Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.612
Peer-review started: October 19, 2021
First decision: December 3, 2021
Revised: December 21, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 27, 2022
Processing time: 156 Days and 11.7 Hours
Nonalcoholic fatty liver disease (NAFLD) is associated with a sedentary lifestyle and depressive symptoms. It is also well established that physical inactivity and depressive symptoms are related. However, an investigation of the interaction between all of these factors in NAFLD has not been previously conducted.
To investigate the interrelationship between physical inactivity and depressive symptoms in individuals with NAFLD.
Data from the Rancho Bernardo Study of Healthy Aging were utilized. 589 individuals were included in the analyses (43.1% male; 95.8% non-Hispanic white; aged 60.0 ± 7.0 years). NAFLD was defined by using the hepatic steatosis index, depression using the Beck Depression Inventory, and physical activity by self-report of number of times per week of strenuous activity. Multivariable generalized linear regression models with Gamma distribution were performed to investigate the proposed relationship.
About 40% of the sample had evidence of NAFLD, 9.3% had evidence of depression, and 29% were physically inactive. Individuals with NAFLD and depression were more likely to be physically inactive (60.7%) compared to individuals with neither NAFLD nor depression (22.9%), individuals with depression without NAFLD (37.0%), and individuals with NAFLD without depression (33.3%). After accounting for various comorbidities (i.e., age, sex, diabetes, hypertension, obesity), individuals with NAFLD and higher levels of physical activity were at a decreased odds of having depressive symptoms [16.1% reduction (95% confidence interval: -25.6 to -5.4%), P = 0.004], which was not observed in those without NAFLD.
Individuals with NAFLD have high levels of physical inactivity, particularly those with depressive symptoms. Because this group is at high risk for poor outcomes, practitioners should screen for the coexistence of depressive symptoms and NAFLD. This group should receive appropriate interventions aimed at increasing both participation and levels of intensity of physical activity.
Core Tip: Physical inactivity and depressive symptoms are common in individuals with nonalcoholic fatty liver disease (NAFLD). Individuals with both NAFLD and depression are more likely to be sedentary than individuals without NAFLD or in individuals with NAFLD without depressive symptoms. Because this group is at high risk for poor outcomes, practitioners should screen for the coexistence of depressive symptoms and NAFLD. This group should receive appropriate interventions aimed at increasing both participation and levels of intensity of physical activity. It is therefore desirable that individuals with NAFLD should be screened for the presence of depressive symptoms to help determine appropriate interventions.
- Citation: Weinstein AA, De Avila L, Kannan S, Paik JM, Golabi P, Gerber LH, Younossi ZM. Interrelationship between physical activity and depression in nonalcoholic fatty liver disease. World J Hepatol 2022; 14(3): 612-622
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/612.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.612
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver condition in the United States and globally[1]. NAFLD is a multisystem disease which can affect many organ systems and increase the risk of cardiovascular disease and type 2 diabetes mellitus, as well as numerous other conditions[2,3]. NAFLD, along with these other conditions, has been associated with a variety of behavioral factors, including a lack of physical activity, poor nutritional status, and substance consumption[4].
Specifically, a sedentary lifestyle has been related to the onset of NAFLD[5,6]. One potential pathway explaining this relationship is that a lack of physical activity is associated with obesity, which in turn is a major risk factor for NAFLD[5,7]. Increases in physical activity and exercise can lead to mobilization of fat from the liver and is suggested as a treatment for NAFLD[8-10]. Therefore, physical activity is an important behavior to understand in the context of NAFLD, both as a risk factor and as a treatment. Another factor that is highly related to physical inactivity is elevated depressive symptoms (both severity and frequency)[11]. In fact, there is a bi-directional relationship between a sedentary lifestyle and depressive symptoms such that physical inactivity is a risk factor for depressive symptoms and depressive symptoms are a risk factor for physical inactivity[5,12].
NAFLD has also been associated with depressive symptoms[13]. Individuals with NAFLD that also have a major depressive disorder are at an increased risk of developing other conditions such as cardiovascular diseases and stroke[14]. In general, individuals with elevated depressive symptoms have worse health outcomes, including increased morbidity and mortality [14]. Since one potential intervention for NAFLD is increasing levels of physical activity, it is important to consider the potential impact of depressive symptoms on the likelihood of participating in physical activity. It has been well established that individuals with depressive symptoms are less adherent to treatment for chronic illness, particularly treatments that involve behavioral changes[15].
Previous research has demonstrated the relationship between NAFLD and physical inactivity, between NAFLD and depressive symptoms, and between physical inactivity and depressive symptoms; however, we were not able to identify previous literature that explored the interaction of NAFLD, physical inactivity, and depressive symptoms together. The current investigation assesses the presence of these three factors in a community sample in order to explore the potential interrelationships.
