Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.602
Peer-review started: October 23, 2021
First decision: December 2, 2021
Revised: December 16, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 27, 2022
Processing time: 151 Days and 23 Hours
Persistent inflammation on histology after successful hepatitis C (HCV) treatment has been reported. However, data regarding the long-term impact in liver transplant recipients is limited, particularly after using direct-acting antiviral (DAA) therapies.
To evaluate the impact of successful treatment with DAAs on histological changes and occult HCV and to describe the clinical course of residual inflammation in liver transplant recipients.
We conducted a case series of 13 chronic HCV infected liver transplant recipients successfully treated with DAAs between December 2013 and May 2014. All patients were treated for 24 wk and had non-detectable serum HCV RNA by the time of biopsy. Only patients with at least one liver biopsy at or after treatment were included. We examined liver biopsies for evidence of residual inflammation and the presence of intrahepatic HCV RNA.
Persistent inflammation was seen in 12/13 patients on end of treatment biopsy. Inflammation was still seen in the available five follow-up biopsies (range 38-48 wk after the end of treatment). Intrahepatic HCV RNA was undetectable in all biopsies. All patients had preserved graft function for a mean follow-up of 2.5 years, except one that developed chronic rejection.
After successful HCV treatment with DAAs, liver transplant recipients may have persistent inflammation on biopsy without evidence of intracellular RNA. The clinical outcome remained favorable in most patients. Further studies with a larger number and longer follow-up are needed to establish the implication of this finding on long-term graft function.
Core Tip: Unexplained residual inflammation can be seen in a subset of liver transplant recipients successfully treated with direct-acting antiviral therapies; however, it does not seem to affect graft function. An extensive clinical and histopathologic workup should still be performed to exclude other potentially treatable conditions.
- Citation: Ismail B, Benrajab KM, Bejarano P, Ruiz P, Sears D, Tzakis A, Zervos XB. Benign course of residual inflammation at end of treatment of liver transplant recipients after sofosbuvir based therapy. World J Hepatol 2022; 14(3): 602-611
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/602.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.602
Until the emergence of direct-acting antivirals (DAAs), hepatitis C virus (HCV) related liver cirrhosis was the most common indication for liver transplant in adults[1,2]. Unfortunately, HCV recurrence after transplant is universal, with immediate exposure after graft reperfusion, leading to accelerated fibrosis, eventually cirrhosis, and graft failure if untreated[3]. Graft survival in HCV infection has been inferior to transplant for other disease etiologies[4], and HCV remains a common indication for re-trans
Although SVR is the critical clinical endpoint, there are mixed results concerning the post-SVR histologic benefit and detection of intra hepatocyte HCV (occult HCV). Prior reports on HCV recurrence following liver transplantation showed histological improvement after achieving SVR with IFN based regimens[9], whereas other studies reported ongoing inflammation in a subset of patients[10,11]. One study included 36 Liver transplant (LT) recipients treated with IFN based regimens showed persistent inflammation in 69% of the post SVR biopsies and identified occult hepatitis C in only one out of 32 biopsies that were tested[11].
Data on inflammation following SVR after DAA are scarce. A study that included nine LT recipients treated with DAAs identified residual inflammation after SVR in four patients and HCV RNA in the tissue sample in four patients[12]. Another study included LT recipients with recurrent HCV and advanced fibrosis (F3-F4) showed improved liver function in the majority of patients; however, regression of fibrosis by elastography (48 wk after treatment) was only seen in 39/77 subjects (51%). Although details on liver histology for these patients were not reported, this remains concerning for residual inflammation in some patients that could have progressed over time[13].
We believe that the finding of persistent inflammation in LT recipients after SVR using DAAs requires further research work as it is of clinical importance to the transplant team. At the same time, many of the available series included a small sample and focused mainly on histology without providing long-term clinical data.
The study aimed to evaluate the impact of successful treatment with DAAs on histologic changes and the occurrence of occult HCV in liver transplant recipients with chronic HCV. The secondary aim was to describe the long-term clinical course of residual inflammation if present.
We reviewed all chronic HCV infected liver transplant recipients treated with DAA regimens between December 2013 and May 2014. We excluded patients who did not achieve SVR or did not have at least one liver biopsy at or after the end of treatment. The study protocol was approved by the Institutional Review Board of the Cleveland Clinic Foundation in Florida. Data collected at baseline and during patient follow-up included; age, gender, race, date of transplant, baseline and end-of-treatment liver enzymes and viral load, HCV treatment regimen, other serologic autoimmune and virological markers, immunosuppression treatment regimen, and liver histology at end-of-treatment. When available, before-treatment and follow-up liver histology were also reported.
SVR: Defined as the absence of HCV RNA by polymerase chain reaction12 and 24 weeks after completion of treatment. The Linear Range of the used assay was 15 IU/mL to 100000000 IU/mL.
End of treatment liver biopsy: for the purpose of our study, this was defined as liver biopsy performed within 12 weeks after HCV RNA becomes undetectable.
Post-treatment liver biopsy: biopsy was done at least 6 mo after the end of treatment.
Biopsy method: All biopsies were performed percutaneously using an 18-gauge coaxial needle via ultrasound guidance. Two core tissue samples were obtained and placed in a formalin container. All samples reviewed by the pathologist contained at least 20 portal tracts to be considered adequate. Tissue sections were processed and stained with hematoxylin-eosin and trichrome stains. For the purpose of this study, the liver biopsies were evaluated by an expert liver pathologist with over 20 years of experience reading liver biopsies in an academic transplant center. The pathologist was blinded to the patients’ clinical data, diagnoses, and previous biopsy interpretation. Evaluation of fibrosis and inflammation was described using Batts-Ludwig grading and staging[14]. Biopsies showing inflammation were carefully examined by the pathologist for the presence of rejection, de novo autoimmune hepatitis, and evidence of hepatotropic and non-hepatotropic viral hepatitis, including cytomegalovirus, Epstein-Barr virus, and Herpes simplex virus.
Method for HCV quantification on liver samples: Total RNA was isolated from five to six 10 µm cuts (curls) from formalin-fixed, paraffin-embedded (FFPE) biopsies in a Maxwell 16/LEV instrument using the Maxwell® 16 LEV RNA FFPE Purification Kit (Promega). The concentration of purified RNA was quantified in NanoDrop 2000. 50 µL of each RNA was diluted in 950 µL of SPEX buffer and ran in a COBAS® AmpliPrep/COBAS® TaqMan® System using the HCV test (Roche Molecular Systems Inc.). The viral load, when detected, was expressed as IU/100 ng of RNA.
Out of 46 patients treated for HCV following liver transplant during the study time, 13 patients met the inclusion criteria. Their baseline and demographic characteristics are summarized in Table 1.
| Gender | Age attreatment start | HCV genotype | Immune suppression | Baseline ALT U/L | Baseline RNA × 106 | Time transplant to treatment (m) | Treatment regimen | End of treatment ALT U/L | End of treatment biopsy | ||||
| Treatment to biopsy (wk) | Inflammation grade | Fibrosis stage | Other findings | ||||||||||
| 1 | F | 59 | 1a | MMF, tacrolimus | 57 | 1.3 | 22 mo | SOF/SIM | 18 | 25 | 2 | 1 | |
| 2 | M | 57 | 1a | Tacrolimus | 12 | 0.3 | 32 d | SOF/RBV | 17 | 22 | 0 | 0 | Mild centrilobular dilatation with focal hemorrhage |
| 3 | M | 59 | 1a | MMF, tacrolimus | 85 | 4.0 | 13 mo | SOF/RBV > SOF/SIM | 11 | 32 | 2 | 0 | |
| 4 | M | 61 | 1a or 1b | Tacrolimus | 20 | 0.1 | 5 mo | SOF/RBV | 16 | 22 | 3 | 0 | |
| 5 | M | 68 | 1a or 1b | Tacrolimus | 44 | 1.2 | 8.5 yr | SOF/RBV | 18 | 25 | 1 | 3 | |
| 6 | F | 76 | 1a | Tacrolimus | 20 | 10.6 | 5.5 mo | SOF/SIM | 48 | 25 | 2 | 0 | Histocytes granuloma |
| 7 | F | 80 | 1a | Tacrolimus | 12 | 5.2 | 18 yr | SOF/SIM | 15 | 24 | 3 | 2 | |
| 8 | M | 62 | 1a | MMF, tacrolimus | 166 | 1.7 | 43 d | SOF/RBV | 9 | 30 | 1 | 0 | Steatosis (< 5%) |
| 9 | M | 60 | 3a | Tacrolimus, prednisone | 23 | 0.9 | 4 mo | SOF/RBV | 82 | 33 | 1 | 0 | Rare councilmen bodies |
| 10 | M | 58 | 1a | Tacrolimus | 25 | 1.7 | 20 mo | SOF/RBV | 30 | 25 | 4 | 3 | Mild TCMR cannot be ruled out |
| 11 | M | 53 | 3a | Cyclosporine | 78 | 5.2 | 4.5 yr | SOF/RBV | 37 | 23 | 4 | 2 | Mild TCMR cannot be ruled out. Two portal tracts show non-necrotizing granulomas |
| 12 | F | 61 | 2b | Tacrolimus | 43 | 7.6 | 6 mo | SOF/RBV | 34 | 20 | 2 | 1 | |
| 13 | M | 65 | 1a | Tacrolimus | 94 | 2.0 | 7.5 yr | SOF/RBV > SOF/SIM | 14 | 29 | 2 | 2 | Mild absence of bile ducts |
One patient started treatment before transplant (125 days before transplant), while 12/13 patients started after transplant (mean 5 years from transplant, range 32 days – 18 years). Eight patients (62%) were treated with Sofosbuvir plus Ribavirin, three patients (23%) were treated with Sofosbuvir plus Simeprevir, and two patients (15%) were started on Sofosbuvir plus Ribavirin then switched to Sofosbuvir plus Simeprevir because of worsening anemia. The total treatment duration in all patients was 24 wk.
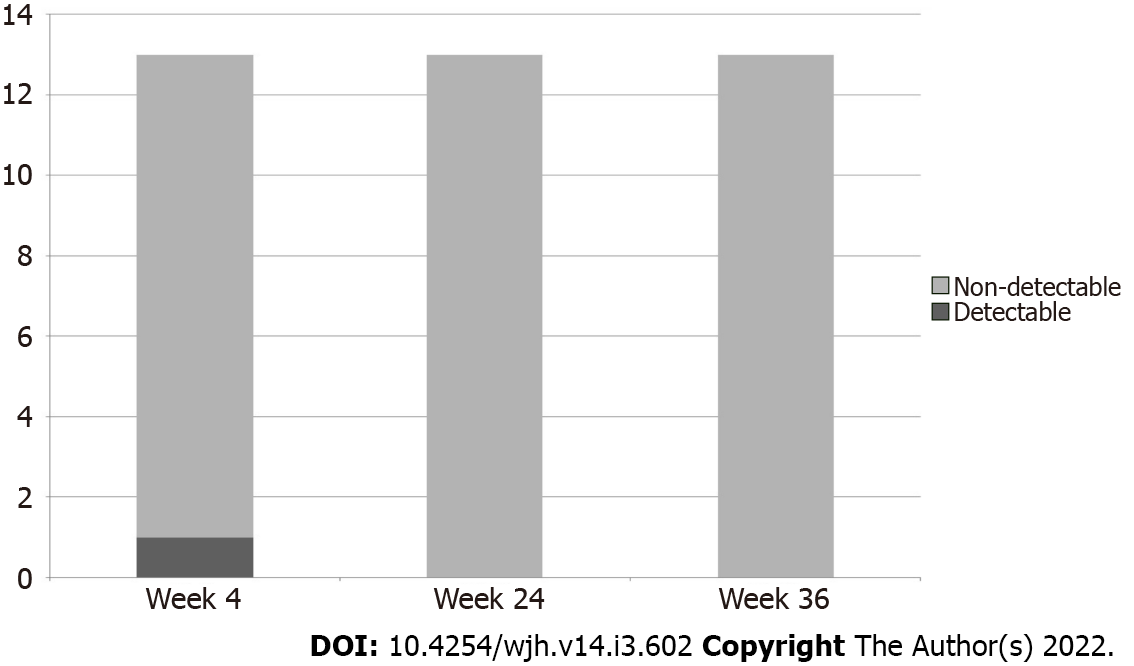
Serum HCV RNA was undetectable in all patients at end-of-treatment and remained undetectable for another 12 and 24 wk post-treatment, consistent with SVR 12 and 24 (Figure 1).
Biopsies at the end of treatment were reviewed in all included patients (mean time from treatment start to biopsy was 25 wk, range 20-33 wk). The biopsies were performed to evaluate abnormal liver function tests or assess the resolution of inflammation after HCV eradication.
Although all patients had undetectable RNA by the time of biopsy, active inflammation was present in 12/13 patients. Eight patients (62%) had grade 1-2 inflammation, and four (31%) had grades 3-4, Table 1.
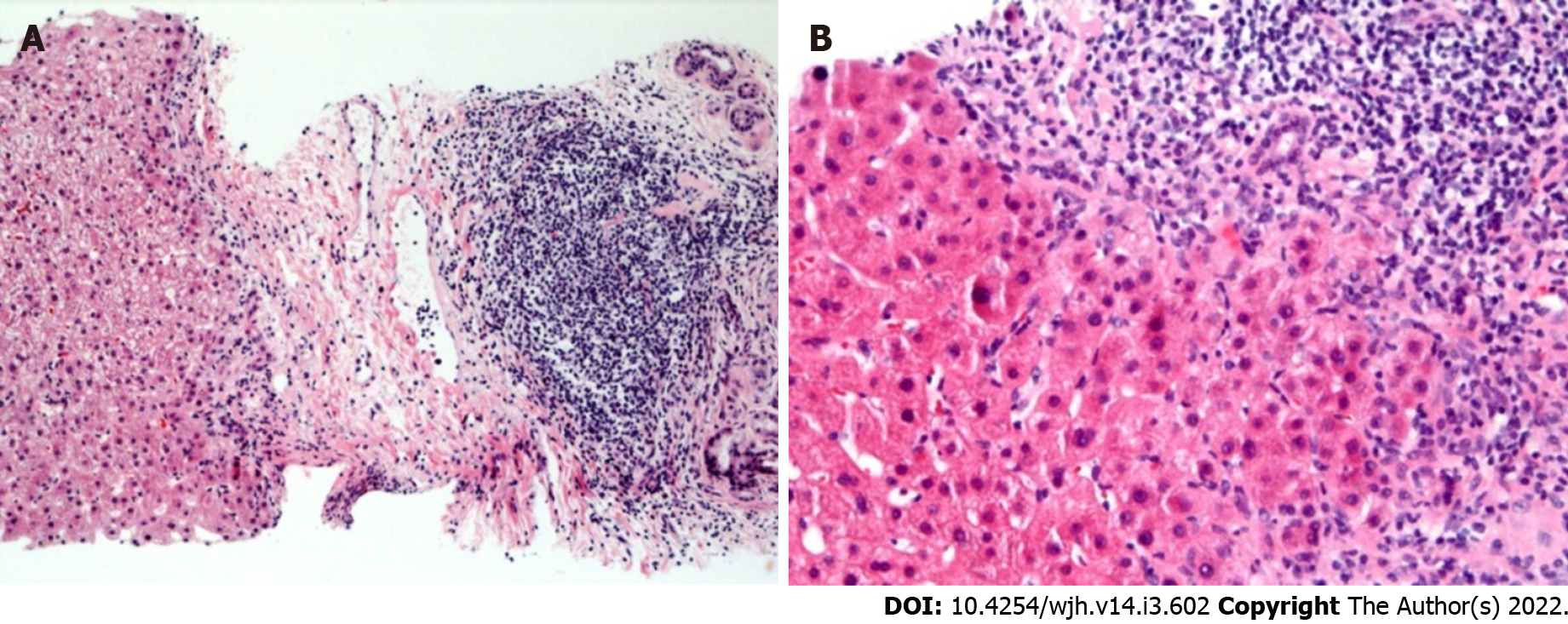
The inflammation observed was consistent with chronic HCV with the presence of chronic portal inflammation, lymphoid aggregates, lobular inflammation, and acidophil bodies (Figure 2). There was a histologic suggestion of mild T cell-mediated rejection on biopsy in 2 patients, but clinically deemed not to have rejection, as one had normal aminotransferases, and the other one had spontaneous normalization of aminotransferases without adjusting their immune suppression regimen. One patient had a mild absence of bile ducts concerning for early chronic rejection. Two patients had occasional non-necrotizing granulomas in portal tracts without an identifiable cause. Steatosis was present in only one patient and was mild < 5%.
We compared pre- and post-therapy histological grades of inflammation in five patients (biopsies were performed at a mean of 17 wk before treatment start, range 10-26 wk). The mean time between pre and end of treatment biopsies was 43 wk, range 35-55 wk. Inflammation increased in three patients (by one point), decreased in one patient (by one point), and remained the same in one, Table 2.
| Patient number | Weeks from biopsy to treatment start | Prior to treatment inflammation grade | End of treatment | |
| Inflammation | ||||
| Grade | Change | |||
| 1 | 10 | 1 | 2 | ↑ |
| 5 | 14 | 2 | 1 | ↓ |
| 10 | 22 | 3 | 4 | ↑ |
| 11 | 18 | 3 | 4 | ↑ |
| 13 | 26 | 2 | 2 | ↔ |
Post-treatment follow-up biopsies were available in five patients (range 38-48 wk after the end of treatment). Compared to end-of-treatment biopsies, inflammation decreased in 4 out of 5 patients (by one point) and increased in one patient (by one point). Fibrosis increased by one point in two patients and remained the same in two. No other causes of inflammation were identified clinically or histologically, Table 3.
| Patient number | End of treatment biopsy | Weeks between both biopsies | ALT | Follow up biopsy | ||
| Inflammation | Fibrosis | Inflammation | Fibrosis | |||
| 3 | 2 | 0 | 38 | 20 | 1 ↓ | 0 ↔ |
| 4 | 3 | 0 | 42 | 15 | 2 ↓ | 1 ↑ |
| 5 | 1 | 3 | 43 | 20 | 2 ↑ | 3 ↔ |
| 10 | 4 | 3 | 48 | 22 | 2 ↓ | 3↔ |
| 11 | 4 | 2 | 44 | 71 | 3 ↓ | 3 ↑ |
HCV RNA was undetected on all available end-of-treatment (13) and post-treatment liver biopsies (5).
The clinical course for all patients was tracked for a mean of 2.5 years after the end of treatment. None of the patients had HCV relapse or worsening liver function. All had preserved graft function, normal aminotransferase, and alkaline phosphatase levels except one that had chronically elevated alkaline phosphatase and was later diagnosed with chronic ductopenic rejection (the patient that had a mild absence of bile ducts on the end of treatment biopsy).
Even after the wide use of DAAs, HCV-related cirrhosis remains one of the leading indications for liver transplant and re-transplant in adults[5]. Due to their safety profile, DAAs allow treating more patients with decompensated cirrhosis prior to transplant; however, treatment is commonly deferred to the post-transplant period to avoid reducing the transplant priority or allowing receipt of a hepatitis C positive organ[15]. DAAs show high SVR rates in transplant recipients; however, data regarding the histological impact of these drugs in terms of inflammatory changes is limited. Throughout our early DAA experience, we evaluated end of treatment and follow-up biopsies after achieving undetectable RNA. We noted that biopsies still showed persistent inflammation at the end of treatment in 12/13 patients, with no improvement from pre-treatment in 4/5 patients.
Additionally, all the available follow-up biopsies (up to 48 wk from the end of treatment) still showed persistent inflammation. We thoroughly evaluated different possibilities that could explain this persistent inflammation. Although no clinically significant drug interactions are reported between immune suppressive regimens and the included DAAs in our study[16], a cure of HCV can potentially influence immune suppression drug levels, and rejection may be a concern. Prior studies have shown an impact of ongoing HCV infection on CYP3A4[17] and eventually on cyclosporine and tacrolimus levels. In one report, lower doses of these immunosuppressants were needed to reach the same therapeutic level compared to non HCV infected patients[18], raising the possibility that resolution of HCV infection can lead to lower immunosuppressant level with subsequent rejection. This possibility was carefully examined in our patients; findings suggestive of mild T cell-mediated rejection were present only in two patients on end of treatment biopsies. The histological changes present were minimal and, when correlated, clinically deemed insignificant as the patients did well clinically without additional interventions or adjustment of immune suppression regimen. Persistent unexplained hepatitis in the liver allograft has been previously reported as idiopathic post-transplant hepatitis (IPTH), chronic hepatitis of unknown etiology, with a variable prevalence ranging from 10%-50% in the adult population[19]. The implication of this diagnosis in a chronic HCV setting is unclear as most of the studies excluded this patient population[20,21].
Furthermore, in our study, inflammation was present in higher frequency (in 12/13 end of treatment biopsies and all 5 post-treatment biopsies), suggesting the presence of another etiology. Prior studies also described a characteristic pattern of plasma cell hepatitis[10,22,23] in patients who achieved SVR after receiving IFN based therapy. This unique pattern was not seen in our patients. One possible explanation is that plasma cell hepatitis is seen more in IFN treated patients, given the immune stimulant effect of IFN leading to exposure of new antigens on hepatocytes[24]. All our patients treated with IFN free regimens can possibly explain the absence of this histologic pattern.
Prior studies reported occult HCV infection, a “controversial” term, indicating persistence of HCV RNA within hepatocytes and/or peripheral blood mononuclear cells despite successfully achieving SVR[25]. Although the active liver disease has not been reported with this finding, it has been shown that this persistent low-level HCV replication promotes persistence of both humoral and T-cellular HCV specific markers, that inversely correlated with time from SVR but can persist for up to 9 years[26]. Our patients, in theory, are at high risk for developing occult HCV for multiple reasons. First, it has been proposed that following treatment with DAA, there is a higher potential for developing occult HCV when compared to IFN based treatment due to the lack of induced immunologic response of the interferon effect[27]. Second, the risk of occult HCV in the immune-compromised patients is likely increased because of the limited ability of the immune system for complete viral clearance, similar to end-stage renal disease patients on dialysis[28]. However, the occurrence of occult infection remains questionable[29], and data following DAA have been inconsistent. One study reported detectable HCV RNA in hepatocytes or peripheral blood mononuclear cells in five out of nine post-transplant patients who were treated with DAA and had elevated liver enzymes despite achieving SVR[12].
In contrast, another study did not find evidence of intracellular RNA in 4 patients with persistent liver enzyme elevation after DAA[30]. The discrepancy in results could be related to the lack of method standardization used among studies to detect HCV RNA in tissue; different sensitivities have been reported depending on the used method and tissue processing before analysis[27,31]. Due to the retrospective nature of our study, we used FFPE specimens. We found no HCV RNA particles on the available end of treatment and post-treatment biopsies indicating that occult infection is not the underlying etiology of residual inflammation in our cohort.
The way viral infections induce liver inflammation is complex; one of the identified triggers of this immune response is the activation of transmembrane and cytosolic receptors that sense both the viral nucleic acid and certain host nucleic acid segments, particularly DNA derived from mitochondrial damage. It has been presumed that this plays a role in some non-viral liver injury models as acetaminophen hepatotoxicity and ischemic injury[32]. The persistent inflammation seen in our subjects could be triggered by the host rather than the remaining viral RNA. However, this is not certain as we did not immunologically characterize the inflammatory cells on liver biopsies for HCV-specific T-cell responses, and this can be an area for future research.
Another likely explanation for the persistent inflammation in our series can be the lag of the histological improvement behind viral clearance and biochemical improvement. Our study did not show complete resolution of inflammation on the end of treatment biopsies nor on post-treatment biopsies. However, most of our patients had post-treatment liver biopsy within 6 mo after completion of treatment, which might not be enough time for inflammation to resolve. Moreover, it should be noted that the change in inflammation grades and fibrosis stages between both biopsies is subtle; hence, we cannot exclude the possibility of this being secondary to sampling variation rather than a true change.
Our study limitations include the small number of patients, mostly genotype 1 treated with some early sofosbuvir-based regimens. However, we believe that our sample is relatively larger than similar studies that evaluated histologic changes post-transplant in this setting and that the findings are likely generalizable to other DAA regimens. Moreover, we did not check for HCV RNA in peripheral blood mononuclear cells due to the retrospective nature of our study, so there is a possibility that we may have missed occult infection in the mononuclear cells. However, in the setting of residual inflammation on liver biopsy that is consistent with HCV activity, we believe it is more important to examine the liver tissue, which was negative for HCV RNA particles, making occult HCV infection a less likely explanation for this persistent inflammation.
Our case series is among the few available that report the histologic findings and clinical outcomes in transplant recipients after achieving SVR using DAAs. We were also able to rule out occult HCV, and we followed the patients clinically for 2.5 years showing a benign course of the residual inflammation in most subjects. Based on our findings, the residual inflammation appears to have a favorable outcome, but it is crucial to exclude other causes of inflammation thoroughly. Moreover, based on our results and prior studies, we believe that checking occult HCV is not routinely necessary from a clinical standpoint. Liver transplant recipients often require liver biopsy for various reasons; recognizing the natural history of this residual inflammation is important to the transplant team. Further studies with a larger and more diverse patient population and longer follow-up will help better characterize the long-term outcome of this persistent inflammation following SVR.
Liver transplant recipients may undergo liver biopsy for different indications, and persistent inflammation in patients who receive DAAs can be seen despite achieving sustained virologic response (SVR).
Data on the significance of persistent inflammation on histology after successful treatment of hepatitis C infection with Direct-acting antiviral (DAA) therapies is scarce.
We aimed to examine the impact of successful treatment with DAAs on histological changes and to describe the clinical course of residual inflammation in liver transplant recipients.
A case series of chronic hepatitis C liver transplant recipients received DAA post-liver transplant and achieved sustained virologic response. Only patients with at least one liver biopsy were included.
Thirteen patients were included in this case series; all achieved SVR. Twelve patients were found to have persistent inflammation at the end of treatment biopsy. Five patients had follow-up biopsies, all of which had persistent inflammation. However, all patients had preserved graft function up to 2.5 years, except one who had chronic rejection.
Persistent inflammation can be seen in liver transplant recipients treated with DAAs; however, it did not appear to affect the outcome.
The findings of our case series shed light on the significance of persistent inflammation in liver transplant recipients post successful DAAs treatment. Further studies are needed to include a more diverse patient population.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Mrzljak A S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Verna EC, Brown RS Jr. Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 3. | Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, Machicao VI, Chaoru C, Nelson DR. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 736] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Berenguer M, López-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Peyton A, Bhamidimarri KR. Hepatitis C treatment in liver transplant setting. Clin Liver Dis (Hoboken). 2015;5:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Coilly A, Roche B, Duclos-Vallée JC, Samuel D. Optimal therapy in hepatitis C virus liver transplant patients with direct acting anti-virals. Liver Int. 2015;35:44-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Dhanasekaran R, Sanchez W, Mounajjed T, Wiesner RH, Watt KD, Charlton MR. Impact of fibrosis progression on clinical outcome in patients treated for post-transplant hepatitis C recurrence. Liver Int. 2015;35:2433-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Whitcomb E, Choi WT, Jerome KR, Cook L, Landis C, Ahn J, Te HS, Esfeh J, Hanouneh IA, Rayhill SC, Gibson W, Plesec T, Koo J, Wang HL, Hart J, Pai RK, Westerhoff M. Biopsy Specimens From Allograft Liver Contain Histologic Features of Hepatitis C Virus Infection After Virus Eradication. Clin Gastroenterol Hepatol. 2017;15:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Elmasry S, Wadhwa S, Bang BR, Cook L, Chopra S, Kanel G, Kim B, Harper T, Feng Z, Jerome KR, Kahn JA, Saito T. Detection of Occult Hepatitis C Virus Infection in Patients Who Achieved a Sustained Virologic Response to Direct-Acting Antiviral Agents for Recurrent Infection After Liver Transplantation. Gastroenterology. 2017;152:550-553.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Martini S, Sacco M, Strona S, Arese D, Tandoi F, Dell Olio D, Stradella D, Cocchis D, Mirabella S, Rizza G, Magistroni P, Moschini P, Ottobrelli A, Amoroso A, Rizzetto M, Salizzoni M, Saracco GM, Romagnoli R. Impact of viral eradication with sofosbuvir-based therapy on the outcome of post-transplant hepatitis C with severe fibrosis. Liver Int. 2017;37:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 15. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 16. | Unique patient populations: Patients WHO develop recurrent HCV infection post-liver transplantation. AASLD-IDSA. Recomm. testing, Manag. Treat. Hepat C. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Morcos PN, Moreira SA, Brennan BJ, Blotner S, Shulman NS, Smith PF. Influence of chronic hepatitis C infection on cytochrome P450 3A4 activity using midazolam as an in vivo probe substrate. Eur J Clin Pharmacol. 2013;69:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Oo YH, Dudley T, Nightingale P, Haydon G, Mutimer D. Tacrolimus and cyclosporin doses and blood levels in hepatitis C and alcoholic liver disease patients after liver transplantation. Liver Transpl. 2008;14:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010;63:47-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Miyagawa-Hayashino A, Haga H, Egawa H, Hayashino Y, Uemoto S, Manabe T. Idiopathic post-transplantation hepatitis following living donor liver transplantation, and significance of autoantibody titre for outcome. Transpl Int. 2009;22:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Syn WK, Nightingale P, Gunson B, Hubscher SG, Neuberger JM. Natural history of unexplained chronic hepatitis after liver transplantation. Liver Transpl. 2007;13:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Cholongitas E, Samonakis D, Patch D, Senzolo M, Burroughs AK, Quaglia A, Dhillon A. Induction of autoimmune hepatitis by pegylated interferon in a liver transplant patient with recurrent hepatitis C virus. Transplantation. 2006;81:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, Schiano TD. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14:861-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Berardi S, Lodato F, Gramenzi A, D'Errico A, Lenzi M, Bontadini A, Morelli MC, Tamè MR, Piscaglia F, Biselli M, Sama C, Mazzella G, Pinna AD, Grazi G, Bernardi M, Andreone P. High incidence of allograft dysfunction in liver transplanted patients treated with pegylated-interferon alpha-2b and ribavirin for hepatitis C recurrence: possible de novo autoimmune hepatitis? Gut. 2007;56:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, Wilkinson J, Adair D, Rakela J, Laskus T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology. 2005;41:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Hedenstierna M, Weiland O, Brass A, Bankwitz D, Behrendt P, Uhnoo I, Aleman S, Cardell K, Fryden A, Norkrans G, Eilard A, Glaumann H, Pietschmann T, Sällberg M, Brenndörfer ED. Long-term follow-up of successful hepatitis C virus therapy: waning immune responses and disappearance of liver disease are consistent with cure. Aliment Pharmacol Ther. 2015;41:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Attar BM, Van Thiel D. A New Twist to a Chronic HCV Infection: Occult Hepatitis C. Gastroenterol Res Pract. 2015;2015:579147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Barril G, Castillo I, Arenas MD, Espinosa M, Garcia-Valdecasas J, Garcia-Fernández N, González-Parra E, Alcazar JM, Sánchez C, Diez-Baylón JC, Martinez P, Bartolomé J, Carreño V. Occult hepatitis C virus infection among hemodialysis patients. J Am Soc Nephrol. 2008;19:2288-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Coppola N, Pisaturo M, Guastafierro S, Tonziello G, Sica A, Sagnelli C, Ferrara MG, Sagnelli E. Absence of occult hepatitis C virus infection in patients under immunosupressive therapy for oncohematological diseases. Hepatology. 2011;54:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Del Bello A, Abravanel F, Alric L, Lavayssiere L, Lhomme S, Bellière J, Izopet J, Kamar N. No evidence of occult hepatitis C or E virus infections in liver-transplant patients with sustained virological response after therapy with direct acting agents. Transpl Infect Dis. 2019;21:e13093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Guerrero RB, Batts KP, Brandhagen DJ, Germer JJ, Perez RG, Persing DH. Effects of formalin fixation and prolonged block storage on detection of hepatitis C virus RNA in liver tissue. Diagn Mol Pathol. 1997;6:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Sun Q, Wang Q, Scott MJ, Billiar TR. Immune Activation in the Liver by Nucleic Acids. J Clin Transl Hepatol. 2016;4:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |