462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-61.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 36. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1796] [Article Influence: 179.6] [Reference Citation Analysis (3)] |
| 37. | Chae YK, Ranganath K, Hammerman PS, Vaklavas C, Mohindra N, Kalyan A, Matsangou M, Costa R, Carneiro B, Villaflor VM, Cristofanilli M, Giles FJ. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2017;8:16052-16074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 38. | Zheng N, Wei W, Wang Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl Cancer Res. 2016;5:1-6. [PubMed] |