The Rancho Bernardo Study (RBS) of Healthy Aging has been previously described in detail[16]. Briefly, between 1972 and 1974, 6339 (82%) adults from the predominantly white and middle to upper middle class southern California community of Rancho Bernardo were enrolled in a longitudinal study focusing on healthy aging. In addition, RBS focused on determining risk factors for cardiovascular disease, diabetes, cognitive function, and bone disease. Participants were followed via 12 subsequent clinic visits occurring approximately every four years as well as annual mailers to follow-up on health status and vital status through July 2019.
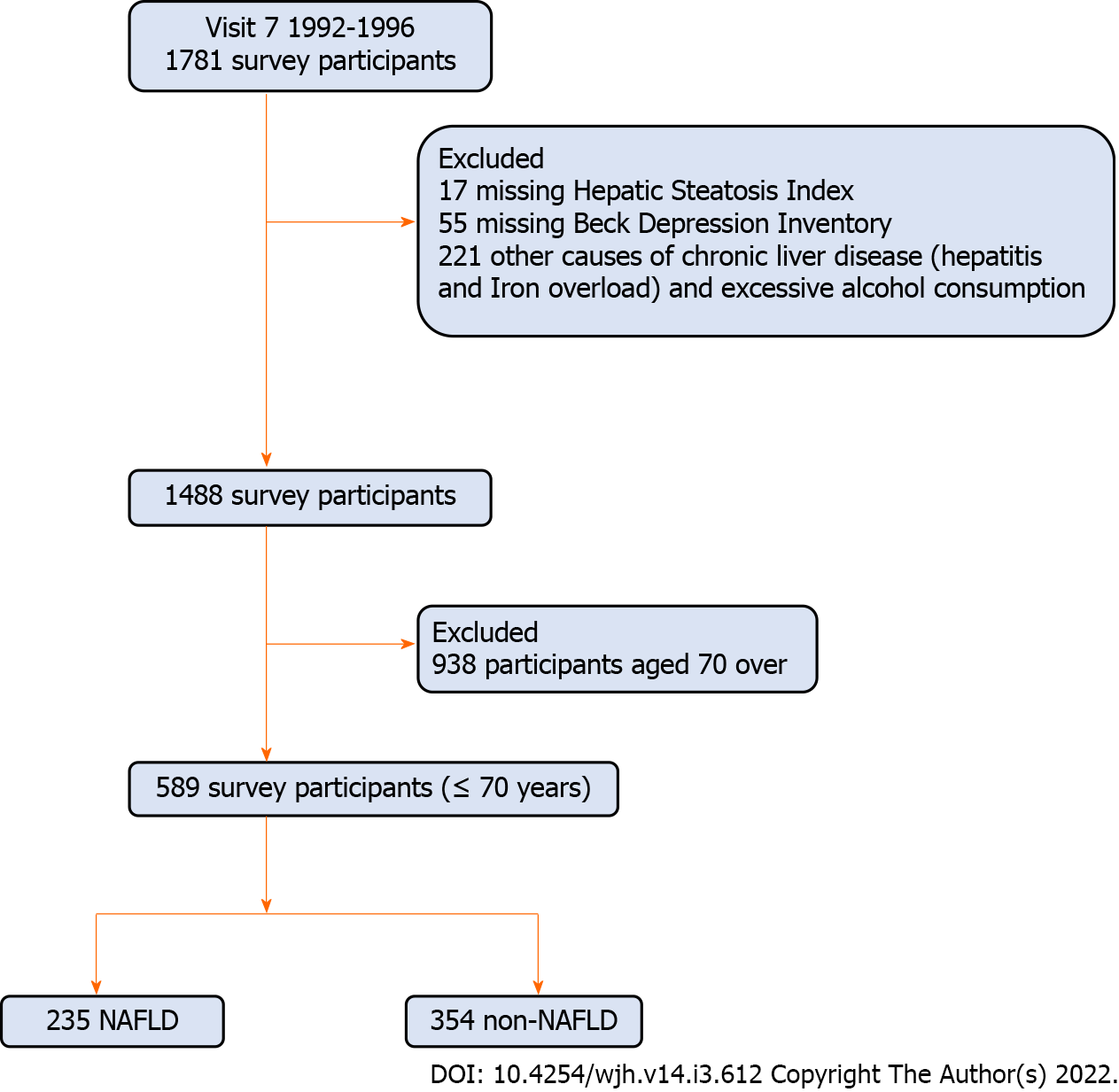
Our study utilized data from 1781 participants who completed clinic visit 7 (1992-1996). Clinic visit 7 was chosen because it assessed the factors necessary to establish presence or absence of NAFLD. Of these, we excluded 17 participants for missing the hepatic steatosis index (HSI), 55 participants for missing Beck Depression Inventory (BDI), and 221 participants who had a history of hepatitis, iron overload (iron ≥ 198 mcg/dL in men and ≥ 170 mcg/dL in women), or excessive alcohol consumption. As depression can manifest differently in older adults[17] and physical activity levels are different in older adults, we further excluded 899 participants aged 70 and over, leaving 589 participants in the final analytical sample (Figure 1). All participants provided written informed consent prior to participation at each visit.
Demographic factors, lifestyle factors, laboratory measures, and medical history data were collected at clinic visit 7 (1992-1996). Lifestyle information was obtained through standard questionnaires and included smoking status [non-smoker, former smoker (quit ≥ 2 years); active smoker], sedentary lifestyle (reported physical activity < 3 times per week) and excessive alcohol consumption (≥ 2 drinks/day in men and ≥ 1 drinks in women). Metabolic components were calculated by the following definitions: (1) Obesity pattern was categorized into lean (BMI: 18.5-25 kg/m2); overweight (25-29.9 kg/ m2) and obese (≥ 30 kg/m2); (2) Hypertension was defined as having a systolic blood pressure of > 140 mmHg or diastolic blood pressure of > 90 mmHg from an average of three measurements and/or use of antihypertensive medications; (3) Hyperlipidemia was defined as a serum cholesterol level of ≥ 200 mg/dL, LDL of ≥ 130 mg/dL, and HDL ≤ 40 mg/dL in men or ≤ 50 mg/dL in women; (4) Diabetes mellitus was defined by a fasting glucose level ≥ 126 mg/dL, post-challenge plasma glucose level of at least 200 mg/dL, and history of physician-diagnosed diabetes or use of diabetes medication; (5) Insulin resistance was defined by the homeostasis model assessment of insulin resistance[18]; and (6) Metabolic syndrome was defined as having at least three of the following: waist circumference > 102 cm in men or > 88 cm in women, fasting plasma glucose > 110 mg/dL, blood pressure > 130/85 mmHg, elevated triglycerides > 150 mg/dL, and HDL ≤ 40 mg/dL in men or ≤ 50 mg/dL in women[19].
We categorized the presence of depression as a BDI score of ≥ 10[20]. Individuals that scored less than 10 were considered to not have depression. We categorized physical activity into 3 groups: (1) “physical inactivity” if participants didn’t engage in any level of physical activity at least three times per week; (2) “ideal physical activity” if participants regularly (≥ 3/week) engaged in strenuous activity; and (3) “moderate physical activity” that encompassed everyone else.
NAFLD was defined by using the HSI, validated previously and used in epidemiologic studies[13,21,22] in the absence of secondary causes of liver disease. HSI was calculated by the following equation: 8 × (alanine aminotransferase/aspartate aminotransferase ratio) + BMI (+2 for diabetes; +2 for female). The published cut-off score of 36 was utilized to define the presence of NAFLD. Participants with a HSI of < 36 and no secondary causes of liver disease were presumed to not have the presence of NAFLD (non-NAFLD).
We compared demographic, lifestyle factors, clinical factors and medical history of the study cohort by the presence of NAFLD, depression and level of physical activity using a non-parametric Kruskal-Wallis test for continuous variables and chi-square test for categorical analysis. Multivariable generalized linear regression model (GLM) with Gamma distribution was performed on BDI score to evaluate the effect of physical activity and NAFLD after adjusting for age, sex, current smoker, diabetes, hypertension, hyperlipidemia, obesity, history of cardiovascular disease and cancer. The adjusted relationship between factors and BDI scores was estimated using coefficients from GLM models, which were exponentiated to yield a percentage change in the outcome associated with each factor. Independent predictors of depression were studied using multivariable logistic regression. All differences reported here are statistically significant otherwise mentioned at the 0.05 Level. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Of 589 study subjects (43.1% male; 95.8% non-Hispanic white; mean (SD) age 60.0 (7.0) years), 235 (39.9%) subjects had evidence of NAFLD and 55 (9.3%) had evidence of depression. Furthermore, 12.6% had diabetes, 75.7% had hyperlipidemia, 20.0% had hypertension, 26.7% had insulin resistance, 29.0% were physically inactive and 11.9% were active smokers.
Compared to individuals without NAFLD, individuals with NAFLD were statistically significantly more commonly male (52.3% vs 37.0%), more likely to be overweight/obese (93.6% vs 28.0%) more likely to have insulin resistance (46.4% vs 13.6%), hyperlipidemia (85.5% vs 69.2%), diabetes (23.4% vs 5.4%), and metabolic syndrome (43.4% vs 6.5%) (all P < 0.02). Among individuals with NAFLD, 36.6% fell into the physical inactivity category and 24.7% were in the ideal physical activity category; whereas among individuals without NAFLD, 24.0% fell into the physical inactivity category and 36.7% were in the ideal physical activity category (P < 0.002) (Table 1). Individuals with NAFLD had a statistically significantly higher mean BDI score than those without NAFLD (4.49 vs 3.67, P = 0.004), although the mean scores were relatively low in each group.
| All (n = 589) | Non-NAFLD (n = 354) | NAFLD (n = 235) | P value | |
| Age, mean ± SD | 59.97 ± 6.97 | 59.99 ± 7.40 | 59.94 ± 6.29 | 0.3959 |
| Male, % | 254 (43.12%) | 131 (37.01%) | 123 (52.34%) | 0.0002 |
| White, % | 564 (95.76%) | 340 (96.05%) | 224 (95.32%) | 0.6686 |
| Smoking status, % | ||||
| Current | 70 (11.90%) | 47 (13.31%) | 23 (9.79%) | 0.1958 |
| Former | 239 (40.65%) | 141 (39.94%) | 98 (41.70%) | 0.6706 |
| Non-smoker | 279 (47.45%) | 165 (46.74%) | 114 (48.51%) | 0.674 |
| Regular exercise, % | ||||
| Physically Inactive | 171 (29.03%) | 85 (24.01%) | 86 (36.60%) | 0.001 |
| Moderate physical activity | 230 (39.05%) | 139 (39.27%) | 91 (38.72%) | 0.8949 |
| Ideal physical activity | 188 (31.92%) | 130 (36.72%) | 58 (24.68%) | 0.0021 |
| Obesity, % BMI | ||||
| Lean | 270 (45.84%) | 255 (72.03%) | 15 (6.38%) | < 0.0001 |
| Overweight | 234 (39.73%) | 99 (27.97%) | 135 (57.45%) | < 0.0001 |
| Obese | 85 (14.43%) | 0 (0.00%) | 85 (36.17%) | < 0.0001 |
| History of CVD, % | 40 (6.79%) | 23 (6.50%) | 17 (7.23%) | 0.7278 |
| History of arthritis, % | 68 (11.54%) | 39 (11.02%) | 29 (12.34%) | 0.6226 |
| History of cancer (any), % | 97 (16.47%) | 48 (13.56%) | 49 (20.85%) | 0.0195 |
| Insulin resistance, % | 157 (26.66%) | 48 (13.56%) | 109 (46.38%) | < 0.0001 |
| Hypertension, % | 117 (19.86%) | 66 (18.64%) | 51 (21.70%) | 0.3623 |
| Hyperlipidemia, % | 446 (75.72%) | 245 (69.21%) | 201 (85.53%) | < 0.0001 |
| Diabetes, % | 74 (12.56%) | 19 (5.37%) | 55 (23.40%) | < 0.0001 |
| Metabolic syndrome1, % | 125 (21.26%) | 23 (6.52%) | 102 (43.40%) | < 0.0001 |
| BDI, mean ± SD | 4.00 ± 3.71 | 3.67 ± 3.62 | 4.49 ± 3.80 | 0.0041 |
Of the entire cohort, 4.8% had both NAFLD and depression, 4.6% had depression without NAFLD, 35.1% had NAFLD without depression and 55.5% had neither depression nor NAFLD. Demographic, lifestyle and general health comorbidities of participants according to the presence of NAFLD and depression status are presented in Table 2. Compared to individuals with NAFLD but no depression, individuals with both NAFLD and depression were more likely to have a history of arthritis (17.6% vs 10.5%). Compared to individuals with depression but no NAFLD, individuals with both NAFLD and depression were less likely to be lean (7.1% vs 85.2%) and have a higher rate of insulin resistance (42.9% vs 3.7%) and metabolic syndrome (39.3% vs 7.4%).
| Individuals with NAFLD | Individuals without NAFLD | |||||
| No depression (n = 207) | Depression (n = 28) | P value | No depression (n = 327) | Depression (n = 27) | P value | |
| Age, mean ± SD | 59.91 ± 6.34 | 60.15 ± 6.00 | 0.9433 | 60.02 ± 7.32 | 59.61 ± 8.44 | 0.9813 |
| Male, % | 115 (55.56%) | 8 (28.57%) | 0.0073 | 125 (38.23%) | 6 (22.22%) | 0.0978 |
| White, % | 197 (95.17%) | 27 (96.43%) | 0.7671 | 316 (96.64%) | 24 (88.89%) | 0.0471 |
| Smoking status, % | ||||||
| Current | 19 (9.18%) | 4 (14.29%) | 0.3934 | 41 (12.58%) | 6 (22.22%) | 0.1563 |
| Former | 86 (41.55%) | 12 (42.86%) | 0.8949 | 133 (40.80%) | 8 (29.63%) | 0.2549 |
| Non-smoker | 102 (49.28%) | 12 (42.86%) | 0.5236 | 152 (46.63%) | 13 (48.15%) | 0.8789 |
| Regular exercise, % | ||||||
| Physically inactive | 69 (33.33%) | 17 (60.71%) | 0.0048 | 75 (22.94%) | 10 (37.04%) | 0.0992 |
| Moderate physical activity | 86 (41.55%) | 5 (17.86%) | 0.0157 | 129 (39.45%) | 10 (37.04%) | 0.8051 |
| Ideal physical activity | 52 (25.12%) | 6 (21.43%) | 0.6706 | 123 (37.61%) | 7 (25.93%) | 0.2259 |
| Obesity, % BMI | ||||||
| Lean | 13 (6.28%) | 2 (7.14%) | 0.8609 | 232 (70.95%) | 23 (85.19%) | 0.1132 |
| Overweight | 118 (57.00%) | 17 (60.71%) | 0.7094 | 95 (29.05%) | 4 (14.81%) | 0.1132 |
| Obese | 76 (36.71%) | 9 (32.14%) | 0.6365 | 0 (0.00%) | 0 (0.00%) | ------- |
| History of CVD, % | 14 (6.76%) | 3 (10.71%) | 0.4488 | 21 (6.42%) | 2 (7.41%) | 0.8417 |
| History of arthritis, % | 21 (10.14%) | 8 (28.57%) | 0.0054 | 37 (11.31%) | 2 (7.41%) | 0.5331 |
| History of any cancer, % | 46 (22.22%) | 3 (10.71%) | 0.1595 | 47 (14.37%) | 1 (3.70%) | 0.1196 |
| Insulin resistance, % | 97 (46.86%) | 12 (42.86%) | 0.6902 | 47 (14.37%) | 1 (3.70%) | 0.1196 |
| Hypertension, % | 44 (21.26%) | 7 (25.00%) | 0.6519 | 61 (18.65%) | 5 (18.52%) | 0.9861 |
| Hyperlipidemia, % | 177 (85.51%) | 24 (85.71%) | 0.9767 | 226 (69.11%) | 19 (70.37%) | 0.8918 |
| Diabetes, % | 50 (24.15%) | 5 (17.86%) | 0.4601 | 16 (4.89%) | 3 (11.11%) | 0.1682 |
| Metabolic syndrome1, % | 91 (43.96%) | 11 (39.29%) | 0.6394 | 21 (6.44%) | 2 (7.41%) | 0.8451 |
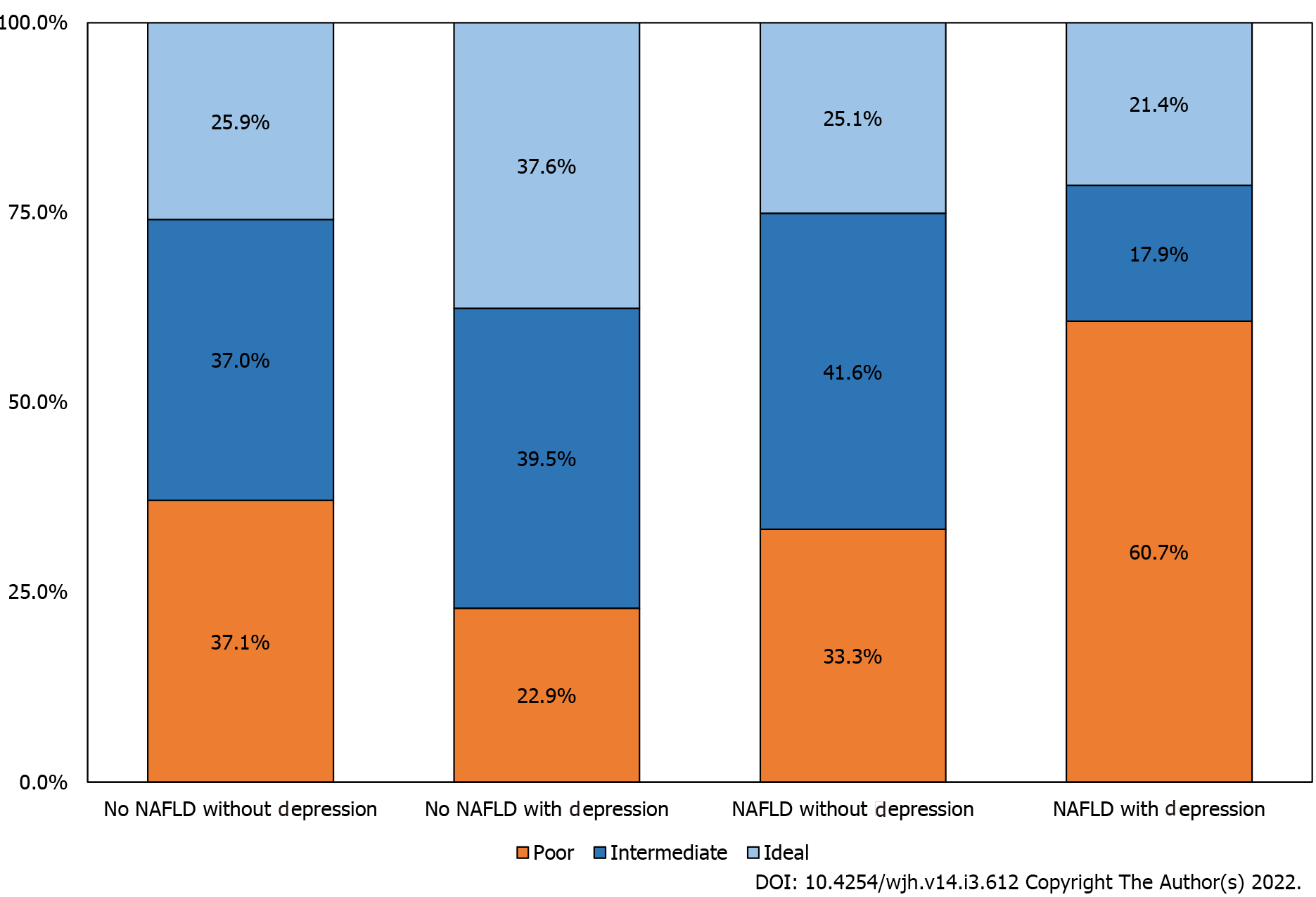
For the individuals that had NAFLD and depression, 60.7% fell within the physical inactivity category which is statistically significantly greater than all of the other groups [individuals with neither NAFLD nor depression (22.9%), individuals with depression without NAFLD (37.0%), and individuals with NAFLD without depression (33.3%)] (Figure 2). Characteristics of individuals according to the presence of NAFLD and physical activity are presented in Supplementary Table 1.
In stratified analyses across the presence of NAFLD, accounting for age, sex, current smoker, diabetes, hypertension, hyperlipidemia, obesity, history of cardiovascular disease and any cancer in GLMs, individuals with NAFLD and higher levels of physical activity experienced greater odds of having a lower BDI score [16.1% reduction (95% confidence interval: -25.6 to -5.4%), P = 0.004]. This association between level of activity and BDI scores was not observed in those without NAFLD (Table 3).
| NAFLD | Non-NAFLD | |||
| % change (95%CI) | P | % change (95%CI) | P | |
| Unadjusted | -16.91 (-26.28 - -6.35) | 0.0024 | -8.96 (-18.25 - 1.38) | 0.0871 |
| Age-sex adjusted | -14.74 (-24.42 - -3.83) | 0.0095 | -6.79 (-16.35 - 3.87) | 0.203 |
| Model 1 | -14.6 (-24.34 - -3.61) | 0.01 | -3.43 (-13.64 - 7.98) | 0.54 |
| Model 2 | -16.12 (-25.6 - -5.44) | 0.004 | -3.27 (-13.49 - 8.15) | 0.5592 |
To assess the association of physical activity and NAFLD on BDI scores, GLMs were performed (Table 4). In the unadjusted model, compared with non-NAFLD individuals with an ideal level of physical activity, NAFLD individuals with physical inactivity had an increased BDI score [46.8% increase (19.3 to 80.8%), P < 0.001]. Even in the fully adjusted model, this result was consistently observed [36.3% increase (9.1% to 70.2%) P < 0.001]. Non-NAFLD Individuals with physical inactivity did not statistically significantly differ from non-NAFLD individuals with an ideal level of physical activity (P = 0.465).
| Unadjusted | Age-sex adjusted | Model 1 | Model 2 | |||||
| Group | % change (95%CI) | P | % change (95%CI) | P | % change (95%CI) | P | % change (95%CI) | P |
| Non-NAFLD with physical ideal | Reference | Reference | Reference | Reference | ||||
| Non-NAFLD with physical moderate | 16.67 (-3.33 - 40.82) | 0.108 | 11.5 (-7.68 - 34.67) | 0.2584 | 7.77 (-11.05 - 30.58) | 0.4447 | 6.99 (-11.65 - 29.56) | 0.4891 |
| Non-NAFLD with physical inactivity | 19.85 (-2.87 - 47.89) | 0.0913 | 14.15 (-7.5 - 40.87) | 0.2174 | 8.25 (-12.74 - 34.29) | 0.4708 | 8.34 | 0.4645 |
| (-12.58 - 34.26) | ||||||||
| NAFLD with physical ideal | 1.23 (-20.04 - 28.15) | 0.919 | 3.31 (-18.23 - 30.53) | 0.7849 | -2.43 (-24.49 - 26.08) | 0.851 | -2.19 (-24.33 - 26.45) | 0.866 |
| NAFLD with physical moderate | 23.08 (-0.09 - 51.63) | 0.051 | 23.99 (0.79 - 52.53) | 0.042 | 15.68 | 0.206 | 16.24 (-7.23 - 45.65) | 0.1911 |
| (-7.7 - 44.98) | ||||||||
| NAFLD with physical inactivity | 46.84 (19.28 - 80.75) | 0.0003 | 43.06 (16.39 - 75.84) | 0.0007 | 35.02 (8.03 - 68.74) | 0.0083 | 36.25 (9.1 - 70.16) | 0.0064 |
In multivariable logistic regression, we included in the model: NAFLD, diabetes, age, sex, smoking status, hypertension, hyperlipidemia, cardiovascular disease, and cancer. The statistically significant risk factors of depression were NAFLD Odds Ratio (OR 2.01 1.08-3.72), P = 0.028), being male [OR 0.37 (0.19-0.72), P = 0.003] and physical inactivity [OR 1.68 (0.78-3.65), P = 0.005] (Supplementary Table 2).
This study investigated the interrelationships between NAFLD, depressive symptoms and physical activity. Our results demonstrate a strong likelihood of physical inactivity in individuals with NAFLD and depression, which was at a higher rate than was seen in individuals without NAFLD or in individuals with NAFLD without depressive symptoms.
Similar findings have been found in individuals with type 2 diabetes[23]. Various symptoms of depression (lack of motivation, low self-esteem, feelings of helplessness, anhedonia) might explain why individuals with depressive symptoms are more often physically inactive[24], but having only depression in this cohort did not explain the inactivity level. The co-existence between NAFLD and depression is likely to associate with physical inactivity.
Depression and NAFLD occur together more often than would be predicted by chance[25]. There are many potential factors that may help to explain this overlap, including the presence of diabetes and obesity, both risk factors for NAFLD and depression[25]. Another area of overlap is the increase in circulating inflammatory cytokines in both depression and NAFLD[26]. In addition, physical inactivity is a risk factor for both depression and NAFLD[5,11]. However, further investigation is needed to clarify this bi-directional relationship between depression and NAFLD.
The findings of the current study show that both physical inactivity and depressive symptoms are common in individuals with NAFLD. In addition, individuals with NAFLD and depressive symptoms are much more likely to be physically inactive than people with depression without NAFLD and those without either. NAFLD is a risk factor for all-cause mortality and exercise is an antidote to this. The combination of depression and NAFLD is significantly associated with low level of physical activity, which in itself is a risk for all-cause mortality. It is therefore desirable that individuals with NAFLD should be screened for the presence of depressive symptoms. Depressive symptoms are likely to contribute to a low level of physical activity, and if treated, may increase participation in more vigorous activity for greater durations. Additionally, increased physical activity has been shown to help mobilize fat from the liver[8,9], and increased physical activity has been shown to have anti-depressive effects[27]. Therefore, it may be important to screen for the combined presence of NAFLD and depression, treat each appropriately, and aim to maximize participation in physical activity. Longitudinal studies investigating these interrelationships are needed to determine if physical inactivity is one of the factors that may link depressive symptoms to subsequent poor health outcomes in NAFLD patients.
Some limitations should be noted. Due to the cross-sectional nature of the current investigation, no causal relationships nor directionality can be inferred between physical inactivity, depressive symptoms, and NAFLD. Another limitation is that we used a noninvasive test (HSI) to identify NAFLD rather than a liver biopsy or other sensitive radiologic tests since these were not available. An objective method of physical activity assessment (i.e., an activity monitor) was not available. In addition, these data were collected in 1992-1996, therefore an older version of the BDI was used and the diagnosis of viral hepatitis was relatively new at the time. We also acknowledge that our findings are not generalizable to the general population, as all participants were well educated, medically insured, predominantly white, and middle to upper-middle-class. Lastly, participants have a relatively low prevalence of obesity, diabetes, and metabolic syndrome compared to the National Health and Nutrition Examination Survey III[28] which may have influenced the results.
Individuals with NAFLD have high levels of physical inactivity, particularly those with depressive symptoms. Because this group is at high risk for poor outcomes, practitioners should screen for the coexistence of depressive symptoms and NAFLD. This group should receive appropriate interventions aimed at increasing both participation and levels of intensity of physical activity.
Since one potential intervention for nonalcoholic fatty liver disease (NAFLD) is increasing levels of physical activity, it is important to consider the potential impact of depressive symptoms on the likelihood of participating in physical activity. It has been well established that individuals with depressive symptoms are less adherent to treatment for chronic illness, particularly treatments that involve behavioral changes.
Previous research has demonstrated the relationship between NAFLD and physical inactivity, between NAFLD and depressive symptoms, and between physical inactivity and depressive symptoms; however, we were not able to identify previous literature that explored the interaction of NAFLD, physical inactivity, and depressive symptoms together.
The current investigation assesses the presence of NAFLD, physical inactivity, and depressive symptoms in a community sample in order to explore the potential interrelationships.
Data from the Rancho Bernardo Study were used. 589 individuals were included in the analyses (43.1% male; 95.8% non-Hispanic white; aged 60.0 ± 7.0 years). NAFLD was defined by using the hepatic steatosis index, depression using the Beck Depression Inventory, and physical activity by self-report of number of times per week of strenuous activity. Multivariable generalized linear regression models with Gamma distribution were performed to investigate the proposed relationship.
About 40% of the sample had evidence of NAFLD, 9.3% had evidence of depression, and 29% were physically inactive. Individuals with NAFLD and depression were more likely to be physically inactive (60.7%) compared to individuals with neither NAFLD nor depression (22.9%), individuals with depression without NAFLD (37.0%), and individuals with NAFLD without depression (33.3%). After accounting for various comorbidities (i.e., age, sex, diabetes, hypertension, obesity), individuals with NAFLD and higher levels of physical activity were at a decreased odds of having depressive symptoms [16.1% reduction (95% confidence interval: -25.6 to -5.4%), P = 0.004], which was not observed in those without NAFLD.
Individuals with NAFLD have high levels of physical inactivity, particularly those with depressive symptoms. Because this group is at high risk for poor outcomes, practitioners should screen for the coexistence of depressive symptoms and NAFLD. This group should receive appropriate interventions aimed at increasing both participation and levels of intensity of physical activity.
Further investigation is needed to clarify this bi-directional relationship between depression and NAFLD. Future work should explore screening for the combined presence of NAFLD and depression to determine if treatment with appropriate physical activity interventions can enhance outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Christodoulou D, El-Gendy HA, Zhang LL S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Li AA, Ahmed A, Kim D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:168-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 4. | Macavei B, Baban A, Dumitrascu DL. Psychological factors associated with NAFLD/NASH: a systematic review. Eur Rev Med Pharmacol Sci. 2016;20:5081-5097. [PubMed] |
| 5. | Croci I, Coombes JS, Bucher Sandbakk S, Keating SE, Nauman J, Macdonald GA, Wisloff U. Non-alcoholic fatty liver disease: Prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Prog Cardiovasc Dis. 2019;62:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Kwak MS, Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 2018;33:64-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 7. | Correction: Dose-response association between physical activity and non-alcoholic fatty liver disease: a case-control study in a Chinese population. BMJ Open. 2020;10:e026854corr1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Gerber LH, Weinstein A, Pawloski L. Role of exercise in optimizing the functional status of patients with nonalcoholic fatty liver disease. Clin Liver Dis. 2014;18:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, Ibrahim AA. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine (Baltimore). 2020;99:e19471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Locklear CT, Golabi P, Gerber L, Younossi ZM. Exercise as an intervention for patients with end-stage liver disease: Systematic review. Medicine (Baltimore). 2018;97:e12774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Achttien R, van Lieshout J, Wensing M, van der Sanden MN, Staal JB. Symptoms of depression are associated with physical inactivity but not modified by gender or the presence of a cardiovascular disease; a cross-sectional study. BMC Cardiovasc Disord. 2019;19:95. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Jung JY, Park SK, Oh CM, Chung PW, Ryoo JH. Non-Alcoholic Fatty Liver Disease and Its Association with Depression in Korean General Population. J Korean Med Sci. 2019;34:e199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Kim D, Yoo ER, Li AA, Tighe SP, Cholankeril G, Harrison SA, Ahmed A. Depression is associated with non-alcoholic fatty liver disease among adults in the United States. Aliment Pharmacol Ther. 2019;50:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Tomeno W, Kawashima K, Yoneda M, Saito S, Ogawa Y, Honda Y, Kessoku T, Imajo K, Mawatari H, Fujita K, Hirayasu Y, Nakajima A. Non-alcoholic fatty liver disease comorbid with major depressive disorder: The pathological features and poor therapeutic efficacy. J Gastroenterol Hepatol. 2015;30:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Lam WY, Fresco P. Medication Adherence Measures: An Overview. Biomed Res Int. 2015;2015:217047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 718] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 16. | Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Haigh EAP, Bogucki OE, Sigmon ST, Blazer DG. Depression Among Older Adults: A 20-Year Update on Five Common Myths and Misconceptions. Am J Geriatr Psychiatry. 2018;26:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 18. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24510] [Article Influence: 612.8] [Reference Citation Analysis (0)] |
| 19. | Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002; 106. Available from: https://pubmed.ncbi.nlm.nih.gov/12485966/. |
| 20. | Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7481] [Cited by in RCA: 7350] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 21. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1064] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 22. | Meffert PJ, Baumeister SE, Lerch MM, Mayerle J, Kratzer W, Völzke H. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. 2014;109:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Koopmans B, Pouwer F, de Bie RA, van Rooij ES, Leusink GL, Pop VJ. Depressive symptoms are associated with physical inactivity in patients with type 2 diabetes. The DIAZOB Primary Care Diabetes study. Fam Pract. 2009;26:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Seime RJ, Vickers KS. The challenges of treating depression with exercise: From evidence to practice. Clin Psychol Sci Pract. 2006;13:194-197. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Xiao J, Lim LKE, Ng CH, Tan DJH, Lim WH, Ho CSH, Tan EXX, Sanyal AJ, Muthiah MD. Is Fatty Liver Associated With Depression? Front Med (Lausanne). 2021;8:691696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Lee JW, Park SH. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. J Affect Disord. 2021;278:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Motl RW, Konopack JF, McAuley E, Elavsky S, Jerome GJ, Marquez DX. Depressive symptoms among older adults: long-term reduction after a physical activity intervention. J Behav Med. 2005;28:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Paik JM, Deshpande R, Golabi P, Younossi I, Henry L, Younossi ZM. The impact of modifiable risk factors on the long-term outcomes of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |